Abstract
Patients with liver diseases acquire complex alterations in their hemostatic system that may lead to abnormalities in routine diagnostic test of hemostasis. Thrombocytopenia, prolongations in the prothrombin time and activated partial thromboplastin time, and decreased plasma fibrinogen are common in patients with advanced liver disease. Historically, liver diseases therefore have been classified as an acquired bleeding disorder. Laboratory and clinical observations have demonstrated that although routine diagnostic tests of hemostasis suggest a hypocoagulable state, patients with liver disease also tend to develop thrombotic events. Overall, patients have commensurate changes in both pro‐ and antihemostatic pathways. This new hemostatic balance, however, appears much more fragile than the hemostatic balance in individuals with normal liver function, and patients with liver disease can readily experience both hemostasis‐related bleeding and thrombotic events. These insights into the hemostatic balance in patients with liver disease have led to revised recommendations for clinical management of hemostasis. In 2020, an SSC working group within the ISTH has been founded with the aim to disseminate new concepts on prevention and treatment of bleeding and thrombosis in patients with liver disease. The current document will outline the hemostatic changes in patients with liver disease, the limitations of routine diagnostic tests of hemostasis, and the concept of rebalanced hemostasis.
Keywords: bleeding, cirrhosis, liver diseases, thrombosis
1. INTRODUCTION
The liver, being the site of synthesis of many hemostatic proteins, plays a central, but often overlooked, role in hemostasis. Consequently, patients with advanced liver disease develop complex hemostatic alterations including reduced platelet number and function and decreased plasma levels of proteins of coagulation and fibrinolysis. 1 Historically, patients with liver disease were thought to have a hemostasis‐related bleeding tendency. Alterations in routine diagnostic tests of hemostasis, such as reductions in the platelet count, or elevations of the prothrombin time (PT), and activated partial thromboplastin time (APTT), in combination with bleeding symptoms, formed the basis for the classification of liver disease as the epitome of the acquired bleeding disorders.
Over the past two decades, careful reanalysis of the bleeding symptoms associated with liver disease in combination with laboratory studies of hemostasis have questioned whether liver diseases truly are associated with an acquired bleeding disorder. 1 , 2 , 3 In contrast, large epidemiological studies demonstrated that liver diseases are a risk factor for development of (venous) thrombosis. 4 The incidence of venous thrombosis in hospitalized patients with cirrhosis is at least 1%, 5 whereas the incidence of portal vein thrombosis is 3% to 4% within the first year of diagnosis, 6 which increases over time to 25% in the sickest patients. These new insights have led to important modifications of hemostatic management of patients with liver disease. For example, recent guidance documents from large hepatological societies increasingly question the need for (prophylactic) prohemostatic management in patients with thrombocytopenia and/or abnormal coagulation test results, prior to surgery or invasive procedures. 6 , 7 In addition, these guidance documents indicate that thromboprophylaxis may not be contraindicated in patients with abnormal routine diagnostic hemostasis tests.
These new insights and subsequent changes in clinical management have been actively disseminated in the hepatology community, but they seem less well absorbed by the thrombosis and hemostasis community. A Scientific and Standardization Committee (SSC) working group was founded in 2020 with the intention to support a re‐evaluation of these changing concepts of hemostasis in patients with liver disease, and to share the implications with the thrombosis and hemostasis community. In particular, it will consider how management strategies to prevent or treat bleeding and thrombotic complications in liver disease are profoundly altered by our current knowledge of hemostatic changes in liver disease.
The current document will outline the hemostatic changes in patients with liver disease, the limitations of routine diagnostic tests of hemostasis, and the concept of rebalanced hemostasis.
2. PATHOPHYSIOLOGY OF HEMOSTATIC DISORDERS IN PATIENTS WITH LIVER DISEASE
Patients with advanced chronic liver disease acquire alterations in all phases of hemostasis (summarized in Figure 1). These alterations are part of scoring systems to classify the severity of illness in patients with chronic liver diseases and are part of the definition of acute liver failure. 8 Although hemostatic changes are not identical between chronic and acute liver disease, and between various etiologies of liver disease, they share many common features. Specifically, alterations in primary hemostasis include thrombocytopenia and alterations in platelet function, 9 , 10 elevated plasma levels of von Willebrand factor (VWF) and decreased levels of A Disintegrin And Metalloprotease with ThromboSpondin‐1 domain (ADAMTS)‐13. 11 , 12 Alterations in secondary hemostasis include concomitantly decreased levels of pro‐ and anticoagulant proteins, except for factor VIII, which is increased. 13 Finally, decreased plasma levels of fibrinolytic proteins except for tissue‐type plasminogen activator (t‐PA) and plasminogen activator inhibitor type 1 (PAI‐1), which are often increased, are found. 14 Elevated levels of VWF, t‐PA, and PAI‐1 are likely a consequence of chronic endothelial cell activation, which may be driven by multiple mechanisms including endotoxemia and other toxins, chronic inflammation, and alterations in blood flow. 11 Plasma levels of VWF are strongly associated to clinically significant portal hypertension in patients with compensated cirrhosis, which may indicate that changes in blood flow are the most important determinant of VWF plasma levels in this setting. 15
FIGURE 1.
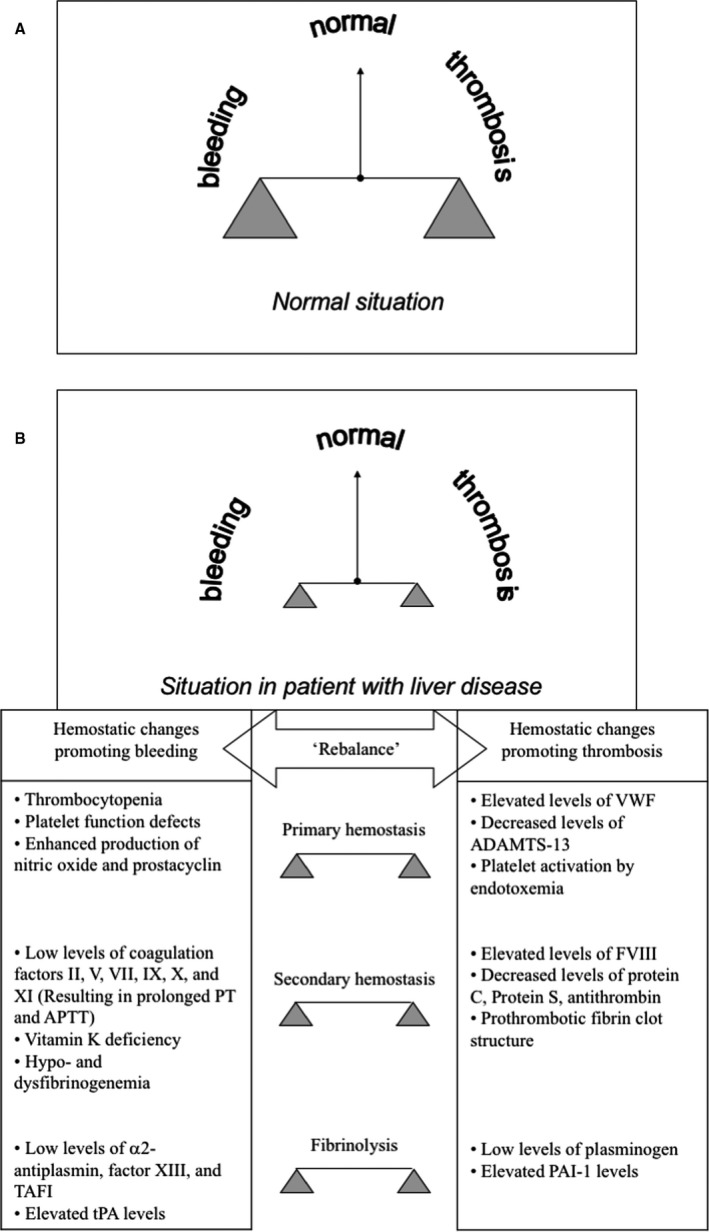
Hemostatic balance in patients with liver disease. Concomitant changes in both pro‐ and anti‐hemostatic pathways result in a ‘rebalanced’ hemostatic state in patients with liver disease. Panel A shows the hemostatic balance in healthy individuals, panel B shows the hemostatic balance in patients with liver disease together with the individual changes in the hemostatic system. The new hemostatic balance in patients with liver disease is much less stable compared to the balance in healthy individuals, as there is much less weight on each end of the hemostatic scale. Simultaneous changes promoting bleeding and promoting thrombosis occur in primary and secondary hemostasis, and fibrinolysis. Modified from Curr Opin Organ Transplant 2008; 13: 298–9 with permission from Wolters Kluwer Health. ADAMTS‐13, A Disintegrin And Metalloprotease with ThromboSpondin‐1 domain; APTT, activated partial thromboplastin time; FVIII, factor VIII; PT, prothrombin time; VWF, von Willebrand factor
Thrombocytopenia of liver disease is likely multifactorial, and the mechanisms involved include decreased platelet production by thrombopoietin deficiency and direct megakaryocyte toxicity, decreased platelet half‐life due to splenomegaly and hypersplenism, and possibly autoantibodies. 9 Although it has long been thought that platelet function was also impaired, more recent studies have suggested that platelet function in patients with liver disease is intact or perhaps even enhanced, recognizing the limitations of assays in earlier studies. 16 Specifically, platelet function testing is unreliable in samples with a reduced platelet count. Importantly, most platelet function tests capture only part of all platelet functions, and it is unclear which test best reflects the in vivo hemostatic capacity of platelets. In addition, it is likely that in vivo, the anemia of cirrhosis impairs platelet function given the role of red blood cells in the process of platelet margination.
Decreased hepatic synthesis is likely and largely responsible for the decrease in plasma levels of coagulation and fibrinolytic proteins although consumption of hemostatic proteins by low‐grade intrahepatic or systemic activation of the hemostatic system may contribute. 17 Similarly, consumption of platelets by intrahepatic or systemic platelet activation may contribute to the thrombocytopenia of liver disease. Evidence for activation of coagulation in the diseased liver is mainly derived from animal models in which liver injury leads to decryption of hepatocyte tissue factor, which drives local coagulation activation. 18 While the apparent procoagulant nature of the (activated) vascular endothelium, combined with elevations of activation markers of coagulation, may suggest low‐grade disseminated intravascular coagulation, elevation of these markers may also be explained by reduced clearance of these proteins by the diseased liver.
3. LIMITATIONS OF ROUTINE DIAGNOSTIC TESTS OF HEMOSTASIS IN PATIENTS WITH LIVER DISEASE
The hemostatic changes in patients with liver disease result in alterations in routine diagnostic tests of hemostasis such as the platelet count, the traditional coagulation tests (PT and APTT), and plasma fibrinogen levels. The combination of low platelet counts, prolonged PT and APTT, and low fibrinogen in patients with liver disease have been and are currently erroneously interpreted as indicative of a bleeding tendency.
Patients with hematological malignancies and thrombocytopenia have been extensively investigated, and data suggest that bleeding occurs across a range of platelet counts and continues to happen despite policies of prophylactic platelet transfusions. Platelet transfusions have also been shown to be harmful in thrombocytopenic critically ill neonates and in adults with intracranial hemorrhage. 19 , 20 , 21 Although prolongations of PT and APTT are associated with bleeding tendency when caused by individual congenital coagulation factor deficiencies, such as the hemophilias and rare bleeding disorders, the PT and APTT can also be prolonged as a consequence of systemic diseases that are not necessarily associated with bleeding. The levels of procoagulant factors in liver disease remain higher than those found in severe hemophilia, which is associated with major spontaneous bleeding and defined by factor levels below 1%, with factor levels of approximately 80% of normal in patients with Child A cirrhosis 22 and levels of 20% to 30% of normal in the sickest patients with acute‐on‐chronic or acute liver failure. 23 , 24 In fact, prolongations of PT or APTT frequently do not have (major) clinical consequences. 25 Finally, isolated quantitative or qualitative deficiencies of fibrinogen do not necessarily lead to bleeding tendency. 26 Notably, a substantial proportion of patients with fibrinogen defects suffer from thrombotic disease.
In patients with liver disease, the low platelet count should be evaluated in the context of the substantially elevated VWF and profoundly decreased ADAMTS‐13 levels that are common in these patients. 11 , 12 In vitro experiments assessing platelet adhesion and aggregation to thrombogenic surfaces have demonstrated that elevated VWF levels (at least partially) compensate for the thrombocytopenia of liver disease, despite functional defects of VWF. 11 Similarly, given the role of ADAMTS‐13 in the regulation of platelet thrombus formation, 27 it is conceivable that low ADAMTS‐13, in part, compensates for the thrombocytopenia of liver disease. Additionally, in vitro experiments demonstrated that platelet numbers as low as 60 × 109/L in platelet‐rich plasma from cirrhotic patients is sufficient to yield thrombin generation equivalent to the lower limit of the normal range. 28 Overall, an isolated low platelet count may have different consequences in patients with or without liver disease.
The value of the PT and APTT in patients with liver disease is limited and may also provide misleading information. On one hand, PT and APTT are sensitive to deficiencies of plasma levels of procoagulant factors and they were in fact developed as screening tools for hemophilia and rare bleeding disorders. They are, however, insensitive to plasma levels of natural anticoagulants. They are in fact normal (not shortened) in patients with congenital deficiency of the naturally occurring anticoagulants (protein C, protein S, and antithrombin deficiency) that are associated with increased levels of thrombin production. Overall, the PT and APTT are not good indicators of hemostatic status in patients with complex alterations of hemostasis, especially in acquired disorders such as liver disease that are characterized by concomitant deficiencies of both pro‐ and anticoagulant factors. Although thrombin‐generation tests are sensitive for plasma levels of anticoagulant proteins, plasma does not contain sufficient amounts of thrombomodulin to facilitate full activation of the protein C pathway. Using a modified thrombin‐generation test that includes the addition of soluble thrombomodulin (or other direct protein C activators) that are responsive to all pro‐ and anticoagulant drivers, it has been demonstrated that the endogenous coagulation potential observed in patients with liver disease is preserved indicating that the deficiency of anticoagulant proteins compensates for the deficiency of procoagulant proteins. 29 Even in those patients with profoundly prolonged PT and APTT, such as patients with acute‐on‐chronic or acute liver failure, thrombomodulin‐modified thrombin generation is normal to increased compared to healthy individuals. 24 , 30
Fibrinogen levels are decreased in patients with advanced liver disease. In addition, fibrin polymerization is delayed due to post‐translational modifications of the fibrinogen molecule (i.e., hypersialylation). 31 These post‐translational modifications contribute to the prolongation in the thrombin time 31 and may lead to underestimation of fibrinogen levels by Clauss fibrinogen assays although this has been poorly studied. However, once the fibrin clot has been formed, it has prothrombotic properties, notably decreased permeability, that may compensate for the low fibrinogen plasma levels. 32 This compensatory aspect is not detected by routine diagnostic assays.
Whole blood viscoelastic tests such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are increasingly used to estimate hemostatic capacity of patients with liver diseases. Interestingly, TEG and ROTEM results are often normal or near‐normal in patients with liver disease despite notable abnormalities in platelet count, PT, APTT, and/or fibrinogen levels. 33 , 34 , 35 Although viscoelastic tests may better reflect the global hemostatic capacity in patients with liver disease compared to traditional hemostatic laboratory tests, a disadvantage of these tests is that they are not responsive to VWF and the protein C anticoagulant system and are therefore still likely to underestimate the hemostatic capacity, as the compensatory effects of elevated VWF and decreased protein C on thrombocytopenia and procoagulant deficiencies are not taken into account. 36 Furthermore, while there are studies showing the benefit of viscoelastic tests in making decisions on transfusion requirements in patients with liver disease undergoing liver transplantation, 37 it is unclear whether viscoelastic tests are predictive of clinical endpoints (bleeding [either spontaneous or provoked] or thrombosis) in patients with liver disease and until more evidence is available cannot be used to guide clinical decision‐making outside the transplant setting.
4. THE CONCEPT OF REBALANCED HEMOSTASIS
Historically, patients with liver disease were thought to have a hemostasis‐related bleeding tendency as evidenced by abnormalities of routine hemostasis tests and severe bleeding, for example variceal bleeding or bleeding during liver transplant surgery. These bleeding manifestations, however, are not necessarily a consequence of hemostatic failure. The most important bleeding complication in patients with liver disease are portal hypertension‐related bleedings, mainly variceal bleeding that up until recently occurred in 25% to 40% of patients with cirrhosis. Interestingly, the incidence of portal hypertension‐related bleeding has profoundly decreased over time mainly due to a better management of portal hypertension with primary and secondary prophylaxis (primarily using non‐selective beta blockers and placement of transjugular intrahepatic portosystemic shunts in selected patients), and by prophylactic endoscopic band ligation. Also, part of the bleeding complications in patients with liver disease relate to inadvertent laceration of blood vessels, for example during invasive procedures. Moreover, liver transplant surgery required massive blood transfusion almost per definition in the 1980s, 38 whereas currently blood product use is relatively modest in many centers, with a substantial proportion of patients not requiring any perioperative blood products. 39 Also, spontaneous bleeding in patients with acute liver failure has decreased tremendously over time as bleeding was a cause of death in approximately 40% of patients in the 1980s, whereas any clinically significant bleeding is rare in contemporary series. 3
Thus, patients with liver disease do not necessarily display a bleeding tendency, and if they bleed, bleeding may be unrelated to hemostatic abnormalities but mainly due to portal hypertension. In addition, studies using advanced hemostasis tests have demonstrated that routine diagnostic test results are unreliable and perhaps misleading in patients with liver disease. In fact, laboratory studies have shown that all the defects of pro‐hemostatic systems are (at least in part) compensated for by the simultaneous changes in antihemostatic systems. Examples of this include:
Thrombocytopenia in liver diseases is balanced by increased VWF and decreased ADAMTS‐13 levels. 11 , 40
Decreased levels of procoagulant proteins are balanced by decreased levels of the anticoagulant proteins protein C and antithrombin and by elevated levels of factor VIII. 13 , 29 , 41 , 42
Decreased fibrinogen levels and delayed fibrin polymerization are balanced by a prothrombotic structure of the fibrin clot. 32
In patients with chronic liver disease, decreased plasma levels of fibrinolytic regulators such as thrombin activatable fibrinolysis inhibitor and antiplasmin and increased circulating t‐PA are balanced by decreased plasminogen levels. 43
The combination of the clinical observations and the insights stemming from laboratory studies have led to the concept of rebalanced hemostasis in liver disease, whereby the net effects of the complex hemostatic changes observed in patients with both chronic and acute liver disease are neutral due to a simultaneous decline in pro‐ and antihemostatic drivers. 1 , 2 , 44 Although hemostatic changes become more pronounced in sicker patients, maintenance of hemostatic balance appears to remain present from compensated patients with only minor hemostatic abnormalities to patients with acute‐on‐chronic or acute liver failure that have profound changes in their hemostatic profile. 22 , 24 , 45 However, this new hemostatic balance in patients with liver disease is more fragile than that observed in healthy individuals, as there is simply much less weight on each end of the hemostatic “scale.” The hemostatic balance may therefore easily flip toward either hyper‐ or a hypocoagulability depending on the clinical context, thus explaining why both bleeding and thrombotic complications may (paradoxically) occur in patients with liver disease.
5. REFINEMENTS IN THE CONCEPT OF REBALANCED HEMOSTASIS
Although the hemostatic status of patients with liver disease is remarkably competent, even in those patients with severe changes of the levels of hemostatic components, there appear to be distinct hypo‐ and hypercoagulable features that may contribute to bleeding or thrombosis.
Distinct hypocoagulable features, for example, include the combination of thrombocytopenia and anemia that impair platelet function, 46 , 47 low fibrinogen levels in combination with delayed fibrin polymerization, 48 and hyperfibrinolysis in a proportion of critically ill patients with chronic liver disease. 49
Distinct hypercoagulable features include a very unfavorable VWF/ADAMTS‐13 ratio with undetectable ADAMTS‐13 in specific situations, 24 , 30 , 50 enhanced thrombin‐generating capacity even in the sickest patients, 24 , 30 a thrombotic fibrin structure, 32 and hypofibrinolysis in a proportion of critically ill patients with chronic liver disease and the vast majority of patients with acute liver failure. 49 , 50
Whether we can use individual profiles of hypo‐ or hypercoagulable features to predict the risk of bleeding or thrombosis is yet unclear.
6. CONCLUSION AND IMPLICATIONS
Patients with chronic and acute liver disease may acquire complex disorders of their hemostatic system. Although alterations in routine diagnostic tests of hemostasis (thrombocytopenia, hypofibrinogenemia, and PT/APTT prolongation) are suggestive for a bleeding tendency, patients with liver disease are in hemostatic balance due to the simultaneous decline of pro‐ and anticoagulant drivers. The concept of rebalanced hemostasis in liver diseases is supported by clinical observations including a lack of predictable hemostasis‐related bleeding and a, perhaps paradoxically, increased risk for thrombosis.
The concept of rebalanced hemostasis has profound practical implications for patient management that will be detailed in upcoming contributions from the Working Group on Hemostatic Management of Patients with Liver Disease. In short, these practical implications include (but are not restricted to):
Prophylactic correction of abnormal laboratory tests of hemostasis with blood products or pharmacological prohemostatic agents aiming to prevent spontaneous or procedure‐related bleeding is not indicated 44 , 51 , 52 ;
Thromboprophylaxis may be indicated in selected patients admitted to hospital, irrespective of thrombocytopenia and prolonged PT and APTT. 53
CONFLICTS OF INTEREST
We have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
TL drafted the manuscript; all other authors provided intellectual input for revisions of the draft. All authors have approved the final version of the manuscript.
Manuscript handled by: Joost Meijers
Final decision: Joost Meijers, 04 January 2021
REFERENCES
- 1. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147‐156. [DOI] [PubMed] [Google Scholar]
- 2. Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878‐885. [DOI] [PubMed] [Google Scholar]
- 3. Stravitz RT, Ellerbe C, Durkalski V, et al. Bleeding complications in acute liver failure. Hepatology. 2018;67:1931‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ambrosino P, Tarantino L, Di Minno G, et al. The risk of venous thromboembolism in patients with cirrhosis: a systematic review and meta‐analysis. Thromb Haemost. 2017;117:139‐148. [DOI] [PubMed] [Google Scholar]
- 5. Qi X, Ren W, Guo X, Fan D. Epidemiology of venous thromboembolism in patients with liver diseases: a systematic review and meta‐analysis. Intern Emerg Med. 2015;10:205‐217. [DOI] [PubMed] [Google Scholar]
- 6. Northup PG, Garcia‐Pagan JC, Garcia‐Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the amercian association for the study of liver diseases. Hepatology. 2021. in press. 10.1002/hep.31646 [DOI] [PubMed] [Google Scholar]
- 7. O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157:34‐43. [DOI] [PubMed] [Google Scholar]
- 8. Wlodzimirow KA, Eslami S, Abu‐Hanna A, Nieuwoudt M, Chamuleau RAFM. Systematic review: acute liver failure ‐ one disease, more than 40 definitions. Aliment Pharmacol Ther. 2012;35:1245‐1256. [DOI] [PubMed] [Google Scholar]
- 9. Witters P, Freson K, Verslype C, et al. Review article: blood platelet number and function in chronic liver disease and cirrhosis. Aliment Pharmacol Ther. 2008;27:1017‐1029. [DOI] [PubMed] [Google Scholar]
- 10. Hugenholtz GGC, Porte RJ, Lisman T. The platelet and platelet function testing in liver disease. Clin Liver Dis. 2009;13:11‐20. [DOI] [PubMed] [Google Scholar]
- 11. Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53‐61. [DOI] [PubMed] [Google Scholar]
- 12. Uemura M, Fujimura Y, Matsumoto M, et al. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99:1019‐1029. [DOI] [PubMed] [Google Scholar]
- 13. Tripodi A, Primignani M, Chantarangkul V, et al. An imbalance of pro‐ vs anti‐coagulation factors in plasma from patients with Cirrhosis. Gastroenterology. 2009;137:2105‐2111. [DOI] [PubMed] [Google Scholar]
- 14. Leebeek FWG, Kluft C, Knot EAR, De Maat MPM, Wilson JHP. A shift in balance between profibrinolytic and antifibrinolytic factors causes enhanced fibrinolysis in cirrhosis. Gastroenterology. 1991;101:1382‐1390. [DOI] [PubMed] [Google Scholar]
- 15. Mandorfer M, Hernández‐Gea V, García‐Pagán JC, Reiberger T. Noninvasive diagnostics for portal hypertension: a comprehensive review. Semin Liver Dis. 2020;40:240‐255. [DOI] [PubMed] [Google Scholar]
- 16. Raparelli V, Basili S, Carnevale R, et al. Low‐grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017;65:571‐581. [DOI] [PubMed] [Google Scholar]
- 17. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost Wiley. 2017;1:150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan BP, Kopec AK, Joshi N, et al. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121:1868‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet‐transfusion thresholds in neonates. N Engl J Med. 2019;380:242‐251. [DOI] [PubMed] [Google Scholar]
- 20. Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997;337:1870‐1875. [DOI] [PubMed] [Google Scholar]
- 21. Stanworth SJ, Estcourt LJ, Powter G, et al. A no‐prophylaxis platelet‐transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771‐1780. [DOI] [PubMed] [Google Scholar]
- 22. Bos S, Van Den Boom B, Kamphuisen PW, et al. Haemostatic profiles are similar across all aetiologies of cirrhosis. Thromb Haemost. 2019;119:246‐253. [DOI] [PubMed] [Google Scholar]
- 23. Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher C, Patel VC, Stoy SH, et al. Balanced haemostasis with both hypo‐ and hyper‐coagulable features in critically ill patients with acute‐on‐chronic‐liver failure. J Crit Care. 2018;43:54‐60. [DOI] [PubMed] [Google Scholar]
- 25. Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864‐873. [DOI] [PubMed] [Google Scholar]
- 26. Casini A, De Moerloose P, Neerman‐Arbez M. Clinical features and management of congenital fibrinogen deficiencies. Semin Thromb Hemost. 2016;42:366‐374. [DOI] [PubMed] [Google Scholar]
- 27. Shida Y, Nishio K, Sugimoto M, et al. Functional imaging of shear‐dependent activity of ADAMTS13 in regulating mural thrombus growth under whole blood flow conditions. Blood. 2008;111:1295‐1298. [DOI] [PubMed] [Google Scholar]
- 28. Tripodi A, Primignani M, Chantarangkul V, et al. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44:440‐445. [DOI] [PubMed] [Google Scholar]
- 29. Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553‐558. [DOI] [PubMed] [Google Scholar]
- 30. Lisman T, Arefaine B, Adelmeijer J, et al. Global hemostatic status in patients with acute‐on‐chronic liver failure and septics without underlying liver disease. J Thromb Haemost. 2020;19(1):85‐95.in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Francis JL, Armstrong DJ. Fibrinogen‐bound sialic acid levels in the dysfibrinogenaemia of liver disease. Haemostasis. 1982;11:215‐222. [DOI] [PubMed] [Google Scholar]
- 32. Hugenholtz GCG, Macrae F, Adelmeijer J, et al. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J Thromb Haemost. 2016;14:1054‐1066. [DOI] [PubMed] [Google Scholar]
- 33. Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Clin Adv Hematol Oncol. 2012;10:513‐520. [PMC free article] [PubMed] [Google Scholar]
- 34. Tripodi A, Primignani M, Chantarangkul V, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124:132‐136. [DOI] [PubMed] [Google Scholar]
- 35. Raeven P, Baron‐Stefaniak J, Simbrunner B, et al. Thromboelastometry in patients with advanced chronic liver disease stratified by severity of portal hypertension. Hepatol Int. 2020;14:1083‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lisman T. Interpreting hemostatic profiles assessed with viscoelastic tests in patients with cirrhosis. J Clin Gastroenterol. 2020;54:389‐391. [DOI] [PubMed] [Google Scholar]
- 37. Kirchner C, Dirkmann D, Treckmann JW, et al. Coagulation management with factor concentrates in liver transplantation: a single‐center experience. Transfusion. 2014;54:2760‐2768. [DOI] [PubMed] [Google Scholar]
- 38. Lewis JH, Bontempo FA, Cornell F, et al. Blood use in liver transplantation. Transfusion. 1987;27:222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Massicotte L, Thibeault L, Roy A. Classical notions of coagulation revisited in relation with blood losses, transfusion rate for 700 consecutive liver transplantations. Semin Thromb Hemost. 2015;41:538‐546. [DOI] [PubMed] [Google Scholar]
- 40. Hugenholtz GCG, Adelmeijer J, Meijers JCM, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology. 2013;58:752‐761. [DOI] [PubMed] [Google Scholar]
- 41. Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol. 2013;59:265‐270. [DOI] [PubMed] [Google Scholar]
- 42. Lisman T, Bos S, Intagliata NM. Mechanisms of enhanced thrombin‐generating capacity in patients with cirrhosis. J Thromb Haemost. 2018;16:1128‐1131. [DOI] [PubMed] [Google Scholar]
- 43. Lisman T, Leebeek FWG, Mosnier LO, et al. Thrombin‐activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121:131‐139. [DOI] [PubMed] [Google Scholar]
- 44. Lisman T, Stravitz RT. Rebalanced hemostasis in patients with acute liver failure. Semin Thromb Hemost. 2015;41:468‐473. [DOI] [PubMed] [Google Scholar]
- 45. Lisman T, Bakhtiari K, Adelmeijer J, Meijers JCM, Porte RJ, Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10:1312‐1319. [DOI] [PubMed] [Google Scholar]
- 46. Escolar G, Cases A, Viñas M, et al. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA‐100(TM)): influence of hematocrit elevation. Haematologica. 1999;84:614‐619. [PubMed] [Google Scholar]
- 47. Lisman T, Adelmeijer J, de Groot PG, Janssen HLA, Leebeek FWG. No evidence for an intrinsic platelet defect in patients with liver cirrhosis ‐ studies under flow conditions. J Thromb Haemost. 2006;4:2070‐2072. [DOI] [PubMed] [Google Scholar]
- 48. Lisman T, Ariëns RAS. Alterations in fibrin structure in patients with liver diseases. Semin Thromb Hemost. 2016;42:389‐396. [DOI] [PubMed] [Google Scholar]
- 49. Blasi A, Patel VC, Adelmeijer J, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute‐on‐chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology. 2020;71:1381‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Driever EG, Stravitz RT, Zhang J, et al. VWF/ADAMTS13 imbalance, but not global coagulation or fibrinolysis, is associated with outcome and bleeding in acute liver failure. Hepatology. 2020. in press. 10.1002/hep.31507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lisman T, Porte RJ. Value of preoperative hemostasis testing in patients with liver disease for perioperative hemostatic management. Anesthesiology. 2017;126:338‐344. [DOI] [PubMed] [Google Scholar]
- 52. Stanworth SJ, Grant‐Casey J, Lowe D, et al. The use of fresh‐frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion. 2011;51:62‐70. [DOI] [PubMed] [Google Scholar]
- 53. Intagliata NM, Henry ZH, Shah N, Lisman T, Caldwell SH, Northup PG. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34:26‐32. [DOI] [PubMed] [Google Scholar]


