Summary
Aspirin and heparin are widely used to reduce the risk of recurrent pregnancy loss in women with antiphospholipid syndrome. This practice is based on only a few intervention studies, and uncertainty regarding benefits and risk remains. In this case‐based review, we summarize the available evidence and address the questions that are most important for clinical practice. We performed a systematic review of randomized controlled trials assessing the effect of heparin (low molecular weight heparin [LMWH] or unfractionated heparin [UFH]), aspirin, or both on live birth rates in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Eleven trials including 1672 women met the inclusion criteria. Aspirin only did not increase live birth rate compared to placebo in one trial of 40 women (risk ratio [RR] 0.94; 95% confidence interval [CI] 0.71–1.25). One trial of 141 women reported a higher live birth rate with LMWH only than with aspirin only (RR 1.20; 95% CI 1.00–1.43). Five trials totaling 1295 women compared heparin plus aspirin with aspirin only. The pooled RR for live birth was 1.27 (95% CI 1.09–1.49) in favor of heparin plus aspirin. There was significant heterogeneity between the subgroups of LMWH and UFH (RR for LWMH plus aspirin versus aspirin 1.20, 95% CI: 1.04–1.38; RR for UFH plus aspirin versus aspirin 1.74, 95% CI: 1.28–2.35; I2 78.9%, p = .03). Characteristics of participants and adverse events were not uniformly reported. Heparin (LMWH or UFH) plus aspirin may improve live birth rates in women with recurrent pregnancy loss and antiphospholipid antibodies, but evidence is of low certainty.
Keywords: antiphospholipid syndrome, heparin, live birth, recurrent pregnancy loss—aspirin
Essentials.
Antithrombotic therapy is used to prevent pregnancy loss in antiphospholipid syndrome.
A meta‐analysis of randomized controlled trials assessed effects of heparin and/or aspirin on live birth rate in women with recurrent pregnancy loss and antiphospholipid antibodies.
Heparin plus aspirin may increase live birth rate in this population.
The available evidence is of low quality and low certainty.
1. INTRODUCTION
Recurrent pregnancy loss, that is, the loss of at least two pregnancies, affects approximately 1% of women and in almost half a cause cannot be identified. 1 Current guidelines suggest testing for antiphospholipid antibodies in women with two or more 2 , 3 or three or more 4 , 5 pregnancy losses, as these can provide a possible explanation for recurrent pregnancy loss. Antiphospholipid syndrome is a heterogeneous autoimmune disorder and clinical features include obstetrical complications and/or thrombotic events, in the persistent (on two separate occasions at least 12 weeks apart) presence of antiphospholipid antibodies. 6 Antiphospholipid antibodies include lupus anticoagulant (LAC), anticardiolipin antibodies (aCL), and anti‐beta‐2‐glycoprotein‐I (aß2GPI) antibodies. Antiphospholipid antibodies are present in approximately 15% of women with recurrent first trimester pregnancy loss. 7 , 8 The mechanisms and triggers inducing the development and persistence of antiphospholipid antibodies and the various clinical manifestations are poorly understood. 9 , 10 Interestingly, 1% to 5.6% of healthy individuals also have antiphospholipid antibodies without clinical manifestations. 7 , 8
In this JTH in Clinic article, we address the most clinically relevant questions about antiphospholipid antibodies in women with recurrent pregnancy loss: “who, what, and how.” In other words, what is the evidence for antithrombotic therapy to prevent recurrent pregnancy loss in antiphospholipid syndrome?
1.1. Case presentations
Case I. A 29‐year‐old woman with three pregnancy losses before 10 weeks’ gestation repeatedly tests positive for anticardiolipin antibodies with titers of 30 and 32 IgG (above 99th percentile) phospholipid units, respectively. Does treatment with aspirin and/or low molecular weight heparin (LMWH) improve her chance of a successful pregnancy?
Case II. A 40‐year‐old woman with two early pregnancy losses is found to have persistent presence of lupus anticoagulant. Should she be counseled for antithrombotic treatment to prevent a third pregnancy loss?
2. OBSTETRIC ANTIPHOSPHOLIPID SYNDROME
Obstetrical complications of the antiphospholipid syndrome can manifest in women with and without a history of thrombotic events. These include recurrent early pregnancy loss, fetal death or (pre)eclampsia, intrauterine growth restriction, and other consequences of placental insufficiency. Traditionally it is hypothesized that pregnancy complications in antiphospholipid syndrome are the result of a hypercoagulable state, partially mediated by thrombosis of the placental vasculature. Recent hypotheses describe a more intertwined pathophysiological mechanism in which the coagulation system as well as inflammation are involved. 9 , 10 , 11 , 12 The inhibitory effect of antiphospholipid antibodies on proliferation of trophoblasts of the placenta has been proposed as the pathogenic mechanism in early pregnancy loss, whereas late obstetrical complications have been attributed to a dysfunctional vasculature of the placenta. 9 , 13 , 14 , 15 These placenta‐mediated complications include preeclampsia, late pregnancy loss, placental abruption, and intrauterine growth restriction.
Possible effects on complement activation may be of more importance and it has been hypothesized that the non‐anticoagulant effects of heparins on inflammatory processes, vascular function, or placental pathology may play a role in prevention of pre‐eclampsia, a disorder strongly associated with antiphospholipid syndrome. 16 , 17 Moreover, antiphospholipid antibodies appear to affect the production of several chemokines and angiogenic factors by human endometrial endothelial cells, which may contribute to impaired placentation and vascular transformation. 18 The risk of (recurrent) pregnancy complications may differ between women with and without previous complications, women with high and low antiphospholipid antibodies titers, and women with positive and negative LAC. 19 , 20 , 21 Antithrombotic therapy reduces the risk of recurrent (either venous or arterial) thrombosis in antiphospholipid syndrome. 4 , 5 Both aspirin and heparin may have a beneficial effect on coagulation and inflammation, 22 , 23 , 24 and are thought to reduce the risk of pregnancy loss in antiphospholipid syndrome.
To answer the questions posed by our patients, we performed a systematic review and meta‐analyses of the evidence available from randomized trials to evaluate the effects of different antithrombotic therapies on pregnancy outcome in women with recurrent pregnancy loss and antiphospholipid antibodies. 25 , 26 As antiphospholipid syndrome is a heterogeneous disease, we chose to focus specifically on women with a history of recurrent pregnancy loss. The primary outcome was defined as live birth. Eleven trials including 1672 women met the inclusion criteria. None of the trials had a no treatment comparator arm. Full details of the methods and extracted data are described in the supporting information. Here, we summarize our findings by addressing the questions most important for clinical practice, “who, what, and how” (Table 1).
TABLE 1.
Prevention of recurrent pregnancy loss in obstetric antiphospholipid syndrome
| Clinical question | Evidence‐based summary | Current management suggestion | Key questions for future research | Evidence synopsis and discussion in paragraph |
|---|---|---|---|---|
| Who should be treated? |
Women with persistent antiphospholipid antibodies AND: Recurrent early pregnancy loss (three or more) Evidence available from randomized controlled trials. Late pregnancy loss or late pregnancy complications No direct evidence available, extrapolated from available evidence and expert opinion. No history of pregnancy complications, two early losses No evidence available, expert opinion. |
Treatment Suggest treatment. Discuss treatment on an individual case‐to‐case basis |
Which subgroups of patients benefit from antithrombotic therapy? | I |
| What is the optimal treatment? |
Aspirin versus placebo Live birth risk ratio 0.94; 95% CI 0.71–1.25 1 trial, 40 women GRADE very low‐certainty evidence Heparin +aspirin versus aspirin only Live birth risk ratio 1.27; 95% CI 1.09–1.49 5 trials, 1295 women GRADE low‐certainty evidence |
LMWH and low dose aspirin are suggested to reduce the risk of pregnancy loss and placenta mediated complications, respectively. | Risk stratification based on clinical and biochemical criteria. | II |
| What is the optimal timing and treatment duration? | No evidence. | In absence of evidence on optimal timing and treatment duration, suggest starting aspirin preconceptionally and LMWH upon confirmation of pregnancy and continue until first signs of labor. |
1) Can treatment be stopped in an earlier stage of pregnancy? 2) Should antithrombotic therapy continue postpartum to prevent thrombosis? |
III |
| What is the optimal dose regimen? | Included studies mostly used prophylactic dose LMWH. No robust evidence regarding comparisons of dose and type of heparin. | Prophylactic dose LMWH | Prophylactic versus intermediate or therapeutic dose LMWH? | IV |
| What is the prognosis if no treatment is given? |
No evidence available. No trials had a no treatment comparator arm; only trials with aspirin only comparator arm. |
In low‐risk groups no treatment can be discussed on an individual case‐to‐case basis |
LMWH +aspirin vs. aspirin only vs. no treatment? |
II, V |
3. I: WHO SHOULD BE TREATED?
Based on the individual history of obstetrical complications, treatment during the subsequent pregnancy can be considered. Table 2 provides an overview of current guidelines and recommendations for preventing pregnancy loss in women with antiphospholipid syndrome, stratified for history of obstetrical complications. It is important to note that all available evidence underlying these recommendations concerns women with persistent antiphospholipid antibodies and recurrent early pregnancy loss. High‐level evidence for the other clinical criteria is virtually absent and management suggestions are extrapolated from mostly observational evidence and expert opinion. Non‐criteria obstetric antiphospholipid syndrome is defined as two early pregnancy losses or delivery after 34 weeks of gestation due to severe (pre)eclampsia. In these women treatment might be considered based on the individual’s risk profile, for instance a high‐risk antiphospholipid antibody profile but no history of thrombosis or pregnancy complications. A high‐risk antiphospholipid antibody profile is defined as presence of lupus anticoagulant, double or triple antiphospholipid antibody positivity, or persistently high antiphospholipid antibody titers. 2
TABLE 2.
Current guidelines to prevent recurrent pregnancy loss in women with antiphospholipid syndrome
| Guidelines | EULAR 2019 2 | ESHRE 2017 3 | ACCP 2012 4 | ACOG 2012 5 |
|---|---|---|---|---|
|
≥3 pregnancy losses <10 weeks gestational age OR ≥1 pregnancy loss ≥10 weeks gestational age |
In women with a history of obstetric APS only (no prior thrombotic events), with or without SLE with a history of ≥3 recurrent spontaneous miscarriages <10th week of gestation and in those with a history of fetal loss (≥10th week of gestation), combination treatment with low‐dose aspirin and heparin at prophylactic dosage during pregnancy is recommended (2b/B). c | For women who fulfil the laboratory criteria of APS and have a history of three or more pregnancy losses, we suggest administration with low‐dose aspirin (75–100 mg/day), starting before conception, and a prophylactic dose heparin (UFH or LMWH) starting at date of a positive pregnancy test, over no treatment (conditional ⊕○○○). d | For women who fulfill the laboratory criteria for APS syndrome and meet the clinical APS criteria based on a history of three or more pregnancy losses, we recommend antepartum administration of prophylactic‐ or intermediate dose UFH or prophylactic LMWH combined with low‐dose aspirin, 75 to 100 mg/day, over no treatment (Grade 1B). e |
In women with APS and a history of stillbirth or recurrent fetal loss but no prior thrombotic history, prophylactic doses of heparin and low‐dose aspirin during pregnancy and 6 weeks of postpartum should be considered. (Grade B). f |
|
Placenta‐mediated pregnancy complications a |
With a history of delivery <34 weeks of gestation due to eclampsia or severe preeclampsia or due to recognized features of placental insufficiency, treatment with low‐dose aspirin or low‐dose aspirin and heparin at prophylactic dosage is recommended considering the individual’s risk profile (2b/B). c | Not discussed. | ||
| “Non‐criteria” obstetric APS b |
Treatment with low‐dose aspirin only or in combination with heparin might be considered based on the individual's risk profile (4/D). c |
The guideline development group suggests offering anticoagulant treatment for women with two pregnancy losses and APS, only in the context of clinical research (GPP). d | Not discussed. | Not discussed. |
| No history of pregnancy complications | In women with a high‐risk antiphospholipid antibody profile but no history of thrombosis or pregnancy complications (with or without SLE), treatment with low‐dose aspirin (75–100 mg daily) during pregnancy should be considered (5/D)3. | Not discussed. |
Abbreviations: APS, antiphospholipid syndrome; GPP, good practice points; LMWH, low molecular weight heparin; RCT, randomized controlled trial; SLE, systemic lupus erythematosus; UFH, unfractionated heparin.
Placenta‐mediated pregnancy complications include preeclampsia, late pregnancy loss, placental abruption, and intrauterine growth restriction.
“Non‐criteria” APS, i.e., two losses <10th week of gestation, or delivery ≥34 weeks of gestation due to severe preeclampsia or eclampsia.
EULAR: Level of evidence: 1a: systematic review of RCTs; 1b: individual RCT; 2a: systematic review of cohort studies; 2b: individual cohort study (and low‐quality RCT); 3a: systematic review of case–control studies; 3b: individual case–control study; 4: case series and poor‐quality cohort and case–control studies; 5: expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles.” Grade of recommendation: A: consistent level 1 studies; B: consistent level 2 or 3 studies, or extrapolations from level 1 studies; C: level 4 studies or extrapolations from level 2 or 3 studies; D: level 5 evidence or troublingly inconsistent or inconclusive studies of any level. 2
ESHRE: Each recommendation was labelled as strong or conditional and a grade was assigned based on the strength of the supporting evidence (High ⊕⊕⊕⊕; Moderate ⊕⊕⊕○; Low ⊕⊕○○; Very low ⊕○○○). In the absence of evidence, the Guidance Development Group formulated no recommendation or a GPP based on clinical expertise. 3
ACCP: recommendations from 1A to 2C. The number (1 or 2) refers to the strength of the recommendation (1: strong recommendation, 2: weak recommendation), and the letters (A, B or C) indicate the quality of the evidence on which the recommendation is based (A: high‐quality evidence, B: moderate quality evidence, C: low‐quality evidence). 4
ACOG: Level A—Recommendations are based on good and consistent scientific evidence. Level B—Recommendations are based on limited or in consistent scientific evidence. Level C—Recommendations are based primarily on consensus and expert opinion. 5
4. II: WHAT IS THE OPTIMAL TREATMENT?
Our search identified 11 randomized trials evaluating antithrombotic treatment in women with recurrent pregnancy loss and antiphospholipid syndrome. Study characteristics are presented in Table 3. The identified trials differed in terms of inclusion criteria and compared a variety of interventions. One trial compared aspirin with placebo, 27 five trials compared heparin (unfractionated heparin [UFH] or LMWH) plus aspirin with aspirin only, 28 , 29 , 30 , 31 , 32 one trial compared LMWH with aspirin, 33 two trials compared LMWH with UFH (both in combination with aspirin), 34 , 35 and two trials investigated the combination of different doses of heparin (either UFH or LMWH) with aspirin. 36 , 37 We did not identify trials with a no treatment comparator arm during pregnancy. Three of 11 trials included women with two or more pregnancy losses. In 8 of 11 trials participants met the clinical criteria for antiphospholipid syndrome with three or more early miscarriages. The mean number of previous pregnancy losses ranged from 3.0 to 4.3. Previous pregnancy losses were mostly early pregnancy losses, but this was only specified in 5 of 11 included studies. All trials included participants with persistent presence of antiphospholipid antibodies, but the timeframe between tests varied.
TABLE 3.
Study characteristics of the included studies
| Study |
No. of patients |
Inclusion criteria for pregnancy loss |
Inclusion criteria for aPL antibodies |
Treatment | Comparator | Ref |
|---|---|---|---|---|---|---|
| Aspirin vs. placebo | ||||||
| Pattison 2000 | 40 | ≥3 pregnancy losses | aCL antibodies or positive LAC on 2 occasions | Aspirin 75 mg/day | Placebo | 27 |
| Heparin +Aspirin vs. Aspirin | ||||||
| Kutteh 1996a | 50 | ≥3 spontaneous consecutive losses | Presence of aPL antibodies on 2 occasions a |
UFH 5000 U bidaily + Aspirin 81 mg/day |
Aspirin 81 mg/day | 28 |
| Rai 1997 | 90 | ≥3 consecutive losses | aCL antibodies or positive LAC on 2 occasions, at least 8 weeks apart |
UFH 5000 U bidaily + Aspirin 75 mg/day |
Aspirin 75 mg/day | 29 |
| Farquharson 2002 | 98 | ≥3 consecutive losses or 2 losses >10 weeks | aCL antibodies or positive LAC on 2 occasions, at least 6 weeks apart |
Dalteparin 5000 U/day + Aspirin 75 mg/day |
Aspirin 75 mg/day | 30 |
| Laskin 2009 | 42 | ≥2 unexplained consecutive losses <32 weeks | aCL antibodies or positive LAC on 2 occasions, at least 8 weeks apart |
Dalteparin 5000 U/day + Aspirin 81 mg/day |
Aspirin 81 mg/day | 31 |
| Bao 2017 | 1015 | ≥2 consecutive losses | Presence of any aPL antibodies on 2 occasions, at least 12 weeks apart |
Nadroparin 4100 IU/day + Aspirin 75 mg/day |
Aspirin 75 mg/day | 32 |
| Heparin vs.ASPIRIN | ||||||
| Alalaf 2012 | 141 | ≥2 consecutive losses <20 weeks | aCL antibodies or positive LAC on 2 occasions, at least 8 weeks apart | Bemiparin 2500 IU/day | Aspirin 100 mg/day | 33 |
| LMWH +Aspirin vs.UFH + Aspirin | ||||||
| Stephenson 2004 | 26 | ≥3 unexplained losses <10 weeks or ≥1 loss ≥10 weeks | aCL antibodies or positive LAC on 2 occasions, at least 6 weeks apart |
Dalteparin 2500–5000–7500 IU/day b + Aspirin 81 mg/day |
UFH 5000–7500–10000 U bid c + Aspirin 81 mg /day |
|
| Fouda 2011 | 60 | ≥3 consecutive losses <10 weeks | aCL antibodies or positive LAC on 2 occasions, at least 12 weeks apart |
Enoxaparin 40 mg/day + Aspirin 75 mg/day |
UFH 5000 U bidaily + Aspirin 75 mg/day |
35 |
| Higher dose Heparin + ASPIRIN vs.lower dose Heparin + Aspirin | ||||||
| Kutteh 1996b | 50 | ≥3 documented losses | Presence of aPL antibodies on 2 occasions a | UFH 5000 U bidaily (higher PTT) c + Aspirin 81 mg/day | UFH 5000 U bidaily (lower PTT) b + Aspirin 81 mg/day | 36 |
| Fouda 2010 | 60 | ≥3 consecutive losses <10 weeks | aCL antibodies or positive LAC on 2 occasions, at least 12 weeks apart |
Enoxaparin 40 mg/day + Aspirin 75 mg/day |
Enoxaparin 20 mg/day + Aspirin 75 mg/day |
37 |
Abbreviations: aCL=anticardiolipin antibodies; aPL, antipohospholipid antibodies; LAC, lupus anticoagulant; LMWH, low molecular weight heparin; PTT, partial thromboplastin time; U, (international) units; UFH, unfractionated heparin.
aPL and timeframe between tests not specified; LAC positivity was an exclusion criterion.
LMWH 2500 IU/day in 1st trimester, 5000 IU/day in 2nd trimester, 7500 IU/day in 3rd trimester; UFH 5000 IU bidaily in 1st trimester, 7500 IU in 2nd trimester, 10.000 IU in 3rd trimester.
UFH dose adjusted to maintain the PTT at 1.2 to 1.5 times the baseline (high‐dose); UFH dose adjusted to maintain PTT at the upper limit of normal (low‐dose).
4.1. Aspirin only
The use of aspirin during pregnancy in antiphospholipid syndrome is widespread. Our search identified one placebo‐controlled trial of 40 women with antiphospholipid antibodies and recurrent pregnancy loss evaluating aspirin treatment. 27 This trial, at high risk of attrition bias due to incomplete reporting of outcome data, found no difference in live birth rate with aspirin compared to placebo (risk ratio [RR] 0.94; 95% confidence interval [CI] 0.71–1.25; GRADE very low‐certainty evidence, Figure 1). 27
FIGURE 1.
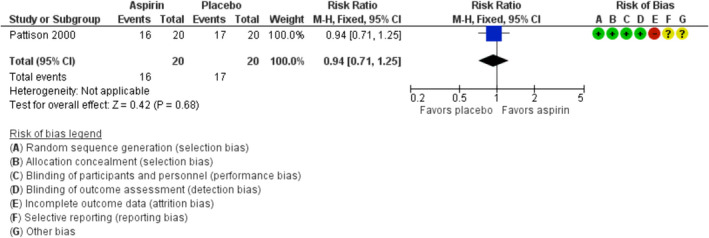
Forest plot of the risk ratio of live birth in trials comparing aspirin with placebo. CI;, confidence interval; M‐H, Mantel‐Haenszel
The small sample size and methodological limitations hamper the conclusions that can be drawn from this study and these results do not provide evidence to support aspirin only for prevention of pregnancy loss in this population. In the general population as well as in women with a history of one to two previous pregnancy losses, preconception aspirin does not increase live births, as shown in the EAGER trial. 38 However, aspirin is effective in reducing the risk of preeclampsia in high‐risk women. 39 , 40 Therefore, considering antiphospholipid antibodies a risk factor for preeclampsia, it is very reasonable to use aspirin for prevention of preeclampsia in women with recurrent pregnancy loss and antiphospholipid syndrome.
4.2. Heparin only
One trial of 141 women with antiphospholipid syndrome reported the results of a head‐to‐head comparison of LMWH only and aspirin only. 33 Women treated with LMWH had a higher live birth rate of 86.3%, compared to a 72.1% live birth rate in the women treated with aspirin only (RR 1.20, 95% CI 1.00–1.43, 1 trial, 141 women, Figure S1 in supporting information). 33 All other trials evaluated heparin in combination with aspirin.
4.3. Heparin plus aspirin
Five trials with a total of 1295 women that compared heparin (either UFH or LMWH) combined with aspirin to aspirin only, were included in a random‐effects meta‐analysis for the primary outcome live birth. The pooled RR for live birth was 1.27 (95% CI 1.09–1.49; Tau2 = 0.01; Chi2 = 7.71, I2 = 48%; GRADE low‐certainty evidence) in favor of heparin plus aspirin compared to aspirin only. 28 , 29 , 30 , 31 , 32 There was significant heterogeneity between the subgroups of LMWH and UFH (RR for LWMH plus aspirin versus aspirin 1.20, 95% CI: 1.04–1.38; RR for UFH plus aspirin versus aspirin 1.74, 95% CI: 1.28–2.35; test for subgroup differences: I2 =78.9%, p = .03, Figure 2A). The observed live birth rate in the aspirin‐only comparator arms of the UFH studies was considerably lower compared to these in the LMWH studies; 42.9% versus 70.4%. We performed a sensitivity analysis excluding two studies for serious methodological limitations; one (n = 50) at high risk of selection bias due to the quasi‐randomized design, 28 one (n = 1015) at high risk of attrition bias due to incomplete reporting of outcome data. 32 This did not materially change the combined (UFH +LMWH) pooled result (RR for live birth 1.20, 95% CI 0.91–1.59; I2 = 58%), but the benefit of LMWH plus aspirin compared to aspirin only was attenuated (RR for live birth 1.07; 95% CI 0.88–1.29, Figure 2B). Furthermore, after excluding the largest and most recent trial, 32 the statistical significance for all heparin trials was lost.
FIGURE 2.
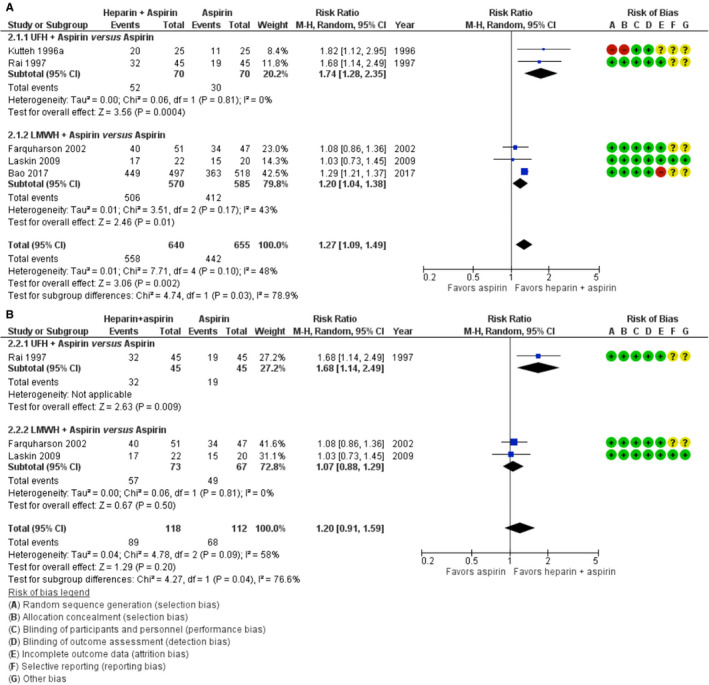
Forest plot of the risk ratio of live birth in trials comparing heparin +aspirin with aspirin only. A, All trials included. B, A sensitivity analysis excluding the trial by Kutteh (1996a)28 and Bao (2017)32 for methodological limitations. CI, confidence interval; LMWH, low‐molecular‐weight heparin; M‐H, Mantel‐Haenszel; UFH, unfractionated heparin
There was no statistically significant difference in live birth between LMWH and aspirin versus UFH and aspirin (RR 1.44, 95% CI 0.80–2.62, 2 trials, 86 women; p = .17; I2 = 48%; Figure S2 in supporting information). 34 , 35 Heparin appears to improve live birth rates, but the low live birth rates in the comparator arms in the UFH studies may lead to an overestimation of the effect of UFH. The observed beneficial effect of LMWH plus aspirin on the other hand is mostly driven by a recently published large single‐center trial (n = 1015) that found a 90.3% live birth rate in women treated with LMWH plus aspirin, compared to 70.1% in those treated with aspirin only. 32 Table 4 provides an overview of study outcomes and certainty of the evidence for the two main comparisons: (1) aspirin versus placebo and (2) heparin plus aspirin versus aspirin only.
TABLE 4.
Study outcomes for two main comparisons: Aspirin versus placebo and heparin plus aspirin versus aspirin only
|
Study intervention No. of participants |
Aspirin vs. Placebo 1 RCT, 40 participants |
Risk ratio |
Heparin +Aspirin vs. Aspirin only 5 RCTs, 1295 Participants |
Risk ratio (95% CI; I2) |
||||
|---|---|---|---|---|---|---|---|---|
| Live birth | 16/20 (80%) | 17/20 (85%) | 0.94 (0.71–1.25 ) |
Any heparin:
|
558/640 (87%) 506/570 (89%) 52/70 (74%) |
442/655 (67%) 412/585 (70%) 30/70 (43%) |
1.27 (1.09–1.49; I2 48%) 1.20 (1.04–1.38) 1.74 (1.28–2.35) |
|
| Pregnancy loss a | 4/20 (20%) | 3/20 (15%) | 1.33 (0.34–5.21) |
Any heparin:
|
82/640 (13%) 64/570 (11%) 18/70 (26%) |
213/655 (33%) 173/585 (30%) 40/70 (57%) |
0.48 (0.32–0.71; I2 53%) 0.55 (0.26–1.16) 0.46 (0.29–0.71) |
|
| Preeclampsia | 3/16 (19%) | 3/17 (18%) | 1.06 (0.25–4.52) | 2/52 (4%) | 2/30 (7%) b | 0.57 (0.10–3.14; I2 0%) | ||
|
Adverse events in the woman
|
9/20 (45%) NA NA NA NA |
7/20 (35%) NA NA NA NA |
1.29 (0.60–2.77) ‐ ‐ ‐ ‐ |
3/20 (15%) 0/70 (0%) 0/45 (0%) 0/92 (0%) 0/92 (0%) |
1/11 (9%) c 0/70 (0%) 0/45 (0%) 0/90 (0%) 0/90 (0%) |
1.65 (0.19–14.03) ‐ ‐ ‐ ‐ |
||
|
Preterm delivery of a live infant |
2/16 (13%) | 0/17 (0%) |
5.29 (0.27–102.49) |
Any heparin:
|
13/92 (14%) 2/40 (5%) 11/52 (21%) |
9/64 (14%) 4/34 (12%) 5/30 (17%) |
0.93 (0.42–2.07; I2 0%) 0.42 (0.08–2.18) 1.27 (0.49–3.27) |
|
| Intrauterine growth restriction | 1/16 (6%) | 4/17 (24%) | 0.27 (0.03–2.13) | 6/52 (12%) | 2/30 (7%) b | 1.73 (0.37–8.04; I2 0%) | ||
|
Adverse events in the child
|
1/16 (6%) NA |
1/17 (6%) NA |
1.06 (0.07–15.60) ‐ |
‐ ‐ |
||||
| Grade | Very low certainty evidence d | Low certainty evidence e | ||||||
Risk ratios were in bold font in order to emphasize our main results.
Abbreviations: CI, confidence interval; HIT, heparin‐induced thrombocytopenia; LMWH, low‐molecular‐weight heparin; NA, not assessed; RCT, randomized controlled trial; UFH, unfractionated heparin.
Both live birth and pregnancy loss are reported to facilitate comparisons with other studies evaluating pregnancy outcome.
Two randomized controlled trials with 82 participants—Kutteh (1996a) and Rai (1997)—both compared UFH +aspirin with aspirin only. 28 , 29
One RCT with 31 participants—Kutteh (1996a)—compared UFH +aspirin versus aspirin only. 28
Downgraded one level due to serious risk of selection, attrition bias, downgraded two levels due to very serious imprecision; few participants and wide CIs crossing the line of no effect.
Downgraded one level due to serious risk of bias for limitations (selection, attrition, reporting bias), downgraded one level due to serious inconsistency; heterogeneity in interventions (I2 > 45%).
Notably, adverse events associated with heparin therapy, easy bruising at injection site or allergies, did not seem to occur frequently or were not reported. LMWH is a reasonable alternative treatment and currently most often used in clinical practice, with its similar efficacy and superior safety profile compared to UFH. 41
5. III: TIMING OF TREATMENT INITIATION AND DURATION?
We observed a wide variation in treatment initiation and duration between trials. Aspirin treatment was started preconceptionally in most trials and continued to 36 weeks of gestation 33 , 34 , 35 , 37 or full‐term pregnancy. 28 , 36 LMWH or UFH was started upon pregnancy confirmation in most studies evaluating heparin treatment. In the trial by Rai et al., aspirin treatment was started upon pregnancy confirmation and when fetal heart activity was confirmed on ultrasound women were randomized to additionally start UFH or continue aspirin only. 29 Four trials initiated treatment at the first confirmation of pregnancy and treatment was continued until 34 weeks of gestation, 29 35 weeks of gestation, 31 , 32 or study duration. 27 One trial started aspirin before conception, with heparin (LMWH or UFH) started in the luteal phase for a maximum of three cycles until delivery and continued postpartum in a prophylactic dose. 34 The mean gestational age at randomization, and thus treatment initiation, varied largely between studies with one study allowing randomization before 12 weeks of gestation. 30 Given the heterogeneity in treatment protocols, it is not possible to provide recommendations on optimal timing of treatment initiation and duration. A recent study that compared early initiation of LMWH (gestational age 5 weeks) to later initiation observed ongoing pregnancy rates of 81% at 12 weeks’ gestation and 61%, respectively. Live birth rates differed between the groups, 70.8% in the early initiation group and 56.5% in the later initiation group, respectively, but this difference was not statistically significant. 42 Also, late pregnancy complications associated with antiphospholipid syndrome, including preeclampsia, intrauterine growth restriction, and intrauterine fetal death, were not statistically significantly different between the two study groups. 42 Similarly, another placebo‐controlled trial reported higher ongoing pregnancy rates at 24 weeks’ gestation in women treated with LMWH and aspirin preconceptionally, but live birth overall was not affected. 43 Initiation of heparin preconceptionally would be undesirable from a patient’s perspective, but whether heparin can be safely discontinued after the first trimester of pregnancy requires further investigation. Three studies continued heparin treatment postpartum; either 3 weeks 28 , 36 or 6 weeks. 34 The incidence of postpartum thrombosis in women with obstetric antiphospholipid syndrome is unknown. Therefore, the aim and duration of postpartum heparin treatment cannot be evidence based. In the absence of evidence, however, women with persistent antiphospholipid antibodies may be at higher risk of thrombosis and postpartum continuation of heparin treatment for prevention of venous thromboembolism can be considered. 44
6. IV: OPTIMAL ANTITHROMBOTIC DOSE REGIMEN?
Various doses of aspirin and heparin were used in the included studies (Table 3). Due to small study sample sizes and limited data it was not possible to account for these differences in the analyses. Two small trials compared a higher and a lower dose of heparin (LMWH or UFH) both combined with aspirin.
A higher dose of LMWH did not improve the live birth rate compared to a lower dose of LMWH (RR 1.10, 95% CI 0.81 to 1.49, 1 trial, 60 women); similar to the effects of a higher dose of UFH compared to a lower dose of UFH (RR 1.05, 95% CI 0.78 to 1.41, 1 trial, 50 women; Figure S3 in supporting information). 36 , 37 Importantly, the study evaluating different doses of UFH lacked the power to detect any significant differences and had methodological limitations due to the quasi‐randomized design. 36 This variation in initiation of treatment, in duration of treatment, as well as different doses and agents used, limits the possibilities of a cross‐study comparison and thus clear treatment recommendations.
7. V: KNOWLEDGE GAPS AND RESEARCH AGENDA
In general, although we focused on women with recurrent pregnancy loss, we observed significant clinical heterogeneity in the study populations. A substantial part of the studied population did not meet the full criteria of antiphospholipid syndrome, due to differences in the definition of prior pregnancy loss used as well as the timing (and interval) of repeat antibody testing. 4 Criteria for antiphospholipid syndrome and pregnancy loss are consensus based and further research regarding which subgroups benefit from antithrombotic therapy should be carried out. It is known that the prognosis varies between subgroups of antiphospholipid syndrome patients. 7 , 19 , 41 , 45 , 46 , 47 Reporting of antibody profiles or the number of previous pregnancy losses was incomplete and not in relation to the primary outcome live birth. For this reason, we were unable to perform subgroup comparisons based on number of previous miscarriages (two vs. three or more), previous placenta‐mediated complications, high‐titer antibodies versus low‐titer antibodies, or positive LAC versus negative LAC.
In light of the limitations of the included studies in this review, the evidence is of low certainty, and a large multicenter randomized controlled trial with clearly defined patients with antiphospholipid antibodies and recurrent pregnancy loss is still warranted. Such a trial should include women with homogenous clinical characteristics and antibody profiles or be powered to analyze subgroups. We realize this is challenging but given the costs, nuisance, and side effects of heparin and aspirin observed in trials in non‐antiphospholipid syndrome patients, 48 , 49 such trials should be performed to obtain a definite answer about the effectiveness in antiphospholipid syndrome. Unfortunately, despite the urgent need to get answers to our important clinical questions, this is challenging. For instance, the well‐designed APPLE pilot study evaluating LMWH plus aspirin versus aspirin only during pregnancy in women with persistent antiphospholipid antibodies and a history of two or more early pregnancy losses or one or more late losses was terminated prematurely for feasibility reasons. 50
8. SUMMARY AND HOW WE TREAT
Our systematic review provides a contemporary and complete synthesis of all available evidence from randomized trials on antithrombotic therapy for improving pregnancy outcomes in women with a history of recurrent pregnancy loss and persistent presence of antiphospholipid antibodies. Based on the available but low‐certainty evidence, heparin plus aspirin appear to improve live birth rates in women with recurrent pregnancy loss and persistent antiphospholipid antibodies.
So how do these findings translate to our own clinical practice? To summarize our “who, what, and how” for women with antiphospholipid antibodies and recurrent pregnancy loss:
Who do we treat? Women with recurrent early pregnancy loss (three or more) and persistent presence of antiphospholipid antibodies tested on two separate occasions at least 12 weeks apart. In women with a late pregnancy loss or late pregnancy complications in persistent presence of antiphospholipid antibodies treatment, treatment will be discussed as we also counsel these women on the absence of evidence on its effectiveness.
What do we prescribe? A combination of low‐dose aspirin and LMWH in prophylactic dose.
How do we treat? Aspirin is started preconceptionally with LMWH added upon pregnancy confirmation. Treatment is continued for the full duration of pregnancy and stopped at either the first signs of labor or 24 hours prior to planned delivery. We consider continuation of LMWH postpartum based on the individual patient's risk profile for venous thromboembolism.
9. CASES REVISITED
Case I. There is insufficient evidence to support use of heparin or aspirin only for increasing subsequent live birth rates after recurrent pregnancy loss. Heparin, either LMWh or UFH in combination with aspirin during pregnancy potentially improves pregnancy outcome, although this is based on low‐certainty evidence.
Case II. Although the clinical criteria for antiphospholipid syndrome are not met (as our patient had two and not three documented early pregnancy losses), given persistent double positive antiphospholipid antibody presence, counseling for LMWH in combination with aspirin during pregnancy can be considered. This is also based on low‐certainty evidence.
CONFLICTS OF INTEREST
S. Middeldorp reports grants and fees paid to her institution from GSK, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. L.J.J. Scheres received funding for the printing of his doctoral thesis from the Dutch Heart Foundation, Dutch Federation of Coagulation Clinics, Stichting tot Steun Promovendi Vasculaire Geneeskunde, Bayer, Daiichi Sankyo, LEO Pharma, and Pfizer, outside the submitted work. M. Goddijn works at the Department of Reproductive Medicine of the Amsterdam UMC (location AMC and location VUmc). Location VUMC has received several research and educational grants from Guerbet, Merck, and Ferring, not related to the presented work. E.N. Hamulyák has nothing to disclose.
AUTHOR CONTRIBUTIONS
L.J.J. Scheres and S. Middeldorp developed the methods for the systematic review and meta‐analyses. All authors participated in the review and selection of included studies. E.N. Hamulyák and L.J.J. Scheres performed the independent data extraction, quality assessment of included trials, all data analyses, and wrote the first draft of the manuscript. All authors reviewed drafts and approved the final draft of the manuscript. S. Middeldorp is the guarantor of the review and was third reviewer for quality assessment.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Mauritia Marijnen for her help in the data preparation and Lynn Hampson, Jim Neilson, Emily Shepherd, Fiona Stewart, and Frances Kellie of the Cochrane Pregnancy and Childbirth Trials Register for their guidance in constructing and finalizing this review. L.J.J. Scheres was a PhD candidate of the CREW project (2013T083) supported by the Netherlands Heart Foundation. S. Middeldorp was supported by a VIDI grant for the Netherlands Organization for Health Research and Development (0.16.126.364). This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth.
REFERENCES
- 1. Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bender Atik R, Christiansen OB, Elson J, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, Thrombophilia, Antithrombotic Therapy, and Pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2):e691S‐e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists . Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514‐1521. [DOI] [PubMed] [Google Scholar]
- 6. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295‐306. [DOI] [PubMed] [Google Scholar]
- 7. Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta 2‐glycoprotein‐I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod. 1995;10(8):2001‐2005. [DOI] [PubMed] [Google Scholar]
- 8. Durcan L, Petri M. Epidemiology of the antiphospholipid syndrome. In: Cervera R, Espinosa G, Khamashta MA, editors. Antiphospholipid Syndrome in Systemic Autoimmune Diseases (2nd edition). Amsterdam: Elsevier; 2016:17‐30. [Google Scholar]
- 9. Meroni PL, Borghi MO, Grossi C, Chighizola CB, Durigutto P, Tedesco F. Obstetric and vascular antiphospholipid syndrome: same antibodies but different diseases? Nat Rev Rheumatol. 2018;14(7):433‐440. [DOI] [PubMed] [Google Scholar]
- 10. Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:18005. [DOI] [PubMed] [Google Scholar]
- 11. Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118(10):3453‐346.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samarkos M, Mylona E, Kapsimali V. The role of complement in the antiphospholipid syndrome: a novel mechanism for pregnancy morbidity. Semin Arthritis Rheum. 2012;42(1):66‐69. [DOI] [PubMed] [Google Scholar]
- 13. Di Simone N, Meroni PL, de Papa N, et al. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered beta2‐glycoprotein I. Arthritis Rheum. 2000;43(1):140‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derksen RH, de Groot PG. The obstetric antiphospholipid syndrome. J Reprod Immunol. 2008;77(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 15. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody‐induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222‐1226. [DOI] [PubMed] [Google Scholar]
- 17. Wat JM, Audette MC, Kingdom JC. Molecular actions of heparin and their implications in preventing pre‐eclampsia. J Thromb Haemost. 2018;16:1510‐1522. [DOI] [PubMed] [Google Scholar]
- 18. Quao ZC, Tong M, Bryce E, Guller S, Chamley LW, Abrahams VM. Low molecular weight heparin and aspirin exacerbate human endometrial endothelial cell responses to antiphospholipid antibodies. Am J Reprod Immunol. 2018;79(1):e12785– 10.1111/aji.12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD. A cross‐sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford). 2002;41(8):924‐929. [DOI] [PubMed] [Google Scholar]
- 20. Ioannou Y, Rahman A. Domain I of beta2‐glycoprotein I: its role as an epitope and the potential to be developed as a specific target for the treatment of the antiphospholipid syndrome. Lupus. 2010;19(4):400‐405. [DOI] [PubMed] [Google Scholar]
- 21. Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. 2012;64(7):2311‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255‐258. [DOI] [PubMed] [Google Scholar]
- 23. Vignoli A, Marchetti M, Balducci D, Barbui T, Falanga A. Differential effect of the low‐molecular‐weight heparin, dalteparin, and unfractionated heparin on microvascular endothelial cell hemostatic properties. Haematologica. 2006;91(2):207‐214. [PubMed] [Google Scholar]
- 24. Kozlowski EO, Pavao MS, Borsig L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P‐selectin. J Thromb Haemost. 2011;9(9):1807‐1815. [DOI] [PubMed] [Google Scholar]
- 25. Scheres LJ, Marijnen MC, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev. 2017;11(11):CD012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev. 2020;5(5):CD012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pattison NS, Chamley LW, Birdsall M, Zanderigo AM, Liddell HS, McDougall J. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol. 2000;183(4):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 28. Kutteh WH. Antiphospholipid antibody‐associated recurrent pregnancy loss: treatment with heparin and low dose aspirin is superior to low‐dose aspirin alone. Am J Obstet Gynecol. 1996;174(5):1584‐1589. [DOI] [PubMed] [Google Scholar]
- 29. Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ. 1997;314(7076):253‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100(3):408‐413. [DOI] [PubMed] [Google Scholar]
- 31. Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol. 2009;36(2):279‐287. [DOI] [PubMed] [Google Scholar]
- 32. Bao SH, Zhou Q, Frempong ST, Tu WY, Sheng SL, Liao H. Use of d‐dimer measurement to guide anticoagulant treatment in recurrent pregnancy loss associated with antiphospholipid syndrome. Am J Reprod Immunol. 2017;78(6):e12770. [DOI] [PubMed] [Google Scholar]
- 33. Alalaf S. Bemiparin versus low dose aspirin for management of recurrent early pregnancy losses due to antiphospholipd antibody syndrome. Arch Gynecol Obstet. 2012;285(3):641‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stephenson MD, Ballem PJ, Tsang P, et al. Treatment of antiphospholipid antibody syndrome (aps) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. J Obstet Gynaecol Can. 2004;26(8):729‐734. [DOI] [PubMed] [Google Scholar]
- 35. Fouda UM, Sayed AM, Abdou AM, Ramadan DI, Fouda IM, Zaki MM. Enoxaparin versus unfractionated heparin in the management of recurrent abortion secondary to antiphospholipid syndrome. Int J Gynaecol Obstet. 2011;112(3):211‐215. [DOI] [PubMed] [Google Scholar]
- 36. Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody‐associated recurrent pregnancy loss with lower dose heparin and aspirin. Am J Reprod Immunol. 1996;35(4):402‐407. [DOI] [PubMed] [Google Scholar]
- 37. Fouda UM, Sayed AM, Ramadan DI, Fouda IM. Efficacy and safety of two doses of low molecular weight heparin (enoxaparin) in pregnant women with a history of recurrent abortion secondary to antiphospholipid syndrome. J Obstet Gyncol. 2010;30(8):842‐846. [DOI] [PubMed] [Google Scholar]
- 38. Schisterman EF, Silver RM, Lesher LL, et al. Preconception low‐dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet. 2014;384:29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA. PARIS Collaborative Group. Antiplatelet agents for prevention of pre‐eclampsia: a meta‐analysis of individual patient data. Lancet. 2007;369(9575):1791‐1798. [DOI] [PubMed] [Google Scholar]
- 40. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 41. Skeith L. Anticoagulating patients with high‐risk acquired thrombophilias. Blood. 2018;132(21):2219‐2229. [DOI] [PubMed] [Google Scholar]
- 42. Eid MI, Abdelhafez MS, El‐Refaie W, et al. Timing of initiation of low molecular weight low‐molecular‐weight heparin administration in pregnant women with antiphospholipid syndrome: a randomized clinical trial of efficacy and safety. Int J Womens Health. 2019;11:41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ismail AM, Hamed AH, Saso S, Abu‐Elhasan AM, Abu‐Elghar MM, Abdelmeged AN. Randomized controlled study of pre‐conception thromboprophylaxis among patients with recurrent spontaneous abortion related to antiphospholipid syndrome. Int J Gynaecol Obstet. 2016;132:219‐223. [DOI] [PubMed] [Google Scholar]
- 44. Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317‐3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meroni PL, Raschi E, Grossi C, et al. Obstetric and vascular APS: same autoantibodies but different diseases? Lupus. 2012;21(7):708‐710. [DOI] [PubMed] [Google Scholar]
- 46. Lockshin MD. Anticoagulation in management of antiphospholipid antibody syndrome in pregnancy. Clin Lab Med. 2013;33(2):367‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buyon JP, Kim MY, Guerra MM, et al. Predictors of Pregnancy Outcomes in Patients with Lupus: A Cohort Study. Ann Intern Med. 2015;163(3):153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaandorp SP, Goddijn M, van der Post JAM, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362(17):1586‐1596. [DOI] [PubMed] [Google Scholar]
- 49. Pasquier E, de Saint Martin L, Bohec C, et al. Enoxaparin for prevention of unexplained recurrent miscarriage: a multicenter randomized double‐blind placebo‐controlled trial. Blood. 2015;125(14):2200‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skeith L, Bates SM, Bates V, Rodger MA. The challenges and lessons learned in conducting clinical trials in pregnant women with antiphospholipid syndrome. Thromb Res. 2020;194:54‐56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


