Abstract
The Vero cell line is the most used continuous cell line in viral vaccine manufacturing. This adherent cell culture platform requires the use of surfaces to support cell growth, typically roller bottles, or microcarriers. We have recently compared the production of rVSV‐ZEBOV on Vero cells between microcarrier and fixed‐bed bioreactors. However, suspension cultures are considered superior with regard to process scalability. Therefore, we further explore the Vero suspension system for recombinant vesicular stomatitis virus (rVSV)‐vectored vaccine production. Previously, this suspension cell line was only able to be cultivated in a proprietary medium. Here, we expand the adaptation and bioreactor cultivation to a serum‐free commercial medium. Following small‐scale optimization and screening studies, we demonstrate bioreactor productions of highly relevant vaccines and vaccine candidates against Ebola virus disease, HIV, and coronavirus disease 2019 in the Vero suspension system. rVSV‐ZEBOV, rVSV‐HIV, and rVSVInd‐msp‐SF‐Gtc can replicate to high titers in the bioreactor, reaching 3.87 × 107 TCID50/ml, 2.12 × 107 TCID50/ml, and 3.59 × 109 TCID50/ml, respectively. Furthermore, we compare cell‐specific productivities, and the quality of the produced viruses by determining the ratio of total viral particles to infectious viral particles.
Keywords: bioreactor production, COVID‐19, rVSV‐ZEBOV, Vero suspension culture, viral vaccine bioprocess
The Vero cell line is the most used continuous cell line in viral vaccine manufacturing. Here, we further explored the Vero suspension system for rVSV‐based vector production. The cells were adapted to a serum‐free commercial medium and cultivated in bioreactors. Following small scale optimization and screening studies, bioreactor productions of highly relevant vaccines and vaccine candidates against Ebola virus disease, HIV and COVID‐19 in the Vero suspension system were successfully demonstrated.
Abbreviations
- COVID‐19
coronavirus disease 2019
- DIP
defective interfering particle
- HIV
human immunodeficiency virus
- hpi
hours postinfection
- MOI
multiplicity of infection
- rVSV
recombinant vesicular stomatitis virus
- TOI
time of infection
1. INTRODUCTION
Recombinant vectored vaccines produced in cell culture are receiving increased attention in the fight against infectious diseases. More and more vaccines are available that are based on this technology and research efforts to develop new vaccines or to improve current manufacturing processes have intensified over the last years (Ura et al., 2020). One such system is based on the recombinant vesicular stomatitis virus (rVSV). In addition to its use as a vaccine vector, VSV has been used extensively in many areas of research, for example, as an oncolytic virus or as a gene delivery tool (Lichty et al., 2004; Munis et al., 2020).
VSV is a replication‐competent virus with a single‐stranded, negative‐sense RNA genome. The native glycoprotein, VSV‐G, is responsible for viral entry into the cell. When genetically engineered to express the glycoprotein of another virus, rVSV can be used as a vaccine vector by delivering foreign antigens (Munis et al., 2020). The advantage of such a vectored vaccine is the increased safety during manufacturing since the production of live‐attenuated or inactivated vaccines of highly pathogenic viruses (e.g., HIV and Ebola) would require stringent biosafety standards. The recent success story of the European Medicines Agency and US Food and Drug Administration‐approved Ebola vaccine rVSV‐ZEBOV showcases the potential of the rVSV platform (Henao‐Restrepo et al., 2017). rVSV‐ZEBOV is a replication‐competent virus in which VSV‐G was replaced by a Zaire Ebolavirus glycoprotein (ZEBOV), which is the main antigen of the Ebolavirus. Several rVSV‐based vaccines are in development, for example against measles, Lassa fever and Middle East respiratory syndrome (MERS) (Henao‐Restrepo et al., 2017; Kiesslich & Kamen, 2020; Munis et al., 2020).
In light of the progress achieved with rVSV‐ZEBOV, three novel rVSV constructs have been described recently, which carry different glycoproteins of the Human Immunodeficiency Virus (HIV) (Mangion et al., 2020). These HIV‐vaccine candidates were produced in adherent Vero cells in tissue culture plates and it was demonstrated that they induced an HIV gp140‐specific antibody response when administered to mice. The rVSV‐B6‐A74Env(PN6)/SIVtm construct was selected for further studies in nonhuman primates.
In the current race for a COVID‐19 vaccine, recombinant vectored vaccines produced in cell culture are amongst the most promising (Ura et al., 2020). For example, ChAdOx1 novel coronavirus disease 2019 (nCoV‐19), developed by the University of Oxford, is based on a chimpanzee adenovirus‐vectored vaccine expressing the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein (Folegatti et al., 2020) and its safety, efficacy, and immunogenicity is being assessed in Phase III clinical trial (NCT04516746). Furthermore, the Oxford–AstraZeneca COVID‐19 vaccine has been authorized by several national agencies, for example in the UK, and has been given WHO Emergency Use Listing (EUL) (World Health Organization, 2021).
Besides, several rVSV‐based COVID‐19 vaccine candidates expressing the SARS‐CoV‐2 spike protein are being evaluated in preclinical trials (University of Manitoba, Canada; University of Western Ontario, Canada; Aurobindo Pharma, India; Israel Institute for Biological Research/Weizmann Institute of Science, Israel; and FBRI SRC VB VECTOR, Russia) and a Phase I clinical trial (Merck Sharp & Dohme/IAVI; NCT04569786) (World Health Organization, 2020). The COVID‐19 vaccine candidate rVSVInd‐msp‐SF‐Gtc, is a temperature‐sensitive construct. It is based on a recombinant VSVInd(GML) mutant, and shows avirulent in vivo reduced cytopathic effect in vitro at 37°C, but replicates well at 31°C (Kim et al., 2015). This attenuation was used as a strategy to further increase the safety of rVSV for its use as a human vaccine. rVSVInd‐msp‐SF‐Gtc is expressing the SARS‐CoV‐2 spike protein gene, the honeybee melittin signal peptide gene, and the VSV‐G protein transmembrane domain gene.
Currently, the rVSV‐ZEBOV vaccine is manufactured under serum‐free conditions in adherent Vero cells using the roller bottle technology (Monath et al., 2019). To improve manufacturing cost‐effectiveness, more scalable bioprocesses involving microcarrier bioreactors and fixed‐bed bioreactors have been studied recently (Kiesslich et al., 2020). However, these adherent cell processes still have scale‐up limitations, for example, the cell expansion steps during the seed train operation require cell detachment from and reattachment to surfaces, usually involving enzymatic solutions such as trypsin. Suspension cell systems are considered superior with regard to process scale‐up since the transfer of cells to successively larger bioreactor vessels is straightforward.
Adherently growing Vero cells are the most used continuous cell line in viral vaccine manufacturing. For example, vaccines against Ebola, influenza, Japanese encephalitis, polio, rabies, rotavirus, and smallpox are available in the market, and vaccines against other infectious diseases are under development, using this cell line. The many advantages of this cell line are its broad susceptibility to many viruses, the long‐term experience in cell culture, and the regulatory portfolio associated with vaccine manufacturing organizations and health authorities worldwide (Kiesslich & Kamen, 2020).
Adaptation of the Vero cell line to grow in suspension culture to significantly improve this cell culture manufacturing platform has been of interest for many years (Litwin, 1992; Paillet et al., 2009). Lately, studies have reported the successful adaptation using proprietary media (Rourou et al., 2019; Shen et al., 2019). Shen et al. (2019) showed that Vero cells can grow in suspension culture in serum‐free batch and perfusion bioreactors, and successfully applied their system to the production of rVSV‐GFP, which uses the native glycoprotein VSV‐G for viral entry into the cell.
In this study, we further explore the Vero suspension system described previously (Shen et al., 2019), and demonstrate its applicability to relevant rVSV‐based vaccine candidates. Using rVSV‐ZEBOV as a model for rVSV, we focus on small‐scale experiments to optimize the multiplicity of infection (MOI) and investigate the effects of different cell densities. Next, we compare the production of rVSV‐ZEBOV in this system to the production in Vero cells that were adapted to grow in suspension culture in a commercially available medium. In addition, we show the production of newly developed candidate vaccines against HIV (rVSV‐HIV) and COVID‐19 (rVSVInd‐msp‐SF‐Gtc). Based on these results, we demonstrate production in a batch bioreactor for all three rVSV variants.
2. MATERIALS AND METHODS
2.1. Cell line and culture media
The suspension‐adapted Vero cell line was provided by the National Research Council (NRC) of Canada, Montreal, Canada, and its adaptation process has been described previously (Shen et al., 2019). For routine passaging, the cells from the late exponential growth phase were harvested by centrifugation for 5 min at 500 g and the cell pellet was resuspended in a fresh medium to a seeding cell density of 2.5–5 × 105 cells/ml in 125 ml polycarbonate shake flasks (TriForest Enterprises) and maintained at 37°C, 135 rpm and 5% CO2 in a humified Multitron orbital shaker (Infors HT). The cells were cultivated in 20 ml working volume of either IHM03 medium, provided by the NRC, or in MDXK medium (Xell AG), supplemented with 4 mM GlutaMAX (Thermo Fisher Scientific).
2.2. Viruses
Origin and viral seed stock amplification of rVSV‐ZEBOV and rVSV‐B6‐A74Env(PN6)/SIVtm (hereafter referred to as rVSV‐HIV) have been described previously (Kiesslich et al., 2020) (Mangion et al., 2020). The viral seed stock of rVSV‐ZEBOV had a titer of 1.49 × 107 TCID50/ml and 2.80 × 109 VG/ml, whereas rVSV‐HIV had a titer of 7.14 × 106 TCID50/ml and 3.13 × 1010 VG/ml, respectively.
The recombinant COVID‐19 vaccine candidate rVSVInd‐msp‐SF‐Gtc was constructed as follows: Codon‐optimized full‐length spike protein gene of SARS‐CoV‐2 (GenBank: JX869059.2) was purchased from Genscript USA Inc, cloned into an avirulent Indiana serotype of vesicular stomatitis virus (VSVInd[GML]) as has been described previously (Kim et al., 2015). The honeybee melittin signal peptide (msp) was inserted at the NH2‐terminus and the transmembrane domain and cytoplasmic tail (Gtc) of the S protein was substituted by the Gtc of VSVInd G protein at the COOH‐terminus. In addition, the VSV intergenic sequences were added in front of the spike protein gene to provide the transcription termination signal, polyadenylation signal, and transcription re‐initiation signal. The modified SARS‐CoV‐2 spike protein gene was inserted into the G and L gene junction of the VSVInd(GML) at Pme I and Mlu I sites. Recombinant rVSVInd‐msp‐SF‐Gtc virus was recovered by VSV reverse genetics as has been described previously (Kim et al., 2015). The recombinant virus was purified by three consecutive plaque picking, and a stock virus was prepared by infecting BHK21 cells. The viral seed stock of rVSVInd‐msp‐SF‐Gtc had a titer of 1.43 × 108 TCID50/ml and 4.64 × 109 VG/ml.
2.3. Shake flask virus studies
For virus infection studies in shake flasks, suspension‐adapted Vero cells from the late exponential growth phase were harvested, if required pooled, and seeded at the indicated cell density in a fresh medium. Vero cells were infected with rVSV at the indicated MOI and the temperature was shifted to either 34°C (rVSV‐ZEBOV, rVSV‐HIV) or 31°C (rVSVInd‐msp‐SF‐Gtc). Samples for virus titration were centrifuged for 5 min at 1200×g to remove cellular debris, aliquoted, and stored at −80°C.
2.4. Bioreactor cultures
Bioreactor cultures were performed in a 1 L bioreactor (Applikon Biotechnology) equipped with a marine impeller, pH sensor, temperature sensor, and dissolved oxygen (DO) concentration sensor. MDXK medium was supplemented 4 mM l‐glutamine (GE Healthcare) instead of GlutaMAX to enable monitoring of l‐glutamine consumption. Vero cell seed cultures were grown in progressively larger polycarbonate shake flasks (TriForest Enterprises), harvested by centrifugation and resuspended in a fresh medium before inoculation. The bioreactor was inoculated at a cell density of 2.5 × 105 cells/ml in 850 ml working volume. The culture was agitated at 100 rpm and kept at 37°C. The DO concentration was kept at 50% air‐saturation by continuous surface aeration of 5 ml/min air and injection of pure oxygen through the sparger when required. The pH was set to 7.2 and regulated by injection of CO2 into the headspace or addition of NaHCO3 (90 g/L) (Sigma). Samples were taken once or twice daily, depending on the progress of the culture, to subsequently determine viable cell density, metabolite concentration and virus titer. Samples for metabolite analysis and virus titration were centrifuged for 5 min at 1200 × g to remove cellular debris, aliquoted, and stored at −80°C.
For virus production, Vero cells were infected with rVSV at an MOI of 0.01 once the targeted cell density was reached and the temperature was shifted to 34°C or 31°C, respectively, during the virus production phase. The glucose concentration was estimated once daily and if required adjusted to 2 g/L by feeding glucose (Sigma) concentrate (180 g/L). In addition, l‐glutamine was maintained at a minimum concentration of 2 mM.
2.5. Analytical methods
Vero cell concentration and viability were determined via the Vi‐CELL XR cell counter (Beckman Coulter). The Median Tissue Culture Infectious Dose (TCID50) assay and digital PCR assay used in this study to quantify the infectious titer and the number of viral genomes, respectively, have been described previously (Gélinas et al., 2020; Kiesslich et al., 2020). For rVSVInd‐msp‐SF‐Gtc, the TCID50 plates were incubated at 31°C, due to the temperature sensitivity of this construct.
2.6. Metabolite analysis
During cell culture cultivations, the glucose concentration was estimated using the d‐Fructose/d‐Glucose Assay Kit (Megazyme). More extensive metabolite analysis was performed offline via Bioprofile 400 (Nova Biomedical) from samples that were stored at −80°C.
3. RESULTS AND DISCUSSION
3.1. Cell growth of Vero cells in suspension cultures
3.1.1. Screening for commercial media
Suspension adapted Vero cells grew well in shake flasks in IHM03 medium up to a cell density of around 2 × 106 cells/ml and with doubling times of around 48 h as previously reported (Shen et al., 2019). IHM03 is an in‐house medium developed and produced in small batches by the NRC, supporting the growth and virus production of Vero suspension cultures. As reported by Shen et al. (2019) and Rourou et al. (2019), media composition is critical for successfully generating a suspension adapted Vero cell line and no commercial media was able to support Vero cell adaptation so far. Despite these recently reported adaptation successes with in‐house media, establishing a process using commercial media could reduce the risk of lot‐to‐lot variations and would make the platform more amenable to work under standard conditions when media supply is assured.
Therefore, efforts have been dedicated to assessing different commercial media. After 20 passages of adaptation in shake flasks, with gradual media replacement during the first five passages, a Vero cell line was obtained that was able to grow in MDXK medium (Xell AG) supplemented with 4 mM Glutamax and which exhibited cell doubling times of around 48–72 h (data not shown). Other commercial media that were tested, but did not support the growth of Vero cells in suspension culture included VP‐SFM, OptiPRO, FreeStyle 293 (Thermo Fisher Scientific), HyClone HyCell TransFx‐H (GE Healthcare), HEK GM (Xell AG), and ProVeroTM −1 serum‐free medium (SFM) (Lonza).
Despite slightly slower growth rates in MDXK than in IHM03 in shake flasks, the cells were able to grow to similar cell densities in batch shake flasks with less formation of cell aggregates. Compared with other mammalian suspension cell lines, like derivatives of HEK293 or CHO with cell doubling times of 24 h, there is still great potential to develop media that can support similar growth rates. With a suspension Vero cell line available, further research can use novel analytical techniques such as transcriptomics and metabolomics to facility media development specifically geared towards this cell line.
3.1.2. Cell growth in batch bioreactor
The suspension‐adapted Vero cells showed similar growth in IHM03 medium in 1 L batch bioreactors as previously reported, reaching 1.78 × 106 cells/ml after 6 days (Shen et al., 2019). Cell viability was above 99% during the whole run. The doubling time was around 51 h for the entire batch process duration between cell seeding and peak in maximum cell density, and around 40 h for the exponential cell growth phase between cell seeding up until 96 h. During the exponential growth phase, the cell growth rate was 0.0174 h−1 (Figure 1a). The substrates glucose and glutamine were almost depleted at the end of the culture. Ammonia was produced throughout and stayed below concentrations of 4 mM. Lactate production reached a concentration of 24.5 mM after 96 h, but declined thereafter which can be explained by uptake of lactate by the cells as previously reported (Quesney et al., 2003).
Figure 1.
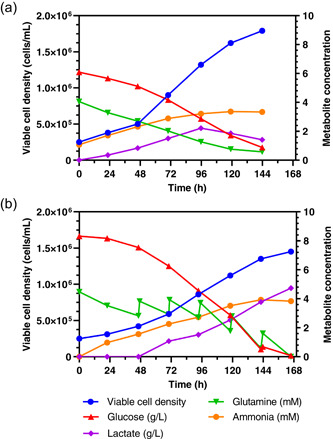
Cell growth of suspension adapted Vero cells in IHM03 (a) and MDXK (b) medium in a 1 L bioreactor in batch mode. The concentration of the main metabolic substrates (glucose, l‐glutamine) and by‐products (lactate, ammonia) are given in mM or g/L [Color figure can be viewed at wileyonlinelibrary.com]
Cell growths in MDXK medium were slightly slower and only reached 1.45 × 106 cells/ml after 7 days. In MDXK, the doubling time was around 65 h for the entire batch process duration between cell seeding and peak in maximum cell density. During the exponential cell growth phase between cell seeding up until 96 h, the cell doubling time was 52 h, resulting in a cell growth rate of 0.0132 h−1 (Figure 1b). However, in contrast to the shake flask experiments, Vero cells in MDXK medium showed a higher degree of aggregate formation in the bioreactor compared with cells in IHM03. This could have led to an underestimation of the cell count, which is also indicated by a higher glucose consumption rate in MDXK. Cell viability again was above 99% throughout the process. As opposed to the previous run, glucose and glutamine were depleted earlier and required feeding, adjusting glucose to 2 g/L and glutamine to 2 mM once daily starting on Day 2 for glutamine and Day 6 for glucose. Ammonia production was similar, never exceeding concentrations of 4 mM. Lactate production, however, was considerably higher and surpassed 50 mM at the end of the culture. Lactate was not consumed by the cells in MDXK medium indicating differences in the cell metabolism in the two media.
A drawback of the current procedure for cell passaging and bioreactor seed preparation is the need for centrifugation. Due to long cell doubling times, it is necessary to exchange spent medium at the end of each passage instead of diluting the culture with fresh medium. Furthermore, the resuspension of the cell pellet after centrifugation breaks apart the majority of loosely aggregated cell clumps. While this procedure works well for small shake flask cultures, scale‐up to larger volumes such as those in bioreactor vessels at the manufacturing stage can be challenging. Further studies will investigate the use of perfusion technologies to achieve higher cell densities so that culture dilution can be applied instead of centrifugation when passaging. In addition, media optimization will be carried out to increase cell growth rates and to reduce cell adherence and aggregation.
3.2. rVSV‐ZEBOV production in shake flask
rVSV‐ZEBOV production experiments were initially carried out at a smaller scale in shake flasks to test multiple conditions simultaneously. In particular, the effects of varying multiplicities of infection (MOI), which is the ratio of infectious particles to the number of cells at the time of infection (TOI), as well as infections at different cell densities and in different growth media were screened.
During the late stages of the cultivation when cell densities exceeded 1 × 106 cells/ml, cells started to adhere to the surface of the shake flask, and cell aggregates were formed, which made it quite difficult to accurately determine the cell count. Therefore, initial experiments to investigate the infection kinetics of rVSV‐ZEBOV in suspension adapted Vero cells, were carried out by seeding a single cell culture in a fresh medium at a cell density of 1 × 106 cells/ml, and cells were infected immediately thereafter.
Previous studies of rVSV‐ZEBOV in adherent Vero cells and suspension cultures of HEK293 cells have shown that infection at a reduced temperature of 34°C led to higher infectious titers compared to 37°C (Kiesslich et al., 2020) (Gélinas et al., 2019). Based on these studies, the temperature was lowered to 34°C after infection in all experiments of this study involving rVSV‐ZEBOV.
3.2.1. Multiplicity of infection
Vero cells grown in IHM03 were infected with rVSV‐ZEBOV at different MOIs and samples were taken every 12 h to determine infection kinetics (Figure 2). For the selected range of MOI, peak production of rVSV‐ZEBOV occurred between 24 and 36 h post infection (hpi) and the titers were in the same range with 1.10 × 107 TCID50/ml, 1.05 × 107 TCID50/ml, and 1.58 × 107 TCID50/ml at an MOI of 0.001, 0.01, and 0.1, respectively. In all cases, the infectivity declined after the maximum titer had been reached. Similar kinetics have been observed for adherent growing Vero cells, however, the titers were more than eight times higher in adherent cell experiments in six‐well tissue culture plates, for example, 8.79 × 107 TCID50/ml at an MOI of 0.01 at 36 hpi (Kiesslich et al., 2020). Further, the cell density at the time of infection was more than three times higher for the suspension cultures than for the adherent cell cultures in the reported study, indicating even lower virus production per cell. It is to mention that adherent Vero cells were cultivated in commercially available media, optimized for cell growth and virus production.
Figure 2.
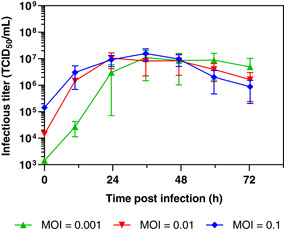
rVSV‐ZEBOV infection of suspension adapted Vero cells at different MOIs ranging from 0.001 to 0.1 in 125 ml shake flask in IHM03 medium at a cell density of 1 × 106 cells/ml. Infectious viral titers expressed as TCID50/ml are plotted against the time postinfection. Bars represent the mean of the samples from three independent shake flask replicates ± standard deviation. MOIs, multiplicity of infections; rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose; ZEBOV, Zaire Ebolavirus [Color figure can be viewed at wileyonlinelibrary.com]
rVSV infections of adherent Vero cells typically lead to a very distinct cytopathic effect, where cells become round‐shaped and eventually lift off from the surface. Of note, since suspension cells are already round‐shaped and not attached to a surface, the cytopathic effect induced by rVSV infections was less noticeable in the early stages of infection and only became more apparent when the cell diameter increased due to viral replication and when cells started to die from lysis caused by the viral release.
Nevertheless, based on these experiments and in accordance with our previous work, it was decided to continue all subsequent rVSV experiments at an MOI of 0.01.
3.2.2. Cell density
rVSV‐ZEBOV replication in Vero cells grown in IHM03 and seeded at different cell densities was investigated to evaluate the effect of varying cell densities and to assess if the production yield was affected by the media capacity at the time of infection. Figure 3 shows rVSV‐ZEBOV infection at 1 × 106 cells/mL, 2 × 106 cells/ml, and 4 × 106 cells/ml. The viral infection kinetics were slower compared with the previous experiment and infectious titers peaked at 48 hpi for all cases, indicating considerable variation between experiments when comparing the data to the experiment presented in Figure 2. In addition, maximum infectious titers were around three times higher for the run at 1 × 106 cells/ml, reaching 3.28 × 107 TCID50/ml. To reduce variations and improve repeatability, cells from similar passages should be used. Further, cell density estimations, especially when the cultures contain aggregates, need to be carried out very carefully since small errors can affect MOI calculations significantly.
Figure 3.
rVSV‐ZEBOV infection of suspension adapted Vero cells at different cell densities ranging from 1 × 106 to 4 × 106 cells/ml in 125 ml shake flask in IHM03 medium. Infectious viral titers expressed as TCID50/ml are plotted against the time postinfection. Bars represent the mean of the samples from three independent shake flask replicates ± standard deviation. rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose; ZEBOV, Zaire Ebolavirus [Color figure can be viewed at wileyonlinelibrary.com]
Throughout the time course of the experiment, titers were even higher at 2 × 106 cells/ml, but the maximum infectious titer was not significantly elevated at 48 hpi. For the run with a seeding cell density of 4 × 106 cells/ml, infectious titers of rVSV‐ZEBOV were significantly higher, reaching 1.32 × 108 TCID50/ml, exceeding infectious titers obtained from adherent Vero studies in six‐well plates (Kiesslich et al., 2020). A similar study carried out in HEK293‐SF obtained two to three times higher titers with 2.92 × 108 TCID50/ml and 2.36 × 108 TCID50/ml, at 2.5 × 106 cells/ml, and 5 × 106 cells/ml, respectively, compared to infection at 1 × 106 cells/ml (Gélinas et al., 2019). Furthermore, for rVSV‐GFP produced in suspension cell cultures of Vero cells at different cell densities in a similar experiment, 3.8 times higher virus titers were obtained at 2.5 × 106 cells/ml compared with 0.8 × 106 cells/ml (Shen et al., 2019). But a further increase in cell density from 2.5 × 106 cells/ml to 5 × 106 cells/ml did not result in higher infectious titers, comparable with the results obtained in HEK293‐SF. This cell density effect has also been observed for the production of other viruses. For example during poliovirus production, a higher cell density leads to lower cell‐specific poliovirus d‐antigen levels (Thomassen et al., 2014).
These results indicate that suspension cultures of Vero cells could be a viable alternative if high cell density processes of suspension cultures can be achieved at a larger scale, but further research is necessary to investigate the effects of high cell density in more detail. One advantage though is that suspension cultures are not limited by the surface area, as is the case for adherent cell cultures using microcarriers, roller bottles, or fixed‐bed bioreactors, which is the prevalent mode of virus production in Vero cells. Nevertheless, these results need to be carefully evaluated since it might be challenging to seed bioreactors in a fresh medium at high cell density. In this context, shake flask studies in HEK293‐SF cells found that if the cultures were grown to high cell densities and infected without medium exchange, the infectious titer of rVSV‐ZEBOV could not be enhanced by increasing the cell density above 2 × 106 cells/ml (Gélinas et al., 2019).
3.3. rVSV‐ZEBOV, rVSV‐HIV, and rVSVInd‐msp‐SF‐Gtc shake flask production in different media
Next, the kinetics of three variants, namely, rVSV‐ZEBOV, rVSV‐HIV, and rVSVInd‐msp‐SF‐Gtc were compared and the effect of different media on rVSV production was studied. Based on temperature study results of rVSV‐ZEBOV infections in Vero cells, rVSV‐HIV infections were carried out at 34°C in a recent study, and this condition was adopted for this study as well (Mangion et al., 2020). The rVSVInd‐msp‐SF‐Gtc construct, however, is temperature sensitive and therefore all infections were carried out at 31°C.
The IHM03 and MDXK adapted cell lines were infected with rVSV to compare the virus production capacities of both media. Despite higher titers obtained at higher cell densities (Figure 3), these experiments were carried out at a seeding cell density of 1 × 106 cells/ml to avoid cell aggregation. Figure 4 shows rVSV‐ZEBOV (a), rVSV‐HIV (b), and rVSVInd‐msp‐SF‐Gtc (c) replication in the MDXK adapted cell line at an MOI of 0.01 in comparison with the corresponding experiment conducted in IHM03.
Figure 4.
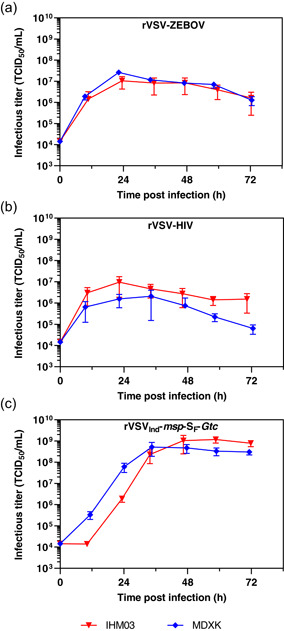
rVSV‐ZEBOV (a), rVSV‐HIV (b), and rVSVInd‐msp‐SF‐Gtc (c) infections of suspension adapted Vero cells cultivated in either IHM03 or MDXK medium at an MOI of 0.01, in 125 ml shake flask at a cell density of 1 × 106 cells/ml. The rVSV‐ZEBOV and rVSV‐HIV infections were carried out at 34°C, whereas the rVSVInd‐msp‐SF‐Gtc infection was carried out at 31°C. Infectious viral titers expressed as TCID50/ml are plotted against the time postinfection. Bars represent the mean of the samples from three independent shake flask replicates ± standard deviation. rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose; ZEBOV, Zaire Ebolavirus [Color figure can be viewed at wileyonlinelibrary.com]
rVSV‐ZEBOV replication in MDXK medium reached slightly higher titers than in IHM03 medium (Figure 4a). Where the infectious titer reached a maximum at 24 hpi in IHM03, the titer in MDXK was with 2.63 × 107 TCID50/ml around 2.5 times higher. Otherwise, almost identical infection kinetics were observed.
rVSV‐HIV replicated better in IHM03 with a higher maximum titer of 9.59 × 106 TCID50/ml reached in a shorter period of time (24 hpi) compared with 2.08 × 106 TCID50/ml in MDXK after 36 hpi (Figure 4b). Titers of rVSV‐HIV were lower in both media than rVSV‐ZEBOV and the infectivity declined faster than for rVSV‐ZEBOV. Besides, rVSV‐HIV produced in adherent Vero cells reached up to 3.91 × 107 TCID50/ml at MOI of 0.01. However, the peak was reached significantly later at 96 hpi (Mangion et al., 2020).
In contrast to these two strains, rVSVInd‐msp‐SF‐Gtc reached significantly higher titers (Figure 4c). In MDXK, 5.19 × 108 TCID50/ml were reached at 36 hpi. In IHM03, almost two‐fold higher titers with 1.17 × 109 TCID50/ml were reached. However, it took additional 24 h to reach this titer, which aligns with the beginning of the replication phase being delayed. In addition to higher infectious titers compared with rVSV‐ZEBOV and rVSV‐HIV, the infectivity of viral particles did not decline significantly over the following sample time points.
Differences in replication kinetics between the three rVSV‐variants can be attributed in part to the use of different glycoproteins. The Ebola virus glycoprotein (Moller‐tank & Maury, 2015), the HIV envelope glycoprotein (Klasse, 2012), and the SARS‐CoV‐2 spike protein (S) (Hoffmann et al., 2020), which are responsible for cell entry of the corresponding rVSV used in this study, all use different types of cell receptors and entry mechanisms. Furthermore, the formation of these proteins during intracellular replication of the virus and the final assembly of the viral particles are unique. These mechanisms have their own rate‐limiting steps and efficiencies, resulting in different amounts of functional rVSV particles and different ratios of infectious to total particles.
Higher infectious titers of rVSVInd‐msp‐SF‐Gtc in Vero cells could also be linked more specifically to the use of different transmembrane domains. Whereas rVSV‐ZEBOV and rVSV‐HIV use Ebola GP or SIV transmembrane domains (Mangion et al., 2020), rVSVInd‐msp‐SF‐Gtc is expressing the native VSV‐G protein transmembrane domain gene. It has been shown that the stem region of the VSV‐G glycoprotein was important for efficient virus assembly, and viruses with shortened sequences were replicated up 20‐fold less (Robison & Whitt, 2000). More research investigating the use of different transmembrane domains with the same extracellular domain could reveal interesting aspects on virus replication rates and identify new targets to improve the rVSV platform. Nevertheless, it might be more appropriate to compare replication of rVSVInd‐msp‐SF‐Gtc to rVSV‐GFP production, which also uses the native VSV‐G protein transmembrane domain, and where titers of up to 8.93 × 109 TCID50/ml have been obtained in shake flask experiments.
Besides, the lower process temperature of 31°C is likely affecting the infectivity. For example, the infectivity of this strain was not declining over the following 36 h after the peak titer had been reached thus potentially stabilizing infectivity. Though for rVSV‐ZEBOV, an optimal production temperature of 34°C was determined in adherent Vero cells (Kiesslich et al., 2020), rVSVInd‐msp‐SF‐Gtc is based on VSVInd(GML) which was adapted to replicate well at the lower temperature of 31°C (Figure 4c).
Another reason for differences in production titers between the three rVSV variants could be linked to defective interfering particles (DIPs) and the quality of the viral seed stocks (Ziegler & Botten, 2020). The ratios of total viral particles to infectious particles for rVSV‐ZEBOV, rVSV‐HIV, and rVSVInd‐msp‐SF‐Gtc are 188 VG/TCID50, 4384 VG/TCID50, and 32 VG/TCID50, respectively. Especially the high ratio of VG/TCID50 of the rVSV‐HIV stock indicates a lower quality of this virus stock and could have led to DIPs influencing the production process.
3.4. Bioreactor processes of suspension adapted Vero cells
3.4.1. Bioreactor production of rVSV‐ZEBOV
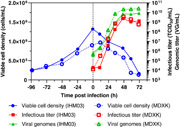
Two bioreactors of Vero cells were infected at a cell density of 1.37 × 106 cells/ml and 1.02 × 106 cells/ml after cells grew for 4 days in IHM03 and MDXK medium, respectively (Figure 5). The cell growth phase corresponded well to the data shown in Figure 1. Despite a lower cell density at the TOI, maximum infectious titers were with 3.87 × 107 TCID50/ml more than one log higher than in IHM03, where only 3.55 × 106 TCID50/ml were obtained. In addition, replication was faster in MDXK, where peak production occurred at 24 hpi compared with 36 hpi, respectively. The MDXK bioreactor also exhibited an almost 15‐times higher cell‐specific productivity with 37.9 TCID50/cell compared with 2.6 TCID50/cell. Moreover, the ratio of total viral particles to infectious particles was lower in MDXK (282 VG/TCID50) than in IHM03 (817 VG/TCID50), further indicating a better quality of the final product if harvested at the time of peak infectious titer. As a result, the MDXK medium appears better suited for rVSV‐ZEBOV production in the bioreactor than IHM03. As shown in Figure 1, the main substrates glucose and glutamine were not at limiting concentrations at the TOI. However, differences in media compositions such as nutrient profile and concentration could have affected rVSV‐ZEBOV production and other metabolites which were not quantified could have been at limiting or inhibiting concentrations.
Figure 5.
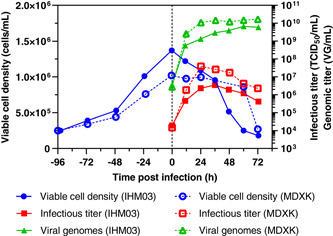
Comparison of the production of rVSV‐ZEBOV in suspension adapted Vero cells cultivated in either IHM03 or MDXK medium in a 1 L bioreactor in batch mode. Infectious viral titers expressed as TCID50/ml and the number of viral genomes in VG/ml are plotted against the time postinfection. rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose; ZEBOV, Zaire Ebolavirus [Color figure can be viewed at wileyonlinelibrary.com]
Compared with the shake flask experiments, rVSV‐ZEBOV replication in MDXK medium reached a similar maximum infectious titer, indicating a successful scale‐up. For IHM03, titers were three times lower than in the shake flask and the peak was reached 12 h later.
Furthermore, in comparison to adherent Vero bioreactor productions of rVSV‐ZEBOV, the production using suspension‐adapted Vero cells in MDXK appears elevated. The infectious titer and the cell‐specific productivities were slightly higher compared with the production in a microcarrier bioreactor (1.42 × 107 TCID50/ml, 7.6 TCID50/cell) and a fixed‐bed bioreactor (2.59 × 107 TCID50/ml, 11.2 TCID50/cell). The ratio of total viral particles to infectious particles was four times lower than in the microcarrier but nine times higher compared with the fixed‐bed process (Kiesslich et al., 2020). Overall, the suspension Vero system is a viable alternative to the current Vero manufacturing system carried out in roller bottles for this Ebola virus disease vaccine (Monath et al., 2019).
When set side by side to a suspension bioreactor production of rVSV‐ZEBOV in HEK293‐SF, where a maximum of 1.19 × 108 TCID50/ml was reached, production in Vero cells in MDXK was three times lower. However, it can be expected that future media development, bioprocess, and cell line engineering of suspension Vero can lead to significantly higher titers comparable to HEK293‐SF (Gélinas et al., 2019).
3.4.2. Bioreactor production of rVSV‐HIV
Two bioreactors were prepared as before, and Vero cell growth phase in IHM03 and MDXK medium was consistent with the data from Figure 1 and Figure 5. The two cultures were infected with rVSV‐HIV after 4 days (Figure 6). In contrast to the previous experiment, virus production was favored in IHM03 over MDXK. In IHM03, rVSV‐HIV reached a maximum titer of 2.12 × 107 TCID50/ml, which was 25‐times higher than in MDXK. However, the production kinetics of viral genomes were almost identical. This is supported by a lower ratio of 143 VG/TCID50 (IHM03) compared with 7041 VG/TCID50 (MDXK). In addition, the fact that the cell‐specific productivity in MDXK was 0.9 TCID50/cell implies that the rVSV‐HIV/MDXK system failed to scale up and is not an adequate production system.
Figure 6.
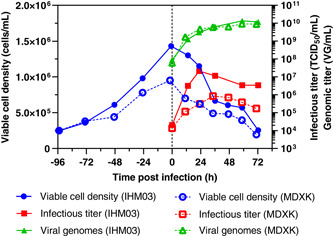
Comparison of the production of rVSV‐HIV in suspension adapted Vero cells cultivated in either IHM03 or MDXK medium in a 1 L bioreactor in batch mode. Infectious viral titers expressed as TCID50/mL and the number of viral genomes in VG/ml are plotted against the time postinfection. rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose [Color figure can be viewed at wileyonlinelibrary.com]
Besides, the shake flask experiment (Figure 4b) already indicated the suitability of IHM03 for rVSV‐HIV replication. Moreover, bioreactor production of rVSV‐HIV in IHM03 exceeded titers from the smaller scale, whereas bioreactor titers in MDXK subsided the small scale.
Differences between the two media were already seen for bioreactor production of rVSV‐ZEBOV. Here, IHM03 appears to be favored for rVSV‐HIV production. However, the production of total rVSV‐HIV particles, estimated from the number of viral genomes, is comparable between the two media. Therefore, one reason of a higher titer of rVSV‐HIV in IHM03 could be that this medium is better suited to stabilize the HIV glycoprotein and to maintain the viral infectivity.
3.4.3. Bioreactor production of rVSVInd‐msp‐SF‐Gtc
Finally, rVSVInd‐msp‐SF‐Gtc production in bioreactors of Vero suspension cell cultures was studied (Figure 7). Again, the cell growth phase was comparable to the previous runs, and cells were infected after 4 days. Infectious titers of rVSVInd‐msp‐SF‐Gtc peaked in both media at 48 hpi. The beginning of the replication phase was delayed in IHM03, as already seen in the shake flask experiments. Nevertheless, titers were similar with 2.38 × 109 TCID50/ml in IHM03 and 3.59 × 109 TCID50/ml in MDXK. Thus, results from the shake flask experiment were exceeded by two‐fold and seven‐fold, respectively. The quality in terms of total viral particles to infectious particles was with 3.0 VG/TCID50 comparable in IHM03 to that in MDXK, where this value was 6.0 VG/TCID50. In addition, the cell‐specific productivity was in the same range in MDXK compared to IHM03, with 3670 TCID50/cell and 1803 TCID50/cell, respectively.
Figure 7.
Comparison of the production of rVSVInd‐msp‐SF‐Gtc in suspension adapted Vero cells cultivated in either IHM03 or MDXK medium in a 1 L bioreactor in batch mode. Infectious viral titers expressed as TCID50/ml and the number of viral genomes in VG/ml are plotted against the time postinfection. rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose [Color figure can be viewed at wileyonlinelibrary.com]
In comparison, rVSV‐ZEBOV and rVSV‐HIV productions in the bioreactor peaked earlier. However, the number of viral genomes continued to increase in those experiments even when the infectious titer declined. Therefore, the rate of viral degradation is higher than the viral production rate after the corresponding peak was reached in the case of these two strains, potentially due to the higher process temperature of 34°C versus 31°C and its impact on viral stability.
Overall, the scale‐up of rVSVInd‐msp‐SF‐Gtc production to the bioreactor was successful, exceeding small‐scale results. In addition, this strain appears to replicate to much higher titers, with a superior cell‐specific productivity and an improved ratio of VG/TCID50 as compared to rVSV‐ZEBOV and rVSV‐HIV. Table 1 shows a summary of the six bioreactor runs, comparing results for the three strains in two different culture media.
Table 1.
Comparison of bioreactor productions of rVSV‐ZEBOV, rVSV‐HIV and rVSVInd‐msp‐SF‐Gtc
| rVSV‐ZEBOV | rVSV‐HIV | rVSVInd‐msp‐SF‐Gtc | ||||
|---|---|---|---|---|---|---|
| Medium | IHM03 | MDXK | IHM03 | MDXK | IHM03 | MDXK |
| Peak infectious titer | 36 hpi | 24 hpi | 24 hpi | 36 hpi | 48 hpi | 48 hpi |
| Infectious titer (TCID50/ml) | 3.55 × 106 | 3.87 × 107 | 2.12 × 107 | 8.45 × 105 | 2.38 × 109 | 3.59 × 109 |
|
Genomic titer (VG/ml) |
2.9 × 109 | 1.09 × 1010 | 3.30 × 109 | 5.95 × 109 | 7.15 × 109 | 2.13 × 1010 |
| Ratio VG/TCID50 | 817 | 282 | 143 | 7041 | 3.0 | 6.0 |
|
Cell‐specific titer (TCID50/cell) |
3 | 38 | 15 | 1 | 1803 | 3670 |
Abbreviations: rVSV, recombinant vesicular stomatitis virus; TCID50, Median Tissue Culture Infectious Dose; ZEBOV, Zaire Ebolavirus.
4. CONCLUSION
In this study, we have demonstrated the feasibility and applicability of suspension adapted Vero cell cultures for the production of highly relevant rVSV‐based vaccines and vaccine candidates. For three rVSV strains, namely, rVSV‐ZEBOV, rVSV‐HIV, and rVSVInd‐msp‐SF‐Gtc, production was successfully scaled up to the bioreactor scale. Furthermore, proof‐of‐concept is provided that rVSV‐ZEBOV and rVSVInd‐msp‐SF‐Gtc can be produced in commercially available media in suspension‐adapted Vero cells.
Previously, the suspension‐adapted Vero cell line was tested for tumorigenicity at passage 163 (Shen et al., 2019). Since the experiments with the Vero cell line in commercial MDXK medium were carried out at a higher passage number, this cell line would also require testing for tumorigenicity to ensure its safety as a substrate for the production of vaccines.
Process parameters developed in suspension Vero and previously in adherent Vero cells for rVSV‐ZEBOV (Kiesslich et al., 2020), have been shown to be applicable to other strains. This is an important observation as rVSV‐ZEBOV can thus serve as a model virus for other rVSV strains. More, this can be of significant value for the production of future rVSV‐based vaccine candidates against emerging infectious diseases.
Moreover, the production of rVSV‐ZEBOV was shown to be elevated compared to previously developed adherent processes in microcarrier and fixed‐bed bioreactors (Kiesslich et al., 2020). Due to the better scalability, the suspension Vero system can serve as a viable alternative to the current Ebola virus disease vaccine manufacturing using roller bottles.
Production of rVSV‐ZEBOV was leading to higher infectious titers in suspension cultures of HEK293‐SF (Gélinas et al., 2019). However, this system used commercially available media and bioprocesses developed with years of experience. The commercially available MDXK medium has only been in the market for a short period of time. Hence, there is great potential for optimization of Vero suspension media and bioprocesses specifically for virus production.
In the context of the current COVID‐19 pandemic, this study shows relevant advancement in the field of bioprocess development for urgently needed vector‐based vaccine candidates. Given that rVSVInd‐msp‐SF‐Gtc grows to titers that are around 100‐fold higher than titers of rVSV‐ZEBOV in the same system, and given that rVSV‐ZEBOV produced in a conventional roller bottle processes has been approved as a vaccine candidate by regulatory agencies, the herein presented bioprocess using suspension adapted Vero cells can serve as a highly efficient system for accelerated and scalable manufacturing of a COVID‐19 vaccine candidate. Further, the quality of the produced viruses in terms of the ratio total particles to infectious particles is better, potentially leading to facilitated downstream processes and ultimately very economical manufacturing.
In the future, fed‐batch and perfusion processes should be developed for high cell density bioreactors. As indicated in shake flask experiments and already demonstrated for VSV‐GFP (Shen et al., 2019), these can be approaches to further push the boundaries and to increase virus productivities of the suspension Vero system.
CONFLICT OF INTERESTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
AUTHOR CONTRIBUTIONS
Sascha Kiesslich: Conceptualization, Investigation, Formal analysis, Writing ‐ Original Draft, Writing ‐ Review & Editing. Gyoung Nyoun Kim: Construction and characterization of rVSVInd‐msp‐SF‐Gtc. Chun Fang Shen: Investigation, Writing ‐ Review & Editing. C. Yong Kang: Conceptualization, Writing ‐ Review & Editing, Supervision, Funding acquisition. Amine A. Kamen: Conceptualization, Writing ‐ Review & Editing, Supervision, Funding acquisition.
ACKNOWLEDGMENT
Dr. Kamen received funding from the Canadian Institutes of Health Research Grant OVV 152411. Sascha Kiesslich was funded by a doctoral scholarship from the Fonds de Recherche du Québec – Santé (FRQS). Dr. Kang received funding from CIHR Grant RES002426 and Sumagen Canada.
Kiesslich, S. , Kim, G. N. , Shen, C. F. , Kang, C. Y. , & Kamen, A. A. (2021). Bioreactor production of rVSV‐based vectors in Vero cell suspension cultures. Biotechnology Bioengineering. 118, 2649–2659. 10.1002/bit.27785
DATA AVAILABILITY STATEMENT
Will be made available through authors upon request.
REFERENCE
- Folegatti, P. M. , Ewer, K. J. , Aley, P. K. , Angus, B. , Becker, S. , Belij‐rammerstorfer, S. , Bellamy, D. , Bibi, S. , Bittaye, M. , Clutterbuck, E. A. , Dold, C. , Faust, S. N. , Finn, A. , Flaxman, A. L. , Hallis, B. , Heath, P. , Jenkin, D. , Lazarus, R. , Makinson, R. … Pollard, A. J. (2020). Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet, 396, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas, J. , Kiesslich, S. , Gilbert, R. , & Kamen, A. A. (2020). Titration methods for rVSV‐based vaccine manufacturing. MethodsX, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas, J.‐F. , Azizi, H. , Kiesslich, S. , Lanthier, S. , Perdersen, J. , Chahal, P. S. , Ansorge, S. , Kobinger, G. , Gilbert, R. , & Kamen, A. A. (2019). Production of rVSV‐ZEBOV in serum‐free suspension culture of HEK 293SF cells. Vaccine, 37, 6624–6632. https://linkinghub.elsevier.com/retrieve/pii/S0264410X19312538 [DOI] [PubMed] [Google Scholar]
- Henao‐Restrepo, A. M. , Camacho, A. , Longini, I. M. , Watson, C. H. , Edmunds, W. J. , Egger, M. , Carroll, M. W. , Dean, N. E. , Diatta, I. , Doumbia, M. , Draguez, B. , Duraffour, S. , Enwere, G. , Grais, R. , Gunther, S. , Gsell, P.‐S. , Hossmann, S. , Watle, S. V. , Kondé, M. K. … Kieny, M.‐P. (2017). Efficacy and effectiveness of an rVSV‐vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open‐label, cluster‐randomised trial (Ebola Ça Suffit!). Lancet, 389, 505–518. http://www.sciencedirect.com/science/article/pii/S0140673616326216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Mü, M. A. , Drosten, C. , Pö, S. , Krü, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , Nitsche, A. , & Pö Hlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor article SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181, 1–10. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesslich, S. , & Kamen, A. A. (2020). Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnology Advances, 44, 107608. 10.1016/j.biotechadv.2020.107608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesslich, S. , Losa, J. P. V. , Gélinas, J. , & Kamen, A. A. (2020). Serum‐free production of rVSV‐ZEBOV in Vero cells: Microcarrier bioreactor versus scale‐X TM hydro fixed‐bed. Journal of Biotechnology, 310, 32–39. 10.1016/j.jbiotec.2020.01.015 [DOI] [PubMed] [Google Scholar]
- Kim, G. N. , Wu, K. , Hong, J. P. , Awamleh, Z. , & Kang, C. Y. (2015). Creation of matrix protein gene variants of two serotypes of vesicular stomatitis virus as prime‐boost vaccine vectors. Journal of Virology, 89, 6338–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse, P. J. (2012). The molecular basis of HIV entry. Cellular Microbiology, 14, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty, B. D. , Power, A. T. , Stojdl, D. F. , & Bell, J. C. (2004). Vesicular stomatitis virus: Re‐inventing the bullet. Trends in Molecular Medicine, 10, 210–216. [DOI] [PubMed] [Google Scholar]
- Litwin, J. (1992). The growth of Vero cells as suspended aggregates in serum‐free medium, Anim. Cell Technol. Butterworth‐Heinemann; 414– 417 p. 10.1016/B978-0-7506-0421-5.50096-9 [DOI] [Google Scholar]
- Mangion, M. , Gélinas, J. F. , Gashti, A. B. Z. , Azizi, H. , Kiesslich, S. , Nassoury, N. , Chahal, P. S. , Kobinger, G. , Gilbert, R. , Garnier, A. , Gaillet, B. , & Kamen, A. (2020). Evaluation of novel HIV vaccine candidates using recombinant vesicular stomatitis virus vector produced in serum‐free Vero cell cultures. Vaccine, 38, 7949–7955. 10.1016/j.vaccine.2020.10.058 [DOI] [PubMed] [Google Scholar]
- Moller‐tank, S. , & Maury, W. (2015). Ebola virus entry: A curious and complex series of events. PLoS Pathogens, 11(4):e1004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath, T. P. , Fast, P. E. , Modjarrad, K. , Clarke, D. K. , Martin, B. K. , Fusco, J. , Nichols, R. , Heppner, D. G. , Simon, J. K. , Dubey, S. , Troth, S. P. , Wolf, J. , Singh, V. , Coller, B.‐A. A. , & Robertson, J. S. (2019). rVSVΔG‐ZEBOV‐GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X, 1, 100009. 10.1016/j.jvacx.2019.100009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munis, A. M. , Bentley, E. M. , & Takeuchi, Y. (2020). A tool with many applications: Vesicular stomatitis virus in research and medicine. Expert Opinion on Biological Therapy, 00, 1–15. 10.1080/14712598.2020.1787981 [DOI] [PubMed] [Google Scholar]
- Paillet, C. , Forno, G. , Kratje, R. , & Etcheverrigaray, M. (2009). Suspension‐Vero cell cultures as a platform for viral vaccine production. Vaccine, 27, 6464–6467. [DOI] [PubMed] [Google Scholar]
- Quesney, S. , Marc, A. , Gerdil, C. , Gimenez, C. , Marvel, J. , Richard, Y. , & Meignier, B. (2003). Kinetics and metabolic specificities of Vero cells in bioreactor cultures with serum‐free medium. Cytotechnology, 42, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison, C. S. , & Whitt, M. A. (2000). The membrane‐proximal stem region of vesicular stomatitis virus G protein confers efficient virus assembly. Journal of Virology, 74, 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourou, S. , Ben Zakkour, M. , & Kallel, H. (2019). Adaptation of Vero cells to suspension growth for rabies virus production in different serum free media. Vaccine, 37, 6987–6995. 10.1016/j.vaccine.2019.05.092 [DOI] [PubMed] [Google Scholar]
- Shen, C. F. , Guilbault, C. , Li, X. , Elahi, S. M. , Ansorge, S. , Kamen, A. A. , & Gilbert, R. (2019). Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine, 37, 6996–7002. 10.1016/j.vaccine.2019.07.003 [DOI] [PubMed] [Google Scholar]
- Thomassen, Y. E. , Rubingh, O. , Wijffels, R. H. , van der Pol, L. A. , & Bakker, W. A. M. (2014). Improved poliovirus D‐antigen yields by application of different Vero cell cultivation methods. Vaccine, 32, 2782–2788. 10.1016/j.vaccine.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura, T. , Yamashita, A. , Mizuki, N. , Okuda, K. , & Shimada, M. (2020). New vaccine production platforms used in developing SARS‐CoV‐2 vaccine candidates. Vaccine, 10.1016/j.vaccine.2020.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. DRAFT landscape of COVID‐19 candidate vaccines ‐ 19 October. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- World Health Organization . 2021. COVAX Statement on WHO Emergency Use Listing for AstraZeneca/Oxford COVID‐19 Vaccine. https://www.who.int/news/item/16-02-2021-covax-statement-on-who-emergency-use-listing-for-astrazeneca-oxford-covid-19-vaccine.
- Ziegler, C. M. , & Botten, J. W. (2020). Defective interfering particles of negative‐strand RNA viruses. Trends in Microbiology, 28, 554–565. 10.1016/j.tim.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Will be made available through authors upon request.


