Abstract
Post-traumatic stress disorder (PTSD) is a psychiatric disorder that afflicts many individuals. However, its molecular and cellular mechanisms remain largely unexplored. Here, we found PTSD susceptible mice exhibited significant up-regulation of alpha-Ca2+/calmodulin-dependent kinase II (αCaMKII) in the lateral amygdala (LA). Consistently, increasing αCaMKII in the LA not only caused PTSD-like behaviors such as impaired fear extinction and anxiety-like behaviors, but also attenuated N-methyl-D-aspartate receptor (NMDAR)-dependent long-term depression (LTD) at thalamo-lateral amygdala (T-LA) synapses, and reduced GluA1-Ser845/Ser831 dephosphorylation and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) internalization. Suppressing the elevated αCaMKII to normal levels completely rescued both PTSD-like behaviors and the impairments in LTD, GluA1-Ser845/Ser831 dephosphorylation, and AMPAR internalization. Intriguingly, deficits in GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization were detected not only after impaired fear extinction, but also after attenuated LTD. Our results suggest that αCaMKII in the LA may be a potential molecular determinant of PTSD. We further demonstrate for the first time that GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization are molecular links between fear extinction and LTD.
Keywords: Posttraumatic stress disorder (PTSD), αCaMKII, Cued fear conditioning, LTD, Anxiety, Amygdala
1. Introduction
Posttraumatic stress disorder (PTSD) is one of the most prevalent and debilitating psychiatric illnesses worldwide (Etkin and Wager 2007; Yehuda et al., 2015). Flashbacks (reliving of natural disaster, military combat, or physical assault-associated traumatic events), impaired fear extinction, increased anxiety, and avoidance of trauma associated stimuli are the typical symptoms of PTSD (Clausen et al., 2020; Li et al., 2020). Accumulating evidence indicates that the pathophysiology of PTSD critically involves lateral amygdala (LA) dysregulation (Castro-Gomes et al., 2016; Ousdal et al., 2020; Fenster et al., 2018). Specifically, PTSD patients show exaggerated LA activity (Etkin and Wager 2007) and smaller than normal LA volume (Morey et al., 2020; Ousdal et al., 2020). Furthermore, LA lesions cause the changes in fear- and anxiety-related behaviors (Fanselow and Gale 2003; Nader et al., 2001; Davis 1992). However, the cellular and molecular mechanisms through which the LA modulates PTSD are largely unknown.
Besides anxiety-related phenotypes, impaired fear extinction is one of the hallmark symptoms of PTSD (Michopoulos et al., 2014; Yehuda et al., 2015), and fear extinction is the basis for psychological exposure therapy (Milad and Quirk 2012). Recent studies have uncovered a link between fear extinction, long-term depression (LTD), and glutamatergic neurotransmission in the LA. Of note, LTD has been implicated in fear extinction (Bennett et al., 2017). Specifically, N-methyl-D-aspartate (NMDA) GluN2B receptor antagonist can abolish both LTD at thalamo-lateral amygdala (T-LA) synapses and fear extinction (Dalton et al., 2012). Similarities occur in the hippocampus with the deletion of kinesin superfamily proteins (KIFs) 21B (Morikawa et al., 2018), while aquaporin-4 deficiency facilitates both fear extinction and NMDAR-dependent hippocampal LTD (Wu et al., 2017). Furthermore, optogenetic delivery of LTD stimuli to the auditory input to the LA facilitates cued fear extinction (Nabavi et al., 2014). Taken together, these findings indicate that there may be a link between LTD and fear extinction.
Previous studies suggest that NMDAR-dependent a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) internalization is involved in fear extinction (Dalton et al., 2008; Kim et al., 2007; Lin et al., 2010; Bai et al., 2014). Specifically, disruption of AMPAR internalization impairs fear extinction (Dalton et al., 2008; Kim et al., 2007), whereas enhanced AMPAR internalization facilitates fear extinction (Lin et al., 2010; Bai et al., 2014). It is worth noting that AMPAR internalization also participates in LTD (Brebner et al., 2005; Collingridge et al. 2004). Hence, we hypothesized that AMPAR internalization may be a link between fear extinction and LTD.
PTSD-like behaviors include impaired fear extinction and heightened anxiety (Règue et al., 2019; Ardi et al., 2016). Here, using a behavioral profiling approach (Ardi et al., 2016), we therefore identified the PTSD susceptible mice by cued fear extinction deficits and anxiety-like behaviors following trauma exposure. Notably, increased alpha-Ca2+/calmodulin-dependent kinase II (αCaMKII) was detected in the LA of PTSD susceptible mice. αCaMKII is an abundant synaptic protein, which plays a crucial role in memory formation (Irvine et al., 2006) and long-term potentiation (LTP) (Lisman et al. 2002). However, whether αCaMKII participates in the processing of fear extinction and LTD remains to be elucidated.
To determine whether increased αCaMKII causes PTSD-like behaviors, we employed an inducible and reversible chemical-genetic technique to temporally and spatially manipulate αCaMKII level in the forebrain of αCaMKII-F89G TG mice, as well as using adeno-associated viral (AAV) vectors to elevate αCaMKII specifically in the LA of C57BL/6J mice. Consistently, the up-regulation of αCaMKII induced PTSD-like behaviors including cued fear extinction deficit and anxiety-like behaviors, which could be reversed by suppressing elevated αCaMKII to normal levels. In addition, we demonstrated that GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization are the links between cued fear extinction and NMDAR-dependent LTD at T-LA synapses. These findings provide novel insights into the mechanisms underlying PTSD and new therapeutic targets.
2. Material and methods
2.1. Animals
2.1.1. Biochemical characterizations of αCaMKII-F89G TG mice
αCaMKII-F89G TG mice were donated by Dr. Tsien's lab (Wang et al., 2003). Mutant αCaMKII-F89G was generated with a silent mutation (i.e. replacing the Phe-89 with Gly in αCaMKII), so that the ATP-binding pocket of αCaMKII-F89G kinase was enlarged. To selectively block exogenous αCaMKII-F89G and leave endogenous αCaMKII intact, we used Naphthylmethyl (1NM-PP1) (donated by Dr. Kevan M. Shokat), designed to fit only this enlarged pocket but not the unmodified pocket of native αCaMKII. By using an αCaMKII promoter-driven construct, we were able to overexpress αCaMKII-F89G in the forebrain neurons. The αCaMKII-F89G could be rapidly and selectively manipulated in the mouse forebrain by intraperitoneal (i.p.) injection or noninvasive oral intake of 1-Naphthylmethyl (NM)-PP1. Specifically, a single i.p. injection of NM-PP1(16.57 ng/g body weight) into freely behaving TG mice could completely suppress αCaMKII-F89G in the forebrain regions of TG mice within 15 min and the complete suppression could be maintained for 40 min. The oral intake (5 μM NM-PP1 in drinking water) could result in partial inhibition of αCaMKII-F89G in the TG mice by 6 h (no inhibition for the initial 3 h) and complete inhibition by 24 h. Incubation of slices derived from TG mice in 0.5 μM NM-PP1 could inhibit αCaMKII-F89G but had no effect on native αCaMKII (Wang et al., 2003).
All experimental procedures were conducted according to Animals Act, 2006 (China) and approved by the Institutional Animal Care and Use Committee (IACUC approval ID #M09018) of the East China Normal University. Since estrous cycle and ovarian hormones could influence the levels of anxiety and fear extinction, female mice were excluded in our study. All mice were male and 3–4 months old. C57BL/6J mice were used for Fig. 1, Fig. 3 αCaMKII-F89G transgenic mice and wild-type littermates were used for the rest of Figures. The mice were housed in 12 h light/12 h dark cycle (lights on at 7 a.m.) with free access to food and water.
Fig. 1.
PTSD susceptible mice exhibit significant up-regulation of αCaMKII and down-regulation of AMPAR internalization in LA. (A1-2) Schematic illustration of PTSD modeling and identifying PTSD susceptible (PS) mice by UWT (A1) or 4-CS/US pairings (A2) exposure. (B-E) PS mice including PS-UWT and PS-4CS/US mice exhibited cued fear extinction deficit (B), and anxiety-like behaviors in OF(C), DL(D), WAZM (E) tests. Both PS-UWT and PS-4CS/US mice spent significantly more time freezing during extinction trials (B, two-way ANOVA followed by multiple comparisons with Bonferroni's correction), less time in the center area of open field chamber, in the light box of DL test and in the open arms of WOM tests (C-E, one-way ANOVA followed by multiple comparisons with Bonferroni's correction) compared to control mice (control, n = 12; PS-UWT, n = 7; PS-4CS/US, n = 7). (F1) Representative blots of LA synaptosomal fractions illustrating an increase in αCaMKII, p-αCaMKII-Thr286, GluA1/2, GluA1-Ser831/Ser845 phosphorylation in PS-UWT and PS-4CS/US mice, but no change in βCaMKII expression. (F2) Ponceau S staining was used as a loading control. (G) Quantifications were based on the average of independent experiments (n = 5 per group). Western blotting was performed after fear extinction and all anxiety-like behavior tests. One-way ANOVA followed by multiple comparisons with Bonferroni's correction. n.s.: not significant, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent s.e.m.
Fig. 3.
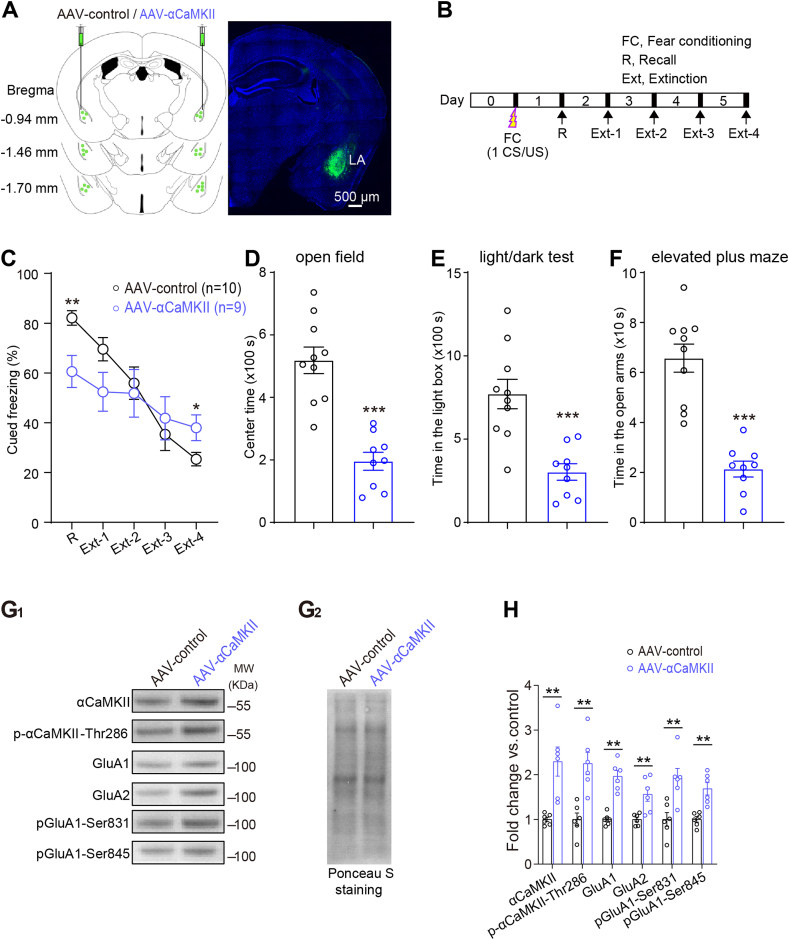
Increasing αCaMKII specifically in the LA impairs the cued fear extinction and AMPAR internalization in AAV-αCaMKII mice. (A) Images of coronal brain slice showing the expression of eGFP (green-colored) 6 weeks after bilateral injections of pAAV-TRE-αCaMKII-P2A-EGFP-CMV-rTA virus into the LA. (B) Schematic of behavioral procedure for cued fear extinction trials. (C) Elevating αCaMKII in the LA impairs cued fear memory and fear extinction (two-way ANOVA followed by multiple comparisons with Bonferroni's correction). (D–F) The higher level of anxiety-like behaviors in AAV-αCaMKII mice in the OF(D), DL(E) and EPM(F) tests (Mann Whitney test). (G1) Representative blots of LA synaptosomal fractions illustrating an increased expression of αCaMKII, p-αCaMKII-Thr286, GluA1/2, phosphorylated GluA1-Ser845/Ser831 in the LA of AAV-αCaMKII mice compared with AAV-control mice. (G2) Ponceau S staining was used as a loading control. (H) Quantifications were based on the average of independent experiment (n = 6 per group). Western blotting was performed after fear extinction and all the anxiety-like behavior tests. Statistical differences were evaluated with Mann Whitney test. *P < 0.05, **P < 0.01, ***P < 0.001 Error bars represent s.e.m. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Behavior experiments
2.2.1. Behavioral profiling for identification of PTSD susceptible mice
We applied a behavioral profiling approach (Ardi et al., 2016) to identify PTSD susceptible mice in either underwater trauma (UWT)-exposed mice (Ritov et al. 2016) or four conditioned stimulus/unconditioned stimulus (4-CS/US)-exposed mice (Milad and Quirk 2011; Mahan and Ressler 2012; Radulovic et al. 2019; Borghans and Homberg 2015; Dębiec et al. 2011; Fenster et al., 2018; Ji et al., 2014).
In detail, the C57BL/6J mice were randomly divided into three groups: control group (n = 12), UWT-exposed group (n = 23) and 4-CS/US-exposed group (n = 23). The control mice were kept in home cages for 4 weeks without any treatment. The UWT-exposed mice were individually allowed to swim freely for 5 s in a water-filled plastic tank, then submerged under water for 35 s using a metal net, next kept in their home cages for 4 weeks (Ritov et al. 2016; Ardi et al., 2016). The 4-CS/US-exposed mice were individually placed in the chamber and allowed to explore the environment freely for 2 min, and then exposed to the conditioned stimulus (CS: 75 dB sound at 2800 Hz) for 30 s. At the last 2 s of tone stimulus, the unconditioned stimulus (US: 0.50 mA footshock, 2 s) was delivered. After 4-CS/US pairings with 2 min intertrial interval, mice were kept in the test chamber for 2 min and then transferred to their home cages for 4 weeks.
Three groups were subjected to the open field (OF), light/dark (LD), water associated zero maze (WAZM), and fear conditioning and extinction paradigms. Freezing behavior was monitored by Freeze Frame system (Coulbourn Instruments, USA).
We calculated six parameters: two parameters represent the level of locomotor activity and four parameters represent anxiety-like performances in the four paradigms. To create the behavioral profiles, firstly we referred to the performance of the control group as the behavior of the normal population and determined the distribution of values in the control group. Standard deviations were used to calculate the upper and lower “cut-off values” for each selected parameter. Secondly, the performance of each mouse in the UWT-exposed group or 4-CS/US-exposed group was compared to the distribution curve of the control group. Mice were considered PTSD susceptible if they exhibited values that are under the lower cut-off value or above the upper cut-off value in at least four out of the six parameters. “Cut-off values” of six parameters: time spent in center in the OF test, 560.32 ± 34.25 s; time spent in the light box in the LD test, 788.60 ± 58.92 s; time spent in the open arms in the OM test, 111.43 ± 8.88 s; freezing percentage in the last day of cued fear extinction, 31.98% ± 3.91%; total distance in the OF test, 7059.99 ± 427.80 cm; total distance in the LD test, 9124.67 ± 220.50 cm.
2.2.2. Cued fear extinction
Four weeks after 4-CS/US parings or 24 h after the 1-CS/US pairing, each mouse was placed in a novel chamber and monitored for 2 min (in the absence of the tone). For the recall test, the cued freezing response to a 3 min tone (75 dB sound at 2800 Hz) without footshock were measured. Then, four cued fear extinction trials were conducted like the recall test in the next 4 following days. Data were presented as mean ± s.e.m. Two-way ANOVA was used for statistical analysis.
2.2.3. Open field
As described previously (Yan et al., 2015), briefly, each mouse was placed in an acrylic open-field chamber (27 cm long × 27 cm wide × 38 cm high) for 30 min. Total moving distance, time spent in the center area, and number of rearing were measured using a TRU-SCAN DigBahv-locomotion Activity Video Analysis System (Coulbourn Instruments, USA). Data were presented as mean ± s.e.m. One-way ANOVA was used for statistical analysis in Fig. 1, Fig. 2C and Mann Whitney test in Fig. S2A.
Fig. 2.
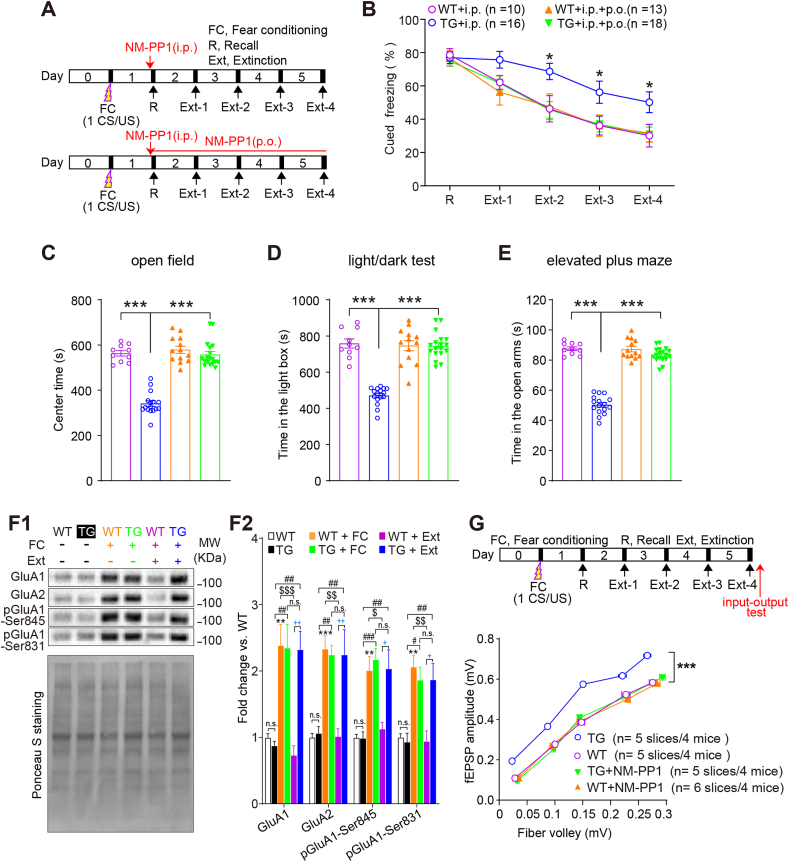
αCaMKII-F89G TG mice exhibit PTSD-like behaviors and reduction in AMPAR internalization after cued fear extinction. (A) Schematic of behavioral procedure for cued fear conditioning and extinction trials. (B) Impaired cued fear extinction in TG mice (two-way ANOVA followed by multiple comparisons with Bonferroni's correction). Intraperitoneal (i.p.) injection and oral (p.o.) administration of NM-PP1 could rescue the cued extinction deficits of TG mice (two-way ANOVA followed by multiple comparisons with Bonferroni's correction). (C-E) The higher levels of anxiety-like behaviors in TG mice in the OF (C), DL (D) and EPM (E) tests after cued fear extinction (one-way ANOVA followed by multiple comparisons with Bonferroni's correction). (F1) Top panel: Representative blots of LA synaptosomal fractions illustrating an increase in GluA1/2, and phosphorylation levels of GluA1-Ser845/Ser831 in both WT and TG mice after cued fear conditioning. A decrease in GluA1/2, phosphorylation level of GluA1-Ser845/Ser831 in WT mice, but not in TG mice after cued fear extinction (n = 5 per group). Bottom panel: Ponceau S staining was used as a loading control. (F2) Quantifications were based on the average of independent experiment. Western blotting in “WT/TG + FC” or “WT/TG + Ext” groups was performed after fear conditioning or fear extinction followed by anxiety-like behavior tests, respectively (one-way ANOVA followed by Bonferroni's multiple comparisons test). (G) Top panel: The schematic of experimental procedure for cued fear conditioning and extinction trials following by input-output recording. Red arrow indicates the time for input-output recordings. Bottom panel: Significantly higher input-output responses in TG slices than that in WT slice. NM-PP1 (0.5 μM) recovered the higher input-output responses in TG slice to normal level (two-way ANOVA followed by multiple comparisons with Bonferroni's correction). n.s.: not significant, *P < 0.05, **P < 0.01 and ***P < 0.001 versus WT group; $ P < 0.05, $$ P < 0.01 and $$$ P < 0.001 versus WT + FC group; #P < 0.05, ##P < 0.01 and ###P < 0.001 versus TG group;  P < 0.05 and
P < 0.05 and  P < 0.01 versus WT + Ext group. Error bars represent s.e.m. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
P < 0.01 versus WT + Ext group. Error bars represent s.e.m. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2.4. Light/dark
The box(27 cm long × 27 cm wide × 38 cm high) was divided into two equal zones - light zone and dark zone. The light zone was painted white and illuminated by white light while the dark zone was painted black and not illuminated. These two zones were connected by a door in the middle divider, and the mice could shuttle freely between the two zones. Total distance and time spent in the light zone were delineated by the TRU-SCAN DigBahv-locomotion Activity Video Analysis System (Coulbourn Instruments, USA) for 30 min. Data were presented as the mean ± s.e.m. One-way ANOVA was used for statistical analysis in Fig. 1, Fig. 2D.
2.2.5. Water-associated zero maze (WAZM)
Experimental protocol and device were as described previously (Ritov and Richter-Levin 2014).This device was composed of an annular platform and a plastic bucket. The annular platform was divided into four equal quadrants: similar to two opposed open arms and two opposed closed arms. The plastic bucket was full of water 40 cm deep. After 5 min habituation, a mouse was put into one of the open arms facing the closed arm for 5 min. The time spent in the open arms vs. closed arms was measured by ANY-maze system (USA, Stoelting). Data were presented as mean ± s.e.m. One-way ANOVA was used for statistical analysis.
2.2.6. Elevated plus maze
The apparatus consists of two opposed open arms (30 cm × 5 cm), two opposed closed arms (30 cm × 5 cm) and one open square (5 cm × 5 cm) in the center, which was elevated above the floor (50 cm). Each mouse was placed in the center of the plus maze facing an open arm and allowed to explore for 5 min. The time spent and moving distances in open and closed arms were automatically recorded by the ANY-maze system (USA, Stoelting). Data were presented as mean ± s.e.m. One-way ANOVA was used for statistical analysis.
2.2.7. Sensitivity to foot shock
This test was performed according to the methods as published (Duan et al., 2015). Mice were individually placed in the conditioning chamber to receive 1 s shocks of gradually increasing current intensity by an increment of 0.01 mA (flinching, 0.05–0.1 mA; vocalization, 0.1–0.2 mA; jumping, 0.45–0.6 mA) with 20 s intervals. The minimum current required to elicit flinching, vocalization and jumping in mice were measured. Data were presented as mean ± s.e.m. Mann Whitney test was used for statistical analysis.
2.3. Animal surgery
To elevate αCaMKII specifically in the LA of C57BL/6J mice, we injected pAAV-TRE- αCaMKII-P2A-EGFP-CMV-rTA (AAV-αCaMKII) or pAAV-TRE-P2A-EGFP-CMV-rTA (AAV-control) virus (2.45 × 10−12 and 2.38 × 10−12 vector genomes/ml, respectively, Obio Technology, China) bilaterally into the LA (AP, −1.60 mm; ML, ±3.35 mm; DV, −4.80 mm) of C57BL/6J mice. After the injection, mice were returned to the home cages to recover for one month before experiments. AAV-αCaMKII mice were fed with doxycycline solution (1 g/L in drinking water) to induce virus expression throughout the behavior tests.
2.4. Dendritic spine analysis
Dendritic spine analysis was performed as previously described (Ming et al., 2018). Briefly, mice were deeply anesthetized and transcardially perfused. Coronal brain sections (200 μm) were cut and collected in 0.1 M PBS. LA neurons were loaded iontophoretically with 5% Lucifer Yellow solution. Images of basal and apical dendrites of LA pyramidal neurons were scanned using a Leica SP2 confocal microscope at 63 × under oil immersion. The number of spines per micrometer along the dendritic longitudinal axis was counted as spine density. Data were presented as mean ± s.e.m. Mann Whitney test was used for statistical analysis.
2.5. Amygdala slice electrophysiology
Protocols were performed as described previously (Kim et al., 2007; Ma et al., 2013). Mice (3–4 months old) were anesthetized with sodium pentobarbital and sacrificed by decapitation. Whole brain coronal slices (370 μm thick for fEPSPs recording) containing the amygdala were cut using a vibroslicer (vibratome 3000) with cold (4 °C) and oxygenated (95% O2/5% CO2) modified artificial cerebrospinal fluid (ACSF) containing (in mM): Choline chloride, 110; KCl, 2.5; CaCl2, 0.5; MgSO4, 7; NaHCO3, 25; NaH2PO4, 1.25; D-glucose, 25; pH 7.4. The slices were recovered in an incubation chamber with normal ACSF containing (in mM): NaCl, 119; CaCl2, 2.5; KCl, 2.5; MgSO4, 1.3; NaHCO3, 26.2; Na2HPO4, 1.0; D-glucose, 11, pH 7.4; 95% O2 and 5% CO2 for 60 min at 31 °C, and then returned to room temperature for at least 1 h before recording.
2.6. Field excitatory postsynaptic potential recording
A stimulating electrode was placed in the fibers from the internal capsule to activate the thalamic input to the lateral amygdala (T-LA) synapses. A recording electrode was positioned in the LA to record field excitatory postsynaptic potential (fEPSP). Test responses were elicited at 0.033 Hz. After obtaining a stable baseline response for at least 15 min, LTP or LTD was induced. LTP was induced by applying 2 trains of high frequency stimulation (100 Hz for 1 s) with 10 s interval or 3 trains theta burst stimulation (10 bursts delivered every 200 ms, each burst consisted of 4 pulses at 100 Hz) with 10 s interval. For LTD induction, the standard 1 Hz protocol (1 Hz for 15 min) and 3 Hz protocol (3 Hz for 5 min) were used. Depotentiation was induced by applying 2 trains of high frequency stimulation (100 Hz for 1s) with 10 s interval followed by the standard 1 Hz protocol (1 Hz for 15 min) after 20 min. For chemical-LTD induction, NMDA (Sigma, 30 μM in ACSF) was infused into the slice chamber for 3 min. Data were presented as mean ± s.e.m. Mann Whitney test (for comparing two different groups with Gaussian distribution) and one-way ANOVA followed by HSD post-hoc test with Bonferroni's correction (for comparing more than two different groups) were used for statistical analysis.
2.7. Protein sample preparation
Combined with the previous protocol (Cui et al., 2011; Yin et al., 2013), synaptosomes were prepared as follows. LA tissues were homogenized in 1.5 ml homogenate-buffer (320 mM sucrose, 5 mM HEPES, pH 7.4) containing freshly added PMSF, PIC and PIC3. Homogenates were centrifuged at 500 g for 5 min to yield insoluble components. Then the supernatant fraction was collected and centrifuged at 10,000 g for 10 min to yield precipitation. The precipitation pellet was resuspended in 2 ml of 0.32 M sucrose, layered onto 2.25 ml of 0.8 M sucrose, and centrifuged at 98,000 g for 15 min using a swinging bucket rotor. Synaptosomes were collected from the 0.8 M sucrose layer and concentrated by centrifugation at 20,800 g for 45 min. Then the precipitation was resuspended in synaptosome lysis buffer (30 mM Tris (pH 8.5), 5 mM magnesium acetate, 8 M Urea, and 4% W/V CHAPS). For total proteins preparation, the LA areas were homogenized with RIPA buffer containing freshly added PMSF, PIC, and PIC3 and lysed on ice for 30 min, centrifuged at 10,000 g at 4 °C for 5 min, and total protein samples were taken as supernatant. Then the protein samples were stored in a −80 °C freezer until used. Protein concentration was quantified by a Pierce BCA Protein Assay kit (Thermo Scientific) after which protein was stored at −20 °C.
2.8. Western blot
Each sample of protein (5 μg/lane) was separated by 10% SDS-PAGE (P40650, NCM Biotech) at 120 V for 120 min. Then the separated proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were blocked in blocking solution (5% skim milk and 1% BSA) at room temperature for 1h. A reversible Ponceau S staining of the membranes was done to normalize the relative amount of each protein on the membrane (just for synaptosomes). After washing with TBST buffer, the PVDF membranes were immunoblotted with the following antibodies: GluA1 antibody (1:2,000, Santa Cruz), GluA2 antibody (1:2,000, Millipore), pGluA1-Ser845 antibody (1:500, Abcam), pGluA1-Ser831 antibody (1:500, Abcam), αCaMKII antibody (1:3,000, Abcam), p-αCaMKII-Thr286 antibody (1:20,000, Santa Cruz), βCaMKII antibody (1:2,000, Invitrogen), β-actin antibody (1:20,000, Sigma), GAPDH antibody (1:20,000, Proteintech), synapsin (SYP) antibody (1:2,000, Proteintech), TfR antibody (1:2,000, Abcam),Tubulin antibody (1:1,000, Millipore) at 4 °C for 12h. After washing with TBST buffer, the blots were reacted with an HRP-conjugated secondary antibody at room temperature for 1 h. Band intensity on the blot was quantified by an ECL immunoblotting detection system (Bio-Rad). Data were shown as mean ± s.e.m. Statistical differences were analyzed using post hoc test with Bonferroni's correction following one-way ANOVA.
2.9. PP2A activity measurement
PP2A activity was measured by using immunoprecipitation phosphatase assay kit according to the manufacturer's instructions (Catalog # 17–313, Millipore). Statistical differences were analyzed using post hoc test with Bonferroni's correction following one-way ANOVA. Data were shown as mean ± s.e.m.
2.10. PP2B activity measurement
The activity of calcineurin (PP2B) was assayed by using a calcineurin cellular activity assay kit (207007, Millipore) by following the manufacturer's instructions. Statistical differences were analyzed using post hoc test with Bonferroni's correction following one-way ANOVA. Data were shown as mean ± s.e.m.
2.11. Statistical analysis
Statistical significance was assessed by one-way ANOVA, two-way ANOVA analysis of variance, or Mann Whitney test, where appropriate. Significant effects in analysis of variances were followed up with Bonferroni post-hoc tests. ANOVA was applied for comparing more than two different groups, followed by multiple comparisons with Bonferroni post-hoc tests. The Mann-Whitney U test was performed for comparing two independent groups without making the assumption that values are normally distributed. Results were considered significantly different when P < 0.05. All data were presented as means ± s.e.m. Detailed information about statistical analysis is provided in figure legends.
3. Results
3.1. PTSD susceptible mice exhibit increased αCaMKII and reduced AMPAR internalization in the LA
PTSD susceptible (PS) individuals were identified in the underwater-trauma (UWT)-exposed group (23 male mice) and 4-CS/US-exposed group (23 male mice) by employing the behavioral profiling approach as described in the MATERIALS AND METHODS section (Fig. 1A1A2). PTSD susceptible mice exhibited persistently higher levels of cued freeze responses through extinction trials (Fig. 1B, PS-UWT vs. Control, F(4, 85) = 6.33, P < 0.001; PS-4CS/US vs. Control, F(4, 85) = 4.70, P < 0.01), spent significantly less time in the center area of the open field (OF) chamber (Fig. 1C, PS-UWT vs. Control, P < 0.01; PS-4CS/US vs. Control, P < 0.001), in the light zone of the light/dark (LD) box test (Fig. 1D, PS-UWT vs. Control, P < 0.01; PS-4CS/US vs. Control, P < 0.001), and in the open arms of water O maze (WOM) test (Fig. 1E, PS-UWT vs. Control, P < 0.001; PS-4CS/US vs. Control, P < 0.001) compared with control mice. Behavioral profiling revealed that only 7 mice each group showed PTSD-like behaviors in 23 mice exposed to UWT or 23 mice exposed repeatedly to US/CS.
The LA is a key brain region for fear extinction and anxiety-like behaviors (Jacques et al., 2019; Erlich et al. 2012; Grosso et al., 2018; Kim et al. 2007, 2015; Krabbe et al. 2018; Mahan and Ressler 2012; Schafe et al. 2005; Forster et al., 2012; Ressler 2010). CaMKII plays an important role in memory extinction (Bevilaqua et al., 2006; Szapiro et al., 2003; Burgdorf et al., 2017). Moreover, GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization contribute to fear extinction (Dalton et al., 2008; Kim et al., 2007; Lin et al., 2010; Bai et al., 2014; Hollis et al., 2016; Talukdar et al., 2018; Lee et al., 2013). Thus, we investigated levels of αCaMKII, βCaMKII, GluA1-Ser845/Ser831 phosphorylation and synaptic GluA1/2 expression in the LA of PTSD susceptible mice, and found that αCaMKII and the phosphorylated (p)-αCaMKII at Thr286 (p-αCaMKII-Thr286) were significantly up-regulated in PTSD susceptible mice that experienced either UWT or 4-CS/US exposure (Fig. 1FG, PS-UWT vs. Control, αCaMKII, P < 0.01, p-αCaMKII-Thr286, P < 0.05; PS-4CS/US vs. Control, αCaMKII, P < 0.05, p-αCaMKII-Thr286, P < 0.05). However, no significant difference was observed in βCaMKII among the three groups (Fig. 1FG, P > 0.05). In addition, PTSD susceptible mice showed significantly higher synaptic expression levels of GluA1/2 and phosphorylated GluA1-Ser845/Ser831 (Fig. 1FG, PS-UWT vs. Control, GluA1: P < 0.01, GluA2: P < 0.01, GluA1-Ser831: P < 0.01, GluA1-Ser845: P < 0.01; PS-4CS/US vs. Control, GluA1: P < 0.001, GluA2: P < 0.05, GluA1-Ser831: P < 0.01, GluA1-Ser845: P < 0.01). Taken together, these results suggest that PTSD susceptible mice display a significantly higher levels of αCaMKII and lower levels of GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization in the LA.
3.2. Increasing αCaMKII in the LA is sufficient to cause PTSD-like phenotypes in both αCaMKII-F89G TG and AAV-αCaMKII mice
To further investigate whether increased αCaMKII in the LA leads to PTSD-like phenotypes such as impaired fear extinction and anxiety-like behaviors, we temporally and spatially manipulated αCaMKII overexpression in αCaMKII-F89G TG mice by employing an inducible and reversible chemical-genetic technique described in MATERIALS AND METHODS section. The TG mice displayed the higher level of αCaMKII/p-αCaMKII-Thr286 (Supplemental information, Fig. S1AB) and normal morphology in the LA (Supplemental information, Fig. S1CD).
Then, cued fear memory recall and cued fear extinction were measured after only 1-CS/US for cued fear conditioning (Fig. 2A). Given that forebrain αCaMKII overexpression impairs fear memory retrieval in our previous study (Cao et al., 2008), to examine the effect of αCaMKII overexpression on cued fear extinction in TG mice, we designed the “normal αCaMKII levels during cued fear memory retrieval but elevated αCaMKII levels during cued fear extinction period” paradigm by a single i.p. injection of NM-PP1 into both TG mice and WT littermates 15 min before the first recall test of cued fear memory (Fig. 2A). Under this paradigm, TG mice exhibited normal retrieval of cued fear memory in comparison to that of wild-type littermate (Fig. 2B, TG + i.p. vs. WT + i.p., P > 0.05). However, during cued fear extinction trials, WT mice had a significantly decreased freezing behavior compared with TG mice (Fig. 2B, TG + i.p. vs. WT + i.p., F (3, 264) = 10.73, P < 0.001). A post hoc analysis revealed that TG mice exhibited significantly higher level of freezing response to the CS in cued fear extinction trial 2, 3 and 4 (Fig. 2B, TG + i.p. vs. WT + i.p., P < 0.05), suggesting that increased αCaMKII may impair cued fear extinction. In addition, TG mice spent significantly less time (Fig. 2C–E, TG + i.p. vs. WT + i.p., P < 0.001) in the center area of the OF chamber (Fig. 2C), in the light zone of the LD test (Fig. 2D), and in the open arms of the elevated plus maze (EPM) test (Fig. 2E) compared with WT mice. Together, these findings indicate that the increased αCaMKII in the LA may cause PTSD-like phenotypes.
To further confirm whether PTSD-like phenotypes in TG mice are due to the overexpression of αCaMKII-F89G protein, we then designed the “normal αCaMKII level during both fear memory recall and extinction period” paradigm by i.p. injection of NM-PP1 15 min before recall test and oral (p.o.) administration throughout the entire fear extinction period (Fig. 2B). Under this paradigm, TG mice had a freezing response similar to WT mice during cued extinction trials (Fig. 2B, TG + i.p. + p.o. vs. WT + i.p., P > 0.05), suggesting impaired cued fear extinction was rescued by NM-PP1 treatment in TG mice. Moreover, NM-PP1 had no effect on cued fear extinction in WT mice (Fig. 2B, WT + i.p. + p.o. vs. WT + i.p., P > 0.05), excluding the possibility that the rescuing effects by NM-PP1 were due to ‘facilitating extinction’ effects. In addition, TG mice treated with NM-PP1 spent comparable amounts of time (Fig. 2C–E, TG + i.p. + p.o. vs. WT + i.p., P > 0.05) in the center area of the open field chamber (Fig. 2C), in the light box of the LD test (Fig. 2D) and in the EPM test (Fig. 2E) compared with WT mice. Furthermore, TG mice without any treatment exhibited normal locomotor activity, exploratory behavior, and pain threshold (Supplemental information, Fig. S2). Taken together, we conclude that increased αCaMKII is indeed sufficient to produce PTSD-like phenotypes including impaired fear extinction and anxiety-like behaviors.
To further examine whether increasing αCaMKII specifically in the LA is sufficient to cause PTSD-like phenotypes, we bilaterally injected viral vectors AAV-αCaMKII (pAAV-TRE-αCaMKII-P2A-EGFP-CMV-rTA) into the LA of C57BL/6J mice to overexpress αCaMKII specifically in the LA (Fig. 3A). As expected, both αCaMKII and p-αCaMKII-Thr286 expression levels significantly increased in the LA of AAV-αCaMKII mice (Fig. 3GH, αCaMKII, P < 0.01; p-αCaMKII-Thr286, P < 0.01). We performed cued fear memory test 24 h after 1-CS/US pairing. AAV-αCaMKII mice exhibited impaired cued fear memory during recall test (Fig. 3C, P < 0.001), which is consistent with our previous finding that αCaMKII overexpression impairs retrieval of fear memory (Cao et al., 2008). In addition, AAV-αCaMKII mice showed significantly impaired fear extinction (Fig. 3C, AAV-αCaMKII vs. AAV-control, F(4, 81) = 2.63, P < 0.05). Post hoc analysis revealed that AAV-αCaMKII mice exhibited freezing responses significantly higher than AAV-control mice on the 4th extinction trial (P < 0.05). In addition to the deficits in cued fear extinction, AAV-αCaMKII mice showed anxiety-like behaviors (Fig. 3D–F, AAV-αCaMKII vs. AAV-control, P < 0.001). These data suggest that increased αCaMKII expression specifically in LA is also sufficient to result in PTSD-like behaviors.
3.3. Increasing αCaMKII in the LA impairs AMPAR internalization and GluA1-Ser845/Ser831 dephosphorylation after cued fear extinction in both αCaMKII-F89G TG and AAV-αCaMKII mice
We quantified the expression of synaptic AMPAR composition subunits (GluA1/2) and GluA1-Ser845/Ser831 phosphorylation in the LA before/after cued fear conditioning and extinction trials. After cued fear extinction trials, compared with cued fear conditioning trial, significant decreases in the GluA1/2 synaptic expression and GluA1-Ser845/Ser831 phosphorylation levels were found only in WT mice (Fig. 2F1-2, WT + FC vs. WT + Ext, GluA1: P < 0.001; GluA2: P < 0.01; pGluA1-Ser845: P < 0.05; pGluA1-Ser831: P < 0.01), but not in TG mice (TG + FC vs TG + Ext, GluA1/A2, pGluA1-Ser845/831: P > 0.05). Furthermore, the GluA1/2 synaptic expression and phosphorylated GluA1-Ser845/Ser831 in TG mice were significantly higher than those in WT mice after cued fear extinction trials (Fig. 2F1-2, TG + Ext vs. WT + Ext, GluA1/A2: P < 0.01; pGluA1-Ser845/Ser831: P < 0.05). Consistently, the input-output curve observed was higher in TG than in WT mice after cued fear extinction trials (Fig. 2G, TG vs. WT, P < 0.001), which could be recovered by 0.5 μM NM-PP1 (Fig. 2G, TG + NM-PP1 vs. TG, P < 0.001; TG + NM-PP1 vs. WT, P > 0.05).
Moreover, consistent with the above western blotting data from αCaMKII-F89G TG mice, synaptic GluA1/2 expression and phosphorylated GluA1-Ser845/Ser831 levels were significantly higher in the LA of AAV-αCaMKII mice than those in AAV-control mice after cued fear extinction trials (Fig. 3GH, GluA1/2, pGluA1-Ser845/Ser831, P < 0.01). Taken together, these results indicate that increasing αCaMKII specifically in the LA disrupts GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization, and may consequently impair cued fear extinction in both αCaMKII-F89G TG and AAV-αCaMKII mice.
3.4. Increasing αCaMKII impairs NMDAR-dependent LTD at T-LA synapses and NM-PP1 can recover the impairments
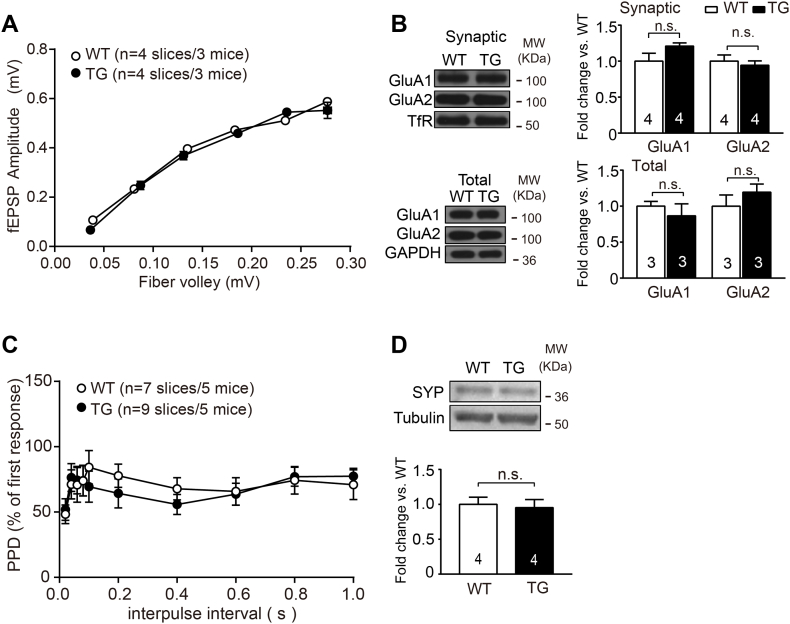
To investigate the cellular mechanism of impaired cued fear extinction and anxiety-like behaviors, we measured the basal synaptic transmission and synaptic plasticity at T-LA synapses in TG mice (Dalton et al., 2012; Xu et al., 2019). No significant difference was observed in input-output curves, synaptic and total GluA1/2 expression of LA (Supplemental information, Fig. S3AB, TG vs. WT, P > 0.05), paired-pulse depression (PPD) and synapsin expression (Fig. S3CD, TG vs. WT, P > 0.05) in LA between TG and WT mice. Moreover, either tetanic or theta burst stimulations induced similar level of LTP at T-LA synapses in two groups (Fig. 4AB, TG vs. WT, P > 0.05). These results indicate that αCaMKII overexpression does not affect basal synaptic transmission and LTP at T-LA synapses.
Fig. 4.
Increasing αCaMKII impairs NMDAR-dependent LTD at T-LA synapses of αCaMKII-F89G TG mice and NM-PP1 rescues the impairments. (A) Similar LTP induced by high frequency stimulations (2 trains of 100 Hz stimulation for 1 s, 10 s interval) in TG slices and WT slices. In this figure and the subsequent ones, insets show sample traces taken at baseline (1) and the last 10 min recording (2). (B) Normal LTP induced by three trains of theta burst stimulations (TBS, each train consisted of 10 bursts delivered at 5 Hz, each burst consisted of 4 pulses at 100 Hz) in TG slices. (C) Significantly weaker LTD induced in TG slices than that in WT slice after 1 Hz (15 min) stimulation. NM-PP1 (0.5 μM) recovered the reduced LTD in TG slice to normal level. (D) LTD was abolished in WT and TG slices exposed to both NM-PP1 (0.5 μM) and APV (50 μM). The solid lines show the duration of both NM-PP1 (red) and APV (blue) application. (E) Strong LTD could be induced by 3 Hz (5 min) stimulation in WT slices but not in TG slices. NM-PP1 (0.5 μM) rescued the impaired LTD in TG slice. (F) Impaired depotentiation can be observed in TG slices. All bar graphs summarize data obtained during last 10 min recording. Statistical differences were evaluated with Mann-Whitney test (A, B, D and F) and one-way ANOVA followed by Bonferroni's multiple comparisons test (C and E). n.s.: not significant, *P < 0.05, **P < 0.01, ***P < 0.001. All values are mean ± s.e.m. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We then analyzed the effects of αCaMKII overexpression on LTD at T-LA synapses. 1Hz-LTD in TG slices was significantly reduced compared to that of WT slices (Fig. 4C, TG vs. WT, P < 0.05), which could be recovered by 0.5 μM NM-PP1 (Fig. 4C, TG + NM-PP1 vs. TG, P < 0.05), while 1Hz-LTD in WT slices was not affected (Fig. 4C, WT + NM-PP1 vs. WT, P > 0.05). Notably, LTD at the T-LA synapses could be blocked by application of APV (50 μM) and NM-PP1 (0.5 μM) (Fig. 4D, TG vs. WT, P > 0.05), suggesting that the LTD at the T-LA synapses is NMDAR-dependent. Additionally, 3Hz-LTD was blocked in TG slices (Fig. 4E; TG vs. WT, P < 0.001), which could also be recovered by NM-PP1 (Fig. 4E, TG + NM-PP1 vs. TG, P < 0.01), while 3Hz-LTD in WT slices was not affected (Fig. 4E, WT + NM-PP1 vs. WT, P > 0.05). Similarly, TG mice exhibited deficits in the depotentiation at T-LA synapses (Fig. 4F, TG vs. WT, P < 0.01). In summary, our results show that αCaMKII overexpression impairs NMDAR-dependent LTD and depotentiation at T-LA synapses in TG mice, which is consistent with the higher input-output responses in TG mice compared with WT mice after impaired cued fear extinction as shown in Fig. 2G.
3.5. Increasing αCaMKII impairs AMPAR internalization and GluA1-Ser845/Ser831 dephosphorylation during NMDAR-dependent LTD, and NM-PP1 can rescue the impairments
Besides low-frequency stimulation (LFS), brief NMDA exposure can chemically induce NMDAR-dependent LTD (Lee et al., 1998). In TG slices, NMDA application (30 μM, 3 min) could elicit a significantly weaker LTD at T-LA synapses than that in WT slices (Fig. 5A, TG + NMDA vs. WT + NMDA, P < 0.01). Furthermore, 0.5 μM NM-PP1 could rescue the reduced NMDA-induced LTD in TG slices to normal level (Fig. 5A, TG + NMDA + NM-PP1 vs. WT + NMDA, P > 0.05; TG + NMDA + NM-PP1 vs. TG + NMDA, P < 0.01), but had no detectable effects on NMDA-induced LTD in WT slices (Fig. 5A, WT + NMDA + NM-PP1 vs. WT + NMDA, P > 0.05). These results suggest that increasing αCaMKII in the LA attenuates NMDAR-dependent chem-LTD at T-LA synapses in TG mice.
Fig. 5.
Increasing αCaMKII impairs AMPAR internalization/dephosphorylation, reduces protein phosphatase (PP) activity, and increases stargazin expression during NMDAR-dependent LTD and NM-PP1 rescues all impairments. (A) Attenuated chem-LTD induced by NMDA (30 μM, 3 min) in TG slices, which could be rescued by 0.5 μM NM-PP1. Top right panel: bar graph summarizing data obtained during last 10 min recording in the different groups depicted. The following Western blotting was performed ~1 h later after 3 min NMDA application. (B) Representative blottings of LA synaptosomal fractions illustrating a smaller reduction in GluA1/2, phosphorylation level of GluA1-Ser845/831 in TG slices after NMDA treatment compared with WT slices. NM-PP1 could rescue these deficits in TG slices. Bottom panel: Ponceau S staining was used as a loading control. (C) Quantifications were based on the average of independent experiments (n = 5 per group). (D) Top panel: Representative blottings of LA synaptosomal fractions illustrating a smaller reduction in phosphorylation level of pPP1-Thr320, indicating an increase in PP1 activity in TG mice after NMDA treatment compared with WT. NM-PP1 rescued such deficit in TG mice. Bottom panel: Quantifications were based on the average of independent experiments (n = 4 per group). (E) A remarkably higher level of stargazin in amygdala synaptosomal fractions in TG slices than that in WT slices after NMDA application, NM-PP1 rescued the deficit in TG mice (n = 5 per group). Bottom panel: Ponceau S staining was used as a loading control. (F) Smaller increases in activity of PP2A and PP2B in TG slices were exhibited after NMDA application compared with that in WT slices, and NM-PP1 rescued these deficits in TG mice (n = 4 per group). Statistical differences were evaluated with one-way ANOVA followed by multiple comparisons with Bonferroni's correction. n.s.: not significant, *P < 0.05, **P < 0.01 and ***P < 0.001 versus WT group; #P < 0.05, ##P < 0.01 and ###P < 0.001 versus TG group;  P < 0.05,
P < 0.05,  P < 0.01 and
P < 0.01 and  P < 0.001 versus WT + NMDA group. Error bars represent s.e.m.
P < 0.001 versus WT + NMDA group. Error bars represent s.e.m.
NMDA-induced LTD elicits more widespread depression of synapse strength and shares certain molecular mechanisms with LFS-LTD such as AMPAR internalization and GluA1-Ser845/Ser831 dephosphorylation (Delgado et al., 2007; He et al., 2011; Kollen et al. 2008; Lee et al., 1998). We found the synaptic expression levels of GluA1/2 and GluA1-Ser845/Ser831 phosphorylation in the LA of TG slices were significantly higher than that in LA of WT slices (Fig. 5BC, TG + NMDA vs. WT + NMDA, GluA1: P < 0.05; GluA2: P < 0.01; pGluA1-Ser845/Ser831: P < 0.05), which is consistent with the higher input-output responses in TG than WT mice after impaired cued fear extinction as shown in Fig. 2G. Furthermore, NM-PP1 (0.5 μM) successfully rescued the impairments of AMPAR internalization and GluA1-Ser845/Ser831 dephosphorylation of the LA in TG slices (Fig. 5BC, TG + NMDA + NM-PP1 vs. TG, GluA1/A2, pGluA1-Ser845/831: P < 0.01), with no effect on that of WT slices (Fig. 5BC, WT + NMDA + NM-PP1 vs. WT + NMDA, GluA1/A2, pGluA1-Ser845/831: P > 0.05). Collectively, these findings indicate that αCaMKII overexpression leads to the impairment of AMPARs internalization and dephosphorylation in the LA, which consequently impairs NMDAR-dependent LTD at T-LA synapses.
3.6. Increasing αCaMKII reduces protein phosphatase (PP) activity and enhances stargazin expression during NMDAR-dependent LTD and NM-PP1 can recover these abnormalities
Activation of protein phosphatase 1 (PP1) contributes to LTD formation (Mansuy and Shenolikar 2006; Mauna et al., 2011). Moreover, stargazin can be dephosphorylated by PP1 to induce the clathrin-dependent AMPAR endocytosis during NMDAR-dependent LTD (Matsuda et al., 2013; Bats et al. 2007). Dephosphorylation of the Thr320 residue on the C-terminal domain of PP1 can enhance PP1 activity during NMDAR-dependent LTD (Dohadwala et al., 1994; Goldberg et al., 1995). Therefore, we investigated PP1-Thr320 phosphorylation (pPP1-Thr320) and stargazin expression in the LA fractions of WT and TG slices with NMDA treatment. With NMDA exposure, the PP1-Thr320 phosphorylation and stargazin expression in LA of TG slices were dramatically higher than that in WT slices (Fig. 5DE, TG + NMDA vs. WT + NMDA, pPP1-Thr320, stargazing, P < 0.05), suggesting that the PP1 activity and stargazin expression were abnormal in TG mice during NMDA-induced LTD. Furthermore, NM-PP1 could recover the abnormalities in PP1 activity and stargazin expression in the LA of TG slices (Fig. 5DE, TG + NMDA + NM-PP1 vs. TG, pPP1-Thr320: P < 0.01; stargazing: P < 0.01; TG + NMDA + NM-PP1 vs. TG + NMDA, pPP1-Thr320: P < 0.05, stargazing: P < 0.05) but did not affect their levels in WT slices (Fig. 5DE, WT + NMDA + NM-PP1 vs. WT + NMDA, pPP1-Thr320: P > 0.05; stargazing: P > 0.05).
Protein phosphatase 2A (PP2A) and calcineurin (PP2B) play important roles in LTD maintenance and induction (Pi and Lisman 2008; Winder and Sweatt 2001). PP2A/2B activity was dramatically lower in the LA of TG slices than in WT slices (Fig. 5F, TG + NMDA vs. WT + NMDA, PP2A: P < 0.01; PP2B: P < 0.001) during NMDA-induced LTD. Furthermore, NM-PP1 (0.5 μM) could also recover down-regulation of PP2A/2B activity in the LA of TG slices (Fig. 5F, TG + NMDA + NM-PP1 vs. TG + NMDA, PP2A, PP2B: P < 0.01) without affecting that of WT slices (Fig. 5F, WT + NMDA + NM-PP1 vs. WT + NMDA, PP2A, PP2B: P > 0.05). Taken together, these results suggest that αCaMKII overexpression can weaken PP1, PP2A/2B activity and increase stargazin expression in LA fractions during NMDAR-dependent LTD, which may cause the impairments in AMPAR internalization and NMDAR-dependent LTD.
4. Discussion
In the present study, we reveal that PTSD susceptible mice exhibit significant up-regulation of αCaMKII, down-regulation of GluA1-Ser845/Ser831 dephosphorylation, and reduction in AMPAR internalization in LA. Consistently, increasing αCaMKII specifically in the LA leads to PTSD-like phenotypes such as fear extinction deficit and anxiety-like behaviors, and impairs AMPAR internalization and dephosphorylation, NMDAR-dependent LTD and depotentiation at T-LA synapses. Furthermore, deficits in AMPAR internalization and dephosphorylation are observed not only after impaired cued fear extinction in vivo, but also after attenuated NMDA-induced LTD in TG slices in vitro. Additionally, the deficits in AMPAR internalization and dephosphorylation are due to down-regulation of PP1/2A, PP2B activity and increased stargazin in TG mice. Importantly, NM-PP1, a specific inhibitor of the exogenous αCaMKII-F89G, could rescue the above deficits in αCaMKII-F89G TG mice. These results identify αCaMKII as a potential molecular determinant of PTSD-like behaviors and a powerful regulator of LTD at T-LA synapses.
4.1. αCaMKII and fear extinction
Previous studies have implicated changes as in CaMKII related to extinction of different memories. Specifically, pharmacological inhibition of CaMKII by KN-62 blocked the extinction of step-down passive avoidance performance (Bevilaqua et al., 2006; Szapiro et al., 2003). Similarly, α/βCaMKII inhibitor KN93 significantly attenuated the extinction of cocaine conditioned place preference (Burgdorf et al., 2017). Furthermore, partial reduction of αCaMKII function due to T286A+/– mutation impaired the extinction of contextual fear and spatial memories (Kimura et al. 2008). Paradoxes also exist, for example, reduction of αCaMKII by phosphorylation at serine 331 in the LA enhances cocaine memory extinction (Rich et al., 2016). Furthermore, increased activation of αCaMKII in the CPEB3-knockout hippocampus reduced the extinction of spatial memories (Berger-Sweeney et al., 2006; Huang et al., 2014). These conflicting findings implied the controversial roles of CaMKII in extinction of different memories. Thus, whether and which CaMKII sub-type are necessary or sufficient for fear extinction remains to be determined. In our study, we found that mouse models of PTSD with cued fear extinction deficit exhibited significant up-regulation of αCaMKII in the LA. Consistently, increasing αCaMKII in the LA impaired cued fear extinction. Furthermore, returning the increased αCaMKII to normal level could completely restore the impaired fear extinction. Our results thus indicate that αCaMKII may serve as a potential molecular determinant of fear extinction.
4.2. αCaMKII and LTD
Endogenous CaMKII plays pivotal roles in synaptic plasticity (Collingridge et al. 2004). Specifically, CaMKII can promote either LTP or LTD (Pi et al., 2010). CaMKII can enhance LTP through promoting the integration of new AMPA receptors at the postsynaptic density (Barria et al., 1997). In our previous studies, αCaMKII up-regulation in the forebrain disrupted LTD in the medial prefrontal cortex (Ma et al., 2015). Similarly, βCaMKII up-regulation blocked LTD in the dentate gyrus (Yin et al., 2017). However, whether αCaMKII overexpression in the LA affects synaptic plasticity at T-LA synapses is unclear. Here we demonstrated that αCaMKII overexpression in the LA did not affect LTP but caused impairments in LTD at T-LA synapses in TG mice, which could be completely rescued by a specific inhibitor (NM-PP1) of exogenous αCaMKII-F89G. We conclude that αCaMKII may be a regulator of LTD at T-LA synapses.
4.3. The molecular links between LTD and fear extinction, and between LTD and anxiety-like behaviors
Previous studies indicated potential links between LTD and fear extinction. Optogenetic delivery of LTD stimuli to the auditory input to the LA facilitates cued fear extinction (Nabavi et al., 2014). Moreover, both hippocampal LTD and fear extinction were enhanced by aquaporin-4 deficiency (Wu et al., 2017). Furthermore, both hippocampal LTD and contextual fear extinction were impaired by kinesin superfamily proteins 21B (Morikawa et al., 2018). Similarly, both LTD at T-LA synapses and fear extinction could be blocked by GluN2B receptor antagonist (Dalton et al., 2012). In addition to the relation between LTD and fear extinction, accumulated evidences also indicated the relation between LTD and anxiety-like behaviors (Zhang et al., 2017; Xu et al., 2019). However, it remains unclear what molecules links between LTD and fear extinction, and between LTD and anxiety-like behaviors are unclear. GluA1-Ser845/Ser831 dephosphorylation (Hollis et al., 2016) and AMPAR internalization (Dalton et al., 2008; Bai et al., 2014) have been implicated in the regulation of fear extinction. Moreover, previous studies have shown that GluA1-Ser845 dephosphorylation and AMPAR internalization are crucial for LTD expression (Lee et al., 2000; Carroll et al., 2001). Although these findings indicate GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization may be a potential link between fear extinction and LTD, a direct evidence remains to be elucidated. In our current study, the deficits in GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization were observed not only after impaired cued fear extinction and increased anxiety-like behaviors in vivo, but also after attenuated NMDA-induced LTD in αCaMKII-F89G TG slices in vitro. Furthermore, a specific inhibitor of the exogenous αCaMKII-F89G (NM-PP1) could completely rescue not only the increased anxiety-like behaviors and deficits in cued fear extinction, but also NMDA-induced LTD, GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization. Notably, the input-output responses in TG slices were significantly higher than WT slices after cued fear extinction and increased anxiety-like behaviors, which could also be reversed by NM-PP1. Besides αCaMKII-F89G TG mice, PTSD susceptible mice and AAV-αCaMKII mice displayed a significantly higher level of αCaMKII, lower levels of GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization in the LA. These mice also exhibited the deficits in cued fear extinction and showed anxiety-like behaviors. Based on the molecular and behavioral characteristics, we hypothesize that PTSD susceptible mice and AAV-αCaMKII mice might also exhibit impairments in LTD at T-LA synapses. Therefore, further studies that examine the LTD in the LA of these mice are required to fully comprehend increased αCaMKII of different mice in mediating synaptic plasticity.
Taken together, these data demonstrate that deficits in Ser845/GluA1-Ser831 dephosphorylation and AMPAR internalization by increased αCaMKII are molecular links not only between impaired NMDAR dependent-LTD and fear extinction, but also between impaired LTD and increased anxiety-like behaviors.
4.4. How does increased αCaMKII impair AMPAR internalization and dephosphorylation during NMDAR-dependent LTD?
CaMKII is the major kinase mediating NMDAR-dependent synaptic plasticity and AMPAR trafficking (Collingridge et al. 2004). In mammals, CaMKII has four isoforms, α, β, γ and δ (Colbran and Soderling 1990; Hell 2014), and the α isoform is predominantly expressed in the forebrain (Kennedy et al. 1983). LTD formation requires PPs (PP1, PP2A and PP2B) activation (Kameyama et al., 1998; Lee et al., 1998). Activated PPs dephosphorylate GluA1-Ser845/Ser831 (Hu et al., 2007; Winder and Sweatt 2001; Mansuy and Shenolikar 2006), which cause a reduction in the probability of AMPAR channels being open or their conductance, and finally contribute to LTD formation. Specifically, PP1 is activated through a Ca2+-PP2B-I1 pathway and has a more predominant role in depressing potentiated synapses, whereas PP2A is activated through PP2B/PP1 cascade or pathways independent on PP2B and mainly depresses naive synapses (Winder and Sweatt 2001). However, in αCaMKII-F89G TG mice, αCaMKII overexpression could exhibit higher potency in the competition with PP2B for Ca2+/CaM, which might decrease the accessibility of PP2B to Ca2+/CaM and inhibit the activity of the PP2B-I1-PP1 pathway, thereby inhibiting PP1 activity. In addition, high concentration of phosphorylated αCaMKII could saturate the dephosphorylation ability of PP1, and thereby weaken PP1 dephosphorylating GluA1-Ser845 or GluA1-Ser831 (Lisman and Zhabotinsky 2001; Lee et al., 2000; Hu et al., 2007; Winder and Sweatt 2001; Mansuy and Shenolikar 2006). Unlike PP1, PP2A can be directly inactivated by CaMKII through phosphorylating its B’ α subunits (Fukunaga et al., 2000; Pi and Lisman 2008), therefore, excessive CaMKII can weaken PP2A activity. Collectively, one explanation for impairment of NMDAR-dependent LTD is that the excessive αCaMKII can reduce the activity of PPs, thereby reducing GluA1-Ser845/Ser831 dephosphorylation and AMPAR internalization, and consequently impairing LTD.
It has been shown that stargazin can be dephosphorylated by PP1 through Ca2+-PP2B-I1 pathway and form a ternary complex with APs to promote AMPAR internalization during NMDAR-dependent LTD (Matsuda et al., 2013; Tomita et al., 2003). Conversely, stargazin can be directly phosphorylated by activated CaMKII and bind to PSD-95 to immobilize AMPARs at synapses, which contributes to LTP (Bats et al. 2007; Opazo et al., 2010). In αCaMKII-F89G TG mice, more stargazin is expressed at the synaptic sites during NMDAR-dependent LTD. Therefore, another explanation for impairment of NMDAR-dependent LTD is that excessive CaMKII weakens AMPAR internalization through directly increasing stargazin phosphorylation and indirectly reducing stargazin dephosphorylation caused by lower PP1 activity, which finally impairs LTD.
Funding
This research was supported by National Natural Science Foundation of China (No: 31771177 and 31471077 to Xiaohua Cao, 31970944 to Shuming An) and fund from MOST China-Israel cooperation (No: 2016YFE0130500 to Xiaohua Cao).
CRediT authorship contribution statement
Shuming An: Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Resources, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, Supervision. Jiayue Wang: Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing. Xuliang Zhang: Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing. Yanhong Duan: Formal analysis, Software, Validation. Yiqiong Xu: Formal analysis, Software, Validation. Junyan Lv: Formal analysis, Software, Validation. Dasheng Wang: Formal analysis, Software, Validation. Huan Zhang: Formal analysis, Software, Validation. Gal Richter-Levin: Writing – review & editing. Oded Klavir: Writing – review & editing. Buwei Yu: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, Supervision. Xiaohua Cao: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Xuechu Zhen and Dr. Liyong Li for their assistance with immunoblotting study, Dr. Kevan M. Shokat of UCSF for providing NM-PP1 inhibitor, and Dr. Bo Wang, Dr. Yihui Cui for their comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100359.
Contributor Information
Buwei Yu, Email: yubuwei_2013@126.com.
Xiaohua Cao, Email: xhcao@brain.ecnu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- Ardi Z., Albrecht A., Richter-Levin A., Saha R., Richter-Levin G. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol. Dis. 2016;88:139–147. doi: 10.1016/j.nbd.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Bai Yanrui, Zhou Limin, Wu Xiaoyan, Dong Zhifang. d-Serine enhances fear extinction by increasing GluA2-containing AMPA receptor endocytosis. Behav. Brain Res. 2014;270:223–227. doi: 10.1016/j.bbr.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Barria A., Muller D., Derkach V., Griffith L.C., Soderling T.R. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bats Cecile, Groc Laurent, Choquet Daniel. The interaction between stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bennett M.R., Arnold J., Hatton S.N., Lagopoulos J. Regulation of fear extinction by long-term depression: the roles of endocannabinoids and brain derived neurotrophic factor. Behav. Brain Res. 2017;319:148–164. doi: 10.1016/j.bbr.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney Joanne, Zearfoss N., Richter Joel. Reduced extinction of hippocampal-dependent memories in CPEB knockout mice. Learn. Mem. 2006;13:4–7. doi: 10.1101/lm.73706. [DOI] [PubMed] [Google Scholar]
- Bevilaqua L.R., Bonini J.S., Rossato J.I., Izquierdo L.A., Cammarota M., Izquierdo I. The entorhinal cortex plays a role in extinction. Neurobiol. Learn. Mem. 2006;85:192–197. doi: 10.1016/j.nlm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Borghans B., Homberg J.R. Animal models for posttraumatic stress disorder: an overview of what is used in research. World J. Psychiatr. 2015;5:387–396. doi: 10.5498/wjp.v5.i4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K., Wong T.P., Liu L., Liu Y., Campsall P., Gray S., Phelps L., Phillips A.G., Wang Y.T. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- Burgdorf C.E., Schierberl K.C., Lee A.S., Fischer D.K., Van Kempen T.A., Mudragel V., Huganir R.L., Milner T.A., Glass M.J., Rajadhyaksha A.M. Extinction of contextual cocaine memories requires Ca(v)1.2 within D1R-expressing cells and recruits hippocampal Ca(v)1.2-Dependent signaling mechanisms. J. Neurosci. 2017;37:11894–11911. doi: 10.1523/JNEUROSCI.2397-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Wang H., Mei B., An S., Yin L., Wang L.P., Tsien J.Z. Inducible and selective erasure of memories in the mouse brain via chemical-genetic manipulation. Neuron. 2008;60:353–366. doi: 10.1016/j.neuron.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R.C., Beattie E.C., von Zastrow M., Malenka R.C. Role of AMPA receptor endocytosis in synaptic plasticity. Nat. Rev. Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Castro-Gomes Vitor, Bergstrom Hadley C., McGuire Jennifer L., Parker Clarissa C., Coyner Jennifer, Landeira-Fernandez J., Ursano Robert J., Palmer Abraham A., Johnson Luke R. A dendritic organization of lateral amygdala neurons in fear susceptible and resistant mice. Neurobiol. Learn. Mem. 2016;127:64–71. doi: 10.1016/j.nlm.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Clausen A.N., Clarke E., Phillips R.D., Haswell C., Morey R.A. Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military Veterans. Neuropsychopharmacology. 2020;45:491–498. doi: 10.1038/s41386-019-0539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran R.J., Soderling T.R. Calcium/calmodulin-dependent protein kinase II. Curr. Top. Cell. Regul. 1990;31:181–221. doi: 10.1016/b978-0-12-152831-7.50007-x. [DOI] [PubMed] [Google Scholar]
- Collingridge G.L., Isaac J.T., Wang Y.T. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Cui Y., Jin J., Zhang X., Xu H., Yang L., Du D., Zeng Q., Tsien J.Z., Yu H., Cao X. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton G.L., Wang Y.T., Floresco S.B., Phillips A.G. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. official publication of the American College of Neuropsychopharmacology. [DOI] [PubMed] [Google Scholar]
- Dalton G.L., Wu D.C., Wang Y.T., Floresco S.B., Phillips A.G. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology. 2012;62:797–806. doi: 10.1016/j.neuropharm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dębiec J., Bush D.E., LeDoux J.E. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats--a possible mechanism for the persistence of traumatic memories in PTSD. Depress. Anxiety. 2011;28:186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado Jary Y., Marcelo Coba, Christopher N.G., Anderson, Kimberly R. Thompson, Erin E. Gray, Heusner Carrie L., Kelsey C., Martin, Seth G.N., Grant, Thomas J. O'Dell. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840'. J. Neurosci. 2007;27:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M., da Cruz e Silva E.F., Hall F.L., Williams R.T., Carbonaro-Hall D.A., Nairn A.C., Greengard P., Berndt N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhou S., Ma J., Yin P., Cao X. Forebrain NR2B overexpression enhancing fear acquisition and long-term potentiation in the lateral amygdala. Eur. J. Neurosci. 2015;42:2214–2223. doi: 10.1111/ejn.13008. [DOI] [PubMed] [Google Scholar]
- Erlich Jeffrey C., Bush David E.A., Ledoux Joseph E. The role of the lateral amygdala in the retrieval and maintenance of fear-memories formed by repeated probabilistic reinforcement. Front. Behav. Neurosci. 2012;6 doi: 10.3389/fnbeh.2012.00016. 16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin Amit, Wager Tor. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M.S., Gale G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fenster Robert J., Lauren A.M., Lebois, Kerry J., Ressler, Suh Junghyup. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci. 2018;19:535–551. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster Gina L., Andrew M., Novick, Jamie L., Scholl, Watt Michael J. The Amygdala - A Discrete Multitasking Manager. 2012. The role of the amygdala in anxiety disorders. [Google Scholar]
- Fukunaga Kohji, Muller Dominique, Ohmitsu Masao, Bakó Eva, Anna A., DePaoli-Roach, Miyamoto Eishichi. Decreased protein phosphatase 2A activity in hippocampal long-term potentiation. J. Neurochem. 2000;74:807–817. doi: 10.1046/j.1471-4159.2000.740807.x. [DOI] [PubMed] [Google Scholar]
- Goldberg J., Huang H.B., Kwon Y.G., Greengard P., Nairn A.C., Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995;376:745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- Grosso Anna, Santoni Giulia, Manassero Eugenio, Renna Annamaria, Benedetto Sacchetti A neuronal basis for fear discrimination in the lateral amygdala. Nat. Commun. 2018;9:1214. doi: 10.1038/s41467-018-03682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Lee A., Song L., Kanold P.O., Lee H.K. AMPA receptor subunit GluR1 (GluA1) serine-845 site is involved in synaptic depression but not in spine shrinkage associated with chemical long-term depression. J. Neurophysiol. 2011;105:1897–1907. doi: 10.1152/jn.00913.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J.W. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F., Sevelinges Y., Grosse J., Zanoletti O., Sandi C. Involvement of CRFR1 in the basolateral amygdala in the immediate fear extinction deficit. Eneuro. 2016;3 doi: 10.1523/ENEURO.0084-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Xiao-dong, Huang Qing, Yang Xian, Xia Houhui. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in Hippocampus. J. Neurosci. 2007;27:4674. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.H., Chao H.W., Tsai L.Y., Chung M.H., Huang Y.S. Elevated activation of CaMKIIα in the CPEB3-knockout hippocampus impairs a specific form of NMDAR-dependent synaptic depotentiation. Front. Cell. Neurosci. 2014;8:367. doi: 10.3389/fncel.2014.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine E.E., von Hertzen L.S., Plattner F., Giese K.P. alphaCaMKII autophosphorylation: a fast track to memory. Trends Neurosci. 2006;29:459–465. doi: 10.1016/j.tins.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Jacques Angela, Chaaya Nicholas, Hettiarachchi Chiemi, Carmody Marie-Louise, Beecher Kate, Belmer Arnauld, Chehrehasa Fatemeh, Bartlett Selena, Battle Andrew R., Johnson Luke R. Microtopography of fear memory consolidation and extinction retrieval within prefrontal cortex and amygdala. Psychopharmacology. 2019;236:383–397. doi: 10.1007/s00213-018-5068-4. [DOI] [PubMed] [Google Scholar]
- Ji Mu-Huo, Jia Min, Zhang Ming-Qiang, Liu Wen-Xue, Xie Zhong-Cong, Wang Zhong-Yun, Yang Jian-Jun. Dexmedetomidine alleviates anxiety-like behaviors and cognitive impairments in a rat model of post-traumatic stress disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2014;54:284–288. doi: 10.1016/j.pnpbp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Kameyama K., Lee H.K., Bear M.F., Huganir R.L. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M.B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J. Neurosci. 1983;3:818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee S., Park K., Hong I., Song B., Son G., Park H., Kim W.R., Park E., Choe H.K., Kim H., Lee C., Sun W., Kim K., Shin K.S., Choi S. Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jihye, An Bobae, Kim Jeongyeon, Park Sewon, Park Sungmo, Hong Ingie, Lee Sukwon, Park Kyungjoon, Choi Sukwoo. mGluR2/3 in the lateral amygdala is required for fear extinction: cortical input synapses onto the lateral amygdala as a target site of the mGluR2/3 action. Neuropsychopharmacology. 2015;40:2916–2928. doi: 10.1038/npp.2015.145. official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R., Silva A.J., Ohno M. Autophosphorylation of alphaCaMKII is differentially involved in new learning and unlearning mechanisms of memory extinction. Learn. Mem. 2008;15:837–843. doi: 10.1101/lm.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollen M., Dutar P., Jouvenceau A. The magnitude of hippocampal long term depression depends on the synaptic location of activated NR2-containing N-methyl-D-aspartate receptors. Neuroscience. 2008;154:1308–1317. doi: 10.1016/j.neuroscience.2008.04.045. [DOI] [PubMed] [Google Scholar]
- Krabbe Sabine, Jan Gründemann, Lüthi Andreas. Amygdala inhibitory circuits regulate associative fear conditioning. Biol. Psychiatr. 2018;83:800–809. doi: 10.1016/j.biopsych.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Barbarosie M., Kameyama K., Bear M.F., Huganir R.L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee Hey-Kyoung, Kameyama Kimihiko, Huganir Richard L., Bear Mark F. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in Hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Lee Sukwon, Song Beomjong, Kim Jeongyeon, Park Kyungjoon, Hong Ingie, An Bobae, Song Sangho, Lee Jiwon, Park Sungmo, Kim Jihye, Park Dongeun, Justin Lee C., Kim Kyungjin, Shin Ki Soon, Tsien Richard W., Choi Sukwoo. GluA1 phosphorylation at serine 831 in the lateral amygdala is required for fear renewal. Nat. Neurosci. 2013;16:1436. doi: 10.1038/nn.3491. [DOI] [PubMed] [Google Scholar]
- Li Fu, Xiang Haitao, Lu Jiashu, Chen Zhuo, Huang Chao, Yuan Xiaomei. Lycopene ameliorates PTSD-like behaviors in mice and rebalances the neuroinflammatory response and oxidative stress in the brain. Physiol. Behav. 2020;224:113026. doi: 10.1016/j.physbeh.2020.113026. [DOI] [PubMed] [Google Scholar]
- Lin H.C., Mao S.C., Su C.L., Gean P.W. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int. J. Neuropsychopharmacol. 2010;13:335–345. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- Lisman J.E., Zhabotinsky A.M. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lisman J., Schulman H., Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Ma J., Duan Y., Qin Z., Wang J., Liu W., Xu M., Zhou S., Cao X. Overexpression of αCaMKII impairs behavioral flexibility and NMDAR-dependent long-term depression in the medial prefrontal cortex. Neuroscience. 2015;310:528–540. doi: 10.1016/j.neuroscience.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Ma T.F., Zhou L., Wang Y., Qin S.J., Zhang Y., Hu B., Yan J.Z., Ma X., Zhou C.H., Gu S.L. A selective M1 and M3 receptor antagonist, penehyclidine hydrochloride, prevents postischemic LTP: involvement of NMDA receptors. Synapse. 2013;67:865–874. doi: 10.1002/syn.21693. [DOI] [PubMed] [Google Scholar]
- Mahan Amy L., Ressler Kerry J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy Isabelle M., Shenolikar Shirish. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Matsuda Shinji, Kakegawa Wataru, Budisantoso Timotheus, Nomura Toshihiro, Kohda Kazuhisa, Yuzaki Michisuke. Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long-term depression. Nat. Commun. 2013;4:2759. doi: 10.1038/ncomms3759. [DOI] [PubMed] [Google Scholar]
- Mauna Jocelyn C., Miyamae Takeaki, Benjamin Pulli, Thiels Edda. Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo. Hippocampus. 2011;21:1093–1104. doi: 10.1002/hipo.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos Vasiliki, Rothbaum Alex O., Jovanovic Tanja, Almli Lynn M., Bradley Bekh, Rothbaum Barbara O., Gillespie Charles F., Ressler Kerry J. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatr. 2014;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63(63):129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad Mohammed R., Quirk Gregory J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2011;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Chen, Shao Da, Fu Yali, Ma Qianqian, Cui Dongyang, Song Jiaojiao, Sheng Huan, Yang Li, Dong Yi, Lai Bin, Zheng Ping. Key determinants for morphine withdrawal conditioned context-induced increase in Arc expression in anterior cingulate cortex and withdrawal memory retrieval. Exp. Neurol. 2018;311 doi: 10.1016/j.expneurol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Morey Rajendra A., Emily K., Clarke, Courtney C., Haswell Rachel D., Phillips, Ashley N., Clausen, Mary S. Mufford, Saygin Zeynep, Brancu Mira, Beckham Jean C., Calhoun Patrick S., Eric Dedert, Elbogen Eric B., Fairbank John A., Hurley Robin A., Kilts Jason D., Kimbrel Nathan A., Kirby Angela, Christine E., Marx Scott D., McDonald Scott D., Moore Jennifer C., Naylor, Rowland Jared, Swinkels Cindy, Szabo Steven T., Taber Katherine H., Tupler Larry A., van Voorhees Elizabeth E., Yoash-Gantz Ruth E., Ryan Wagner H., LaBar Kevin S. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol. Psychiatr.: Cognit. Neurosci. Neuroimag. 2020;5:281–290. doi: 10.1016/j.bpsc.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Tanaka Y., Cho H.S., Yoshihara M., Hirokawa N. The molecular motor KIF21B Mediates synaptic plasticity and fear extinction by terminating Rac1 activation. Cell Rep. 2018;23:3864–3877. doi: 10.1016/j.celrep.2018.05.089. [DOI] [PubMed] [Google Scholar]
- Nabavi S., Fox R., Proulx C.D., Lin J.Y., Tsien R.Y., Malinow R. 'Engineering a memory with LTD and LTP'. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K., Majidishad P., Amorapanth P., LeDoux J.E. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn. Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo Patricio, Labrecque Simon, Tigaret Cezar M., Arnaud Frouin, Wiseman Paul W., De Koninck Paul, Choquet Daniel. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Ousdal Olga Therese, Anne Marita Milde, Gertrud Sofie Hafstad, Hodneland Erlend, Dyb Grete, Craven Alexander R., Melinder Annika, Endestad Tor, Hugdahl Kenneth. The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Transl. Psychiatry. 2020;10:288. doi: 10.1038/s41398-020-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H.J., Otmakhov N., Lemelin D., De Koninck P., Lisman J. Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J. Neurosci. 2010;30:8704–8709. doi: 10.1523/JNEUROSCI.0133-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi Hyun Jae, Lisman John E. Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression. J. Neurosci. 2008;28:13132. doi: 10.1523/JNEUROSCI.2348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J., Ren L.Y., Gao C. N-Methyl D-aspartate receptor subunit signaling in fear extinction. Psychopharmacology (Berlin) 2019;236:239–250. doi: 10.1007/s00213-018-5022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Règue Mathilde, Poilbout Corinne, Martin Vincent, Franc Bernard, Lanfumey Laurence, Raymond Mongeau. Increased 5-HT2C receptor editing predisposes to PTSD-like behaviors and alters BDNF and cytokines signaling. Transl. Psychiatry. 2019;9:100. doi: 10.1038/s41398-019-0431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler Kerry J. Amygdala activity, fear, and anxiety: modulation by stress. Biol. Psychiatr. 2010;67:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich M.T., Abbott T.B., Chung L., Gulcicek E.E., Stone K.L., Colangelo C.M., Lam T.T., Nairn A.C., Taylor J.R., Torregrossa M.M. Phosphoproteomic analysis reveals a novel mechanism of CaMKIIα regulation inversely induced by cocaine memory extinction versus reconsolidation. J. Neurosci. 2016;36:7613–7627. doi: 10.1523/JNEUROSCI.1108-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov G., Boltyansky B., Richter-Levin G. A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol. Psychiatr. 2016;21:630–641. doi: 10.1038/mp.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov Gilad, Richter-Levin Gal. Water associated zero maze: a novel rat test for long term traumatic re-experiencing. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00001. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe Glenn E., Doyère Valérie, LeDoux Joseph E. Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J. Neurosci. 2005;25:10010. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G., Vianna M.R., McGaugh J.L., Medina J.H., Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Talukdar G., Inoue R., Yoshida T., Mori H. Impairment in extinction of cued fear memory in syntenin-1 knockout mice. Neurobiol. Learn. Mem. 2018;149:58–67. doi: 10.1016/j.nlm.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Tomita Susumu, Chen Lu, Kawasaki Yoshimi, Petralia Ronald S., Wenthold Robert J., Nicoll Roger A., Bredt David S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Shimizu E., Tang Y.P., Cho M., Kyin M., Zuo W., Robinson D.A., Alaimo P.J., Zhang C., Morimoto H., Zhuo M., Feng R., Shokat K.M., Tsien J.Z. 'Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder Danny G., Sweatt J. David. Roles of serine/threonine phosphatases in hippocampel synaptic plasticity. Nat. Rev. Neurosci. 2001;2:461. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Wu Xin, Zhang Jie-Ting, Li Di, Zhou Jun, Yang Jun, Zheng Hui-Ling, Chen Jian-Guo, Wang Fang. Aquaporin-4 deficiency facilitates fear memory extinction in the hippocampus through excessive activation of extrasynaptic GluN2B-containing NMDA receptors. Neuropharmacology. 2017;112:124–134. doi: 10.1016/j.neuropharm.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Xu Zhi-Xiang, Tan Ji-Wei, Xu Haifei, Hill Cassandra J., Ostrovskaya Olga, Martemyanov Kirill A., Xu Baoji. Caspase-2 promotes AMPA receptor internalization and cognitive flexibility via mTORC2-AKT-GSK3β signaling. Nat. Commun. 2019;10:3622. doi: 10.1038/s41467-019-11575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W.J., Tan Y.C., Xu J.C., Tang X.P., Zhang C., Zhang P.B., Ren Z.Q. Protective effects of silibinin and its possible mechanism of action in mice exposed to chronic unpredictable mild stress. Biomol. Therapeut. 2015;23:245–250. doi: 10.4062/biomolther.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda Rachel, Hoge Charles W., McFarlane Alexander C., Vermetten Eric, Lanius Ruth A., Nievergelt Caroline M., Hobfoll Stevan E., Koenen Karestan C., Neylan Thomas C., Hyman Steven E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Yin D.M., Chen Y.J., Lu Y.S., Bean J.C., Sathyamurthy A., Shen C., Liu X., Lin T.W., Smith C.A., Xiong W.C., Mei L. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78:644–657. doi: 10.1016/j.neuron.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Pengcheng, Xu Hao, Wang Qi, Wang Jiayue, Yin Liang, Xu Meichen, Xie Zhenyang, Liu Wenzhao, Cao Xiaohua. Overexpression of βCaMKII impairs behavioral flexibility and NMDAR-dependent long-term depression in the dentate gyrus. Neuropharmacology. 2017;116:270–287. doi: 10.1016/j.neuropharm.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen T., Chen P., Zhang B., Hong J., Chen L. MPTP-Induced dopamine depletion in basolateral amygdala via decrease of D2R activation suppresses GABA(A) receptors expression and LTD induction leading to anxiety-like behaviors. Front. Mol. Neurosci. 2017;10:247. doi: 10.3389/fnmol.2017.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.