Summary
The tuberculosis (TB) vaccine Bacillus Calmette‐Guérin (BCG) was introduced 100 years ago, but as it provides insufficient protection against TB disease, especially in adults, new vaccines are being developed and evaluated. The discovery that BCG protects humans from becoming infected with Mycobacterium tuberculosis (Mtb) and not just from progressing to TB disease provides justification for considering Mtb infection as an endpoint in vaccine trials. Such trials would require fewer participants than those with disease as an endpoint. In this review, we first define Mtb infection and disease phenotypes that can be used for mechanistic studies and/or endpoints for vaccine trials. Secondly, we review the evidence for BCG‐induced protection against Mtb infection from observational and BCG re‐vaccination studies, and discuss limitations and variation of this protection. Thirdly, we review possible underlying mechanisms for BCG efficacy against Mtb infection, including alternative T cell responses, antibody‐mediated protection, and innate immune mechanisms, with a specific focus on BCG‐induced trained immunity, which involves epigenetic and metabolic reprogramming of innate immune cells. Finally, we discuss the implications for further studies of BCG efficacy against Mtb infection, including for mechanistic research, and their relevance to the design and evaluation of new TB vaccines.
Keywords: BCG, epigenetics, innate immunity, phenotypes, tuberculosis, vaccine
1. INTRODUCTION
Bacillus Calmette‐Guérin (BCG) is a live‐attenuated vaccine derived from Mycobacterium bovis of the Mycobacterium tuberculosis (Mtb) complex and was first used in medical practice in 1921. It is administered intradermally after birth, while repeat dosing in adolescence and at other stages of life have been adopted inconsistently in different parts of the world. Efficacy trials have yielded hugely variable results, ranging from 0‐80% efficacy against TB disease across different locations. 1 Furthermore, the mechanisms of BCG protection remain poorly understood after 100 years of research and practice, making it difficult to determine what new generation TB vaccines need to induce to provide improved protection.
The discovery that BCG protects against Mtb infection (as defined by a positive interferon‐γ release assay (IGRA) result) and not just progression from Mtb infection to TB disease 2 , 3 has significant implications for basic and applied TB research. For basic research, it means that both innate and adaptive immune protective responses play a role in BCG protection. In addition to T cell–mediated responses, BCG‐induced protection may involve humoral 4 and innate immune memory responses termed “trained immunity.” 5 For applied research, it means that Mtb infection, not just disease, should be considered as an endpoint for TB vaccine trials. The advantage of this is that trials using Mtb infection as the primary endpoint can be much smaller than those focused on TB disease, potentially including only hundreds rather than many thousands of participants. 6
To facilitate research in this field, first we identify and justify five phenotypes of Mtb infection and disease, which are, or have potential to be, useful for basic and applied research, including vaccine trials. Secondly, we explore the evidence for BCG efficacy against Mtb infection using these phenotypes as a reference. As part of this, we explore evidence for limitations around BCG efficacy. Thirdly, we review the evidence around the possible mechanisms of BCG efficacy against Mtb infection phenotypes from animal and human studies. And finally, we discuss the implications for future research studies and for the design and evaluation of new vaccines.
2. EVIDENCE FOR BCG‐INDUCED PROTECTION AGAINST M. TUBERCULOSIS INFECTION
2.1. Understanding the phenotypes of M. tuberculosis infection and disease
Historically, latent Mtb infection was regarded as a distinct phenotype whereby, in those who are exposed to, and infected by Mtb, and do not progress quickly to TB disease, the pathogen enters into a dormant state which either continues indefinitely or, with a new susceptibility in the host, reactivates to cause TB disease. 6 It is now thought that this is simplistic, and that Mtb is associated with a spectrum of phenotypes that can occur after exposure to the pathogen. 7 , 8 To be fit for purpose to discuss BCG‐induced protection, the range of phenotypes associated with Mtb infection need to be (or have the potential to be) measurable as potential endpoints for assessing efficacy and identifying mechanisms. We propose five such phenotypes, summarized in Table 1.
TABLE 1.
Proposed phenotypes associated with M. tuberculosis infection
| Mtb infection phenotype | Definition | Possible subphenotypes | Identification | Issues as endpoint for vaccine trials |
|---|---|---|---|---|
| Early clearance | Eradication of Mtb before establishment of an infection | ‘Resister’: Failure of Mtb to establish an infection upon repeated exposure | Persistent IGRA or TST negativity upon testing at least 3 months apart post‐exposure |
May include unexposed individuals Repeated TST causes boosting |
| Infection | Established Mtb infection. | Incipient disease: the presence of lesions that will inevitably progress to TB disease |
IGRA or TST positive Biosignature positive |
IGRA and TST are both imperfect tests for Mtb infection |
| Delayed clearance | Clearance of an established Mtb infection | Repeated delayed Mtb clearance after repeated infections |
IGRA or TST reversion Biosignature change |
IGRA and TST reversion are imperfect biomarkers of Mtb clearance |
| Subclinical disease | Asymptomatic TB disease diagnosed through routine diagnostic test | Non‐progressor: individuals who never develop symptomatic disease | Routine test for TB disease | Phenotype may expand through advances in diagnostics and may overlap with incipient disease |
| Clinical disease | Symptomatic TB disease diagnosed through routine diagnostic tests | A spectrum from mild to severe disease, may prove to be relevant | Routine test for TB disease | Large numbers of trial participants needed |
IGRA, Interferon Gamma Release Assay; TST, tuberculin skin test; Mtb, Mycobacterium tuberculosis.
2.1.1. Early clearance
The first phenotype is early clearance, which we have defined as the eradication of Mtb infection before an adaptive immune response develops. 9 Clear examples of individuals with evidence of early clearance include nursing students who never become tuberculin skin test (TST) positive in work environments with high Mtb transmission, 10 and sailors sharing a cabin for six months on a ship with others with pulmonary TB. 11 Early clearance may be achieved through physical barriers, such as nasal hairs or particular physical and chemical properties of saliva or mucous, or it may be through the innate immune system with or without involvement of other components of the immune system. We have defined early clearance of Mtb as persistent IGRA negativity over a three‐month period after exposure. 3 It now appears that early clearance may be achieved with some Mtb exposures and not with others, depending on the presence or absence of associated variables, or it may occur in a smaller subset of individuals after every exposure that they may have. The latter “resisters” have been defined as having a repeatedly negative TST or IGRA over at least a two‐year period after an initial exposure in a setting where ongoing exposure is likely, as reflected by ongoing conversion in other members of the same cohort. 12
One concern about the early clearance phenotype is whether a large proportion of such individuals are simply unexposed. To assist with this issue, several measures of exposure have been identified, including the characteristics and diagnostic findings of a symptomatic case in relation to their contacts (age, sex, sputum smear status, extent of disease on X‐ray, duration and intensity of contact, and Mtb strain) and aerosolization of the pathogen. These factors can be combined into an exposure score. 3 Another concern is whether the IGRA or TST are adequate to classify individuals as truly uninfected. Recently, Lu et al. 4 followed a cohort of 82 Ugandan case contacts of patients with TB who tested negative by IGRA and TST over an average of more than nine years of follow‐up. There were no differences in antibodies to classic immunological targets between these “resisters” and controls who were positive by Mtb infection test. However, the “resisters” possessed IgM, class‐switched IgG antibody responses, and non‐IFN‐γ T cell responses to the Mtb proteins ESAT‐6 and CFP‐10. Of course, these individuals were subject to tuberculin injection by regular TST tests, which itself can induce an, albeit weak, immune response. There have also been no longitudinal studies to show whether such individuals are more likely than others to progress to TB disease.
2.1.2. Infection
The second phenotype is Mtb infection, whereby the pathogen has established an infection, but is not causing TB disease. These individuals are defined as positive by either one or both of TST or IGRA. Reviewed extensively elsewhere, 13 these two tests have similar performance characteristics, but there is significant discordance between them. TSTs are subject to a false‐positive result, especially early in life, due to prior BCG vaccination. A TST can also cause boosting of subsequent TSTs. 14 IGRA tests are more likely to undergo reversion. 15 Both tests incur a drop in sensitivity in immunocompromised individuals.
Rather than considering these individuals as hosting Mtb in a dormant state, it is probably better to regard them as hosting an ongoing engagement between their immune system and the pathogen. Within these individuals, multiple subpopulations of the pathogen exist in different states—some are actively engaging the immune system, whereas some are in a dormant state. Post mortem studies in humans with Mtb infection have demonstrated lesions that represent a subset of those seen in active TB disease, with variable recovery rates and physiological states of viable mycobacteria. 16 When the engagement of the immune system shifts in favour of the pathogen, these individuals may progress to develop disease. If this happens early, it is called progressive primary disease. If it occurs later, it is called re‐activation disease.
A “subphenotype”‐labelled “incipient TB” may prove to be distinguishable following advances in immune profiling and biomarker research. For example, the TB case‐contact study platform, which we developed in The Gambia, 17 was replicated across multiple African sites as part of the Bill & Melinda Gates funded GC6‐74 biomarker study. Samples were taken to profile gene expression at baseline and over the following two years to compare those who progress to disease (n = 79) with matched non‐progressors (n = 328) using a training‐test‐validation approach. 18 A four‐gene signature predicted risk of progression with similar accuracy in four cohorts from three sub‐Sahara African populations. None of the genes in this, or other signatures, relate to a Mtb‐specific response; rather, they represent specific components of an inflammatory response. This signature was equally predictive from samples more or less than a year prior to diagnosis of TB, whereas a different signature originated from profiles generated in South African adolescents, which was most predictive in the months prior to diagnosis. 19 Proteomic and metabolomic signatures were also identified in the GC6‐74 study cohort, with modest predictive values for disease progression in the year before a TB diagnosis. 20
2.1.3. Delayed clearance
The third phenotype is delayed clearance of Mtb infection. Interest in this phenotype has revived recently as the commonly held assumption of “lifelong infection” has been challenged. 21 However, the possibility of the existence of this phenotype has been recognized for many decades, as it was indicated from age‐stratified prevalence studies. 22 Further, longitudinal follow‐up studies have shown that TST reversion occurs over time after an exposure to Mtb in some individuals who have an initial positive TST. We showed in adult Gambian case‐contacts, that 9% of 56 initially TST‐positive individuals underwent reversion after 18 months. 15 In Uganda, Johnson et al. found that 20.5% of 123 initially TST‐positive household contacts of all ages reverted to TST negative after 1 year, with reversion most prominent in children. 23 Further, the marked drop in the incidence of TB disease in the years after an exposure, 24 , 25 consistent across birth cohorts, 26 supports both the existence of the delayed clearance phenotype, and the premise that some people with a persistently positive TST may have cleared their infection. On the other hand, the assumption that TST reversion reflects waning cell‐mediated immunity has been used to guide re‐vaccination of BCG in TB control programmes 27 and in healthcare workers. 28
IGRA tests have provided further insights into delayed clearance of Mtb. IGRAs measure a predominantly effector T cell response, which generally lasts only a few days in the absence of antigen stimulation. We followed a cohort of 341 Gambian TB case contacts for IGRA test (ELISpot) conversion and reversion. 15 Reversion was defined as both a change to a negative test and at least a 6‐spot count reduction. Remarkably, of 134 initially ELISpot‐positive contacts, 54 (40.2%) underwent ELISpot reversion at three months, in the absence of any intervention. It was hoped that reversion of a positive IGRA test might show utility as a biomarker for delayed clearance of Mtb. We assessed this by randomizing 211 ELISpot and TST‐positive TB case‐contacts to isoniazid preventive treatment to affect Mtb clearance, or to placebo, and followed up with repeated ELISpot tests at one, three, six, and twelve months of follow‐up. 29 There were no significant differences in qualitative or quantitative ELISpot changes over time between the two study arms. Biraro et al. randomized 47 Quantiferon positive Ugandan case contacts to six months of isoniazid or no treatment. 30 They found a relative decline, in the isoniazid arm compared to the no‐treatment arm, of Mtb‐specific production of IFN‐γ (p = 0.01) and IL‐2 (p = 0.04) as well as a decline in CFP‐10 antibodies (p = 0.04). Of note, rifampicin may be better at effecting clearance of Mtb infection than isoniazid, on the basis of studies in macaques. 31
Therefore, in humans, the proportion of those who become infected with Mtb that actually clear their infection is not accurately reflected by TST or IGRA. Further insights come from studies in cynomolgus macaques, who develop the full spectrum of Mtb infection outcomes, with manifestations similar to humans. Combining high‐resolution computed tomography (CT) and positron emission tomography (PET) and genomically bar‐coded strains of Mtb, 32 it has been possible to track the pathogen through infection and clearance, while immune responses can be tracked in parallel, including at the level of the granuloma. 33 Gideon et al. showed that a particular combination of pro‐ and anti‐inflammatory factors, rather than a strong Th1 response, are associated with sterilization of granulomas, offering hope that a biomarker of delayed clearance may be identified. 33 The animals with latent infection were followed for up to 601 days, and while many had at least one sterile granuloma, only approximately 5% had all of their evaluated granulomas sterile by the end of follow‐up. Furthermore, systemic responses such as those measured by IGRA did not reliably reflect T cell responses at the level of the granuloma.
2.1.4. Subclinical and clinical TB disease
The fourth phenotype is subclinical TB disease. These individuals are completely asymptomatic but are positive on routine investigation for TB disease. Subclinical TB is identified most frequently in TB prevalence surveys and other active case‐finding initiatives. Often, these people will progress to symptomatic disease over time. PET/CT provide new insights into this phenotype. 34 There may be a case for including readouts from these investigations as part of the phenotype definition, although there may be some blurring of the boundary with Mtb infection and especially with the emerging incipient TB disease subphenotype described above. Of note, ongoing changes on PET scan in individuals who have had curative TB treatment remain unexplained. 35 It is possible that they are “viable pathogen‐free,” purely localized immunological processes.
The fifth major phenotype is clinical TB disease. These individuals are symptomatic and are diagnosed with TB, often by a diagnostic test. If treated appropriately, over 90% will be cured and return to normal health. This article focuses on the first three phenotypes; the two disease phenotypes are not discussed further.
2.2. Evidence of BCG‐induced protection against M. tuberculosis infection
2.2.1. Observational studies
TB case‐contact studies using IGRA as the readout have provided strong evidence of BCG‐induced protection against acute Mtb infection. This was previously difficult to demonstrate because BCG can cause a false‐positive TST, so any reduction in Mtb infection due to BCG vaccination is countered by BCG‐induced TST positivity. In 979 child household contacts of 414 adult index patients with sputum smear‐positive pulmonary tuberculosis in Turkey, Soysal et al. showed that BCG‐vaccinated children (as indicated by the presence of a scar) had an odds ratio of 0.60 (95% CI 0.43‐0.83, p = 0.003) for Mtb infection (as defined by ELISpot assay), compared with unvaccinated children. 36 BCG scars are often used as an indicator of prior vaccination, especially where vaccination records are unreliable or unavailable. This induces the potential for bias, as a minority of BCG‐vaccinated individuals do not make a scar. Misclassification of these scar‐negative individuals as unvaccinated may cause an underestimation of the protective effect of BCG against Mtb infection.
Further, in a systematic review of 14 studies and 3855 child participants, the estimated overall risk ratiot was 0.81 (95% CI 0.71‐0.92), indicating a protective efficacy of 19% against infection among vaccinated children after exposure compared with unvaccinated children. 2 In the Gambia, to assess ELISpot conversion and reversion, we followed a cohort of 207 ELISpot‐negative adult contacts of sputum smear‐positive TB cases. 15 Those with a BCG scar were half as likely as those without to undergo ELISpot conversion after three months (OR = 0.5; 95% CI 0.2‐1.0), p = 0.06). Similarly, in Indonesia, we followed 317 IGRA (QuantiFERON‐TB Gold) negative contacts of sputum smear‐positive TB cases, with a repeat test after 14 weeks. 3 Those with a BCG scar were just under half as likely to undergo IGRA conversion as those without scars (RR = 0.56; 95% CI 0.40‐0.77, p < 0.001). Longitudinal studies may provide the most accurate estimate of the extent of BCG‐induced protection against Mtb infection, at least to a recent exposure. The question then arises as to whether it is preferable to have an accurate readout of the level of protection against a recent known exposure, or protection from all exposures in the past. In our view, BCG protection may be best understood as depending on a combination of factors that influence whether it is adequate for a particular exposure to Mtb. In other words, under certain circumstances, it may be possible to overcome BCG‐mediated protection. It is of course possible that some persistently IGRA negative contacts are anergic to Mtb antigens, although a negative IGRA is associated overall with a lower rate of progression to active disease. 37
There is some evidence that BCG enhances delayed clearance of Mtb. Mancuso et al., in a 55‐year follow‐up of a randomized trial, showed that BCG vaccination after infancy was associated with an increased risk of TST positivity relative to placebo over a 55‐year follow‐up period, with the strongest risk in the first 15 years post‐vaccination. 38 However, positive TST results were also more likely to revert to negative in the BCG group during the first 15 years of follow‐up, possibly reflecting enhanced delayed clearance of the infection. There was no difference between the groups after 15 years.
Additionally, BCG offers protection against other mycobacterial infections, most notably leprosy. A meta‐analysis of seven experimental studies showed that BCG reduced the risk of development of clinical leprosy by 26%, and this effect was stronger with multiple doses of BCG. 39
2.2.2. BCG re‐vaccination studies
In South Africa, Nemes et al. randomly assigned 990 adolescents, who had received neonatal BCG vaccination, to receive the H4:IC31 vaccine, BCG re‐vaccination or placebo. 6 All participants had negative results on testing for Mtb infection by IGRA test (QuantiFERON‐TB Gold IGRA In‐tube assay; QFT), and for human immunodeficiency virus (HIV). While BCG re‐vaccination did not reduce the rate of initial IGRA conversion, it did reduce the rate of sustained QFT conversion to a positive test without reversion to negative status at three months and six months after initial conversion (this was a secondary outcome measure), with an efficacy of 45.4% (p = 0.03).
There have been no randomized trials to assess whether BCG, given to those who have evidence of Mtb infection, increases the rate of IGRA or TST reversion to reflect efficacy to enhance delayed clearance.
2.3. Limitations of, and variation in, BCG‐induced protection against M. tuberculosis infection
2.3.1. Duration of protection
The duration of BCG‐induced protection is not known, although it has previously been regarded as limited to the first few years of life. 40 Recent studies suggest that BCG is effective against TB for at least 20 years when given at birth or school age. 41 , 42 Further, in our case‐contact study in Indonesia, we stratified the association of BCG with IGRA test results, by age group and found a significant interaction. 3 Those in the lowest age tertile had the strongest evidence of BCG‐induced protection on their baseline IGRA (prevalence ratio (PR) 0.76; 95% CI 0.67‐0.87), while for those in the highest age tertile (over the age of 33 years), the odds of baseline IGRA positivity were 1.01 (95% CI 0.89‐1.15).
2.3.2. Exposure dependency
As mentioned above, it seems likely that particular circumstances, such as particularly high or prolonged exposure to Mtb, or increased host vulnerability may favour “immune evasion” by the pathogen over long‐term host‐mediated immune protection. In Indonesia, to assess BCG protection by level of Mtb exposure, we created one summary measure of exposure, calculating exposure risk scores predicted from a logistic regression of Mtb exposure variables (index case: sputum smear grade, cavities, extent of radiographic disease; contacts: hours spent with, and sleeping proximity to, the case). These exposure scores were compared against IGRA results. 3 We found an interaction between exposure and BCG vaccination in relation to IGRA conversion (p = 0.05). There was stronger BCG protection at lower levels of exposure: for those in the lowest exposure tertile, the relative risk (RR) of IGRA conversion was 0.37 (95% CI 0.22‐0.61), while it was 0.61 (0.46‐0.96) in the highest tertile. These findings were supported by replicating the exposure‐based analysis on a cohort of adult TB contacts in the Gambia 43 and suggest that BCG‐mediated protection against Mtb infection may be overcome by a high “dose” of pathogen. In the pre‐antibiotic era, Brailey showed that BCG vaccinated children had increasing rates of TST conversion with time of exposure to a sputum positive TB case 44 : 37% of children had a positive TST after exposure to a case for less than one month, with TST positivity rising steadily up to 85% of those exposed for over 12 months.
2.3.3. Host factors including genomics
With equal exposure, certain TB contacts may be more likely to become infected than others. In our household contact study in Indonesia, besides those who were older, those with lower haemoglobin levels were at significantly higher risk of IGRA conversion, as were those who smoked (adjusted OR 1.47; 95% CI 0.96‐2.26; p=0.08). 3 Other factors that were rarer in our contact study, such as diabetes may also increase susceptibility to infection. 45 , 46
Host genetic factors may also influence susceptibility to Mtb infection. Studies have shown higher concordance of TB disease in monozygotic twins compared to dizygotic twins, 47 robust associations with variation in several candidate genes, 48 and several loci from genome‐wide association studies. 49 , 50 , 51 , 52 , 53 , 54 Fewer studies have focussed explicitly on heritability and genetics of susceptibility to Mtb infection. In a study of household members with similar TB exposure, TST reactivity was correlated among siblings but not among unrelated children. 55 In a study of TST reactivity in household contacts of TB in Colombian population, a single locus accounted for 65% of TST variability. 56 In a study including 128 families in South Africa, two loci were shown to influence TST reactivity, including TST1 locus on chromosome 11p14 involved in resistance to Mtb infection, and TST2 locus on chromosome 5p15 that controlled the intensity of positive TSTs. 57 Interestingly, TST1 lies in the vicinity of the TNF1 locus that controls TNF production after stimulation by BCG and BCG plus IFN‐γ, and this suggested the connection between TNF production response and negative TST. 58 In another study, genome‐wide linkage analysis in Uganda found regions on chromosome 2q21‐2q24 (mapped to GTDC1 and ZEB2) 59 and on 5p13‐5q22 (mapped to SLC6A3) 60 to be associated with resistance to Mtb infection. Further, a genome‐wide association study among HIV‐positive subjects in Tanzania and Uganda identified an association between chromosome 5q31 (including the IL9 gene) and TST reactivity, while this study also replicated the previously mentioned linked loci on chromosome 2, 5, and 11. 52 Finally, whole‐genome sequencing in an Icelandic population identified HLA class II sequence variants that were associated with an increased risk of Mtb infection, and a decreased risk of pulmonary TB disease. 61
2.3.4. Pathogen genomics
Genomic variation of Mtb may also be relevant. In Indonesia, we conducted whole‐genome sequencing of the Mtb isolates of the index cases, and used a SNP‐based “barcode” to group the strains. Two‐fifths of the isolates were of the Beijing genotype family. We found a significant interaction between strain and BCG vaccination with respect to IGRA test results at 14 weeks (p=0.01). For those exposed to a non‐Beijing strain, there was strong BCG protection against Mtb infection (RR 0.42; 95% CI 0.28‐0.63). 62 However, for those exposed to a Beijing strain, the risk of IGRA conversion was 1.04 (95% CI 0.54‐2.01), suggesting that some Mtb strains can overcome vaccine‐induced, host‐mediated protection. Similarly, in a rabbit model, prior BCG vaccination did not protect against infection with the Mtb strain HN878, 63 which induces an increased pro‐inflammatory response compared to other strains. 64 These findings have broad implications for understanding the epidemiology of TB in relation to BCG vaccination in populations and on the importance of testing new vaccines against multiple Mtb strains.
3. MECHANISMS OF BCG‐INDUCED PROTECTION AGAINST M. TUBERCULOSIS INFECTION
The immunological mechanisms of BCG‐induced protection against Mtb infection are incompletely understood. BCG‐mediated protection against TB has historically been attributed to vaccine‐induced memory CD4+ T cells which rapidly secrete Th1 cytokines and control secondary infection with Mtb. 65 However, there is little evidence that vaccine‐induced memory CD4+ T cells confer protection against TB in immune‐competent hosts (reviewed by Steigler et al. 66 ). Many new candidate vaccines against TB have entered the development pipeline, but few have progressed to clinical trials in humans, where they have failed to show greater efficacy than BCG.
The MVA85A vaccine induced robust, Ag85A‐specific IFN‐γ, TNF‐α, IL‐2, and IL‐17 production by T cells in both infants and adults, but did not offer protection against incident Mtb infection or active disease. 67 , 68 Despite these disappointing results, several promising TB vaccine candidates are currently undergoing clinical testing. VPM1002 is a recombinant strain of BCG expressing listeriolysin O to promote phagosome escape and improve antigen release into the cytosol. 69 Phase I/II clinical trials have demonstrated that this vaccine is safe, and elicits similar immune responses to BCG in adults and infants. 70 , 71 An efficacy trial in adolescents and adults previously treated for pulmonary TB is underway (NCT03152903). Another whole‐cell vaccine candidate is MTBVAC, a strain of Mtb attenuated by the deletion of virulence factors phoP and faD26, controlling expression of ESAT‐6 and virulence‐associated cell wall lipids, respectively. 72 A clinical trial of adults and neonates in South Africa has demonstrated that MTBVAC is safe, and generates durable Mtb‐specific Th1 responses. 73 Finally, the subunit vaccine M72/AS01E is a fusion protein of Mtb antigens Mtb32A and Mtb39A, combined with the adjuvant ASO1, and has been shown to reduce progression to active TB disease in latently infected adults. 74
The lack of immune correlates of protection against Mtb—that is, a characterized immune response associated with protection—represents the most significant challenge to the development of new TB vaccines. 75 , 76 Robust, vaccine‐induced Th1‐type T cell responses have failed to improve the protection against TB already elicited by BCG. We propose that characterizing the immunological events of early clearance and how this phenotype is influenced by BCG vaccination may uncover new correlates of vaccine‐induced immune protection against Mtb infection, potentially informing future vaccination strategies.
3.1. Proposed mechanisms of early clearance
Early clearance is defined by the absence of specific IFN‐γ producing T cells, making it seemingly impossible for these cells to mediate this phenotype. However, IGRA and TST are incomplete measures of T cell responses, considering that they are unable to detect IFN‐γ independent T cells and non‐conventional T cell responses. Therefore, these alternative T cell responses might still contribute to early clearance of Mtb infection. In addition, antibodies produced by B cells are among the proposed immunological mechanisms to explain early clearance. Innate immune responses mediated by monocytes, macrophages, neutrophils and NK cells are also likely to play a role in the early clearance of Mtb infection. In this section, we will briefly discuss these proposed mechanisms of early clearance.
3.1.1. Alternative T cell–mediated resistance
IFN‐γ independent or unconventional T cell responses might play role in the early clearance mechanism. BCG re‐vaccination in humans was recently shown to boost the populations of Th1‐type CD4+ T cells, expressing IFN‐γ, IL‐2, and/or TNF, as well as CD4+ T cells expressing IL‐22, highlighting the importance of unbiased analyses of vaccination responses to discover previously neglected populations. 77 Th17 cells, a subset of CD4+ T cells, have been shown to confer protection against Mtb infection in murine adoptive transfer models. RAG‐deficient mice, which lack both T cells and B cells, were transferred with BCG‐specific Th17 cells from immunized IFN‐γ‐deficient mice, and these mice had a better survival rate and reduced bacterial load compared to RAG‐deficient mice that received naïve T cells. 78 Another adoptive transfer study shows that transferred ESAT‐6‐specific Th17 CD4+ T cells partially inhibited Mtb growth. 79 Mice transferred with Th17 cells have increased inflammation and neutrophil infiltration in the lungs. 80 This Th17‐mediated inflammation and neutrophil recruitment may explain how Th17 cells contribute to clearance of Mtb infection as will be discussed in the next section.
Mucosal‐associated invariant T (MAIT) cells, which are a subset of non‐conventional T cells, are enriched in the respiratory tract, and are thus uniquely positioned for rapid responses to pulmonary infections such as Mtb. 81 These CD8+ T cells recognize metabolites of the riboflavin synthesis pathway through MR1‐restricted TCR interactions. In response to infected cells, they exert cytotoxicity and produce inflammatory cytokines. MAIT cells activated by BCG produce IFN‐γ and TNF‐α as well as granulysin in response to subsequent mycobacterial stimulation. 82 γδ T cells are another subset of non‐conventional T cells, and are also present in the alveolar space, and these recognize non‐peptide, phosphorylated antigens presented by Mtb‐infected alveolar macrophages. 83 γδ T cells can recognize and exert cytotoxicity against Mtb‐infected macrophages by producing granulysin and perforin. 84 These non‐conventional T cell subsets may contribute to early clearance of Mtb infection through these mechanisms. Indeed, among household contacts who had spent at least one month sleeping close to an active tuberculosis case in Haiti, persistent IGRA negativity over six months was associated with increased activation of peripheral MAIT cells. 85 Additionally, CD4+ γδ T cell activation was impaired in IGRA‐positive case contacts, only becoming active after infection, while these responses among IGRA negative contacts did not differ compared to healthy community controls. This suggests that impaired activation of γδ T cells may increase susceptibility to Mtb infection, and that MAIT cells may contribute to early clearance.
3.1.2. Antibody‐mediated resistance
The role of antibodies in protection against Mtb is not completely understood. However, growing evidence suggests that antibodies have key contributions to protection against Mtb. 4 , 86 , 87 , 88 A study conducted in healthy, heavily exposed healthcare workers in Beijing showed that a minority of the subjects had protective antibodies against Mtb. 89 Interestingly, three out of seven subjects that produced these protective antibodies were IGRA negative. Another study from South Africa in HIV‐infected patients with no TB, and persistently negative TST/IGRA despite living in an area of TB hyperendemicity showed the presence of antibodies specific for Mtb. 90
There are several potential mechanisms of antibody‐mediated resistance against Mtb in the context of early clearance. Antibody could bind to Mtb bacteria to prevent entry into cells, drive antibody‐dependent cellular phagocytosis to increase bacterial killing, mediate antibody‐dependent cellular cytotoxicity to kill infected cells, or mediate the recruitment of innate immune cells that express the Fc receptor. 86 , 91 , 92 Some studies showed evidence that antibody‐mediated resistance may play a role in early clearance. Recent findings showed that resisters possess IgM, class‐switched IgG antibody responses to the Mtb‐specific proteins ESAT6 and CFP10. 4 In a recent non‐human primate study, intravenous BCG vaccination before Mtb challenge resulted in superior protection against Mtb and induced higher titers of IgG, IgM, and IgA antibody specific for Mtb whole‐cell lysate in plasma and bronchoalveolar lavage compared with other BCG vaccination routes. 93
3.1.3. Innate immune cell–mediated resistance
Innate immune mechanisms likely play a role in protection against Mtb infection. In the initial stage of infection, inhaled aerosolized Mtb encounters the lung‐resident alveolar macrophages as the first line of defence against pathogens in the lung alveoli. 92 , 94 Alveolar macrophages are a self‐renewing population permanently residing in the lungs, while shorter‐lived, monocyte‐derived macrophages (MDMs) are recruited to the lungs during infection. 95 , 96 , 97 Alveolar macrophages are the initial hosts for Mtb, and recruited MDMs assume this role as infection progresses. 98 Macrophages are endowed with the ability to kill internalized Mtb and produce pro‐inflammatory cytokines and chemokines to recruit other immune cells to the lungs, and therefore are highly influential in the eventual outcome of Mtb infection.
Innate immune cells recognize Mtb through germline‐encoded pattern recognition receptors (PRRs), both on the cell surface and in the cytosol, which leads to phagocytosis of Mtb and immune activation. 99 , 100 , 101 Engagement of various toll‐like receptors (TLRs), a subgroup of PRRs, by mycobacterial cell wall components triggers the production of pro‐inflammatory cytokines such as TNF‐α, 102 IL‐1β, 103 and IL‐6. 104 Previous studies have shown that mice deficient in one or more of these TLR signalling pathways are more susceptible to mycobacterial infections than wildtype mice. 105 , 106 , 107 , 108 , 109 Engagement of intracellular NOD‐like receptors, another type of PRR, such as NOD2 by mycobacterial peptidoglycans also induces the production of IL‐1β and has synergistic effects on TLR2‐induced production of TNF‐α and IL‐6. 110 , 111 NOD2 stimulation also directly enhances TNF‐α and IL‐1β production in response to subsequent infection with Mtb and other pathogens, and improves mycobacterial killing. 112 , 113 When the infection cannot be cleared, the infected alveolar macrophages will eventually undergo apoptosis or necrosis. 9 Infected cells that undergo apoptosis express ATP and phosphatidylserine, which promote efferocytosis of apoptotic macrophages by uninfected monocytes and neutrophils, enhancing Mtb killing by improved delivery to the lysosomal compartment. 114 While apoptosis has been shown to inhibit Mtb replication, necrosis facilitates the dissemination of Mtb in the lung interstitium, causing infection of other recruited interstitial macrophages, leading to Mtb outgrowth. 115 In this way, early clearance may be influenced by the fate of Mtb‐infected macrophages and their intracellular killing capacity.
When macrophages are unable to kill the internalized pathogens and clear the infection, they produce chemokines to attract other cell types to the infection site. Chemokines such as CCL2, CCL3, CCL4, CCL5, and MCP‐1 recruit monocytes and MDMs, NK cells, dendritic cells (DCs), and neutrophils. 116 Several studies have shown the importance of neutrophils in early responses against Mtb infection. In a rat study, inducing neutrophilia by intratracheal injection of LPS before Mtb infection resulted in reduced Mtb growth, and impeded granuloma formation in the lungs. 117 Another study in mice showed that intraperitoneal injection of Mtb led to extensive neutrophil recruitment to the infection site. 118 In the same study, induced neutropenia by intravenous injection of an antineutrophil monoclonal antibody in the first week of Mtb infection resulted in increased mycobacterial growth in the liver, spleen, and lung. 118
Activated neutrophils secrete an array of antimicrobial enzymes, as well as cytokines and chemokines to combat the infection. 9 , 119 Neutrophils are capable of internalizing and killing Mtb by releasing granule‐associated antimicrobial peptides such as cathelicidin and lipocalin‐2. 120 Neutrophils can also augment the intracellular growth restriction of Mtb by macrophages. As macrophages internalize apoptotic neutrophils, neutrophil granules fuse with Mtb‐containing phagosomes, leading to enhanced anti‐Mtb activity. 121 Supporting a role for neutrophils in restriction of Mtb growth, it was recently demonstrated by Lowe et al. that depletion of neutrophils from whole blood before infection with Mtb resulted in impaired growth restriction. 122 In contrast, the addition of viable neutrophils restored the restrictive capacity. Further, in a cohort of 117 TB case contacts in London, higher peripheral neutrophil counts were associated with a reduced risk of a positive IGRA result. 120 Additionally, persistently IGRA negative case contacts in the Gambia displayed greater Th17 cytokine responses (associated with neutrophil recruitment) to whole‐blood stimulation with mycobacterial antigens compared to IGRA converters. 123
Natural killer (NK) cells are another cell type that may be involved in early clearance. NK cells can promote Mtb killing and macrophage apoptosis through the production of IFN‐γ and IL‐22. 124 , 125 These cells can also exert cytotoxicity against Mtb‐infected cells to mediate mycobacterial killing 126 and can kill Mtb directly via granulysin and perforin. 127 Additionally, NK cells display a specific memory‐like ability and are capable of mounting an enhanced recall response. 128 NK cells activated by cytomegalovirus infection display a long‐lasting, T cell–independent memory response against re‐infection, characterized by rapid degranulation and cytokine production. Further, adoptive transfer of these activated NK cells to naïve recipient mice offered significant protection against viral infection. In concordance with this, BCG vaccination has been shown to enhance long‐term responsiveness of NK cells against unrelated pathogens such as Candida albicans. 129
3.2. BCG‐induced trained immunity as a mechanism of early clearance
Although it is clear from the previous section that different cell types and immune mechanisms contribute to protection against Mtb infection, one question remains unanswered: what accounts for the observed protection of BCG against Mtb infection? Based on our work and that of others, we think that the answer may lie in BCG‐induced trained immunity. Vaccination is traditionally based on the induction of specific adaptive immune memory against a particular pathogen, which leads to enhanced responsiveness of lymphocytes upon subsequent infection with the same pathogen. However, an increasing body of evidence suggests that a number of live‐attenuated vaccines, including BCG and measles vaccine, also provide protection against unrelated infectious diseases. A number of randomized controlled trials have confirmed that BCG vaccination indeed lowers all‐cause morbidity and mortality through protection against unrelated infections, so‐called non‐specific protection. 130 It has been proposed that BCG may also offer protection against infection with SARS‐CoV‐2 and reduce severity of COVID‐19 disease, 131 with some studies suggesting an association between universal BCG vaccination and reduced COVID‐19 mortality, 132 while others did not. 133 Several randomized trials in healthcare workers 134 and elderly patients that are underway or pending final analysis will provide final proof for a protective effect of BCG against COVID‐19.
In search of an immunological mechanism explaining these observations, it is unlikely that cross‐reactive lymphocytes are able to protect against such a broad range of pathogens. A more likely explanation of these non‐specific effects is the education of the innate immune system. While it is well established that adaptive immune cells develop immunological memory upon stimulation, recent studies have shown that an infection or vaccination can also leave an imprint on innate immune cells. This de facto innate immune memory has been termed trained immunity 135 , 136 and is briefly outlined in Fig. 1. The mechanism of BCG‐induced trained immunity and its possible relevance for protection against Mtb is described in more detail in the following sections.
FIGURE 1.
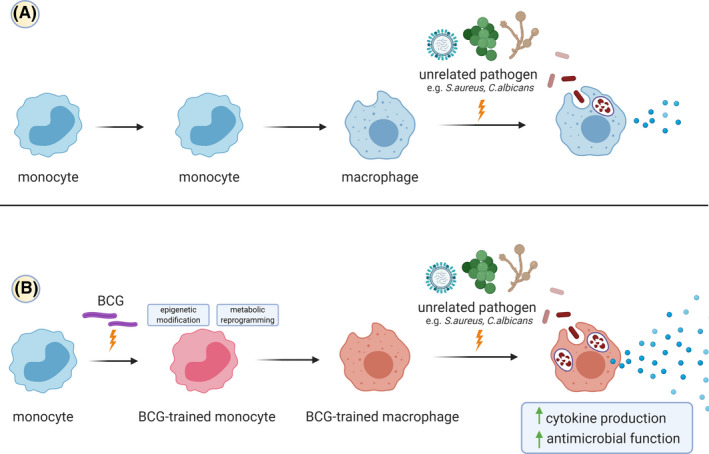
BCG vaccination induces trained immunity in monocytes and enhances subsequent responses to unrelated pathogens. (A) Interaction of macrophages with various pathogens induces the release of cytokines and activation of several antimicrobial functions to clear the infection. (B) BCG vaccination induces persistent epigenetic modifications and metabolic reprogramming in innate immune cells (depicted here in monocytes, giving rise to trained macrophages). These changes allow the trained innate immune cells to exhibit enhanced level of cytokine production and antimicrobial functions in response to unrelated pathogens, leading to better protection compared to untrained innate immune cells. Created with BioRender.com
3.2.1. BCG vaccination enhances innate responses to unrelated pathogens
We have conducted a series of studies to characterize the mechanisms involved in the induction of trained immunity after BCG vaccination. PBMCs isolated from BCG‐vaccinated healthy adults produce increased levels of the innate cytokines TNF‐α, IL‐6, and IL‐1β in response to stimulation with Mtb lysate, but also upon stimulation with unrelated pathogens such as Staphylococcus aureus and C. albicans. 113 , 145 The elevated capacity for IL‐1β and TNF‐α production persisted for three months post‐vaccination, returning to baseline levels within 12 months. 143 After vaccination, monocytes also displayed increased surface expression of activation markers CD11b, CD14, CD206, and TLR4, and these changes persisted for 12 months. 113 , 143 BCG vaccination also enhanced cytokine responses from NK cells at two weeks and three months post‐vaccination. 129 Importantly, BCG vaccination leads to temporarily increased inflammation but no increased inflammation at 90 days post‐vaccination, rather it enhances the inflammatory responsiveness to a secondary stimulation. 144
BCG vaccination also induces trained immunity in newborn infants. In a study in Guinea‐Bissau, BCG vaccination at birth led to increased production of TNF‐α, IL‐1β, and IL‐6 in response to PPD and the TLR2 agonist Pam3CSK4 at four weeks post‐vaccination. 146 Additionally, whole‐blood stimulation with Mtb lysate induced greater production of IFN‐γ, TNF‐α, IL‐6, and GM‐CSF among BCG‐vaccinated infants compared to unvaccinated infants at four months post‐vaccination. 147 Monocytes from these infants also had greater expression of CD11b and CD206, and NK cells displayed greater expression of the activation marker CD69. Finally, neonatal BCG vaccination induced greater IL‐6 and TNF‐α responses to stimulation with BCG at seven months post‐vaccination. 148 Together, these data indicate that BCG vaccination in infants and adults enhances the capacity for cytokine production by innate immune cells in response to secondary stimulation with mycobacterial or other antigens.
Next, we asked ourselves which receptors and intracellular signalling pathways are involved in BCG‐induced trained immunity. This process was shown to be dependent on engagement of the NOD2 receptor and the receptor‐interacting protein kinase (Rip2) as monocytes from individuals with genetic deficiencies in the NOD2 receptor are incapable of mounting trained immunity in response to BCG. 113 , 137 NOD2 recognizes muramyl dipeptide (MDP), a key component of the mycobacterial cell wall, 149 and stimulation of monocytes with MDP alone was sufficient to induce trained immunity. 113 Further, the levels of circulating MDP before BCG vaccination were associated with the strength of trained immunity responses, 145 demonstrating the importance of this pathway for mounting trained immunity in response to BCG. NOD2 is also a crucial PRR for the control of Mtb infection in human macrophages. 112 Because NOD2 is essential for both trained immunity and effective restriction of Mtb growth by macrophages, trained immunity might also have a crucial role in protective responses against Mtb infection.
3.2.2. BCG‐induced trained immunity is independent from T and B lymphocytes
To address question whether BCG‐induced trained immunity is indeed mediated by monocytes or other innate immune cells and is independent from T and B lymphocytes, we injected severe combined immunodeficiency (SCID) mice, which lack T cells and B cells, with either BCG or saline 14 days before inoculation with a lethal dose of C. albicans. 113 BCG conferred complete protection against this unrelated infection, with lower outgrowth of fungi, and higher LPS‐induced production of pro‐inflammatory cytokines. This effect was replicated in another mouse study. 129 In line with these results, a recent study examining intravenous BCG vaccination in mice showed that macrophages from BCG‐vaccinated mice have stronger ex vivo control of Mtb growth compared to naïve macrophages, in the absence of any other cells including B and T cells. 150
3.3. Molecular mechanisms of BCG‐induced trained immunity
Several studies have helped elucidate the molecular mechanisms underlying BCG‐induced trained immunity, showing that it is mediated by metabolic and epigenetic changes that affect transcription of particular genes, resulting in increased responsiveness of cells. 135 , 151 This process is briefly outlined in Fig. 2 and is discussed in more depth in this section.
FIGURE 2.
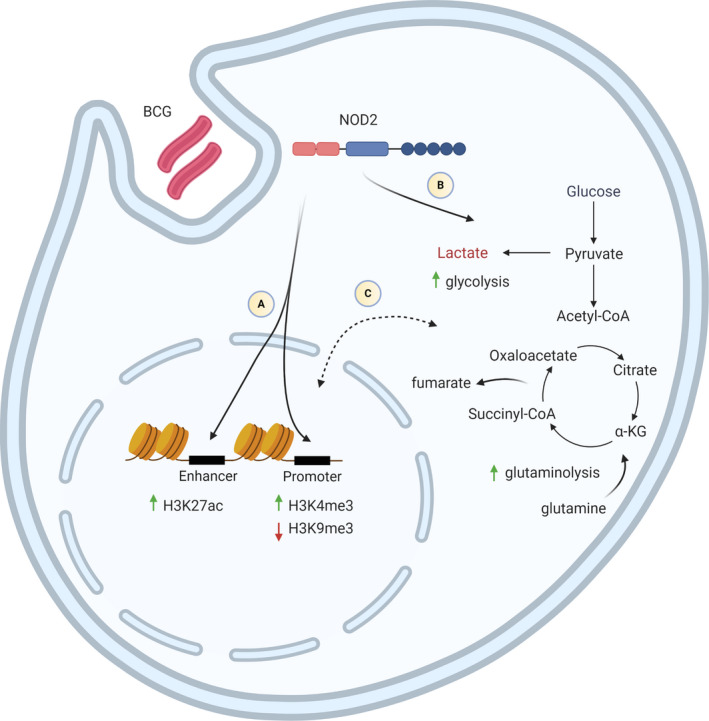
Molecular mechanisms of BCG‐induced trained immunity. (A) Muramyl dipeptide (MDP) from BCG interacts with the cytosolic NOD2 receptor. NOD2‐Rip2 signalling mediates epigenetic modifications such as increased H3K4me3 and decreased H3K9me3 at the promoter regions, and increased H3K27ac at the enhancer regions of pro‐inflammatory genes, leading to increased chromatin accessibility and transcriptional activity. (B) In addition, metabolic reprogramming through the activation of the Akt/mTOR signalling pathway results in increased glycolysis and glutaminolysis. (C) Fumarate and metabolites from glutaminolysis accumulate, acting as a link between metabolic and epigenetic changes by inhibiting KD5M demethylases and promoting the deposition of H3K4me3 and H3K27ac. Created with BioRender.com
3.3.1. Epigenetic modification
The altered transcriptional programme in trained immunity is mediated by multiple regulatory processes, including histone modifications, alterations in DNA methylation, and transcription of long non‐coding RNAs (lncRNAs). Histone modifications at the promoter and enhancer regions of pro‐inflammatory genes are a hallmark of BCG‐induced trained immunity. Tri‐methylation of lysine‐4 of the H3 histone protein (H3K4me3) is upregulated at the promoters for TNFA, IL6, and TLR4 in BCG‐trained monocytes, accompanied by increased mRNA expression for these genes. 113 , 138 , 142 , 152 In addition, retinoic acid has been shown to inhibit BCG‐induced trained immunity in monocytes in vitro, through the methyltransferases SUV39H2, resulting in downregulation of H3K4me3 and upregulation of the repressive histone mark H3K9me3 at the promoters of several cytokine genes. 138
Acetylation of lysine‐27 of histone protein 3 (H3K27ac) is also a key histone mark in BCG‐induced trained immunity. Whole‐epigenome analysis of monocytes before BCG vaccination and one month post‐vaccination revealed a differential pattern of H3K27ac, and this trained response was associated with enhanced protection against experimental infection with yellow fever virus. 140 In particular, H3K27ac at the NOD2 gene was associated with the strongest anti‐viral response induced by BCG, in line with the essential role for NOD2 in BCG‐induced trained immunity.
DNA methylation has also been suggested to regulate BCG‐induced immune responses. After BCG vaccination, macrophages from people who showed enhanced containment of Mtb, defined as “responders,” displayed altered DNA methylation patterns on promoters of immune genes compared to non‐responders. 153 In addition, PBMCs from infants after BCG vaccination revealed differentially methylated genes between high and low responders based on their cytokine responses, and these genes were enriched in immune pathways and cellular processes, such as glutamate signalling and WNT pathways. 154
Recently, Fanucchi et al. showed that epigenetic reprogramming of genes in trained immunity is influenced by a novel subset of lncRNAs called immune gene priming lncRNA (IPLs). 155 Upon β‐glucan priming, which is a fungal cell wall component and another known inducer of trained immunity, IPLs were upregulated and coordinated H3K4me3 accumulation at target gene promoters. 156 One such IPL called UMLILO (upstream master lncRNAs of the inflammatory chemokine locus) was found to mediate H3K4me3 accumulation at the promoters of IL8, CXCL1, CXCL2, and CXCL3 during β‐glucan‐induced trained immunity. 156 Similar mechanisms may be involved in BCG‐induced trained immunity. Together, these data show that BCG‐induced trained immunity is mediated by a changing epigenetic landscape, involving a balance of transcriptionally permissive and repressive histone marks, resulting in an altered transcriptional program upon secondary stimulation.
3.3.2. Metabolic reprogramming
The epigenetic modifications observed in BCG‐induced trained immunity occur in concert with changes to intracellular metabolism, involving a metabolic shift towards glycolysis. In vitro stimulation of human monocytes with BCG leads to an increase in glucose consumption and lactate production, indicating the upregulation of glycolysis. 139 , 157 H3K4me3 was increased and H3K9me3 was decreased at the promoters for key glycolysis enzymes HK2, PFKP, and the master regulator of glycolysis, mTOR, which led to increased mRNA expression of these genes. 139 Further, BCG‐induced trained immunity was prevented when mTOR was inhibited by metformin treatment, indicating that increased glycolysis is essential for trained immunity.
Additionally, genes involved in glutamine metabolism, such as the glutaminolysis enzymes glutaminase and glutamate dehydrogenase, are also upregulated in monocytes after BCG training. 139 Furthermore, inhibiting glutamine metabolism, or reducing glutamine concentration in the culture medium during in vitro training, prevented the potentiation of secondary TNF‐α, IL‐1β, and IL‐6 responses. 139 Fumarate is a metabolite of glutaminolysis and has been shown to affect histone methylation and acetylation in trained immunity. Fumarate inhibits KDM5 histone demethylases enzymes (responsible for H3K4 demethylation), and stimulation of monocytes with fumarate alone resulted in increased H3K4me3 and H3K27ac, hallmark histone changes of trained immunity. 158 Accumulation of fumarate through glutaminolysis therefore links the metabolic and epigenetic changes in trained immunity. 158 Clearly, changes in intracellular metabolism are crucial for the generation of trained immunity by BCG and are intertwined with BCG‐induced changes to the epigenomic landscape.
3.3.3. Functional changes in various innate immune cells
Most of the research performed so far has focussed on unravelling the mechanisms of trained immunity in monocytes. However, the ability of BCG vaccination to induce trained immunity in other cell types of the innate immune system remains largely unexplored. As already discussed, BCG vaccination can induce long‐term functional reprogramming of NK cells. NK cells from BCG vaccinated healthy subjects produce increased levels of pro‐inflammatory cytokines upon ex vivo stimulation with mycobacterial or unrelated pathogens. 129 NK cells were also shown to play a role in the protective effect of BCG vaccination against unrelated pathogens. After BCG vaccination, NOD/SCID/IL2Rγ (NSG) mice lacking T, B, and NK cells have lower survival rates following C. albicans infection compared to SCID mice, which only lack B and T cells. 129 Trained immunity was also induced in NK cells from BCG‐vaccinated infants, as these NK cells exhibited increased expression of activation markers and secreted higher concentrations of IL‐12 and IL‐10 following stimulation with Pam3Cys. 147
Microbial exposure has also been shown to elicit memory‐like responses by dendritic cells (DCs). DCs isolated from mice vaccinated with Cryptococcus neoformans produce higher levels of IFN‐γ, IL‐2, IL‐4, and TNF‐α following secondary challenge. 159 DCs from these vaccinated mice also express NOS2, CXCL9, and CXCL10, pro‐inflammatory markers associated with M1 macrophages. These changes were linked to epigenetic changes as the effects were reduced by treatment with a methyltransferase inhibitor. Additionally, increased CXCL9 and CXCL10 production has been associated with trained immunity and enhanced anti‐mycobacterial activity in a cohort of recently exposed individuals in the Netherlands. 160
Recently, it was also observed that neutrophils undergo long‐term immunophenotypic changes after BCG vaccination in humans. After BCG vaccination, neutrophils showed enhanced expression of activation markers CD66b and myeloperoxidase, as well as increased production of IL‐8 and the antimicrobial enzyme elastase after ex vivo stimulation. 161 In addition, neutrophils also displayed enhanced reactive oxygen species (ROS) production, and increased capacity for phagocytosis and C. albicans killing. This increased responsiveness persisted for at least 3 months after vaccination. Finally, these changes were accompanied by genome‐wide epigenetic changes at the level of H3K4me3 in promoter regions of pro‐inflammatory and glycolysis genes. In addition, in a study of BCG‐vaccinated adults, the bone marrow was skewed towards granulocytic cell lineage priming and the transcriptome of these progenitor cells was also enriched in genes involved in neutrophil‐mediated immunity. 141 These findings were corroborated by higher neutrophil numbers in BCG‐vaccined infants, suggesting a possible role for neutrophils in BCG‐induced trained immunity.
3.3.4. Long‐term epigenetic changes of trained immunity are mediated by progenitor cells
BCG vaccination enhances the responsiveness of innate immune cells for three months and even up to one year. 113 , 143 These effects persist far beyond the typical one‐day lifespan of monocytes in the peripheral circulation after emergence from the bone marrow. 162 This suggests that trained immunity may be induced at the level of myeloid progenitors in the bone marrow. Indeed, intravenous BCG vaccination induced IFN‐γ‐dependent expansion of haematopoietic stem and progenitor cells (HSPCs) in mice, with a bias towards myeloid differentiation, at the expense of lymphoid differentiation. 150 Bone marrow–derived macrophages (BMDMs) from BCG vaccinated mice had a greater capacity to restrict Mtb growth in vitro, demonstrating that BCG can imprint an enhanced anti‐mycobacterial programme in myeloid progenitors. Intradermal BCG vaccination in humans also induced a transcriptional shift towards myelopoiesis. 141 HSPCs taken from volunteers at three months post‐vaccination were enriched for mRNA transcripts of multiple macrophage and neutrophil‐associated genes. This training programme in HSPCs was mediated by hepatic nuclear family (HNF) transcription factors and increased chromatin accessibility at upregulated genes, facilitating the persistent renewal of trained peripheral CD14+ monocytes.
Trained immunity induced by β‐glucan also acts through shifting HSPC differentiation towards myelopoiesis. In mice, this effect is dependent on the action of GM‐CSF and IL‐1β in the bone marrow. 163 Further, in β‐glucan training, GM‐CSF signalling caused upregulation of the dectin‐1 receptor, suggesting that GM‐CSF can intensify the β‐glucan signal and improve the induction of trained immunity. 164 It is unclear whether GM‐CSF signalling is involved in the induction of trained immunity by BCG in myeloid progenitors; however, genes implicated in GM‐CSF signalling were overrepresented in human macrophages that were restrictive of Mtb growth in vitro, compared to those that were more permissive. 165 Additionally, the addition of GM‐CSF made these macrophages more restrictive of Mtb growth, while blockage of GM‐CSF made them more permissive. Further, GM‐CSF production by macrophages is associated with their ability to control intracellular Mtb infection. 166 If GM‐CSF is involved in BCG‐induced training of myeloid precursors, this may generate differentiated macrophages with enhanced anti‐mycobacterial activity and contribute to early clearance of Mtb infection.
3.4. BCG‐induced trained immunity and early clearance of M. tuberculosis infection
3.4.1. Human studies
Since early clearance has been associated with BCG vaccination, and BCG induces trained immunity, we hypothesize that BCG confers protection against Mtb infection through the induction of trained immunity. 5 This hypothesis is supported by results from a randomized trial evaluating BCG re‐vaccination in South Africa. 6 BCG re‐vaccination reduced the risk of sustained IGRA conversion over two years by 45.5% compared to placebo vaccination, 6 but also reduced the incidence of upper respiratory tract infections by more than 70% (2.1% in BCG re‐vaccinated subjects versus 7.9% in placebo arm). This protection against heterologous, non‐mycobacterial infections is a hallmark of BCG‐induced trained immunity. 130
Indirect evidence may also come from our study of household contacts of TB patients in Indonesia, which has shown differences in innate immune signatures between those who were persistently IGRA negative over 14 weeks (early clearers) and IGRA converters. 167 Among early clearers, peripheral monocytes, granulocytes, and innate‐like T cells became less frequent over 14 weeks, while this contraction of innate immune populations was not observed in IGRA converters. This may reflect elimination of the infection in early clearers, with ongoing inflammation in Mtb infection explaining the lack of contraction in IGRA converters. Whole‐blood stimulation with E. coli also elicited greater production of TNF‐α, IL‐6, and IL‐8 from early clearers than from IGRA converters. This heterologous response is consistent with trained immunity induced by BCG. Further, in individuals who had a BCG scar, the magnitude of these effects was increased, suggesting that BCG vaccination may enhance early clearance by inducing trained immunity, facilitating more robust, protective innate immune responses to incident Mtb infection.
Other immunological evidence also supports the idea that trained immunity induced by mycobacterial exposure may confer host‐mediated protection against Mtb. A study of previously mycobacteria‐naïve donors in the Netherlands showed that PBMCs from those who were recently exposed to Mtb had greater capacity to control the outgrowth of BCG than PBMCs from naïve controls, or from active/latent TB patients. 160 The culture supernatants of these individuals also had increased levels of TNF‐α, IL‐1β, and IL‐6, the hallmark cytokines of BCG‐induced trained immunity. The increased capacity for BCG control was dependent on CXCR3 signalling and was associated with the frequency of non‐classical CD14dim monocytes which produced CXCL10. This CD14dim monocyte population was also identified as a contracting cell population over 14 weeks among early clearers in Indonesia, suggesting a key role. 167
3.4.2. Experimental evidence for trained immunity and protection against M. tuberculosis
In addition to studies with BCG, 150 studies with β‐glucan provide strong evidence that trained immunity can protect against Mtb. Human monocytes treated with β‐glucan in vitro displayed enhanced TNF‐α, IL‐1β, and IL‐6 responses to Mtb and were more restrictive of Mtb growth compared with naïve monocytes. 168 Intraperitoneal injection of mice with β‐glucan four or seven days prior to infection with Mtb also improved protection, reducing bacterial burden in the lung and improving survival. 168 This was mediated by shifting HSPC differentiation to myelopoiesis, increasing expression of anti‐mycobacterial genes in an IL‐1‐dependent manner. IL‐1 signalling is crucial for trained immunity, as genetic variants in this pathway modulate the induction of trained immunity by BCG, and IL‐1β alone is even capable of inducing trained immunity. 140 IL‐1β production has also been shown to improve anti‐Mtb activity by macrophages, 169 , 170 , 171 intersecting trained immunity and anti‐mycobacterial activity. These data suggest that the induction of trained immunity in myeloid precursors by BCG and β‐glucan, mediated by cytokine signalling, may elicit improved protection against Mtb. The studies that showed the capacity of trained immunity to protect against Mtb are summarized in Table 2.
TABLE 2.
Experimental evidence of trained immunity and protection against M. tuberculosis
| Author(s), year | Model | Type of experiment | Type of training | Outcome |
|---|---|---|---|---|
| Kaufmann et al., 2018144 | Mice parabiosis and adoptive transfer | In vivo | Intravenous BCG | Lower Mtb burden in target organs |
| Mice BMDMs | Ex vivo | Intravenous BCG | Differences in expression pattern compared to control in response to infection | |
| Moorlag et al., 2020162 | Mice | In vivo | Intraperitoneal β‐Glucan | Higher survival rate compared to control and lower Mtb burden in the lung |
| Human PBMCs | Ex vivo | In vitro β‐Glucan | Increased proinflammatory cytokines and restriction of Mtb growth | |
| Khan et al. 2020166 | Mice BMDMs | Ex vivo | Intravenous BCG | Lowest colony forming units compared to other groups |
| Mice adoptive transfer | In vivo | Intravenous BCG | Lower lung bacterial burden | |
| Mice BMDMs | Ex vivo | Intraperitoneal β‐Glucan | Lower colony forming units compared to PBS | |
| Mice | In vivo | Intraperitoneal β‐Glucan | Better survival rate compared to PBS |
3.4.3. M. tuberculosis prevents the induction of trained immunity
Virulent Mtb promotes a different immune response to BCG and prevents the induction of trained immunity. 172 C57BL/6J mice were injected intravenously with Mtb or BCG four weeks before bone marrow harvest and BMDM differentiation. Mtb imprinted a unique transcriptomic profile in HSPCs that impairs myelopoiesis and innate immunity against Mtb. The Mtb and BCG groups also displayed a different transcriptional signature in the IFN‐I signalling and iron metabolism pathways. Mtb induced RIPK3‐dependent necroptosis in the myeloid progenitors through the IFN‐I/Fe axis, leading to impairment of myelopoiesis and the trained immunity response. The study also demonstrated that both BCG and Mtb imprinting of HSPCs can last for at least one year.
3.5. Unanswered questions regarding trained immunity
A number of questions remain unanswered about BCG‐induced trained immunity. First, the large interindividual variability that we have observed in the induction of trained immunity after BCG vaccination has not been explained. 113 , 139 , 140 , 152 , 173 Two experimental human infection studies depicted the large interindividual variation in the ability of BCG vaccination to protect against infections, as was studied for yellow fever 139 and malaria 173 . Both studies identified “responders,” people in whom BCG vaccination led to increased infection control, and “non‐responders.” The biological mechanism underlying this variation in BCG‐induced protection is not clearly understood, and understanding this process is crucial to harness trained immunity for vaccination strategies against Mtb.
One factor that might drive the interindividual variation is host genetics, as it was shown previously that genetic variants influence cytokine responses. 174 Indeed, genetic variation related to glycolysis, autophagy, and the production of pro‐inflammatory cytokines such as IL‐1β influences the induction of trained immunity by BCG. 139 , 140 , 152 Variation in the promoter region of IL1B and polymorphisms in other genes of the IL‐1β pathway such as IL‐18 receptors and inflammasome components PYCARD/ASC, 141 as well as the glycolysis rate‐limiting enzymes HK2 and PFKP influenced the production of IL‐6 and TNF‐α by monocytes in response to LPS stimulation in BCG‐induced trained immunity. 139 , 140 Polymorphisms in the autophagy genes ATG2B or ATG5 also dampened the induction of trained immunity by BCG. 152 Individuals infected with Mtb who carry these genetic variations may not mount strong enough innate immune responses to clear the infection.
Interindividual variation in the epigenome, possibly induced by environmental factors, also influences the induction of trained immunity by BCG. Verma et al. found alterations in the DNA methylome of MDMs isolated from a subset of BCG‐vaccinated “responders.” 153 Macrophages isolated from these responders restricted the growth of Mtb to a greater extent than those from non‐responders. They also observed that MDMs from responders produced greater amounts of IL‐1β in response to Mtb infection than non‐responders, even before BCG vaccination. These preliminary findings prompted a subsequent study by Das et al., mapping 43 differentially methylated genes from PBMCs prior to vaccination, enriched in genes involved in regulating phagocytosis. 175 Macrophages from responders were more effective at internalizing fluorescent Mtb, a process which precedes mycobacteria‐induced production of IL‐1β.
Other factors, including age, sex, diet, and time of vaccination, might also impact trained immunity responses. It was recently shown that morning administration of BCG vaccination induced stronger trained immunity with higher cytokine production (IL‐1β and TNF‐α) after ex vivo stimulation with Mtb as well as S. aureus compared to evening administration. 176 This result was validated by in vitro experiments using peripheral blood from healthy volunteers. Monocytes isolated in the morning had a higher capability of trained immunity compared to those isolated in the evening. This suggests that the intrinsic molecular clock of monocytes is an important regulator of BCG‐induced trained immunity.
Other factors that are known to impact the immune response, including the metabolome, the gut microbiome, and immune cell subset frequencies, could also impact the magnitude of BCG‐induced trained immunity responses, and could be the subject of future research.
It is not known whether different BCG strains equally induce trained immunity. Multiple strains of BCG exist, which are all subcultures of the original BCG strain, resulting in BCG vaccine heterogeneity that differ in phenotype and genotype. In terms of cytokine production after BCG vaccination, BCG‐Denmark and BCG‐Japan seem to be more immunogenic than other BCG strains. 177 In the context of trained immunity, studies that used BCG‐Denmark 113 seem to show a higher fold‐increase of cytokine after secondary stimulation with unrelated stimuli such as S. aureus, compared to studies that used BCG‐Bulgaria. 144 One could hypothesize that the more immunogenic BCG strains may more robustly induce trained immunity.
It is unclear whether the route of BCG administration could influence the induction of trained immunity in humans and how this affects protection against unrelated pathogens and Mtb. Previously, intravenous BCG vaccination rather than subcutaneous BCG has been shown to induce trained immunity through imprinting of the HPSCs in mice, giving rise to macrophages which confer enhanced protection against Mtb. 150 Another recent study showed that intravenous BCG vaccination promotes better protection against TB disease compared with intradermal and aerosol BCG administration in non‐human primates. 93 However, there were no significant increases in TNF, IL‐1β, IL‐6, or other trained immunity‐associated cytokines in response to ex vivo stimulation of PBMCs with Mtb, heat‐killed S. aureus, or LPS in any vaccination group. In mice studies exploring non‐specific BCG‐mediated protection, intranasal BCG vaccination elicited stronger protection against influenza virus A (H1N1), compared to subcutaneous vaccination 178 or intraperitoneal vaccination. 179 In contrast, intravenous BCG did not result in protection against avian influenza A/Anhui/1/2013 (H7N9) challenge in mice, despite splenocytes and peritoneal macrophages showing characteristics of trained immunity in response to ex vivo stimulation. 180
3.6. Potential mechanisms of delayed clearance
We have defined delayed clearance as the elimination of Mtb infection after it has been established (Table 1). Gaining understanding of the mechanisms of delayed clearance phenotype is challenging, primarily because diagnostic tests rely on immune reactivity to mycobacterial antigens and do not test for bacterial presence directly. Further, reversion of a positive IGRA result to negative does not reliably predict clearance, 22 , 29 and individuals may remain TST positive for up to 10 years without developing disease, even in a state of immunosuppression, suggesting many of these individuals have previously cleared their infection. 21
In cynomolgus macaques, those that develop active TB disease have more lesions in the lung, with increased bacterial burden and dissemination, while those with Mtb infection have fewer lesions and no extrapulmonary involvement. 181 Macaques with active disease or infection both contain granulomas with the capacity for sterilization. Those with active disease simultaneously have localized areas of extensive tissue pathology with bacterial growth as well as sterile granulomas. 181 The observed heterogeneity between granulomas may be a crucial determinant of the infection outcome, as the capacity for bacterial killing by individual lesions dictates the bacterial burden. 32 , 182
Further, genomic barcoding of individual Mtb bacilli reveals that each granuloma in an infected host is seeded by a single organism, with considerable variation in their developmental trajectory and capacity for sterilization. 182 Rather than a globally permissive or restrictive response, each individual granuloma in a host represents a distinct, localized environment. Failure of individual granulomas to contain or eliminate bacteria, while rare, contributes to dissemination and sustained infection with Mtb, with those who eventually clear the infection having sterile granulomas, theoretically with the potential for all granulomas in an individual to be sterile.
Granulomas in macaques capable of sterilization displayed slightly higher production of IL‐17, TNF, and other Th1‐type cytokines by T cells, although most T cells were single‐functional. 33 This suggests that sterilization requires a combination of T cells with different functional profiles. Indeed, sterilization was associated with a combination of pro‐ (IFN‐γ, TNF, IL‐2, IL‐17) and anti‐inflammatory (IL‐10) cytokine production by T cells within the granuloma. 33 Finally, intravenous BCG vaccination of macaques six months prior to challenge with Mtb resulted in almost complete protection. 93 Six of ten macaques had no Mtb in any tissues, and three more had fewer than 45 CFU, all contained within one granuloma. In the lungs of these protected animals, there were an increased proportion of CD3+ T cells, and CD11c+ antigen‐presenting cells. Further, approximately 80% of these T cells were tissue‐derived, and expressed CD69 and CD103, indicating that they may represent tissue‐resident memory T cells induced by intravenous BCG vaccination. Delayed clearance may be mediated through the development and subsequent sterilization of granulomas as seen in cynomolgus macaques, mediated by a combination of innate and adaptive immune mechanisms that alter the trajectory of individual granulomas. Potentiating the local immune responses through vaccination may increase the killing capacity of individual granulomas and contribute to bacterial clearance.
4. IMPLICATIONS FOR DEVELOPMENT AND EVALUATION OF NEW‐GENERATION TB VACCINES
Clearly, improved understanding of the mechanisms underlying BCG‐induced protection and the possible role of trained immunity in early clearance and the prevention of Mtb infection should lead to more effective TB‐preventive strategies, including vaccination. In this section, we discuss preclinical and clinical aspects of development of TB vaccines focused on Mtb infection.
4.1. Early clearance and trained immunity in TB vaccine development
4.1.1. Establishing a biomarker signature for early and delayed clearance
The early and delayed clearance phenotypes represent examples of effective, host‐mediated protection against Mtb infection. In our TB household study in Indonesia, we have found that early clearance is associated with increased ex vivo cytokine production in response to unrelated stimuli, as seen in trained immunity. 167 To further characterize the biosignature of early clearance and to examine whether it indeed has similarities with trained immunity, further immunological phenotyping of circulating innate cell populations and multi‐omics comparison between early clearers and IGRA converters is now ongoing. 3 , 167 Similar phenotyping studies should be performed in other well‐characterized cohorts to further develop and refine a signature of early clearance, which could help unravel possible underlying mechanisms and be used as an indicator of protective efficacy in future vaccine studies.
Many studies in TB have used blood transcriptomics, but to our knowledge, no such studies have specifically focused on Mtb clearance. Somewhat related however, IGRA‐positive individuals in London who received TB‐preventive therapy showed divergent longitudinal blood transcriptomic profiles. 183 One subgroup displayed a similar gene expression profile over time to unexposed, IGRA negative controls, while the second subgroup did not. Differentially expressed genes were largely involved in immune responses, many of which had previously been identified in transcriptomic studies of TB patients vs healthy controls, suggesting a lack of viable Mtb infection in the first subgroup. If confirmed, this signature may represent a biological marker of delayed clearance to be used as a readout for vaccine efficacy studies. Another study examined the transcriptional response of ex vivo stimulated monocytes from Ugandan TB contacts who were either IGRA/TST positive or “resisters.” 184 Differential expression included pathways controlled by histone deacetylases (HDACs), while treatment of monocytes with HDAC inhibitors increased cytokine production in response to Mtb infection. These data and studies that have shown that HDAC inhibitors increase glycolysis and IL‐1β production of human MDMs infected with Mtb, 185 and improve pro‐inflammatory cytokine production and restriction of intracellular Mtb growth by macrophages, 186 suggest that epigenetic and metabolic changes reminiscent of trained immunity 140 are also involved in Mtb clearance.
Proteomic or metabolomic profiling may complement transcriptomic studies and add to our understanding of early and delayed clearance. Plasma proteomic signatures can help distinguish active TB and LTBI, and predict progression of LTBI to active TB. 20 With regard to early clearance, Bark et al. recently applied a proteomic approach in 97 TB household contacts. 187 Using discovery and validation groups, they identified a number of proteins that were upregulated differentially between HIV‐uninfected contacts in Uganda who were “resisters,” compared to contacts who became TST positive. Albeit relatively small, this study clearly shows the potential of proteomics for better understanding of early clearance, and the need for replication in other cohorts. Metabolomic studies in TB have mainly focused on diagnosis of LTBI and active TB, or on predicting progression to active disease, 188 , 189 , 190 but one study in India found significantly higher concentrations of particular metabolites in household contacts compared to controls. 191 This is an interesting result, but unfortunately no data were provided on IGRA/TST among household contacts, so no conclusions can be made regarding early clearance.
Future studies, with stronger data on exposure, infection status, and traditional risk factors (age, smoking, diabetes, etc.) should integrate ‘omics’ data, host genotyping and functional immunological data, 192 as has been done in candidemia for instance. 193 , 194 Finally, early clearance and trained immunity signatures should be compared, to examine what pathways are overlapping. This can help further experimental work, but can also select possible correlates of protection against Mtb infection in TB contact or TB vaccination studies.
4.1.2. Examining trained immunity effects of new TB vaccines
It is largely unknown if new TB vaccine candidates, induce trained immunity similarly to BCG. MTBVAC is the only TB vaccine based on live‐attenuated Mtb that has entered clinical trials, where it demonstrated a similar safety profile to BCG in neonates and adults, along with similar induction of Th1‐type T cell responses. 73 MTBVAC has shown better protection against Mtb infection compared to BCG in mice, 195 and it improved the pre‐existing BCG‐mediated protection in guinea pigs. 196 Interestingly, MTBVAC vaccination also conferred heterologous protection against S. pneumoniae infection, both in wildtype mice and SCID mice that lack T cells and B cells, and increased cytokine responses to LPS by human PBMCs in vitro, providing clear evidence of its trained immunity effects. 197 It is urgent to assess whether MTBVAC vaccination induces trained immunity in humans, and whether this accounts for its protective effects. However, it may be difficult to characterize the effects of MTBVAC on early clearance, because the vaccine contains Mtb antigens and causes IGRA conversion in a dose‐dependent manner, although reversion is common, especially at lower doses. 73 Studies of MTBVAC‐induced protection against Mtb infection may adopt clinical endpoints of sustained IGRA conversion, similar to the definition used by Nemes et al. 6 as IGRA positivity may be vaccine‐induced, rather than reflecting incident infection. Other vaccine candidates besides MTBVAC such as M72/ASO1E, 198 and the adjuvant ASO1 alone should also be examined for their capacity to induce trained immunity, and whether their capacity to do so is associated with protection against Mtb.
4.1.3. Improving vaccine‐induced trained immunity
If markers of trained immunity show a consistent association with early clearance, this may direct future vaccine strategies to enhance the induction of trained immunity by BCG vaccination. From our studies in healthy adult volunteers, it is clear that BCG does not induce trained immunity equally well in all individuals. 113 , 139 , 140 , 152 , 153 It may be possible to modify vaccination strategies to improve the consistency with which vaccines induce trained immunity between individuals. These novel “trained immunity‐based vaccines” may capitalize on innate immunological memory through the incorporation of innate immune adjuvants to already‐existing vaccines such as BCG. 199 Such adjuvants that engage PRRs may amplify the trained immune response already induced by BCG, and because different stimuli induce different cell activation pathways, adjuvants may induce trained immunity pathways beyond those elicited by BCG. 200 If pathways involved in trained immunity show a consistent association with early clearance, this may direct future vaccine strategies to enhance the induction of trained immunity.
There may be ways to enhance the trained immunity effects of TB vaccines. MDP may engage the NOD2 receptor and enhance the activation of this pathway beyond what is achieved by vaccination alone. 111 , 113 , 145 β‐glucan may amplify the vaccine‐induced trained immune response through engagement of the dectin‐1 receptor and enhance anti‐mycobacterial responses as observed in the mouse model of Mtb infection. 168 Further, intravesical BCG in combination with recombinant Th1 cytokines such as IFN‐γ, IL‐2, and GM‐CSF have shown stronger efficacy than BCG alone in inducing anti‐tumour responses and preventing recurrence in patients with bladder cancer. 201 IFN‐γ 202 , 203 , 204 and GM‐CSF 165 , 166 are both known to enhance anti‐mycobacterial activity by monocytes and macrophages, and are also essential for the induction of trained immunity at the level of myeloid precursors in the bone marrow in murine models. 150 , 163 , 164 In mice, BCG strains expressing GM‐CSF have been shown to increase the quantity of pulmonary APCs compared to BCG alone, leading to enhanced T cell responses and improved protection against disseminated Mtb infection. 205 , 206 Further, using DNA vaccine expressing Ag85A and GM‐CSF as a boost following BCG vaccination also improves protection against Mtb infection in mice. 207 These data suggest that incorporating inflammatory mediators in vaccines to modulate the local immune response by enhancing trained immunity may contribute to protection against Mtb infection.
Given that trained immunity is mediated by epigenomic modulation and changes to intracellular metabolism, Dominguez‐Andrés et al. (Mbio, in press) propose incorporating metabolic and epigenetic modulators to amplify vaccine‐induced immune responses. These “amplifiers” would be incorporated in addition to the primary immunogenic antigen or organism, and any adjuvants, ultimately enhancing effector responses and amplifying the induction of trained immunity. Cellular metabolism may be modulated by compounds analogous to metabolites of the TCA cycle, such as succinate and fumarate, leading to inhibition of KDM5 histone demethylase enzymes and improving the deposition of permissive histone marks characteristic of trained immunity. 158 Histone modifying enzymes such as HDACs, histone methyltransferases, and histone acetyltransferases may also be targeted directly to modulate the deposition and removal of histone marks and improve chromatin accessibility at the promoters of pro‐inflammatory genes after vaccination. 208 Modulating the molecular mechanism of trained immunity in this way may amplify the signals induced by vaccination and improve the induction of trained immunity and further enhance protection against Mtb infection.
4.2. Clinical evaluation: Epidemiological and immunological characterization
Essentially, trials of vaccine efficacy against Mtb infection should assess the ability of a vaccine to prevent an initial or subsequent Mtb infection. In an endemic setting, the first/initial infection may occur early in life in most people. However, since infection may be transient, there is likely to be value in assessing the ability of a vaccine to prevent a new infection in anyone who is TST or IGRA negative, especially if they are entering a period or setting whereby they are at increased risk of developing infection and/or disease. For example, children entering into adolescent years are at increased risk of developing TB disease, while it is unclear if they are at increased risk of Mtb infection; and those entering healthcare work in a TB‐endemic setting for the first time are at increased risk of Mtb infection and disease.
4.2.1. Pre‐infection intervention trials
We consider three particular approaches to pre‐infection trials—vaccination at birth, in adolescents, and in healthcare students. There are other populations that are at increased risk of infection, such as people with diabetes and people living with HIV. While it will be important for a new vaccine to show efficacy in these groups, they may not be ideal groups for initial trials because they may have impaired immune responses, leading to lower, non‐generalizable efficacy estimates.
Vaccination at birth
Since BCG is given at birth, it would seem reasonable for new vaccines to be trialed at birth or as an early booster for BCG. In a high TB‐endemic setting, it would be reasonably straightforward to downsize historical BCG trials for an Mtb infection endpoint. A vaccine, or vaccine combination, given at birth could be assessed for efficacy against initial Mtb infection, including with different levels of Mtb exposure in those who become contacts of known TB cases, and in enhancing delayed clearance in those who do develop Mtb infection.
In their long‐term follow‐up of a BCG trial, Mancuso et al. 38 found that approximately 20% of TST converters in the BCG arm underwent TST reversion within five years of a positive TST, compared to less than 5% of TST converters in the placebo arm. Given that some TST conversion may be due to BCG vaccination itself, and these individuals may be expected to more readily revert, differences in IGRA reversion between arms may be smaller than differences in TST reversion.
Vaccination of adolescents
Vaccination of adolescents is a strategy adopted in South Africa by Nemes et al. 6 A full confirmatory trial has been funded by the Bill and Melinda Gates Foundation. This is essentially a trial of BCG re‐vaccination in a high burden/high incidence country. While the adolescents need to be IGRA negative for randomization, because of the limitations of any test for Mtb infection, and the high exposure setting, it is likely that a significant proportion have been previously exposed, infected, or even remain infected. Therefore, this study should be seen as reflecting protection against a new exposure and new infection, in the context of likely exposures in the past. The South African confirmatory trial in approximately 1800 randomized adolescents will assess the efficacy of BCG against the primary endpoint of sustained IGRA conversion based on an IFN‐γ concentration cutoff value of 0.35 IU/mL (A. Schmidt, personal communication).
Vaccination of healthcare students
One approach is to identify a study population transitioning into a high exposure situation. The obvious population for this is healthcare worker trainees. In Indonesia we have identified that nursing and medical students entering clinical training are such a group. They are also knowledgeable and motivated to find solutions in relation to their exposure to Mtb. In an initial study (Apriani et al., submitted for publication) we enrolled 379 students entering clinical training into a cohort study; 70 (18.5%) were IGRA positive at baseline. Of 293 IGRA negative students tested at one year, 26 (8.9%) underwent IGRA conversion. Participation in sputum collection or bronchoscopy procedures were significantly associated with IGRA conversion. We will now proceed to a proof‐of‐principle randomized controlled trial of BCG re‐vaccination, incorporating multiple IGRA tests over a 12‐month follow‐up period, and integrated sampling and bio‐archiving for immunological studies focused on innate immune training.
It is possible to provide an estimate of the required size for a trial of a vaccine against Mtb infection in healthcare students. In the study of healthcare students, the adjusted relative risk of IGRA conversion for those who were BCG vaccinated was 0.68, with relatively wide confidence intervals in keeping with the size of the study (95% CI 0.25‐1.83). Using a more stringent cutoff for conversion, there were 24 converters and the adjusted relative risk for IGRA conversion among BCG vaccinated students was 0.53 (0.19‐1.53). Assuming the absolute proportion of initially IGRA negative students who are IGRA positive at 12 months is 0.09, and the proportion of students that have IGRA conversion at any time before that which converts to negative at 12 months is 0.05, the cumulative proportion of students with IGRA conversion is estimated to be 0.14 in the placebo arm. With a conservative estimate of 30% protection, the cumulative proportion of IGRA converters in the BCG intervention arm is estimated to be 0.098. Assuming 95% completion, with 1300 individuals in each arm, there would be 90% power to detect this difference at the p = 0.05 (two‐sided) level of significance. If the efficacy is 40% or greater, the numbers required in each arm would be well under 1000 students.
4.2.2. Post‐infection intervention trials
On balance, our judgment is that there is not enough evidence that a post‐infection vaccine will promote delayed clearance of Mtb to warrant designing trials of post‐infection vaccines focused on a delayed clearance endpoint. However, such a trial would enrol those who have evidence of Mtb infection and randomize them to vaccine or placebo. Considering that post‐infection vaccines are currently in development, it would be relatively straightforward to include repeated tests for Mtb infection as part of follow‐up.
4.2.3. Sampling in trials to facilitate interlocking microbiological and immunological studies
In intervention studies of early clearance in humans, it is essential that samples are collected before, and at various time points after intervention to capture peak responsiveness for immunological and epigenomic or transcriptomic analysis. Typically, to facilitate sample collection from a large number of individuals, responses will be measured in peripheral blood. While it must be acknowledged that peripheral blood responses do not robustly reflect the local responses in the lungs, 33 if an intervention is demonstrated to have a protective effect on infection with Mtb, peripheral blood represents a valuable, easily accessible resource to identify biomarkers that are correlated with increased protection or susceptibility to infection. Immunological assays may be performed on peripheral blood samples to assess changes in immune cell populations, cytokine responsiveness, and the capacity for mycobacterial growth inhibition. Other assays may test for whole‐blood or cell‐specific transcriptomic, metabolic, and epigenomic changes. These methods allow for an evaluation of the biological response to any intervention that may correlate with susceptibility or protection against Mtb infection. Trials in humans should be conducted in a variety of locations, particularly those where a high enough proportion of Mtb strains are of the Beijing genotype family.
5. CONCLUDING REMARKS
The discovery that BCG is likely to protect against Mtb infection and not just progression from infection to TB disease has presented new opportunities to characterize this protection, identify associated protective immune responses, and to design and assess possible interventions. Key opportunities to improve on BCG protection against Mtb infection include the duration of the effect, susceptibility to high pathogen infecting dose, and protection across different Mtb strains. The infection phenotypes discussed in this review allow for robust definition and analysis of epidemiological cohorts with known, quantifiable exposure levels to Mtb. It is likely that IFN‐γ‐independent and non‐conventional T cells, humoral immunity, and innate immune mechanisms including BCG‐induced trained immunity all contribute to early clearance of Mtb, and examining their role in early clearance may identify correlates of immune‐mediated protection against Mtb to inform future vaccination strategies. Such vaccines may aim specifically to boost trained immunity responses through inflammatory mediators and amplifiers. Further, identifying correlates of protection may aid in the development of a well‐defined biomarker signature of early clearance which, if validated, may be used as an endpoint in future vaccine efficacy trials, enabling evaluation of new or modified vaccines at a lower cost, reducing the necessity for large trials with clinical endpoints.
CONFLICT OF INTEREST
We have no conflict of interest to declare.
Foster M, Hill PC, Setiabudiawan TP, Koeken VACM, Alisjahbana B, van Crevel R. BCG‐induced protection against Mycobacterium tuberculosis infection: Evidence, mechanisms, and implications for next‐generation vaccines. Immunol Rev. 2021;301:122–144. 10.1111/imr.12965
This article is part of a series of reviews covering Immunity to Mycobacteria appearing in Volume 301 of Immunological Reviews.
REFERENCES
- 1. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):1339–1345. [DOI] [PubMed] [Google Scholar]
- 2. Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta‐analysis. BMJ. 2014;349:g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verrall AJ, Alisjahbana B, Apriani L, et al. Early clearance of Mycobacterium tuberculosis: the INFECT case contact cohort study in Indonesia. J Infect Dis. 2020;221(8):1351–1360. [DOI] [PubMed] [Google Scholar]
- 4. Lu LL, Smith MT, Yu KKQ, et al. IFN‐γ‐independent immune markers of Mycobacterium tuberculosis exposure. Nat Med. 2019;25(6):977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koeken V, Verrall AJ, Netea MG, Hill PC, van Crevel R. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin Microbiol Infect. 2019;25(12):1468–1472. [DOI] [PubMed] [Google Scholar]
- 6. Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Modlin RL, Bloom BR. TB or not TB: that is no longer the question. Sci Transl Med. 2013;5(213):213sr6. [DOI] [PubMed] [Google Scholar]
- 8. Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17(5):183–188. [DOI] [PubMed] [Google Scholar]
- 9. Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141(4):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israel HL, Hetherington HW, Ord JG. A study of tuberculosis among students of nursing. JAMA. 1941;117(10):839–844. [Google Scholar]
- 11. Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16(1):26–35. [DOI] [PubMed] [Google Scholar]
- 12. Stein CM, Zalwango S, Malone LL, et al. Resistance and susceptibility to Mycobacterium tuberculosis infection and disease in tuberculosis households in Kampala, Uganda. Am J Epidemiol. 2018;187(7):1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou G, Luo Q, Luo S, et al. Interferon‐γ release assays or tuberculin skin test for detection and management of latent tuberculosis infection: a systematic review and meta‐analysis. Lancet Infect Dis. 2020;20(12):1457–1469. [DOI] [PubMed] [Google Scholar]
- 14. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99(2):131–138. [DOI] [PubMed] [Google Scholar]
- 15. Hill PC, Brookes RH, Fox A, et al. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Medicine. 2007;4(6):e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandiviere HM, Loring WE, Melvin I, Willis S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am J Med Sci. 1956;232(1):30–37. [DOI] [PubMed] [Google Scholar]
- 17. Hill PC, Ota MO. Tuberculosis case‐contact research in endemic tropical settings: design, conduct, and relevance to other infectious diseases. Lancet Infect Dis. 2010;10(10):723–732. [DOI] [PubMed] [Google Scholar]
- 18. Suliman S, Thompson EG, Sutherland J, et al. Four‐gene pan‐African blood signature predicts progression to tuberculosis. Am J Respir Crit Care Med. 2018;197(9):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zak DE, Penn‐Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet (London, England). 2016;387(10035):2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penn‐Nicholson A, Hraha T, Thompson EG, et al. Discovery and validation of a prognostic proteomic signature for tuberculosis progression: a prospective cohort study. PLoS Medicine. 2019;16(4):e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Behr MA, Edelstein PH, Ramakrishnan L. Is Mycobacterium tuberculosis infection life long? BMJ. 2019;367:l5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narain R, Nair SS, Rao GR, Chandrasekhar P. Distribution of tuberculous infection and disease among households in a rural community. Bull World Health Organ. 1966;34(4):639–654. [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson DF, Malone LL, Zalwango S, et al. Tuberculin skin test reversion following isoniazid preventive therapy reflects diversity of immune response to primary Mycobacterium tuberculosis infection. PLoS One. 2014;9(5):e96613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125(3 Pt 2):8–15. [DOI] [PubMed] [Google Scholar]
- 25. Horwitz O, Wilbek E, Erickson PA. Epidemiological basis of tuberculosis eradication. 10. Longitudinal studies on the risk of tuberculosis in the general population of a low‐prevalence area. Bull World Health Organ. 1969;41(1):95–113. [PMC free article] [PubMed] [Google Scholar]
- 26. Wiker HG, Mustafa T, Bjune GA, Harboe M. Evidence for waning of latency in a cohort study of tuberculosis. BMC Infect Dis. 2010;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drobniewski F, Tayler E, Ignatenko N, et al. Tuberculosis in Siberia: 1. An epidemiological and microbiological assessment. Tuber Lung Dis. 1996;77(3):199–206. [DOI] [PubMed] [Google Scholar]
- 28. Heimbeck J. Tuberculous infection: attempts to prevent it by subcutaneous vaccination with BCG. Arch Intern Med. 1931;47(6):901–916. [Google Scholar]
- 29. Adetifa IM, Ota MOC, Jeffries DJ, et al. Interferon‐γ ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am J Respir Crit Care Med. 2013;187(4):439–445. [DOI] [PubMed] [Google Scholar]
- 30. Biraro IA, Egesa M, Kimuda S, et al. Effect of isoniazid preventive therapy on immune responses to Mycobacterium tuberculosis: an open label randomised, controlled, exploratory study. BMC Infect Dis. 2015;15:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin PL, Coleman T, Carney JPJ, et al. Radiologic responses in cynomolgus macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother. 2013;57(9):4237–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin CJ, Cadena AM, Leung VW, et al. Digitally barcoding Mycobacterium tuberculosis reveals in vivo infection dynamics in the macaque model of tuberculosis. MBio. 2017;8(3):e00312–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gideon HP, Phuah JiaYao, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro‐ and anti‐inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11(1):e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malherbe ST, Kleynhans L, Walzl G. The potential of imaging tools as correlates of infection and disease for new TB vaccine development. Semin Immunol. 2018;39:73–80. [DOI] [PubMed] [Google Scholar]
- 35. Malherbe ST, Shenai S, Ronacher K, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med. 2016;22(10):1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soysal A, Millington KA, Bakir M, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community‐based study. Lancet. 2005;366(9495):1443–1451. [DOI] [PubMed] [Google Scholar]
- 37. Gao L, Li X, Liu J, et al. Incidence of active tuberculosis in individuals with latent tuberculosis infection in rural China: follow‐up results of a population‐based, multicentre, prospective cohort study. Lancet Infect Dis. 2017;17(10):1053–1061. [DOI] [PubMed] [Google Scholar]
- 38. Mancuso JD, Mody RM, Olsen CH, et al. The long‐term effect of Bacille Calmette‐Guérin vaccination on tuberculin skin testing: a 55‐year follow‐up study. Chest. 2017;152(2):282–294. [DOI] [PubMed] [Google Scholar]
- 39. Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta‐analysis. Lancet Infect Dis. 2006;6(3):162–170. [DOI] [PubMed] [Google Scholar]
- 40. Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2(3):200–207. [PubMed] [Google Scholar]
- 41. Mangtani P, Nguipdop‐Djomo P, Keogh RH, et al. The duration of protection of school‐aged BCG vaccination in England: a population‐based case‐control study. Int J Epidemiol. 2018;47(1):193–201. [DOI] [PubMed] [Google Scholar]
- 42. Nguipdop‐Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population‐based cohort study. Lancet Infect Dis. 2016;16(2):219–226. [DOI] [PubMed] [Google Scholar]
- 43. Campbell N, Verrall AJ, Donkor S, Sutherland JS, Hill PC. BCG protection against Mycobacterium tuberculosis infection by level of exposure in The Gambia. J Infect Dis. 2020;223(4):719–720. [DOI] [PubMed] [Google Scholar]
- 44. Brailey M. A study of tuberculous infection and mortality in the children of tuberculous households. Am J Epidemiol. 1940;31‐SectionA(1):1–43. [Google Scholar]
- 45. Koesoemadinata RC, McAllister SM, Soetedjo NNM, et al. Latent TB infection and pulmonary TB disease among patients with diabetes mellitus in Bandung, Indonesia. Trans R Soc Trop Med Hyg. 2017;111(2):81–89. [DOI] [PubMed] [Google Scholar]
- 46. Lee MR, Huang YP, Kuo YT, et al. Diabetes mellitus and latent tuberculosis infection: a systematic review and metaanalysis. Clin Infect Dis. 2017;64(6):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Comstock GW. Tuberculosis in twins: a re‐analysis of the Prophit survey. Am Rev Respir Dis. 1978;117(4):621–624. [DOI] [PubMed] [Google Scholar]
- 48. Stein CM, Sausville L, Wejse C, et al. Genomics of human pulmonary tuberculosis: from genes to pathways. Curr Genet Med Rep. 2017;5(4):149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chimusa ER, Zaitlen N, Daya M, et al. Genome‐wide association study of ancestry‐specific TB risk in the South African coloured population. Hum Mol Genet. 2014;23(3):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curtis J, Luo Y, Zenner HL, et al. Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat Genet. 2015;47(5):523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sobota R, Stein C, Kodaman N, et al. A locus at 5q33.3 confers resistance to tuberculosis in highly susceptible individuals. Am J Hum Genet. 2016;98(3):514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stein CM, Zalwango S, Malone LL, et al. Genome scan of M. tuberculosis infection and disease in Ugandans. PLoS One. 2008;3(12):e4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thye T, Owusu‐Dabo E, Vannberg FO, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet. 2012;44(3):257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thye T, Vannberg FO, Wong SH, et al. Genome‐wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet. 2010;42(9):739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sepulveda RL, Heiba IM, King A, et al. Evaluation of tuberculin reactivity in BCG‐immunized siblings. Am J Respir Crit Care Med. 1994;149(3 Pt 1):620–624. [DOI] [PubMed] [Google Scholar]
- 56. Cobat A, Barrera LF, Henao H, et al. Tuberculin skin test reactivity is dependent on host genetic background in Colombian tuberculosis household contacts. Clin Infect Dis. 2012;54(7):968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cobat A, Gallant CJ, Simkin L, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206(12):2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cobat A, Hoal EG, Gallant CJ, et al. Identification of a major locus, TNF1, that controls BCG‐triggered tumor necrosis factor production by leukocytes in an area hyperendemic for tuberculosis. Clin Infect Dis. 2013;57(7):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Igo RP, Hall NB, Malone LL, et al. Fine‐mapping analysis of a chromosome 2 region linked to resistance to mycobacterium tuberculosis infection in Uganda reveals potential regulatory variants. Genes Immun. 2019;20(6):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall NB, Igo RP, Malone LL, et al. Polymorphisms in TICAM2 and IL1B are associated with TB. Genes Immun. 2015;16(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sveinbjornsson G, Gudbjartsson DF, Halldorsson BV, et al. HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nat Genet. 2016;48(3):318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Verrall AJ, Chaidir L, Ruesen C, et al. Lower Bacillus Calmette‐Guérin protection against Mycobacterium tuberculosis infection after exposure to Beijing strains. Am J Respir Crit Care Med. 2020;201(9):1152–1155. [DOI] [PubMed] [Google Scholar]
- 63. Tsenova L, Fallows D, Kolloli A, et al. Inoculum size and traits of the infecting clinical strain define the protection level against Mycobacterium tuberculosis infection in a rabbit model. Eur J Immunol. 2020;50(6):858–872. [DOI] [PubMed] [Google Scholar]
- 64. Subbian S, Singh P, Kolloli A, et al. BCG vaccination of infants confers Mycobacterium tuberculosis strain‐specific immune responses by leukocytes. ACS Infect Dis. 2020;6(12):3141–3146. [DOI] [PubMed] [Google Scholar]
- 65. Andersen P, Scriba TJ. Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol. 2019;19(9):550–562. [DOI] [PubMed] [Google Scholar]
- 66. Steigler P, Verrall AJ, Kirman JR. Beyond memory T cells: mechanisms of protective immunity to tuberculosis infection. Immunol Cell Biol. 2019;97(7):647–655. [DOI] [PubMed] [Google Scholar]
- 67. Ndiaye BP, Thienemann F, Ota M, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV‐1: a randomised, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2015;3(3):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo‐controlled phase 2b trial. Lancet. 2013;381(9871):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nieuwenhuizen NE, Kulkarni PS, Shaligram U, et al. The recombinant Bacille Calmette‐Guérin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol. 2017;8:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grode L, Ganoza CA, Brohm C, et al. Safety and immunogenicity of the recombinant BCG vaccine VPM10002 in a phase 1 open‐label randomized clinical trial. Vaccine. 2013;31(9):1340–1348. [DOI] [PubMed] [Google Scholar]
- 71. Loxton AG, Knaul JK, Grode L, et al. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV‐unexposed newborn infants in South Africa. Clin Vaccine Immunol. 2017;24(2):e00439–e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arbues A, Aguilo JI, Gonzalo‐Asensio J, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live‐attenuated M. tuberculosis‐based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–4873. [DOI] [PubMed] [Google Scholar]
- 73. Tameris M, Mearns H, Penn‐Nicholson A, et al. Live‐attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double‐blind dose‐escalation trial. Lancet Respir Med. 2019;7(9):757–770. [DOI] [PubMed] [Google Scholar]
- 74. Van Der Meeren O, Hatherill M, Nduba V, et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2018;379(17):1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sable SB, Posey JE, Scriba TJ. Tuberculosis vaccine development: progress in clinical evaluation. Clin Microbiol Rev. 2019;33(1):e00100‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schrager LK, Vekemens J, Drager N, Lewinsohn DM, Olesen OF. The status of tuberculosis vaccine development. Lancet Infect Dis. 2020;20(3):e28–e37. [DOI] [PubMed] [Google Scholar]
- 77. Rozot V, Nemes E, Geldenhuys H, et al. Multidimensional analyses reveal modulation of adaptive and innate immune subsets by tuberculosis vaccines. Commun Biol. 2020;3(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG‐specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010;78(10):4187–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gallegos AM, van Heijst JW, Samstein M, et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo . PLoS Pathog. 2011;7(5):e1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liang SC, Long AJ, Bennett F, et al. An IL‐17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–7799. [DOI] [PubMed] [Google Scholar]
- 81. Gold MC, Napier RJ, Lewinsohn DM. MR1‐restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis . Immunol Rev. 2015;264(1):154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sharma M, Zhang S, Niu L, et al. Mucosal‐associated invariant T cells develop an innate‐like transcriptomic program in anti‐mycobacterial responses. Front Immunol. 2020;11:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meraviglia S, El Daker S, Dieli F, Martini F, Martino A. γδ T cells cross‐link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:587315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dieli F, Troye‐Blomberg M, Ivanyi J, et al. Granulysin‐dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184(8):1082–1085. [DOI] [PubMed] [Google Scholar]
- 85. Vorkas CK, Wipperman MF, Li K, et al. Mucosal‐associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight. 2018;3(19):e121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kawahara JY, Irvine EB, Alter G. A case for antibodies as mechanistic correlates of immunity in tuberculosis. Front Immunol. 2019;10:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lu LL, Chung AW, Rosebrock TR, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–43.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lu LL, Das J, Grace PS, et al. Antibody Fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis. 2020;222(12):2093–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li H, Wang XX, Wang B, et al. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis . Proc Natl Acad Sci USA. 2017;114(19):5023–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kroon EE, Kinnear CJ, Orlova M, et al. An observational study identifying highly tuberculosis‐exposed, HIV‐1‐positive but persistently TB, tuberculin and IGRA negative persons with M. tuberculosis specific antibodies in Cape Town, South Africa. EBioMedicine. 2020;61:103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. [DOI] [PubMed] [Google Scholar]
- 92. Simmons JD, Stein CM, Seshadri C, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018;18(9):575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577(7788):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. The Lancet. 2016;387(10024):1211–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cai Y, Sugimoto C, Arainga M, et al. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol. 2014;192(6):2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Choreño‐Parra JA, Weinstein LI, Yunis EJ, Zúñiga J, Hernández‐Pando R. Thinking outside the box: innate‐ and B cell‐memory responses as novel protective mechanisms against tuberculosis. Front Immunol. 2020;11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yona S, Kim K‐W, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wolf AJ, Linas B, Trevejo‐Nuñez GJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo . J Immunol. 2007;179(4):2509–2519. [DOI] [PubMed] [Google Scholar]
- 99. Hossain MM, Norazmi MN. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection–the double‐edged sword? Biomed Res Int. 2013;2013:179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis . Clin Dev Immunol. 2011;2011:405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Queval CJ, Brosch R, Simeone R. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis . Front Microbiol. 2017;8:2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll‐like receptor‐2 mediates mycobacteria‐induced pro‐inflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96(25):14459–14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kleinnijenhuis J, Joosten LA, van de Veerdonk FL, et al. Transcriptional and inflammasome‐mediated pathways for the induction of IL‐1beta production by Mycobacterium tuberculosis . Eur J Immunol. 2009;39(7):1914–1922. [DOI] [PubMed] [Google Scholar]
- 104. Bulut Y, Michelsen KS, Hayrapetian L, et al. Mycobacterium tuberculosis heat shock proteins use diverse toll‐like receptor pathways to activate pro‐inflammatory signals. J Biol Chem. 2005;280(22):20961–20967. [DOI] [PubMed] [Google Scholar]
- 105. Abel B, Thieblemont N, Quesniaux VJ, et al. Toll‐like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169(6):3155–3162. [DOI] [PubMed] [Google Scholar]
- 106. Bafica A, Scanga CA, Feng CG, et al. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis . J Exp Med. 2005;202(12):1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fremond CM, Togbe D, Doz E, et al. IL‐1 receptor‐mediated signal is an essential component of MyD88‐dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179(2):1178–1189. [DOI] [PubMed] [Google Scholar]
- 108. Fremond CM, Yeremeev V, Nicolle DM, et al. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114(12):1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reiling N, Hölscher C, Fehrenbach A, et al. Cutting edge: toll‐like receptor (TLR)2‐ and TLR4‐mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis . J Immunol. 2002;169(7):3480–3484. [DOI] [PubMed] [Google Scholar]
- 110. Ferwerda G, Girardin SE, Kullberg B‐J, et al. NOD2 and toll‐like receptors are nonredundant recognition systems of Mycobacterium tuberculosis . PLoS Pathog. 2005;1(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ferwerda G, Kullberg BJ, de Jong DJ, et al. Mycobacterium paratuberculosis is recognized by toll‐like receptors and NOD2. J Leukoc Biol. 2007;82(4):1011–1018. [DOI] [PubMed] [Google Scholar]
- 112. Brooks MN, Rajaram MVS, Azad AK, et al. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13(3):402–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette‐Guérin induces NOD2‐dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martin C, Booty M, Rosebrock T, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12(3):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Immunol. 2010;8(9):668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol. 2014;26(6):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sugawara I, Udagawa T, Yamada H. Rat neutrophils prevent the development of tuberculosis. Infect Immuno. 2004;72(3):1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pedrosa J, Saunders BM, Appelberg R, et al. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68(2):577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. O'Garra A, Redford PS, McNab FW, et al. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. [DOI] [PubMed] [Google Scholar]
- 120. Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil‐mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177(3):1864–1871. [DOI] [PubMed] [Google Scholar]
- 122. Lowe DM, Demaret J, Bangani N, et al. Differential effect of viable versus necrotic neutrophils on Mycobacterium tuberculosis growth and cytokine induction in whole blood. Front Immunol. 2018;9:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Coulter F, Parrish A, Manning D, et al. IL‐17 production from T helper 17, mucosal‐associated invariant T, and γδ cells in tuberculosis infection and disease. Front Immunol. 2017;8:1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brill KJ, Li Q, Larkin R, et al. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule‐independent mechanisms. Infect Immun. 2001;69(3):1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Guerra C, Johal K, Morris D, et al. Control of Mycobacterium tuberculosis growth by activated natural killer cells. Clin Exp Immunol. 2012;168(1):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Prager I, Watzl C. Mechanisms of natural killer cell‐mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319–1329. [DOI] [PubMed] [Google Scholar]
- 127. Lu C‐C, Wu T‐S, Hsu Y‐J, et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J Leukoc Biol. 2014;96(6):1119–1129. [DOI] [PubMed] [Google Scholar]
- 128. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG‐induced trained immunity in NK cells: role for non‐specific protection to infection. Clin Immunol. 2014;155(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. de Bree L, Koeken VACM, Joosten LAB, et al. Non‐specific effects of vaccines: current evidence and potential implications. Semin Immunol. 2018;39:35–43. [DOI] [PubMed] [Google Scholar]
- 131. O'Neill LAJ, Netea MG. BCG‐induced trained immunity: can it offer protection against COVID‐19? Nat Rev Immunol. 2020;20(6):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Escobar LE, Molina‐Cruz A, Barillas‐Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci USA. 2020;117(30):17720–17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wassenaar TM, Buzard GS, Newman DJ. BCG vaccination early in life does not improve COVID‐19 outcome of elderly populations, based on nationally reported data. Lett Appl Microbiol. 2020;71(5):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Junqueira‐Kipnis AP, dos Anjos LRB, Barbosa LCdS, et al. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID‐19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Netea MG, Domínguez‐Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. [DOI] [PubMed] [Google Scholar]
- 137. Arts RJW, Blok BA, Aaby P, et al. Long‐term in vitro and in vivo effects of γ‐irradiated BCG on innate and adaptive immunity. J Leukoc Biol. 2015;98(6):995–1001. [DOI] [PubMed] [Google Scholar]
- 138. Arts RJW, Blok BA, van Crevel R, et al. Vitamin A induces inhibitory histone methylation modifications and down‐regulates trained immunity in human monocytes. J Leukoc Biol. 2015;98(1):129–136. [DOI] [PubMed] [Google Scholar]
- 139. Arts RJW, Carvalho A, La Rocca C, et al. Immunometabolic pathways in BCG‐induced trained immunity. Cell Rep. 2016;17(10):2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Arts RJW, Moorlag SJCFM, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. [DOI] [PubMed] [Google Scholar]
- 141. Cirovic B, de Bree LCJ, Groh L, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322–34.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Giamarellos‐Bourboulis EJ, Tsilika M, Moorlag S, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–23.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kleinnijenhuis J, Quintin J, Preijers F, et al. Long‐lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6(2):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Koeken VA, de Bree LCJ, Mourits VP, et al. BCG vaccination in humans inhibits systemic inflammation in a sex‐dependent manner. J Clin Invest. 2020;130(10):5591–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mourits VP, Koeken V, de Bree LCJ, et al. BCG‐induced trained immunity in healthy individuals: the effect of plasma muramyl dipeptide concentrations. J Immunol Res. 2020;2020:5812743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jensen KJ, Larsen N, Biering‐Sørensen S, et al. Heterologous immunological effects of early BCG vaccination in low‐birth‐weight infants in Guinea‐Bissau: a randomized‐controlled trial. J Infect Dis. 2015;211(6):956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Smith SG, Kleinnijenhuis J, Netea MG, Dockrell HM. Whole blood profiling of Bacillus Calmette‐Guérin‐induced trained innate immunity in infants identifies epidermal growth factor, IL‐6, platelet‐derived growth factor‐AB/BB, and natural killer cell activation. Front Immunol. 2017;8:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Freyne B, Messina NL, Donath S, et al. Neonatal BCG vaccination reduces interferon‐γ responsiveness to heterologous pathogens in infants from a randomized controlled trial. J Infect Dis. 2020;221(12):1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Coulombe F, Divangahi M, Veyrier F, et al. Increased NOD2‐mediated recognition of n‐glycolyl muramyl dipeptide. J Exp Med. 2009;206(8):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kaufmann E, Sanz J, Dunn JL, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1–2):176–90.e19. [DOI] [PubMed] [Google Scholar]
- 151. van der Heijden CDCC, Noz MP, Joosten LAB, et al. Epigenetics and trained immunity. Antioxid Redox Signal. 2018;29(11):1023–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG‐induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10(10):e1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Verma D, Parasa VR, Raffetseder J, et al. Anti‐mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG‐vaccinated subjects. Sci Rep. 2017;7(1):12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hasso‐Agopsowicz M, Scriba TJ, Hanekom WA, Dockrell HM, Smith SG. Differential DNA methylation of potassium channel KCa3.1 and immune signalling pathways is associated with infant immune responses following BCG vaccination. Sci Rep. 2018;8(1):13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fanucchi S, Domínguez‐Andrés J, Joosten LAB, Netea MG, Mhlanga MM. The intersection of epigenetics and metabolism in trained immunity. Immunity. 2020;S1074–7613(20):30452. [DOI] [PubMed] [Google Scholar]
- 156. Fanucchi S, Fok ET, Dalla E, et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet. 2019;51(1):138–150. [DOI] [PubMed] [Google Scholar]
- 157. Bekkering S, Blok BA, Joosten LA, et al. In vitro experimental model of trained innate immunity in human primary monocytes. Clin Vaccine Immunol. 2016;23(12):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Arts RJW, Novakovic B, ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hole CR, Wager CML, Castro‐Lopez N, et al. Induction of memory‐like dendritic cell responses in vivo . Nat Commun. 2019;10(1):2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Joosten SA, van Meijgaarden KE, Arend SM, et al. Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest. 2018;128(5):1837–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Moorlag SJCFM, Rodriguez‐Rosales YA, Gillard J, et al. BCG vaccination induces long‐term functional reprogramming of human neutrophils. Cell Rep. 2020;33(7):108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Patel AA, Zhang Y, Fullerton JN, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214(7):1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mitroulis I, Ruppova K, Wang B, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172(1–2):147–61.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Walachowski S, Tabouret G, Fabre M, Foucras G. Molecular analysis of a short‐term model of β‐glucans‐trained immunity highlights the accessory contribution of GM‐CSF in priming mouse macrophages response. Front Immunol. 2017;8:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bryson BD, Rosebrock TR, Tafesse FG, et al. Heterogeneous gm‐csf signaling in macrophages is associated with control of Mycobacterium tuberculosis . Nat Commun. 2019;10(1):2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mishra A, Singh VK, Actor JK, et al. GM‐CSF dependent differential control of Mycobacterium tuberculosis infection in human and mouse macrophages: is macrophage source of GM‐CSF critical to tuberculosis immunity? Front Immunol. 2020;11:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Verrall AJ, Schneider M, Alisjahbana B, et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J Infect Dis. 2020;221(8):1342–1350. [DOI] [PubMed] [Google Scholar]
- 168. Moorlag SJCFM, Khan N, Novakovic B, et al. β‐glucan induces protective trained immunity against Mycobacterium tuberculosis infection: a key role for IL‐1. Cell Rep. 2020;31(7):107634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Eklund D, Welin A, Andersson H, et al. Human gene variants linked to enhanced NLRP3 activity limit intramacrophage growth of Mycobacterium tuberculosis . J Infect Dis. 2014;209(5):749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mishra BB, Moura‐Alves P, Sonawane A, et al. Mycobacterium tuberculosis protein ESAT‐6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12(8):1046–1063. [DOI] [PubMed] [Google Scholar]
- 171. Welin A, Eklund D, Stendahl O, Lerm M. Human macrophages infected with a high burden of ESAT‐6‐expressing M. tuberculosis undergo caspase‐1‐ and cathepsin B‐independent necrosis. PLoS One. 2011;6(5):e20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Khan N, Downey J, Sanz J, et al. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell. 2020;183(3):752–70.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Walk J, de Bree LCJ, Graumans W, et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun. 2019;10(1):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Li Y, Oosting M, Smeekens SP, et al. A functional genomics approach to understand variation in cytokine production in humans. Cell. 2016;167(4):1099–110.e14. [DOI] [PubMed] [Google Scholar]
- 175. Das J, Verma D, Gustafsson M, Lerm M. Identification of DNA methylation patterns predisposing for an efficient response to BCG vaccination in healthy BCG‐naïve subjects. Epigenetics. 2019;14(6):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. de Bree LCJ, Mourits VP, Koeken VA, et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Invest. 2020;130(10):5603–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Schaltz‐Buchholzer F, Bjerregaard‐Andersen M, Øland CB, et al. Early vaccination with Bacille Calmette‐Guérin‐Denmark or BCG‐Japan versus BCG‐Russia to healthy newborns in Guinea‐Bissau: a randomized controlled trial. Clin Infect Dis. 2020;71(8):1883–1893. [DOI] [PubMed] [Google Scholar]
- 178. Mukherjee S, Subramaniam R, Chen H, et al. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS One. 2017;12(7):e0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Spencer JC, Ganguly R, Waldman RH. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette‐Guérin. J Infect Dis. 1977;136(2):171–175. [DOI] [PubMed] [Google Scholar]
- 180. de Bree LCJ, Marijnissen RJ, Kel JM, et al. Bacillus Calmette‐Guérin‐induced trained immunity is not protective for experimental influenza A/Anhui/1/2013 (H7N9) infection in mice. Front Immunol. 2018;9:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77(10):4631–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lin PL, Ford CB, Coleman MT, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within‐host variability in bacterial killing. Nat Med. 2014;20(1):75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Broderick C, Cliff JM, Lee J‐S, Kaforou M, Moore DA. Host transcriptional response to TB preventive therapy differentiates two sub‐groups of IGRA‐positive individuals. bioRxiv. 2020;2020(07):20.202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Seshadri C, Sedaghat N, Campo M, et al. Transcriptional networks are associated with resistance to Mycobacterium tuberculosis infection. PLoS One. 2017;12(4):e0175844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Cox DJ, Coleman AM, Gogan KM, et al. Inhibiting histone deacetylases in human macrophages promotes glycolysis, IL‐1β, and T helper cell responses to Mycobacterium tuberculosis . Front Immunol. 2020;11:1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Moreira JD, Koch BEV, van Veen S, et al. Functional inhibition of host histone deacetylases (HDACs) enhances in vitro and in vivo anti‐mycobacterial activity in human macrophages and in zebrafish. Front Immunol. 2020;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bark CM, Manceur AM, Malone LL, et al. Identification of host proteins predictive of early stage Mycobacterium tuberculosis infection. EBioMedicine. 2017;21:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Duffy FJ, Weiner J, Hansen S, et al. Immunometabolic signatures predict risk of progression to active tuberculosis and disease outcome. Front Immunol. 2019;10:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. van Laarhoven A, Dian S, Aguirre‐Gamboa R, et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis. 2018;18(5):526–535. [DOI] [PubMed] [Google Scholar]
- 190. Weiner J, Maertzdorf J, Sutherland JS, et al. Metabolite changes in blood predict the onset of tuberculosis. Nature Commun. 2018;9(1):5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Albors‐Vaquer A, Rizvi A, Matzapetakis M, et al. Active and prospective latent tuberculosis are associated with different metabolomic profiles: clinical potential for the identification of rapid and non‐invasive biomarkers. Emerg Microbes Infect. 2020;9(1):1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Netea MG, Joosten LAB, Li Y, et al. Understanding human immune function using the resources from the human functional genomics project. Nature Med. 2016;22(8):831–833. [DOI] [PubMed] [Google Scholar]
- 193. Jaeger M, Matzaraki V, Aguirre‐Gamboa R, et al. A genome‐wide functional genomics approach identifies susceptibility pathways to fungal bloodstream infection in humans. J Infect Dis. 2019;220(5):862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Smeekens SP, Ng A, Kumar V, et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans . Nat Commun. 2013;4:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Aguilo N, Uranga S, Marinova D, et al. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis. 2016;96:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Clark S, Lanni F, Marinova D, et al. Revaccination of guinea pigs with the live attenuated Mycobacterium tuberculosis vaccine MTBVAC improves BCG’s protection against tuberculosis. J Infect Dis. 2017;216(5):525–533. [DOI] [PubMed] [Google Scholar]
- 197. Tarancón R, Domínguez‐Andrés J, Uranga S, et al. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog. 2020;16(4):e1008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Tait DR, Hatherill M, Van Der Meeren O, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2019;381(25):2429–2439. [DOI] [PubMed] [Google Scholar]
- 199. Sánchez‐Ramón S, Conejero L, Netea MG, et al. Trained immunity‐based vaccines: a new paradigm for the development of broad‐spectrum anti‐infectious formulations. Front Immunol. 2018;9:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Angelidou A, Diray‐Arce J, Conti MG, et al. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Luo Y, Henning J, O'Donnell MA. Th1 cytokine‐secreting recombinant mycobacterium bovis Bacillus Calmette‐Guérin and prospective use in immunotherapy of bladder cancer. Clin Dev Immunol. 2011;2011:728930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Flynn JL, Chan J, Triebold KJ, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Khan TA, Mazhar H, Saleha S, et al. Interferon‐gamma improves macrophages function against M. tuberculosis in multidrug‐resistant tuberculosis patients. Chemother Res Pract. 2016;2016:7295390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wong K‐W, Jacobs WR Jr. Mycobacterium tuberculosis exploits human interferon‐γ to stimulate macrophage extracellular trap formation and necrosis. J Infect Dis. 2013;208(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Nambiar JK, Ryan AA, Kong CU, Britton WJ, Triccas JA. Modulation of pulmonary DC function by vaccine‐encoded GM‐CSF enhances protective immunity against Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40(1):153–161. [DOI] [PubMed] [Google Scholar]
- 206. Ryan AA, Wozniak TM, Shklovskaya E, et al. Improved protection against disseminated tuberculosis by Mycobacterium bovis bacillus Calmette‐Guerin secreting murine GM‐CSF is associated with expansion and activation of APCs. J Immunol. 2007;179(12):8418–8424. [DOI] [PubMed] [Google Scholar]
- 207. Dou J, Tang Q, Yu F, et al. Investigation of immunogenic effect of the BCG priming and Ag85A‐ GM‐CSF boosting in Balb/c mice model. Immunobiology. 2010;215(2):133–142. [DOI] [PubMed] [Google Scholar]
- 208. Zhang Q, Cao X. Epigenetic regulation of the innate immune response to infection. Nat Rev Immunol. 2019;19(7):417–432. [DOI] [PubMed] [Google Scholar]


