Abstract
Objective
To estimate time to onset of cannabidiol (CBD) treatment effect (seizure reduction and adverse events [AEs]), we conducted post hoc analyses of data from two randomized, placebo‐controlled, Phase 3 trials, GWPCARE3 (NCT02224560) and GWPCARE4 (NCT02224690), of patients with Lennox–Gastaut syndrome.
Methods
Patients received plant‐derived pharmaceutical formulation of highly purified CBD (Epidiolex, 100 mg/ml oral solution) at 10 mg/kg/day (CBD10; GWPCARE3) or 20 mg/kg/day (CBD20; both trials) or placebo for 14 weeks. Treatment started at 2.5 mg/kg/day for all groups and reached 10 mg/kg/day on Day 7 and 20 mg/kg/day (CBD20 and matching placebo only) on Day 11. Percentage change from baseline in drop seizure frequency was calculated by cumulative day (i.e., including all previous days). Time to onset and resolution of AEs were evaluated.
Results
Overall, 235 patients received CBD (CBD10 [GWPCARE3 only], n = 67; CBD20 [pooled GWPCARE3&4], n = 168) and 161 received placebo. Mean (range) age was 15.3 years (2.6–48.0). Patients had previously discontinued a median (range) of six (0–28) antiepileptic drugs (AEDs) and were currently taking a median of three (0–5) AEDs. Differences in drop seizure reduction between placebo and CBD emerged during the titration period and became nominally significant by Day 6 (p = .008) for pooled CBD treatment groups. Separation between placebo and CBD in ≥50% responder rate emerged by Day 6. Onset of the first reported AE occurred during the titration period in 45% of patients (CBD10, 46%; CBD20, 52%; placebo, 38%). In patients with AEs, resolution occurred within 4 weeks of onset in 53% of placebo and 39% of CBD patients and by end of study in 63% of placebo and 61% of CBD patients.
Significance
Treatment effect (efficacy and AEs) of CBD may occur within 1 week of starting treatment. Although AEs lasted longer for CBD than placebo, most resolved within the 14‐week period.
Keywords: antiepileptic drug, antiseizure medication, childhood onset epilepsy, drop seizures, treatment‐resistant epilepsy
Key Points.
This was an post hoc analysis of data from two trials of CBD versus placebo in patients with LGS, evaluating time to onset of treatment effect
Patients were randomized to receive CBD at 10 or 20 mg/kg/day, or matching placebo; all patients reached the 10‐mg/kg/day dose on Day 7
Significantly greater reduction in drop seizures with CBD versus placebo emerged as early as Day 6 and was maintained throughout the study
Overall, 81% of patients had an adverse event, and 45% of patients had onset during the 2‐week titration period
In patients with adverse events, resolution occurred within 4 weeks of onset in 44% of patients and by the end of treatment in 62%
1. INTRODUCTION
Lennox–Gastaut syndrome (LGS) is a severe developmental epileptic encephalopathy, with a reported prevalence rate of 1%–5% of all childhood epilepsies. 1 , 2 , 3 , 4 , 5 The onset is typically between the ages of 1 and 7 years, most commonly between 3 and 5 years. 6 , 7 Seizures associated with LGS are treatment resistant in more than 90% of cases. 6 The disease is characterized by multiple types of seizures, including tonic, atonic, and atypical absence seizures, and an abnormal electroencephalogram (EEG) consisting primarily of interictal diffuse, slow spike–wave complexes at less than 2.5 Hz, occurring during wakefulness, and fast rhythms in sleep. 6 , 7 Cognitive impairment is present in an estimated 20%–60% of patients at the time of diagnosis and becomes more apparent over time, with more than 90% of patients showing moderate to severe cognitive impairment and intellectual disability by adulthood. 8 , 9 Additionally, many patients may develop psychiatric and behavioral disorders as the disease progresses. 10 Patients with LGS have a high potential for injury caused by drop attacks from tonic and atonic seizures, 3 and the generalized tonic–clonic seizures (present in LGS) are associated with an increased risk of sudden unexpected death in epilepsy. 11
Treatments for LGS include antiepileptic drugs (AEDs) and nonpharmacologic options such as ketogenic diet, resective surgery, vagus nerve stimulation, and corpus callosotomy. 7 Antiepileptic medications approved in the United States and Europe for treatment of LGS are lamotrigine, rufinamide, topiramate, clobazam, clonazepam, felbamate, and most recently, cannabidiol (CBD). 7 , 12 , 13 , 14 , 15 , 16 Although not approved for LGS, valproate, based on clinical experience, is one of the first AEDs prescribed for epilepsy characterized by generalized or multiple seizure types. 7 , 12 , 13 , 14
Highly purified CBD demonstrated efficacy against seizures associated with LGS, Dravet syndrome (DS), and tuberous sclerosis complex (TSC) when given in addition to patients' current AEDs in five randomized placebo‐controlled trials. 15 , 16 , 17 , 18 , 19 CBD is approved as Epidiolex in the United States for treatment of seizures associated with LGS, DS, or TSC in patients ≥1 year of age and as Epidyolex in the European Union in conjunction with clobazam for LGS and DS in patients ≥2 years of age. 20 , 21
CBD treatment produced significantly greater reductions than placebo in seizures associated with LGS when evaluated for the full treatment period in two 14‐week (2‐week titration and 12‐week maintenance periods), randomized, double‐blind, placebo‐controlled, Phase 3 trials (GWPCARE3, NCT02224560; GWPCARE4, NCT02224690). 15 , 16 Although the superiority of CBD over placebo in reduction in drop seizures was established during the first 4 weeks of the maintenance period, the precise timing of the onset of CBD treatment effect, including the titration period, was not assessed. Here, we used the combined data from GWPCARE3 and GWPCARE4 to evaluate cumulative seizure reduction and incidence of adverse events (AEs) for each cumulative day of treatment, starting with the first day of titration. This provides more precise timing of the onset of CBD treatment effect.
2. MATERIALS AND METHODS
GWPCARE3 and GWPCARE4 were international, randomized, double‐blind, placebo‐controlled, Phase 3 trials. Trial design and patient eligibility criteria were published previously. 15 , 16 Briefly, the trials consisted of a 4‐week baseline period followed by a 14‐week treatment time, which included 2‐week dose escalation (titration) and 12‐week treatment maintenance periods. Treatment period was followed by a 10‐day tapering phase. Patients who completed the blinded study were allowed to transition to an open‐label extension study. 22
Patients with clinically confirmed LGS were eligible to enroll if they were 2–55 years of age, had an EEG showing a pattern of slow (<3.0 Hz) spike‐and‐wave complexes, and had at least two types of generalized seizures, including drop seizures, for at least 6 months. Patients must have been taking 1–4 AEDs at a stable dose for at least 4 weeks before screening and must have had at least two drop seizures per week during the baseline period.
Patients were randomly assigned to receive either a pharmaceutical formulation of highly purified CBD derived from Cannabis sativa L. plant in oral solution (100 mg/ml; Epidiolex in the United States and Epidyolex in the European Union; GW Research Ltd.) or matching placebo. Patients received either 10 or 20 mg/kg/day of add‐on CBD in GWPCARE3 or 20 mg/kg/day in GWPCARE4. CBD, administered twice daily in equally divided doses, started at 2.5 mg/kg/day and was titrated up to 10 mg/kg/day by Day 7 and to 20 mg/kg/day by Day 11 of the titration period (Table 1).
TABLE 1.
Titration schedule
| Total CBD dose received per day, mg/kg a | ||
|---|---|---|
| Day | 10 mg/kg/day target dose | 20 mg/kg/day target dose |
| 1 | 2.5 | 2.5 |
| 2 | 2.5 | 2.5 |
| 3 | 5.0 | 5.0 |
| 4 | 5.0 | 5.0 |
| 5 | 7.5 | 7.5 |
| 6 | 7.5 | 7.5 |
| 7 | 10.0 | 10.0 |
| 8 | 10.0 | 10.0 |
| 9 | 10.0 | 15.0 |
| 10 | 10.0 | 15.0 |
| 11–14 | 10.0 | 20.0 |
Abbreviation: CBD, cannabidiol.
The study medication was taken twice daily in equally divided doses.
Patients or their caregivers recorded the type and number of seizures, including drop seizures, daily using an interactive voice‐response system. Drop seizures, the seizure type used for the primary endpoint of the trials, were defined as an attack or spell (atonic, tonic, or tonic–clonic) involving the entire body, trunk, or head that led or could have led to a fall, injury, slumping in a chair, or the patient's head hitting a surface; on average, 59% of patients' seizures in these studies were drop seizures. Antiseizure effect of CBD was assessed by evaluating reduction from baseline in drop seizure frequency compared with placebo. Median percentage change from baseline in drop and total seizures per 28 days was calculated for the 2‐week titration period and for Weeks 1–4, 5–8, and 9–12 of the maintenance period. To determine a more precise onset of antiseizure effect, starting with Day 1 of treatment, median percentage reductions in cumulative drop seizure frequency (i.e., including all previous treatment days) for each day of the treatment period were calculated. Percentage of patients with ≥50% reduction from baseline in drop seizure frequency during the treatment period by cumulative day was also measured. Because evaluation of treatment effect by day was an post hoc analysis, it is subject to multiplicity; therefore, nominal p values are reported only for the primary endpoint of percentage reduction in drop seizure frequency.
Incidences of AEs by time to onset during the 2‐week titration period and for Weeks 1–4, 5–8, and 9–12 of the maintenance period were assessed. The time to first onset of an AE was calculated as the start date of the AE minus date of first dose of study medication plus 1. Time‐to‐first‐event analysis by day was also conducted for the most frequent AEs of special interest. Time‐to‐AE‐resolution analysis summarized incidence of AEs that resolved within 4 weeks or after 4 weeks of onset. If any of the AEs did not resolve, then the AE was categorized as ongoing. The time to AE resolution was calculated as the stop date of an AE minus start date of the AE plus 1.
Efficacy and safety data for CBD 10 mg/kg/day was taken from GWPCARE3 and pooled across GWPCARE3 and GWPCARE4 for placebo and CBD 20 mg/kg/day; effect of CBD 10 mg/kg/day was not evaluated in GWPCARE4.
3. RESULTS
3.1. Patients
Of the total 396 patients enrolled in the two trials, 235 patients were randomized to receive CBD, either 10 or 20 mg/kg/day, and 161 to receive placebo (Figure S1). Two hundred ten patients in the CBD group and 158 in the placebo group completed the studies. Overall, 28 patients (7.1%) withdrew from the studies (CBD, 25 [10.6%]; placebo, 3 [1.9%]); 15 of these patients (53.6%) had AEs as the primary reason for withdrawal (13 [52.0%] in the CBD group and 2 [66.7%] in the placebo group). Five patients in the CBD group and none in the placebo group met the withdrawal criteria. Four patients receiving CBD and one patient receiving placebo were withdrawn by subject/caregivers or the principal investigator. Two patients in the CBD group withdrew for other reasons. One patient taking CBD withdrew because of a protocol deviation. The majority of patients (366 [99.5%]) who completed the studies entered the open‐label extension trial of CBD in LGS.
The mean age of patients was 15.5 years, and 31.6% of them were adults. Baseline characteristics were consistent between the CBD and placebo groups (Table 2). Overall, the median (minimum, maximum) number of drop seizure frequency for the 28‐day period at baseline was 80.0 (8.7, 7494.0), and the total seizure frequency was 169.7 (11.0, 13 607.0). The most frequently reported seizure subtypes were tonic (308 patients [77.8%]), atonic (237 [59.8%]), and tonic–clonic seizures (214 [54.0%]) across all treatments. Patients had discontinued a median (minimum, maximum) of six (0, 28) AEDs that had failed to control seizures and were currently receiving a median (minimum, maximum) of three (0, 5) AEDs at baseline. Clobazam (49.0% of patients) and valproate (38.9%) were the most common concomitant AEDs.
TABLE 2.
Patient demographics and baseline characteristics
| Parameter | Placebo, n = 161 | All CBD doses, n = 235 |
|---|---|---|
| Age, years | ||
| Mean (min, max) | 15.3 (2.6, 45.1) | 15.6 (2.6, 48.0) |
| Age group, n (%) | ||
| 2–5 years | 21 (13.0) | 28 (11.9) |
| 6–11 years | 51 (31.7) | 75 (31.9) |
| 12–17 years | 38 (23.6) | 58 (24.7) |
| 18–55 years | 51 (31.7) | 74 (31.5) |
| Sex, n (%) | ||
| Male | 87 (54.0) | 130 (55.3) |
| Female | 74 (46.0) | 105 (44.7) |
| Race, n (%) | ||
| White | 148 (91.9) | 204 (86.8) |
| Other | 13 (8.1) | 31 (13.2) |
| Weight at baseline, kg | ||
| Mean (min, max) | 44.2 (11.9, 112.6) | 42.6 (10.8, 140.2) |
| Region | ||
| United States | 128 (79.5) | 181 (77.0) |
| Rest of the world | 33 (20.5) | 54 (23.0) |
| Drop seizures per 28 days | ||
| Median (min, max) | 79.0 (8.7, 3174.6) | 81.0 (10.3, 7494.0) |
| Total seizures per 28 days | ||
| Median (min, max) | 177.7 (11.0, 4357.4) | 167.2 (13.0, 13 607.0) |
| Seizure subtypes, n (%) | ||
| Tonic | 122 (75.8) | 186 (79.1) |
| Atonic | 100 (62.1) | 137 (58.3) |
| Tonic–clonic | 87 (54.0) | 127 (54.0) |
| Number of failed AEDs | ||
| Median (min, max) | 6 (0, 28) | 6 (0, 21) |
| Number of current AEDs | ||
| Median (min, max) | 3 (1, 5) | 3 (0, 5) |
| Current AEDs [>20%], n (%) | ||
| Clobazam | 79 (49.1) | 115 (48.9) |
| Valproate | 63 (39.1) | 91 (38.7) |
| Lamotrigine | 56 (34.8) | 75 (31.9) |
| Levetiracetam | 58 (36.0) | 69 (29.4) |
| Rufinamide | 41 (25.5) | 70 (29.8) |
Abbreviations: AED, antiepileptic drug; CBD, cannabidiol; max, maximum; min, minimum.
3.2. Efficacy
During the trials, greater reduction in the median percentage change from baseline in drop and total seizure frequency for CBD treatment compared with placebo was first observed during the titration period (Figure S2). In the analysis by cumulative days, clear separation between placebo and CBD in reduction in drop seizures was observed as early as Day 6 (nominal p = .008; Figure 1A), when all patients were at 7.5‐mg/kg/day dosage. When the analysis was repeated in the cohorts taking CBD with and without clobazam, a clear separation between placebo and CBD emerged during the first week of the titration period in both subgroups (Figure S3), with the magnitude of reduction in drop seizures greater in patients taking concomitant clobazam than in those not on clobazam. Overall reduction in drop seizure frequency by cumulative day was maintained throughout the treatment period (Figure S4A).
FIGURE 1.
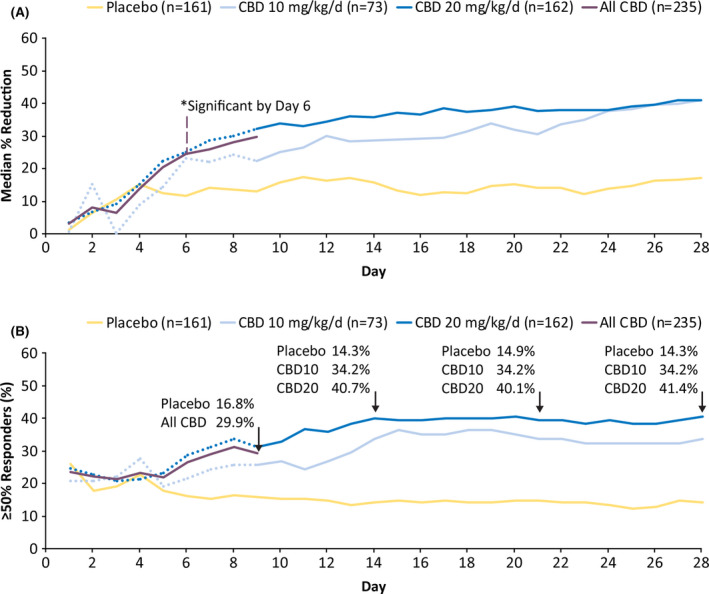
Efficacy outcomes: cumulative percentage reduction from baseline in drop seizure frequency by day (A) and percentage of patients with ≥50% reduction in drop seizure frequency by day (B). *Difference between pooled cannabidiol (CBD) groups and placebo became nominally significant on Day 6 at p = .008. The dotted line for each dose group represents the data until Day 9, when all patients were receiving the same dose of study drug, reflecting small variations in response not attributable to difference in dosage. The solid purple line represents all CBD patients until Day 9, at which point patients in CBD10 remained on 10 mg/kg/day and those in CBD20 continued titration up to 20 mg/kg/day. CBD10, cannabidiol 10 mg/kg/day; CBD20, cannabidiol 20 mg/kg/day
Overall, the median percentage reduction in drop seizure frequency per 28 days during the full treatment period was 37.2% (a reduction from a median of 86.9 seizures per 28 days at baseline to 50.0 per 28 days) for CBD 10 mg/kg/day (GWPCARE3 only), 42.8% (reduction from 78.1 seizures per 28 days to 38.4 seizures per 28 days) for CBD 20 mg/kg/day, and 20.1% (reduction from 79.0 seizures per 28 days to 63.2 seizures per 28 days) for placebo. The median percentage reduction in total seizure frequency per 28 days during the full treatment period was 36.4% (a decrease from a median of 165.0 seizures per 28 days at baseline to 76.1 per 28 days) for CBD 10 mg/kg/day, 38.7% (reduction from 167.6 seizures per 28 days to 85.6 seizures per 28 days) for CBD 20 mg/kg/day, and 17.0% (reduction from 177.7 seizures per 28 days to 129.0 seizures per 28 days) for placebo.
In the analysis by cumulative day of patients with ≥50% reduction in drop seizure frequency, clear separation between placebo and CBD was observed as early as the first week (Day 6) of treatment (Figure 1B). The proportion of patients taking CBD with ≥50% reduction in drop seizure frequency increased during the first 28 days of treatment and was then maintained throughout the study (Figure S4B).
3.3. Safety
Treatment‐emergent AEs, across the two studies, were reported in 114 (70.8%) patients taking placebo, 56 (83.6%) taking CBD 10 mg/kg/day, and 151 (89.9%) taking CBD 20 mg/kg/day (Table S1). The most commonly reported AEs were somnolence (16.2%), decreased appetite (12.9%), and diarrhea (12.1%). Somnolence was more frequently reported in patients taking concomitant clobazam compared with those who were not in all three treatment groups (placebo, 8 [10.0%] vs. 4 [4.9%]; CBD 10 mg/kg/day, 11 [31.4%] vs. 3 [9.4%]; CBD 20 mg/kg/day, 24 [30.4%] vs. 14 [15.7%]). Serious AEs were reported in 14.6% of patients. AEs were listed as one of the reasons for treatment discontinuation in 21 patients (5.3%). There was one death reported during the trial (acute respiratory distress syndrome in the CBD 20‐mg/kg/day group), which was deemed not treatment related by the investigator.
Most patients reported AE onset early in the treatment, during the titration period in 180 patients (45.5%) and during the first 4 weeks of the maintenance period in 95 patients (24.0%; Figure 2). In the time‐to‐first‐event analysis of the most common AEs, clear separation in onset of somnolence or sedation between placebo and CBD emerged by Day 2 of titration, when patients were receiving the 2.5‐mg/kg/day dose (Figure 3A); most patients reported the first occurrence of decreased appetite (Figure 3B) within 20 days and the first occurrence of diarrhea (Figure 3C) within 40 days of starting CBD or placebo treatment.
FIGURE 2.
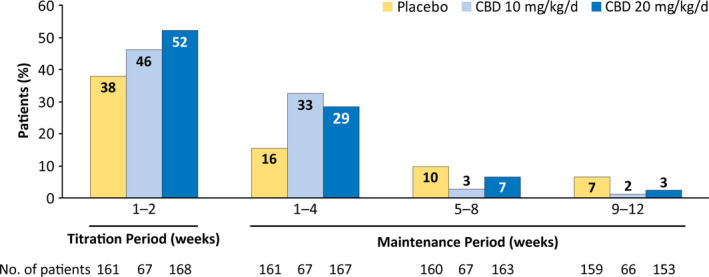
Adverse events by time of onset. If a patient had multiple occurrences of an adverse event (AE), then the AE was counted once for the first occurrence only. Percentage of patients was calculated based on the number of patients in the safety analysis set who had a visit or follow‐up call within each time period. One patient in the placebo group first experienced an AE after the 14‐week treatment period. CBD, cannabidiol
FIGURE 3.
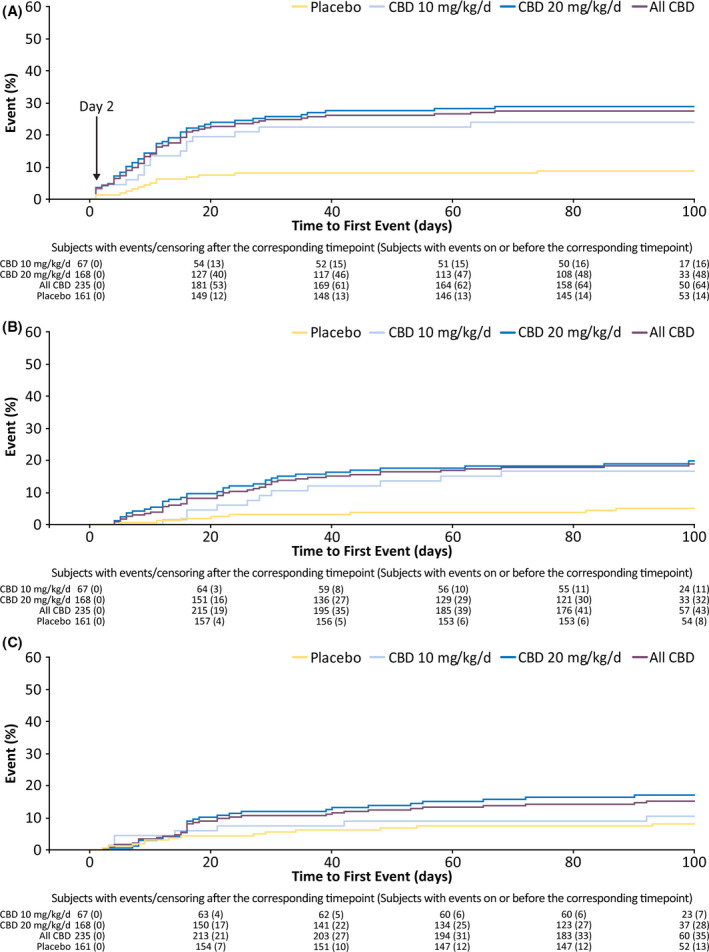
Time to first occurrence of somnolence or sedation (A), decreased appetite (B), and diarrhea (C) by day. The arrow in panel A shows the time (Day 2) when a clear separation in the time to first occurrence between placebo and cannabidiol (CBD) was observed. During the first 2 days of treatment, 10 patients taking CBD and one patient taking placebo had a treatment‐emergent adverse event of somnolence; seven of 10 patients on CBD were also taking clobazam. No incidence of sedation was reported during Days 1–2
Overall, AEs resolved within 4 weeks of onset in more than 40% of patients and by the end of the studies in more than 60% of patients who experienced an AE (Table 3). The most common AE, somnolence, resolved within 4 weeks in 67% of patients on placebo, 64% of patients on CBD10, and 37% of patients on CBD20. For combined CBD groups, decreased appetite and diarrhea events resolved within 4 weeks in 35% and 60% of patients, respectively. Diarrhea resolved sooner in patients taking CBD10 than CBD20; resolution of decreased appetite was not notably affected by CBD dosage. These three most common AEs resolved by end of study in more than 70% of patients (Table 3).
TABLE 3.
Time to resolution for all AEs and most common AEs
| Placebo, n = 161 | CBD 10 mg/kg/day, n = 67 | CBD 20 mg/kg/day, n = 168 | |
|---|---|---|---|
| All AEs, n (%) a | 114 (70.8) | 56 (83.6) | 151 (89.9) |
| Within 4 weeks | 60/114 (52.6) | 22/56 (39.3) | 59/151 (39.1) |
| After 4 weeks, by end of study | 12/114 (10.5) | 10/56 (17.9) | 36/151 (23.8) |
| Ongoing | 42/114 (36.8) | 24/56 (42.9) | 56/151 (37.1) |
| Somnolence, n (%) | 12 (7.5) | 14 (20.9) | 38 (22.6) |
| Within 4 weeks | 8/12 (66.7) | 9/14 (64.3) | 14/38 (36.8) |
| After 4 weeks, by end of study | 3/12 (25.0) | 1/14 (7.1) | 17/38 (44.7) |
| Ongoing | 1/12 (8.3) | 4/14 (28.6) | 7/38 (18.4) |
| Decreased appetite, n (%) | 8 (5.0) | 11 (16.4) | 32 (19.0) |
| Within 4 weeks | 7/8 (87.5) | 3/11 (27.3) | 12/32 (37.5) |
| After 4 weeks, by end of study | 0 | 4/11 (36.4) | 11/32 (34.4) |
| Ongoing | 1/8 (12.5) | 4/11 (36.4) | 9/32 (28.1) |
| Diarrhea, n (%) | 13 (8.1) | 7 (10.4) | 28 (16.7) |
| Within 4 weeks | 11/13 (84.6) | 6/7 (85.7) | 15/28 (53.6) |
| After 4 weeks, by end of study | 0 | 1/7 (14.3) | 5/28 (17.9) |
| Ongoing | 2/13 (15.4) | 0 | 8/28 (28.6) |
Abbreviations: AE, adverse event; CBD, cannabidiol.
For patients with multiple AEs, the longest time to resolution was used.
Measurement of transaminases was first performed on Day 15 of treatment (i.e., after the titration period) and then on Days 29, 57, and 99 (end of treatment) and at safety follow‐up. Increase to more than three times the upper limit of normal in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels occurred in three of 67 (4.5%) patients in the CBD 10‐mg/kg/day group, 31 of 168 (18.5%) in the CBD 20‐mg/kg/day group, and one of 161 (.6%) in the placebo group (Table S2); 26 (74.3%) of these 35 patients were on concomitant valproic acid or its derivatives, and 16 (45.7%) were on clobazam. All elevations in patients taking CBD 10 mg/kg/day and most in those taking CBD 20 mg/kg/day (19 [61.3%]) occurred within 30 days of treatment initiation. No patient met the standard criteria for severe drug‐induced liver injury (Hy's law). Elevated ALT/AST levels resolved in all patients during the 14‐week trials.
4. DISCUSSION
Results from this post hoc analysis of efficacy and safety data from the two randomized, placebo‐controlled trials of add‐on CBD in patients with LGS suggest that the CBD treatment effect (seizure reduction and incidence of AEs) may occur as early as during the first week of treatment. Following multiple twice‐daily dosing of CBD, plasma levels reach steady state after approximately 2 days. 23 Separation between CBD and placebo groups in the median percentage reduction in drop seizures and ≥50% responder rate emerged within the first 6–8 days of the titration period, when patients in this study were just reaching the 10‐mg/kg/day dose (Table 1), suggesting that a treatment effect may emerge with doses lower than 10 mg/kg/day.
Bidirectional pharmacokinetic drug–drug interaction has been previously demonstrated between CBD and clobazam, which leads to increased exposure in each other's active metabolite and a potential synergistic effect 24 ; therefore, to explore whether concomitant clobazam may be required for the early onset of effect, the analysis by cumulative day was repeated in patients taking CBD with and without clobazam. Similar to the overall population, clear separation between CBD and placebo was evident within the first week of titration in both subgroups, suggesting that the early onset of effect occurs regardless of clobazam. However, because these trials were not designed to compare patients on versus off clobazam, comparisons between the two subgroups should be interpreted with caution. Reliability of these results is further limited because of the reduced sample size in the subgroups and the limited days of data during the titration period, compared with the full 14‐week treatment period used in estimating the overall treatment effect. The clobazam‐independent treatment effect of CBD has been previously demonstrated in several meta‐analyses. 25 , 26 These analyses had a larger sample size and evaluated response over a longer period than in the analysis by cumulative day, providing a more reliable estimate of treatment effect in the on‐ versus off‐clobazam subgroup. Drug–drug interactions between CBD and other AEDs such as valproate and stiripentol are less notable and have been previously reviewed. 27 Although we could not investigate the effect of other AEDs on the onset of CBD treatment effect in this study, it is possible that interactions with other medications could affect the onset of both efficacy and AEs.
In addition to the interactions with other AEDs, which may affect the timing of CBD treatment effect, there is an established food effect on CBD exposure, in which taking CBD with a high‐fat meal leads to a four‐ to five‐fold increase in exposure, with no notable effect on Tmax or terminal half‐life. 23 CBD exposure was also shown to increase with a low‐fat/calorie meal or whole milk, although to a lesser extent. 28 Although patients enrolled in the GWPCARE studies were instructed to take CBD consistently with or without food, whether they chose to take it fed or fasted was not recorded. Presumably, taking CBD with food could lead to a higher exposure sooner, and thus may translate to a more rapid onset of effect.
The early onset of antiseizure effect is of particular importance, as a faster onset would reduce cumulative seizure burden and may reduce the number of accidents associated with drop attacks. Although on a population level, the majority of effects appeared to occur by 4 weeks, on an individual patient level, the maximum time to observe an effect may depend on differences in the underlying epilepsy and dose optimization of CBD and background AEDs. Future studies and/or real‐world experience is required to better understand the full effect of patient variability on time to onset of response with this new AED.
Onset of AEs further support a relatively fast CBD treatment effect; more than 50% of patients receiving CBD reported AEs within the 2‐week titration period. For the three most common AEs, somnolence, decreased appetite, and diarrhea, the slope of time‐to‐first‐event graphs was similar for the two CBD dose groups and steeper than placebo, suggesting that the AEs were related to CBD. Furthermore, somnolence events started occurring in the CBD groups as early as 2 days into the titration period, when patients were at the 2.5‐mg/kg/day dosage; concomitant clobazam use may have contributed to these early events, as 70% of these patients were also taking clobazam. In clinical practice, it may be important to individualize the titration schedule for patients, because a faster titration and high dosage could lead to higher rates of AEs.
The majority of AEs (60%) resolved during the study, with approximately 40% resolving within 4 weeks of onset. Some AEs, such as somnolence, may have occurred early, when patients were adapting to the treatment effect, and resolved as patients became habituated, and some AEs may not have been treatment related and thus resolved spontaneously. Additionally, it is possible that because most (>80%) of the AEs reported in any treatment group were of mild or moderate severity, they resolved quickly or were mild enough in severity that they were not reported later during the trial. Other AEDs have also shown early onset of AEs; the four top perampanel treatment‐emergent AEs occurred more frequently during the 6‐week titration period (the full treatment period was 19 weeks) among patients with refractory partial seizures enrolled in three randomized, placebo‐controlled, Phase 3 trials. 29 Overall, CBD was generally well tolerated with AEs that were mostly mild or moderate in severity and self‐limited, which is consistent with the safety profile of other AED treatments for LGS. 30 , 31 , 32 , 33 , 34
The precise timing for the elevation in ALT/AST levels could not be determined, because testing did not start until Day 15; however, among patients who had an elevation in ALT/AST levels, all patients taking CBD 10 mg/kg/day and 61% of those taking CBD 20 mg/kg/day experienced onset during the first 30 days of treatment. In these trials, although different titration schemes were not assessed, theoretically, the relatively fast titration (2.5‐mg/kg/day increase every 2 days) could have contributed to the higher incidence of liver enzyme elevations in patients assigned to CBD 20 mg/kg/day; a slower titration than that used in these trials is recommended in the prescribing information. 20 All ALT/AST elevations resolved, 60% of them without treatment discontinuation.
There are limited data available for time to onset of efficacy for other AEDs in LGS; data availability for some of the AEDs may be limited, because the patient paper diaries may not have been saved in the database. In one randomized, double‐blind, placebo‐controlled trial of patients ≥13 years of age with a diagnosis of epilepsy with primary generalized tonic–clonic seizures, adjunctive lamotrigine treatment demonstrated a significant reduction in seizure frequency and ≥50% reduction in seizure frequency that became significantly different from placebo by Day 8 of the treatment escalation phase. 35 In another analysis of pooled data from three randomized, placebo‐controlled, Phase 3 trials of brivaracetam in patients with well‐characterized focal seizures, a significantly higher proportion of patients taking brivaracetam achieved sustained ≥50% responder status on treatment Day 1 than patients taking placebo. 36 However, in this analysis, only patients who had ≥50% reduction in seizure frequency that was sustained throughout the study period were included. Thus, it is possible that the antiseizure effect of many AEDs may be observed while the drug is being titrated up and before the target dose is reached. Titration phase for most AEDs approved for LGS is 2–3 weeks, except lamotrigine, which is titrated over a period of 6 weeks. 30 , 31 , 32 , 33 , 37
Our study has several limitations. Because this was an post hoc analysis, the interpretability and generalizability of the results are limited. Although the primary endpoint effect size at early time points was nominally significant, it has not been replicated in a prospectively designed trial assessing early onset of action. However, evidence for an early treatment effect has been observed in trials of CBD in DS and TSC, supporting the robustness of the results here. 38 , 39 Interpatient variability may mean early onset is not evident in some patients. The onset of spontaneous AE reporting assigned to the day of treatment has limitations but likely underestimates the treatment‐related reports.
In conclusion, in this post hoc analysis of the pooled efficacy and safety data (CBD 10 mg/kg/day was tested only in GWPCARE3) from the two randomized, placebo‐controlled, Phase 3 trials of add‐on CBD in patients with LGS, we demonstrated that both seizure reductions and onset of AEs emerged early during the first week of the titration period. However, this analysis does not exclude the effect of the treatment (seizure reduction or AEs) occurring later in some patients. Results of the pooled analysis concur with the individual trials and further confirm the effectiveness of CBD in reducing seizures associated with LGS with an acceptable safety profile.
CONFLICT OF INTEREST
M.P. has received research grants from SK Life Science, Xenon Pharmaceuticals, Epilepsy Foundation, and GW Pharmaceuticals, and has served as a consultant for SK Life Science, GW Pharmaceuticals, and Neurelis. H.B. was an investigator on trials sponsored by GW Research. M.W. received funds from GW Pharmaceuticals associated with conducting this study. J.H.C.'s institution received grants from Zogenix, Marinius, and Vitaflo and other support from GW Pharmaceuticals, Nutricia, and Biomarin. Her research is supported by the National Institute of Health Research Biomedical Research Centre at Great Ormond Street Hospital. E.W. has participated on advisory boards for Biomarin and Sunovian and has received personal fees from UpToDate. E.M. has served as a consultant for Eisai Pharma and Cydan, and as a study investigator for GW Pharmaceuticals. M.M.‐B. has served as a study investigator for GW Pharmaceuticals. V.V. has received grants from Eisai and UCB, and has participated on advisory boards for Eisai, UCB, Bial, Esteve, GW Pharmaceuticals, Arvelle Therapeutics, and Novartis. D.C. is a full‐time employee of GW Research. V.K. is a full‐time employee of Greenwich Biosciences and owns shares in the company. K.V. was an employee of Greenwich Biosciences at the time this study was conducted.
AUTHOR CONTRIBUTIONS
All authors provided substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; and provided final approval of the version to be published.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants, their families, and the study sites that participated in these trials. Medical writing and editorial support were provided to authors by Ritu Pathak, and Dena McWain of Ashfield MedComms, Middletown, Connecticut, an Ashfield Health company, and funded by Greenwich Biosciences, Inc.
Funding information
This study was sponsored by GW Research Ltd., Cambridge, UK.
REFERENCES
- 1. Trevathan E, Murphy CC, Yeargin‐Allsopp M. Prevalence and descriptive epidemiology of Lennox‐Gastaut syndrome among Atlanta children. Epilepsia. 1997;38:1283–8. [DOI] [PubMed] [Google Scholar]
- 2. Camfield P, Camfield C. Long‐term prognosis for symptomatic (secondarily) generalized epilepsies: a population‐based study. Epilepsia. 2007;48:1128–32. [DOI] [PubMed] [Google Scholar]
- 3. Camfield PR. Definition and natural history of Lennox‐Gastaut syndrome. Epilepsia. 2011;52:3–9. [DOI] [PubMed] [Google Scholar]
- 4. Wirrell EC, Grossardt BR, Wong‐Kisiel LC, Nickels KC. Incidence and classification of new‐onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: a population‐based study. Epilepsy Res. 2011;95:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg AT, Levy SR, Testa FM. Evolution and course of early life developmental encephalopathic epilepsies: focus on Lennox‐Gastaut syndrome. Epilepsia. 2018;59:2096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostendorf AP, Ng YT. Treatment‐resistant Lennox‐Gastaut syndrome: therapeutic trends, challenges and future directions. Neuropsychiatr Dis Treat. 2017;13:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cross JH, Auvin S, Falip M, Striano P, Arzimanoglou A. Expert opinion on the management of Lennox‐Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arzimanoglou A, French J, Blume WT, Cross JH, Ernst JP, Feucht M, et al. Lennox‐Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8:82–93. [DOI] [PubMed] [Google Scholar]
- 9. Kerr M, Kluger G, Philip S. Evolution and management of Lennox‐Gastaut syndrome through adolescence and into adulthood: are seizures always the primary issue? Epileptic Disord. 2011;13(Suppl 1):S15–26. [DOI] [PubMed] [Google Scholar]
- 10. Glauser TA. Following catastrophic epilepsy patients from childhood to adulthood. Epilepsia. 2004;45(Suppl 5):23–6. [DOI] [PubMed] [Google Scholar]
- 11. Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors. Neurology. 2017;88:1674–80. [DOI] [PubMed] [Google Scholar]
- 12. Doring JH, Lampert A, Hoffmann GF, Ries M. Thirty years of orphan drug legislation and the development of drugs to treat rare seizure conditions: a cross sectional analysis. PLoS One. 2016;11:e0161660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancock EC, Cross JH. Treatment of Lennox‐Gastaut syndrome. Cochrane Database Syst Rev. 2013;(2):CD003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodie MJ, Chung S, Wade A, Quelen C, Guiraud‐Diawara A, François C, et al. Clobazam and clonazepam use in epilepsy: results from a UK database incident user cohort study. Epilepsy Res. 2016;123:68–74. [DOI] [PubMed] [Google Scholar]
- 15. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 16. Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 17. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 18. Miller I, Scheffer IE, Gunning B, Sanchez‐Carpintero R, Gil‐Nagel A, Perry MS, et al. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add‐on cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurol. 2021:78(3):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. EPIDIOLEX (cannabidiol) oral solution [prescribing information]. Carlsbad, CA: Greenwich Biosciences. October 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210365s008lbl.pdf. Accessed 18 Nov 2020.
- 21. Epidyolex (cannabidiol) oral solution [summary of product characteristics]. Amersfoort, the Netherlands: GW Pharma (International). October 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex#product‐information‐section. Accessed 23 Jan 2020.
- 22. Thiele E, Marsh E, Mazurkiewicz‐Beldzinska M, Halford JJ, Gunning B, Devinsky O, et al. Cannabidiol in patients with Lennox‐Gastaut syndrome: interim analysis of an open‐label extension study. Epilepsia. 2019;60:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8:1009–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta‐analysis. Epilepsia. 2020;61:1090–8. [DOI] [PubMed] [Google Scholar]
- 26. Devinsky O, Thiele EA, Wright S, Checketts D, Morrison G, Dunayevich E, et al. Cannabidiol efficacy independent of clobazam: meta‐analysis of four randomized controlled trials. Acta Neurol Scand. 2020;142:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patsalos PN, Szaflarski JP, Gidal B, VanLandingham K, Critchley D, Morrison G. Clinical implications of trials investigating drug‐drug interactions between cannabidiol and enzyme inducers or inhibitors or common antiseizure drugs. Epilepsia. 2020;61:1854–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crockett J, Critchley D, Tayo B, Berwaerts J, Morrison G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia. 2020;61:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ko D, Yang H, Williams B, Xing D, Laurenza A. Perampanel in the treatment of partial seizures: time to onset and duration of most common adverse events from pooled phase III and extension studies. Epilepsy Behav. 2015;48:45–52. [DOI] [PubMed] [Google Scholar]
- 30. Felbamate Study Group in Lennox‐Gastaut Syndrome . Efficacy of felbamate in childhood epileptic encephalopathy (Lennox‐Gastaut syndrome). N Engl J Med. 1993;328:29–33. [DOI] [PubMed] [Google Scholar]
- 31. Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox‐Gastaut syndrome. Lamictal Lennox‐Gastaut Study Group. N Engl J Med. 1997;337:1807–12. [DOI] [PubMed] [Google Scholar]
- 32. Sachdeo RC, Glauser TA, Ritter F, Reife R, Lim P, Pledger G. A double‐blind, randomized trial of topiramate in Lennox‐Gastaut syndrome. Topiramate YL Study Group. Neurology. 1999;52:1882–7. [DOI] [PubMed] [Google Scholar]
- 33. Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox‐Gastaut syndrome. Neurology. 2008;70:1950–8. [DOI] [PubMed] [Google Scholar]
- 34. Conry JA, Ng Y‐T, Paolicchi JM, Kernitsky L, Mitchell WG, Ritter FJ, et al. Clobazam in the treatment of Lennox‐Gastaut syndrome. Epilepsia. 2009;50:1158–66. [DOI] [PubMed] [Google Scholar]
- 35. Biton V, Di Memmo J, Shukla R, Lee YY, Poverennova I, Demchenko V, et al. Adjunctive lamotrigine XR for primary generalized tonic‐clonic seizures in a randomized, placebo‐controlled study. Epilepsy Behav. 2010;19:352–8. [DOI] [PubMed] [Google Scholar]
- 36. Klein P, Johnson ME, Schiemann J, Whitesides J. Time to onset of sustained ≥50% responder status in patients with focal (partial‐onset) seizures in three phase III studies of adjunctive brivaracetam treatment. Epilepsia. 2017;58:e21–5. [DOI] [PubMed] [Google Scholar]
- 37. Ng YT, Conry JA, Drummond R, Stolle J, Weinberg MA. Randomized, phase III study results of clobazam in Lennox‐Gastaut syndrome. Neurology. 2011;77:1473–81. [DOI] [PubMed] [Google Scholar]
- 38. Madan Cohen J, Checketts D, Dunayevich E, Gunning B, Hyslop A, Madhavan D, et al. Time to onset of cannabidiol (CBD) treatment effect and resolution of adverse events in patients with Dravet syndrome: pooled analysis of 2 randomized controlled trials. 2019. AES 2019 Annual Meeting Abstract Database. https://www.AESnet.org. Accessed 25 Jun 2020.
- 39. Wu J, Cock H, Devinsky O, Joshi C, Miller I, Roberts C, et al. Time to onset of cannabidiol (CBD) treatment effect and resolution of adverse events (AEs) in the tuberous sclerosis complex (TSC) phase 3 randomized controlled trial (GWPCARE6) (674). Neurology. 2020;94:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material


