Summary
A universal anti‐Xa assay for the determination of rivaroxaban, apixaban and edoxaban drug concentrations would simplify laboratory procedures and facilitate widespread implementation. Following two pilot studies analysing spiked samples and material from 698 patients, we conducted a prospective multicentre cross‐sectional study, including 867 patients treated with rivaroxaban, apixaban or edoxaban in clinical practice to comprehensively evaluate a simple, readily available anti‐Xa assay that would accurately measure drug concentrations and correctly predict relevant levels in clinical practice. Anti‐Xa activity was measured by an assay calibrated with low‐molecular‐weight heparin (LMWH) in addition to ultra‐high performance liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). As an external validation, LMWH‐calibrated anti‐Xa activity was also determined in nine external laboratories. The LMWH‐calibrated anti‐Xa activity correlated strongly with rivaroxaban, apixaban or edoxaban drug levels [r s = 0·98, 95% confidence interval (CI) 0·98–0·98]. The sensitivity for the clinically relevant cut‐off levels of 30, 50 and 100 µg/l was 96·2% (95% CI 94·4–97·4), 96·4% (95% CI 94·4–97·7) and 96·7% (95% CI 94·3–98·1) respectively. Concordant results were obtained in the external validation study. In conclusion, a universal, LMWH‐calibrated anti‐Xa assay accurately measured rivaroxaban, apixaban and edoxaban concentrations and correctly predicted relevant drug concentrations in clinical practice.
Keywords: factor Xa inhibitors, rivaroxaban, apixaban, edoxaban, heparin, low‐molecular‐weight, drug monitoring
Introduction
Rapid and accurate determination of anticoagulant drug levels is essential in critical clinical situations such as urgent surgery, planned thrombolysis or major bleeding. 1 Any anticoagulation treatment is inevitably associated with an increased risk of bleeding. This risk is as high as 15%/year in certain patient populations. 2 , 3 Besides, ~25% of intracranial haemorrhages, which are the most dangerous adverse events, are linked to anticoagulation treatment. 4 The bleeding risk associated with anticoagulants is particularly important in trauma patients because uncontrolled bleeding is the leading cause of death in these patients. 5 In any patient with massive bleeding, early and targeted treatment is the most critical measure to save lives because 80% of trauma deaths occur within the first hour after trauma. 1 , 5 , 6 Laboratory tests that can rapidly verify the presence of a relevant anticoagulant drug level will support a fast treatment with reversal agents. 1 Besides, knowledge of the anticoagulant drug level is essential in case of urgent surgery and planned thrombolysis. 7 Rapid intervention is required in both cases, but an increased bleeding risk exposes patients to high procedural risks. Thus, simple laboratory tests that accurately determine the anticoagulant drug level would improve care in patients with anticoagulation treatment. 1
The proportion of the population taking direct oral anticoagulants (DOACs) such as rivaroxaban, apixaban or edoxaban is rapidly increasing and critical clinical situations occur frequently. 8 , 9 A simple and readily available laboratory test, accurately determining these anticoagulants’ concentration in various healthcare settings, would improve care in this vulnerable population. Routine coagulation tests, which are widely available, are not suitable for determining DOAC drug levels. 10 , 11 , 12 Anti‐Xa assays, which are successfully used for the monitoring of heparin and low‐molecular‐weight heparin (LMWH) 13 were introduced and a nice linear relationship was demonstrated in samples spiked with rivaroxaban. 14 , 15 DOAC‐calibrated anti‐Xa activity correlated well with drug concentrations if measured in healthy volunteers and even patients. 11 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 However, widespread implementation in clinical practice is hampered by two major problems. First, a large study investigating the accuracy of all Xa inhibitors over the full spectrum of concentrations in clinical practice is still missing. Second, providing different assays for the determination of rivaroxaban, apixaban, and edoxaban drug levels in a ‘24/7’ service is elaborate and expensive. It would be very convenient if laboratories could use a test that is already available in 24/7 service to determine all three Xa inhibitors. Such a universal anti‐Xa assay for the determination of rivaroxaban, apixaban and edoxaban drug levels would simplify laboratory procedures and foster widespread implementation.
We hypothesised that a readily available LMWH‐calibrated anti‐Xa assay would accurately measure rivaroxaban, apixaban and edoxaban drug concentrations and correctly predict relevant levels in clinical practice. In the present study, we aimed to test this hypothesis in a comprehensive set of evaluation studies.
Methods
Overall study design
A phased approach was chosen to evaluate the universal test. The sensitivity and linearity of measurements were studied in spiking experiments. The feasibility of the test for the measurement of rivaroxaban, apixaban and edoxaban was observed in a retrospective analysis. The diagnostic accuracy was tested in a large prospective cross‐sectional study in clinical practice. External validation in nine laboratories was additionally conducted.
Pilot study: spiking experiments
Standardised human plasma (Cryocheck pooled normal plasma, Precision Biologic, Dartmouth, Canada) was spiked with increasing concentrations of rivaroxaban or apixaban respectively. Stock solutions containing rivaroxaban and apixaban were prepared using dimethylsulphoxide (DMSO) following the manufacturer’s internal instructions. Seven concentrations between 10 and 200 µg/l were prepared and monitored using a drug‐specific and drug‐calibrated anti‐Xa assay (Biophen® DiXaI, Hyphen BioMed, Neuilly‐sur Oise, France). Final DMSO concentrations were <1%. Rivaroxaban and apixaban pure substances were provided by Bayer AG (Wuppertal, Germany) and Bristol Myers Squibb Company (New Brunswick, NJ, USA). Samples were snap‐frozen at −80°C after preparation and sent on dry ice to seven specialised haemostasis laboratories. The LMWH‐calibrated anti‐Xa activity was measured within 2 weeks using local reagents and analysers (Biophen® Heparin LRT on Sysmex CS5100, Siemens BCS XP, or Stago STA‐R evolution). Edoxaban pure substance was not yet available at the time of the spiking experiments.
Pilot study: retrospective analysis
In a retrospective analysis, laboratory data collected between 2014 and 2017 in one university hospital were retrieved. During this period, drug‐specific anti‐Xa activity was measured once daily in batches, and LMWH‐calibrated anti‐Xa activity was used as a proxy in 24/7 service. Inclusion criteria were: (a) anti‐Xa activity for rivaroxaban or apixaban requested (edoxaban was not yet available), (b) age >18 years and (c) signed general consent. An exclusion criterion was the application of anticoagulants other than rivaroxaban or apixaban. Venous blood was drawn into tubes containing 0·109 M sodium citrate (BD Vacutainer, Plymouth, UK). A standardised protocol was implemented to ensure adequate pre‐analytical conditions. Citrated samples were snap‐frozen at −80°C after determining LMWH‐calibrated anti‐Xa activity until analysis of drug‐specific specific anti‐Xa activity (using rivaroxaban and apixaban calibrators). The LMWH‐calibrated anti‐Xa activity was done using Biophen® Heparin LRT on a Sysmex CS5100 analyser. Drug‐specific anti‐Xa activity was determined using Biophen DiXaI® on a Siemens BCS XP (Siemens Healthineers, Erlangen, Germany). Strict management guidelines are implemented and the laboratory is accredited to the Swiss Accreditation Service (SAS). The appropriate Ethics Committee approved the study protocol and all patients signed a general informed consent.
Cross‐sectional study: design, setting and population
We conducted a prospective, multicentre cross‐sectional study to test the hypothesis that a LMWH‐calibrated anti‐Xa assay would accurately measure rivaroxaban, apixaban and edoxaban drug levels in clinical practice and correctly predict clinically relevant concentrations. The flow chart of the patients is given in Fig 1. Consecutive in‐ and outpatients treated with rivaroxaban, apixaban or edoxaban in clinical practice were included in 2018 and 2019 in nine major haemostasis laboratories affiliated to Swiss tertiary hospitals. Inclusion criteria were: (a) age >18 years, (b) use of rivaroxaban, apixaban or edoxaban, (c) drug‐level requested and (d) signed general informed consent, if required by local authorities. Exclusion criteria were: (a) refused general informed consent, (b) additional use of heparin, (c) more than one DOAC used, (d) pre‐analytical issues and (e) insufficient sample material. The appropriate Ethics Committee approved the protocol and the study was conducted in accordance with the declaration of Helsinki.
Fig 1.
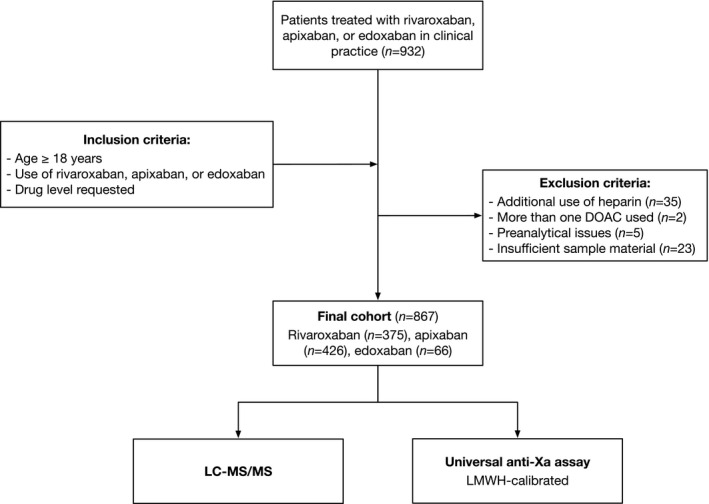
Flow of the patients. A prospective, multicentre cross‐sectional study was conducted to test the hypothesis that a universal anti‐Xa assay would accurately measure rivaroxaban, apixaban, or edoxaban drug levels in clinical practice, and correctly predict clinically relevant drug levels.
Ultra‐high performance liquid chromatography‐tandem mass spectrometry (UPLC‐MS/MS) targeting rivaroxaban, apixaban, edoxaban and edoxaban M4 metabolite was conducted as the reference standard in parallel to the LMWH‐calibrated anti‐Xa assay at Inselspital, Bern University Hospital; details are discussed below. Clinically relevant drug concentrations were defined as 30, 50 and 100 mg/l. 24
To determine the limit of detection (LOD), 20 blank samples were determined on 20 consecutive days. 25 Samples with low drug concentration (~80 µg/l) and high drug concentration (200 µg/l) were measured 15 times to determine the within‐run imprecision and on 15 consecutive days to verify the day‐to‐day imprecision.
External validation
As an external validation, the LMWH‐calibrated anti‐Xa measurements were conducted in nine referring laboratories using the same sample for the cross‐sectional study mentioned above. We aimed to confirm the applicability of the specific cut‐offs to other laboratories. Measurements were conducted after inclusion, but before freezing the samples. Local reagents and analysers established for the determination of LMWH drug levels were utilised. The following reagents and analysers were used: Biophen® Heparin LRT on a Sysmex CS‐5100 (four laboratories), Biophen® Heparin LRT on a BCS XP (Siemens Healthineers; one laboratory), Biophen® Heparin LRT on an ACL Top 750 (Instrumentation Laboratory; two laboratories), STA‐LIQUID anti‐Xa on a STA‐R (Stago; one laboratory), and HemosIL liquid anti‐Xa on ACL Top 750 (one laboratory).
Data collection and handling of samples
Anonymised data were collected in a password protected RedCAP database. The following data were retrieved: age, sex and drug. Samples were de‐identified before shipment. Protocols ensuring adequate pre‐analytical conditions were implemented at all institutions. 26 Briefly, venous blood was drawn in plastic syringes containing 1 ml trisodium citrate (0·106 or 0·109 mol/l respectively) per ml of blood. Citrated samples were collected with a different syringe to avoid tissue factor contamination. Tourniquet application was limited to a minimum and removed before blood withdrawal. Samples were transported and processed immediately. After aliquoting, samples were snap‐frozen at −80°C and shipped on dry ice in one batch to the central laboratory (delivery time 3–4 h). Samples were stored continuously at −80°C until testing without any freeze–thaw cycle. The storage period varied between 1 and 13 months. Laboratory test results were exported automatically to avoid typing errors.
Determination of the universal anti‐Xa assay
Concluding from the two pilot studies and previous research, we selected the Biophen® Heparin LRT with LMWH calibrators. 14 , 16 This assay is implemented in many Swiss institutions for the determination of LMWH drug levels. 13 All analyses were conducted in batches on an Atellica COAG 360 analyser (Siemens Healthineers). 27 A five‐step calibration curve with the following concentrations of LMWH was applied: 0·0, 0·42, 0·86, 1·26 and 1·67 iu/ml (Biophen® Heparin Calibrator). Samples were rapidly thawed and gently mixed at 37°C. The patient’s plasma was pre‐diluted (1/2; 100 µl) and added to the solution containing the chromogenic substrate (250 µl). Factor Xa (250 µl) was added after incubation at 37°C. The reaction was stopped after 120 s and the absorption was measured at 405 nm. All instructions of the manufacturers were strictly followed. Following these instructions, the maximum level was 3·34 iu/ml. Clinical information and reference standard test results were not available to the performer of the anti‐Xa assay.
Determination by LC‐MS/MS
The LC‐MS/MS was conducted to quantify rivaroxaban, apixaban, edoxaban and edoxaban M4 metabolite. For protein precipitation and analyte extraction, 10 µl acetonitrile:water 1:1 (v/v), 25 µl extraction buffer (MassTox TDM Series A, Chromsystems Instruments and Chemicals GmbH, Gräfelfing, Germany) and 240 µl precipitation reagent (MassTox TDM Series A, Chromsystems Instruments and Chemicals) containing the internal standards (rivaroxaban 13C6, apixaban 13C,D3 and edoxaban 13C,D2) were added to 50 µl plasma. After vortexing, the samples were centrifuged at 14 000 g and 20°C for 4 min. A 20‐µl aliquot of the supernatant was diluted with 80 µl of water:methanol 8:2 (v/v) and stored at 10°C until analysis. Calibrators and quality controls were prepared in pooled plasma (Innovative Research, Novi, MI, USA); 3 µl of the extracted samples were analysed by reversed‐phase chromatography (Cortecs UPLC C18 column, 2·1 × 75 mm, 1·7 µm; Waters Corp., Milford, MA, USA) on a triple quadrupole mass spectrometer (Xevo TQ‐S, Waters) coupled to a UPLC Acquity I‐Class system (Waters). Rivaroxaban, apixaban, edoxaban and edoxaban M4 were separated at 0·4 ml/min with a gradient using water (A) and methanol (B) acidified with 0·1% (v/v) formic acid as mobiles phases (0·0–0·5 min, 20% B; 0·5–2·5 min, 20–99% B; 2·5–3·5 min, 99% B; 3·5–3·51 min, 99–20% B; 3·51–4·5 min, 20% B). The source offset and transition parameters were optimised for each analyte. The raw data were processed with TargetLynx available in the MassLynx software (version 4.1, Waters). Edoxaban M4 metabolite was summed with edoxaban for further analysis. Rivaroxaban, apixaban and edoxaban pure substances were provided by Bayer AG, Bristol Myers Squibb Company and Daichi Sankyo Co, Ltd. (Tokyo, Japan). Clinical information and index test results were not available to the performer of the LC‐MS/MS.
Statistical analysis
Descriptive statistics were used to describe the study population (numbers, percentages, or median and range, as appropriate). The Spearman correlation coefficient was calculated to determine the level of agreement between the universal anti‐Xa assay and the LC‐MS/MS. Spearman correlations of ≥0·90 were regarded as high. 16 A modified Bland–Altman plot was obtained to determine a possible systematic bias over the measurement range (ratios rather differences were used to account for different scales). Deming regression was additionally calculated. Sensitivities and specificities were calculated regarding the clinically relevant drug concentrations of 30, 50 and 100 mg/l. 24 The corresponding cut‐off points of the universal anti‐Xa assay were determined using Deming regression. Sensitivity and specificity >90% was regarded as high. 16 The LOD was calculated in 20 blank samples (3 × standard variation) and the coefficient of variation (SD/mean) in 15 measurements of samples with low and high drug concentrations (within‐run and day‐to‐day imprecision). 25 We conducted a power analysis for a one‐sample correlation test. A power of 0·9 and an alpha level of 0·05 was applied. We further considered that a sufficient number of patients should be distributed among the full range of measurements. However, as long as many patients were taking rivaroxaban and apixaban, and only a few patients edoxaban, patients’ inclusion was continued until 932 patients were included. Analyses were performed using the Statistical Package for the Social Sciences (SPSS®) version 24 (IBM Corp., Armonk, NY, USA) and Stata version 14.1 (Stata Corp LP, College Station, TX, USA). Figures were created using Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Spiking experiments
Calculated and realised concentrations of the spiked samples are shown in Table SI of the supplemental material. Figure 2 illustrates the association between LMWH‐calibrated anti‐Xa measurements and drug concentrations in all seven laboratories. The Spearman correlation coefficient between anti‐Xa activity and drug concentrations was 1·0 in all laboratories.
Fig 2.
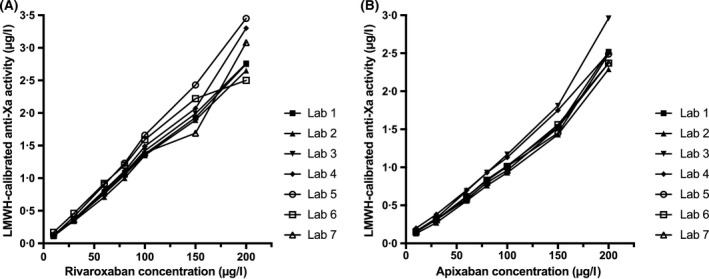
Low‐molecular‐weight heparin (LMWH)‐calibrated anti‐Xa activity in standardised plasma samples spiked with rivaroxaban, and apixaban, as measured in seven haemostasis laboratories. Results of pilot study 1 are shown; Spearman correlation coefficient was 1·0 in all laboratories.
Retrospective analysis
A total of 698 samples from 474 patients were available for analysis. Among them, 432 patients were taking rivaroxaban and 73 apixaban. The median (range) age was 68·2 (18–99) years and 70·1% were female. The correlations between LMWH‐ and drug‐calibrated anti‐Xa activities are shown in Figure S1 of the supplemental material. The Spearman correlation coefficient was 0·96 for rivaroxaban [95% confidence interval (CI) 0·95–0·97] and 0·98 for apixaban (95% CI 0·97–0·99). A deviation from linearity was observed in patient samples >483 µg/l.
Patient characteristics
A total of 932 patients were included in nine study centres (Fig 1). In all, 35 patients were excluded because of heparin use, two were excluded because more than one DOAC was used, five were excluded due to pre‐analytical issues, and 23 were excluded due to insufficient sample material. Thus, 867 patients were eventually available for analysis. Among those, 375 patients were taking rivaroxaban, 426 apixaban and 66 edoxaban. The median [interquartile range (IQR)] age was 76 (66–82) years and 42·8% of the patients were female. Rivaroxaban, apixaban and edoxaban drug levels were balanced distributed over the full range of measurements (Fig 3).
Fig 3.
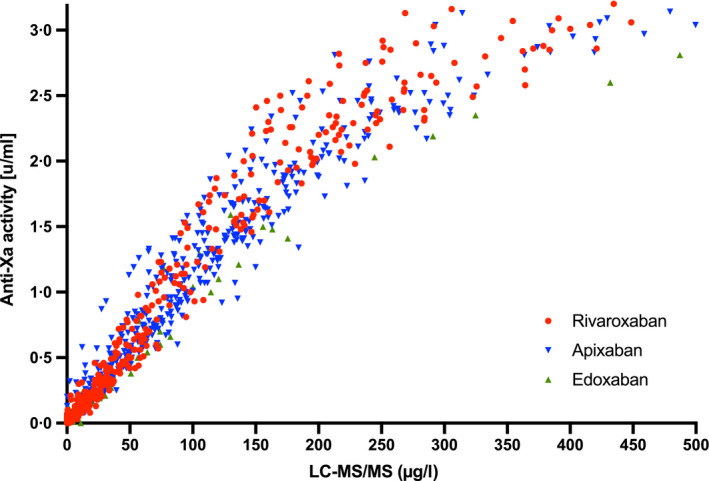
Measurements of a universal, low‐molecular‐weight heparin (LMWH)‐calibrated anti‐Xa assay in relation to drug concentrations in a multicentre cross‐sectional study conducted in clinical practice (n = 867). Drug concentrations were determined using ultra‐high performance liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Of the patients, 375 were taking rivaroxaban, 426 apixaban and 66 edoxaban. Spearman correlation coefficient was 0·99 for rivaroxaban (95% CI 0·98–0·99), 0·97 for apixaban (95% CI 0·96–0·97), and 0·98 for edoxaban (95% CI 0·95–0·99).
Diagnostic accuracy
The association between measurements of the LMWH‐calibrated anti‐Xa assay and drug concentrations is shown in Fig 3. The Spearman correlation coefficient r s was 0·99 for rivaroxaban (95% CI 0·98–0·99), 0·97 for apixaban (95% CI 0·96–0·97) and 0·98 for edoxaban (95% CI 0·95–0·99). Below 50 µg/l, the r s was 0·90 (95% CI 0·88–0·93) and above 200 µg/l, the r s was 0·80 (95% CI 0·74–0·86). Above 483 µg/l, the r s was 0·40 and the association was not linear anymore (Fig 3).
The slope of the regression equation was 0·012 for rivaroxaban (95% CI 0·011–0·013), 0·011 for apixaban (95% CI 0·010–0·012) and 0·010 for edoxaban (95% CI 0·008–0·013). The y‐intercept was −0·006 (rivaroxaban, 95% CI −0·025 to 0·011), 0·050 (apixaban, 95% CI 0·015 to 0·084) and −0·001 (edoxaban, 95% CI −0·037 to 0·034).
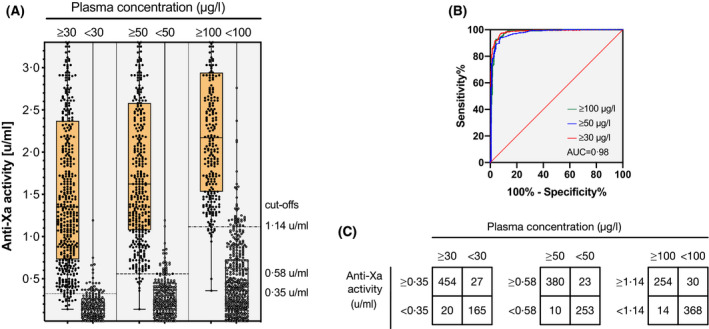
The diagnostic accuracy of the LMWH‐calibrated anti‐Xa assay against the clinically relevant drug concentrations is illustrated in Fig 4. Drug levels of >30 µg/l were detected with a sensitivity of 96·2% (95% CI 94·4–97·4; cut‐off value 0·35 u/ml), drug levels of >50 µg/l with a sensitivity of 96·4% (95% CI 94·4–97·7; cut‐off value 0·58 u/ml) and drug levels of >100 µg/l with a sensitivity of 96·4% (95% CI 94·0–97·9; cut‐off value 1·14 u/ml). The specificities were 92·9% (95% CI 88·9–95·5), 93·3% (95% CI 90·1–95·5) and 92·0% (95% CI 89·4–94·0) respectively. The area under the receiver‐operating characteristic (ROC) curve (AUC) was 0·99 for all three cut‐offs.
Fig 4.
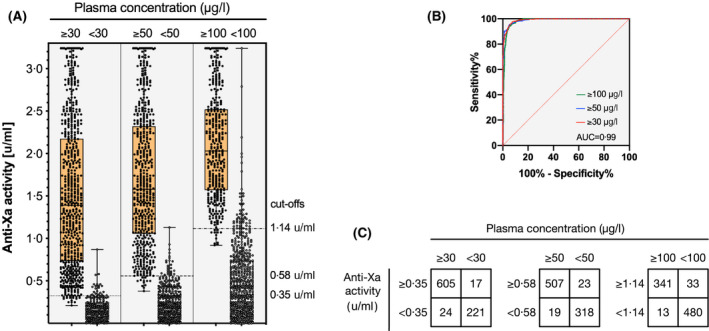
Diagnostic accuracy of a universal, low‐molecular‐weight heparin (LMWH)‐calibrated anti‐Xa assay for the measurement of rivaroxaban, apixaban, and edoxaban drug concentrations as determined in a multicentre cross‐sectional study in clinical practice (n = 867). (A) Box plots illustrating the distribution of measurements (median, interquartile range, minimum to maximum, all results) at clinically relevant cut‐offs (> and < 30, 50, and 100 µg/l). (B) Receiver‐operating characteristic (ROC) curves showing the diagnostic accuracy. The area under the ROC curve was 0·99 for all cut‐offs. (C) Confusion matrices at each cut‐off. Drug levels of >30 µg/l were detected with a sensitivity of 96·2% (95% CI 94·4–97·4; cut‐off value 0·35 u/ml), drug levels of >50 µg/l with a sensitivity of 96·4% (95% CI 94·4–97·7; cut‐off value 0·58 u/ml) and drug levels of >100 µg/l with a sensitivity of 96·4% (95% CI 94·0–97·9; cut‐off value 1·14 u/ml). The specificities were 92·9% (95% CI 88·9–95·5), 93·3% (95% CI 90·1–95·5) and 92·0 (95% CI 89·4–94·0) respectively.
The LOD was 14·85 µg/l, the within‐run imprecision was 1·4 [coefficient of variation (CV)], and the day‐to‐day imprecision 2·1 (CV). No differences were observed in samples with ≥6 months of storage time compared with samples with <6 months storage time.
External validation
A total of 666 measurements were available for the external validation (rivaroxaban, n = 322; apixaban, n = 289; edoxaban, n = 55), results are shown in Fig 5. Correlation with the drug level (r s) was 0·96 for rivaroxaban (95% CI 0·95–0·97), 0·95 for apixaban (95% CI 0·94–0·96) and 0·97 for edoxaban (95% CI 0·95–0·98). Drug levels of >30 µg/l were detected with a sensitivity of 95·8% (95% CI 93·0–97·3), drug levels of >50 µg/l with a sensitivity of 97·4% (95% CI 95·4–98·6) and drug levels of >100 µg/l with a sensitivity of 94·8% (95% CI 91·4–97·1). The specificities were 85·9% (95% CI 80·3–90·2), 91·7% (95% CI 87·8–94·4) and 92·5% (95% CI 89·4–94·9) respectively. The AUC was 0·98 for all cut‐offs.
Fig 5.
Results of an external validation of a universal, low‐molecular‐weight heparin (LMWH)‐calibrated anti‐Xa assay for the measurement of rivaroxaban, apixaban, and edoxaban (n = 666). (A) Box plots illustrating the distribution of measurements (median, interquartile range, minimum to maximum, all results) at clinically relevant cut‐offs (> and < 30, 50, and 100 µg/l). (B) Receiver‐operating characteristic (ROC) curves showing the diagnostic accuracy. The area under the ROC curve was 0·98 for all cut‐offs. (C) Confusion matrices at each cut‐off. Drug levels of >30 µg/l were detected with a sensitivity of 95·8% (95% CI 93·0–97·3), drug levels of >50 µg/l with a sensitivity of 97·4% (95% CI 95·4–98·6), and drug levels of >100 µg/l with a sensitivity of 94·8% (95% CI 91·4–97·1). The specificities were 85·9% (95% CI 80·3–90·2), 91·7% (95% CI 87·8–94·4) and 92·5% (95% CI 89·4–94·9), respectively.
Discussion
In a comprehensive set of evaluation studies, a universal LMWH‐calibrated anti‐Xa assay accurately estimated concentrations of rivaroxaban, apixaban and edoxaban, and correctly predicted relevant drug levels. We demonstrated a sensitive and linear relationship between drug concentrations and the assay measurements in spiking experiments. In an extensive retrospective analysis, we confirmed the method’s feasibility by comparing drug‐specific measurements with LMWH‐calibrated results. A large prospective multicentre cross‐sectional study utilising LC‐MS/MS as a reference standard established a high accuracy and sound sensitivity and specificity for clinically relevant thresholds. Also, external validation conducted in nine laboratories confirmed that the assay can be applied to other institutions.
A few preliminary studies proposing a single calibration to determine all anti‐Xa inhibitors have been conducted; our present results are essentially consistent with those results. A high level of correlation was observed in a retrospective study measuring an LMWH‐calibrated assay and LC‐MS/MS in 210 patients taking rivaroxaban or apixaban. 28 Similar results were also reported by a retrospective analysis by Douxfils et al. 18 in 52 patients and another cross‐sectional analysis in 30 patients. 29 More recently, rivaroxaban and apixaban‐calibrated anti‐Xa measurements were compared with possible LMWH‐calibrated measurements as inferred from the same measurement. 30 In addition, in vitro or ex vivo spiking experiments have been done by several other authors, reporting correlation coefficients ranging between 0·98 and 1·00. 14 , 15 , 31 However, these studies’ methodology is limited by the number of participants, the choice of the reference standard, the retrospective design, the range of measurements or the selection of drugs.
Our present study’s strength is that we have conducted a comprehensive set of evaluation studies that were carefully designed, thus clarifying all essential questions associated with the implementation. In particular, the cross‐sectional study was prospectively designed and conducted in a multicentre design, including many patients. LC‐MS/MS was utilised in all patients as the most valid reference standard. Moreover, a clinical practice rather than an artificial study setting was chosen. External validation was done as well, confirming the potential application of the specific cut‐offs to other laboratories.
The present study has several potential limitations. First, edoxaban was not studied in the spiking experiments and the retrospective analysis because it was not yet available in Switzerland at the time of these experiments. As long as the accuracy of the measurements was equivalent to rivaroxaban and apixaban in the cross‐sectional study and the external validation, we believe that findings inferred from spiking experiments and retrospective analysis can be extrapolated to edoxaban as well. Second, the number of patients taking edoxaban was limited because of its limited use in Switzerland. However, we estimate the risk of bias as low because the accuracy measures are consistent between drugs and in the external validation study. Third, one particular LMWH‐calibrated assay was studied and it might be possible that other anti‐Xa assays behave differently. Thus, these results cannot straightforwardly be applied to other LMWH‐calibrated anti‐Xa assays. Fourth, external validation focussed on the applicability of the specific cut‐offs to other laboratories, rather than the applicability to different patient populations. However, we studied a large and diverse population, making relevant differences unlikely.
How should the universal assay be applied? Two different options appear feasible and are illustrated in Table I. First, cut‐off values for the clinical thresholds 30, 50 and 100 µg/l can be given. The corresponding categorical result is the most crucial information, which might directly prompt clinical decision making. Second, the regression equations can be used to determine drug levels as accurate as possible.
Table I.
Application of the low‐molecular‐weight heparin (LMWH)‐calibrated, universal assay.
| Measure | Result |
|---|---|
| DOAC cut‐off value 30 µg/l | 0·35 U/ml |
| DOAC cut‐off value 50 µg/l | 0·58 U/ml |
| DOAC cut‐off value 100 µg/l | 1·14 U/ml |
| Regression equation rivaroxaban | 120 × [U/ml]−19 |
| Regression equation apixaban | 115 × [U/ml]−22 |
| Regression equation edoxaban | 164 × [U/ml]−24 |
Two options appear. First, cut‐off values for the clinical thresholds can be given. The corresponding categorial result is the most crucial information, which can prompt clinical decision making. Second, the regression equations can be used to calculate plasma concentrations of the individual drugs.
Our present findings confirm that LMWH‐calibrated anti‐Xa assays can be used as a universal anti‐Xa assay to determine rivaroxaban, apixaban and edoxaban drug concentrations in clinical practice. Even though a high accuracy was observed at all clinically relevant cut‐offs, a wider spread and a non‐linear curve was observed in samples with high drug levels. The reason for this effect was the maximum level, which can be measured with the LMWH‐calibrated assay (3·34 u/ml corresponding to a drug level of 483 µg/l). Although this effect will not alter most clinical decisions, drug levels might be underestimated in case of intoxications and accumulation. However, LMWH‐calibrated assays might not only simplify laboratory procedures and save healthcare costs; it might foster the implementation of anti‐Xa assays in a variety of institutions. However, whether this universal assay can be implemented for the determination of DOAC drug levels depends on national in vitro diagnostics (IVD) regulations and potential Conformité Européene (CE) labelling of the manufacturer. In our laboratory, we implemented it as a laboratory‐developed in‐house test.
Rapid determination of DOAC drug levels is essential in critical clinical situations such as significant bleeding, urgent surgery or planned thrombolysis. 1 Given that the proportion of patients with DOAC is rapidly increasing worldwide, universal anti‐Xa assays have the potential to improve care in many patients and various healthcare settings. Future research shall confirm our observations in different healthcare settings, patient populations and using other LMWH‐calibrated assays. While LC‐MS/MS is expensive and might not be available in certain institutions, drug‐specific anti‐Xa assays or even the Biophen® Heparin LRT can be utilised as a surrogate reference standard as well.
Conclusion
In conclusion, a universal LMWH‐calibrated anti‐Xa assay accurately measured concentrations of rivaroxaban, apixaban and edoxaban and correctly predicted relevant drug levels in clinical practice. A sensitive and linear relationship was established, the feasibility confirmed, a high accuracy and sound sensitivity and specificity demonstrated and the test verified in an external validation. Implementation of the universal assay might not only simplify laboratory processes and save healthcare costs, but foster implementation of anti‐Xa assays for the determination of rivaroxaban, apixaban and edoxaban in various healthcare settings. Future research shall confirm our observations in other settings using different reagents and analysers.
Conflict of interest
The study was supported by a research grant of the Research Fund Haematology Cantonal Hospital Lucerne. Michael Nagler is supported by a research grant of the Swiss National Science Foundation (#179334). Lorenzo Alberio is supported by a grant of the Swiss National Science Foundation (#320030‐197392). Implementation of the LC‐MS/MS measurements was supported by the Gottfried & Julia Bangerter‐Rhyner Stiftung (applicant Ursula Amstutz). We thank the following companies for the provision of pure substances: Bayer Healthcare AG, Bristol‐Myers Squibb, and Daiichi Sankyo. These companies had no role in study design, data collection and analysis, the decision to publish, or manuscript preparation. Michael Nagler reports research grants from Bayer Healthcare, outside of the submitted work, lecture honoraria from Bayer Healthcare, and Daiichi Sankyo. Lorenzo Alberio reports research grants from Bayer, CSL‐Behring, Novartis, Novo Nordisk, Roche, Sobi, and Takeda. Walter A. Wuillemin reports research grants from Bayer Healthcare, BMS‐Pfizer, Daiichi Sankyo and Sanofi, and honoraria for participating in scientific advisory boards from Bayer, Pfizer, and from Alexion Pharma GmbH, all outside the submitted. Jan‐Dirk Studt reports lecture fees and advisory honoraria from Bayer Healthcare, Pfizer, Takeda, Siemens, and Sanofi.
Supporting information
Fig S1. Association of low‐molecular‐weight heparin (LMWH)‐calibrated and drug‐specific anti‐Xa activity measurements in 698 patients taking rivaroxaban or apixaban. Results of a retrospective analysis conducted in one University hospital are shown (pilot study 2). Spearman correlation coefficient was 0·96 in case of rivaroxaban [95% confidence interval (CI) 0·95–0·97] and 0·98 in case of apixaban (95% CI 0·97–0·99).
Fig S2. A modified Bland–Altman plot using ratios to determine a possible systematic bias over the measurement range.
Table SI. Calculated and realised concentrations of spiked samples. Concentrations were measured using a drug‐specific anti‐Xa assay (Biophen® DiXaI, Hyphen BioMed, Neuilly‐sur Oise, France).
Acknowledgments
Guido Willekens analysed the data and wrote the manuscript. Jan‐Dirk Studt, Adriana Mendez, Lorenzo Alberio, Pierre Fontana, Walter A. Wuillemin, Adrian Schmidt, Lukas Graf, Bernhard Gerber, Cedric Bovet, and Thomas C. Sauter collected data, and contributed to study design, protocol, and preparation of the manuscript. Cedric Bovet contributed essential tools and reagents. Michael Nagler designed the study, wrote the protocol, collected data, analysed the data, and wrote the manuscript. We thank a number of individuals who supported this study: Jessica Sangalli‐Baruffaldi for doing the spiking experiments, Evelyne Giabbani for organising the sample collection, Gabriela Monika Maeder for conducting all LC‐MS/MS measurements, Marc Gisi, Francisco Gomez, and Vincent Benites for doing the anti‐Xa measurements, Sarah Phillip for technical support, and Martin Fiedler for providing the infrastructure at Inselspital.
References
- 1. Sauter TC, Eberle B, Wuillemin WA, Thiele T, Angelillo‐Scherrer A, Exadaktylos AK, et al. How I manage patients with anticoagulation‐associated bleeding or urgent surgery. Swiss Med Wkly. 2018;148:w14598. [DOI] [PubMed] [Google Scholar]
- 2. Enriquez A, Lip GY, Baranchuk A. Anticoagulation reversal in the era of the non‐vitamin K oral anticoagulants. Europace. 2016;18:955–64. [DOI] [PubMed] [Google Scholar]
- 3. Milling TJ Jr, Frontera J. Exploring indications for the use of direct oral anticoagulants and the associated risks of major bleeding. Am J Manag Care. 2017;23(4 Suppl):S67–S80. [PMC free article] [PubMed] [Google Scholar]
- 4. Schols AM, Schreuder FH, van Raak EP, Schreuder TH, Rooyer FA, van Oostenbrugge RJ, et al. Incidence of oral anticoagulant‐associated intracerebral hemorrhage in the Netherlands. Stroke. 2014;45:268–70. [DOI] [PubMed] [Google Scholar]
- 5. Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith W, Williams A, Agudelo J, Shannon M, Morgan S, Stahel P, et al. Early predictors of mortality in hemodynamically unstable pelvis fractures. J Orthop Trauma. 2007;21:31–7. [DOI] [PubMed] [Google Scholar]
- 7. Moner‐Banet T, Alberio L, Bart PA. Does one dose really fit all? On the monitoring of direct oral anticoagulants: a review of the literature. Hamostaseologie. 2020;40:184–200. [DOI] [PubMed] [Google Scholar]
- 8. van den Heuvel JM, Hövels AM, Büller HR, Mantel‐Teeuwisse AK, de Boer A, Maitland‐van der Zee AH. Maitland‐van der Zee AH. NOACs replace VKA as preferred oral anticoagulant among new patients: a drug utilization study in 560 pharmacies in The Netherlands. Thromb J. 2018;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beyer‐Westendorf J, Gelbricht V, Forster K, Ebertz F, Kohler C, Werth S, et al. Peri‐interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur. Heart J. 2014;35:1888–96. [DOI] [PubMed] [Google Scholar]
- 10. Fontana P, Alberio L, Angelillo‐Scherrer A, Asmis LM, Korte W, Mendez A, et al. Impact of rivaroxaban on point‐of‐care assays. Thromb Res. 2017;153:65–70. [DOI] [PubMed] [Google Scholar]
- 11. Francart SJ, Hawes EM, Deal AM, Adcock DM, Gosselin R, Jeanneret C, et al. Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross‐sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost. 2014;111:1133–40. [DOI] [PubMed] [Google Scholar]
- 12. Gosselin RC, Adcock D, Hawes EM, Francart SJ, Grant RP, Moll S. Evaluating the use of commercial drug‐specific calibrators for determining PT and APTT reagent sensitivity to dabigatran and rivaroxaban. Thromb Haemost. 2015;113:77–84. [DOI] [PubMed] [Google Scholar]
- 13. Burki S, Brand B, Escher R, Wuillemin WA, Nagler M. Accuracy, reproducibility and costs of different laboratory assays for the monitoring of unfractionated heparin in clinical practice: a prospective evaluation study and survey among Swiss institutions. BMJ Open. 2018;8:e022943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asmis LM, Alberio L, Angelillo‐Scherrer A, Korte W, Mendez A, Reber G, et al. Rivaroxaban: quantification by anti‐FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res. 2012;129:492–8. [DOI] [PubMed] [Google Scholar]
- 15. Beyer J, Trujillo T, Fisher S, Ko A, Lind SE, Kiser TH. Evaluation of a heparin‐calibrated antifactor Xa assay for measuring the anticoagulant effect of oral direct Xa inhibitors. Clin Appl Thromb Hemost. 2016;22:423–8. [DOI] [PubMed] [Google Scholar]
- 16. Studt J‐D, Alberio L, Angelillo‐Scherrer A, Asmis LM, Fontana P, Korte W, et al. Accuracy and consistency of anti‐Xa activity measurement for determination of rivaroxaban plasma levels. J Thromb Haemost. 2017;15:1576–83. [DOI] [PubMed] [Google Scholar]
- 17. Bardy G, Fischer F, Appert A, Baldin B, Stève M, Spreux A, et al. Is anti‐factor Xa chromogenic assay for Rivaroxaban appropriate in clinical practice? Advantages and comparative drawbacks. Thromb Res. 2015;136:396–401. [DOI] [PubMed] [Google Scholar]
- 18. Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogne JM, et al. Comparison of calibrated chromogenic anti‐Xa assay and PT tests with LC‐MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110:723–31. [DOI] [PubMed] [Google Scholar]
- 19. Gosselin RC, Funk DM, Taylor JM, Francart SJ, Hawes EM, Friedman KD, et al. Comparison of anti‐Xa and dilute Russell viper venom time assays in quantifying drug levels in patients on therapeutic doses of rivaroxaban. Arch Pathol Lab Med. 2014;138:1680–4. [DOI] [PubMed] [Google Scholar]
- 20. Königsbrügge O, Quehenberger P, Belik S, Weigel G, Seger C, Griesmacher A, et al. Anti‐coagulation assessment with prothrombin time and anti‐Xa assays in real‐world patients on treatment with rivaroxaban. Ann Hematol. 2015;94:1463–71. [DOI] [PubMed] [Google Scholar]
- 21. Rathbun S, Tafur A, Grant R, Esmon N, Mauer K, Marlar RA. Comparison of methods to determine rivaroxaban anti‐factor Xa activity. Thromb Res. 2015;135:394–7. [DOI] [PubMed] [Google Scholar]
- 22. Schellings M, Boonen K, Schmitz E, Jonkers F, van den Heuvel DJ, Besselaar A, et al. Determination of dabigatran and rivaroxaban by ultra‐performance liquid chromatography‐tandem mass spectrometry and coagulation assays after major orthopaedic surgery. Thromb Res. 2016;139:128–34. [DOI] [PubMed] [Google Scholar]
- 23. Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, et al. Determination of dabigatran, rivaroxaban and apixaban by ultra‐performance liquid chromatography ‐ tandem mass spectrometry (UPLC‐MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost. 2014;12:1636–46. [DOI] [PubMed] [Google Scholar]
- 24. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:623–7. [DOI] [PubMed] [Google Scholar]
- 25. Magnusson B, Örnemark U eds. Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related Topics, 2nd ed. 2014. ISBN 978‐91‐87461‐59‐0. Available from: www.eurachem.org.
- 26. Clinical and Laboratory Standards Institute (CLSI) . Collection, Transport, and Processing of Blood Specimens for Testing Plasma‐Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline ‐ Fifth Edition. CLSI document H21‐A5. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 27. Horber S, Lehmann R, Peter A. Evaluation of the Atellica COAG 360 coagulation analyzer in a central laboratory of a maximum care hospital. Int J Lab Hematol. 2020;42:28–36. [DOI] [PubMed] [Google Scholar]
- 28. Billoir P, Barbay V, Joly LM, Fresel M, Chretien MH, Le Cam DV. Anti‐Xa oral anticoagulant plasma concentration assay in real life: rivaroxaban and apixaban quantification in emergency with LMWH calibrator. Ann Pharmacother. 2019;53:341–7. [DOI] [PubMed] [Google Scholar]
- 29. Gosselin RC, Francart SJ, Hawes EM, Moll S, Dager WE, Adcock DM. Heparin‐calibrated chromogenic anti‐Xa activity measurements in patients receiving rivaroxaban: can this test be used to quantify drug level? Ann Pharmacother. 2015;49:777–83. [DOI] [PubMed] [Google Scholar]
- 30. Boissier E, Senage T, Babuty A, Gouin‐Thibault I, Rozec B, Roussel JC, et al. Heparin anti‐Xa activity, a readily available unique test to quantify apixaban, rivaroxaban, fondaparinux, and danaparoid levels. Anesth Analg. 2021;132:707–16. [DOI] [PubMed] [Google Scholar]
- 31. Lindhoff‐Last E, Perzborn E, Schwers S, Herth N, Hesse C, Stratmann G, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost. 2017;108:191–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Association of low‐molecular‐weight heparin (LMWH)‐calibrated and drug‐specific anti‐Xa activity measurements in 698 patients taking rivaroxaban or apixaban. Results of a retrospective analysis conducted in one University hospital are shown (pilot study 2). Spearman correlation coefficient was 0·96 in case of rivaroxaban [95% confidence interval (CI) 0·95–0·97] and 0·98 in case of apixaban (95% CI 0·97–0·99).
Fig S2. A modified Bland–Altman plot using ratios to determine a possible systematic bias over the measurement range.
Table SI. Calculated and realised concentrations of spiked samples. Concentrations were measured using a drug‐specific anti‐Xa assay (Biophen® DiXaI, Hyphen BioMed, Neuilly‐sur Oise, France).


