Abstract
Background:
The feasibility of measuring β-hydroxybutyrate in ISF using a continuous ketone monitoring (CKM) sensor using a single calibration without further adjustments over 14 days is described.
Methods:
A CKM sensor was developed using wired enzyme technology with β-hydroxybutyrate dehydrogenase chemistry. In vitro characterization of the sensor was performed in phosphate buffered saline at 37°C. In vivo performance was evaluated in 12 healthy participants on low carbohydrate diets, who wore 3 ketone sensors on the back of their upper arms to continuously measure ketone levels over 14 days. Reference capillary ketone measurements were performed using Precision Xtra® test strips at least 8 times a day.
Results:
The sensor is stable over 14 days and has a linear response over the 0-8 mM range. The operational stability of the sensor is very good with a 2.1% signal change over 14 days. The first human study of the CKM sensor demonstrated that the sensor can continuously track ketones well through the entire 14 days of wear. The performance with a single retrospective calibration of the sensor showed 82.4% of data pairs within 0.225 mM/20% and 91.4% within 0.3 mM/30% of the capillary ketone reference (presented as mM at <1.5 mM and as percentage at or above 1.5 mM). This suggests that the sensor can be used with a single calibration for the 14 days of use.
Conclusions:
Measuring ketones in ISF using a continuous ketone sensor is feasible. Additional studies are required to evaluate the performance in intended patient populations, including conditions of ketosis and diabetic ketoacidosis.
Keywords: continuous ketone monitoring, β-hydroxybutyrate, diabetes ketoacidosis, factory calibration, ketogenic
Introduction
When cells do not receive sufficient glucose, that they need for typical energy production, the body begins to burn fat and utilize the breakdown products of fat to generate an alternate energy source, ketone bodies (ketones).1 The production of ketones as a source of fuel can be both physiologic such as in the case of fasting or low-carbohydrate (ketogenic) diets or can be detrimental such as in the case of diabetic ketoacidosis.
In individuals who are on ketogenic diets, where the carbohydrate intake is drastically reduced and replaced with fat, the body uses ketones instead of glucose for energy.2 A significant reduction in carbohydrates puts the body in a metabolic state called ketosis.3 Ketogenic diets have been used for a variety of reasons in medicine, including the management of pediatric epilepsy as well as weight loss.4,5 In patients with type 2 diabetes (T2D), nutritional ketosis is associated with sustained improvement in the atherogenic lipid and lipoprotein profile.6,7
Most cells require insulin to shuttle glucose into the cell. When the body has insufficient insulin, an intracellular shortage of glucose occurs, forcing the body to produce ketones for fuel. However, if the ketones build up in blood, faster than they can be metabolized, then the body becomes acidic resulting in diabetic ketoacidosis (DKA).8 Ketoacidosis can occur in T2D rarely, but is a significant risk in those people living with type 1 diabetes (T1D). In people with diabetes (PWD) managed with insulin pumps with about 3% of those between 13 and 49 years have experienced at least 1 episode of DKA in the previous 3 months.9 Following the introduction of SGLT2 (Sodium-Glucose co-Transporter-2) inhibitor therapy for T2D and off-label use in T1D, incidents of euglycemic diabetic ketoacidosis (EDKA) have been reported.10
Ketones produced during ketosis or ketoacidosis are water soluble ketones, namely, acetoacetate, β-hydroxybutyrate (BHB), and the spontaneous breakdown product of acetoacetate, which is acetone. In a setting of acidosis, acetoacetate is converted into BHB. With resolution of the ketoacidosis, BHB levels fall, with an increase in the conversion of BHB to acetoacetate that remains detectable in the urine.11 Therefore, BHB is the primary ketone body that indicates the onset and resolution of a ketoacidosis and levels are commonly sampled over time in the critical care setting to help guide therapy.8
Currently, measurement of ketone levels is most frequently performed using urine or blood ketone test strips.12 Assays using blood are based on BHB utilizing nicotinamide adenine dinucleotide (NAD+) dependent β-hydroxybutyrate dehydrogenase (β-HBDH) enzyme.13 These measurements do not indicate the onset of ketosis or ketoacidosis, but rather confirm if ketosis or ketoacidosis is already in progress and may require medical attention. In real world clinical care, patients seldom check their ketone levels as recommended by the health care professional because of the lack of availability of the in-date strips at the time of need14 and the fingerstick blood testing requires a conscious action by the patient. Under multiple scenarios, such as failure of insulin delivery, sick days, low carbohydrate intake, use of SGLT2 inhibitors, high risk patients such as people with T1D, or recurring DKA, and so on, patients would benefit from a continuous ketone sensor. Further, continuous monitoring such as how glucose is measured, has been shown to provide real-time behavior feedback to help guide treatment decisions.15 Measurement of analytes beyond glucose may provide additional information for self-management decisions, potentially reducing the incidence and severity of DKA and perhaps serving as a tool to inform those following a ketogenic diet.
Lee et al. suggest that a multianalyte system incorporating a ketone sensor could provide an additional input to a closed loop system’s safety algorithm, warning of impending DKA to allow appropriate action to be taken.16 Attempts have been made to measure ketones continuously using a spectroscopy-based approach and have demonstrated that this concept works in an animal model.17 A microneedle-based continuous ketone sensor based on β-HBDH was demonstrated in a skin mimicking model.18
While it is feasible to build a continuous ketone monitoring (CKM) sensor utilizing the well understood glucose sensor process, the question remains whether a subcutaneous CKM sensor could accurately track changes in blood ketone levels over multiple days, specifically because the coenzyme (NAD+) of β-HBDH is a free molecule and difficult to immobilize. In this report, we demonstrate the feasibility of a subcutaneous CKM sensor, it’s in vitro performance and in vivo performance over 14 days with a single calibration.
Materials and Methods
Sensor System
The sensor used in this study has a structure and dimensions similar to the glucose sensor described previously,19 but it has been modified for ketone chemistry. It consists of a 3-electrode system, where the sensing electrode is poised at 40 mV against a silver-silver chloride reference electrode. NAD+ dependent β-HBDH and a proprietary redox mediation chemistry containing osmium complex are deposited on the sensing layer to allow for selective oxidation of BHB and transport of electrons from the enzyme to the electrode using the wired enzyme technology. An outer membrane limits the BHB flux to the sensor and forms the interface to the tissue. Proprietary manufacturing methods were used to control the area of the sensing layer on the working electrode and to control the thickness of the BHB limiting membrane.
The sensor system consists of a sensor and an applicator. The applicator used in the study is identical to that of FreeStyle Libre® applicator.20 The sensor measures BHB in the subcutaneous tissue and stores the ketone signal in its memory, which is transferred to a receiver. The data from the receiver were uploaded to a computer using a proprietary software for data processing. A single lot of ketone sensors was used for these in vitro and in vivo studies. The sensors were sterilized, and a negative endotoxin test was confirmed before releasing the sensor for in vivo studies.
In vitro Studies
The in vitro sensor sensitivity was measured by submerging 16 sensors in a solution of 100 mM phosphate buffer at a controlled temperature of 37°C. Aliquots of 1 M ketone were sequentially injected into the solution to achieve the ketone concentrations: 1, 2, 3, 4, 6, and 8 mmol/L in the solution. The current from each sensor was monitored with a proprietary potentiostat, and sensor sensitivity was quantified by the slope of a least square regression through the current versus ketone concentration. This sensitivity was used to generate calibrated sensor response for all in vitro studies. The response time of the sensor was calculated as the time required for the sensor response to change from 10% above baseline and to 90% of the plateau for each aliquot addition.
The stability of the sensor over the intended wear period was assessed under simulated conditions where 16 sensors were submerged in phosphate buffer with 8 mM of ketone at 37°C for 14 days. The stability of the sensor response was measured by measuring the drift in the sensor response over the test period.
The interference profile is expected to be similar to that of the FreeStyle Libre sensor since the mediator chemistry is identical to that of the FreeStyle Libre sensor, which is shown to have interference from ascorbic acid at high concentrations.20 To assess the interference from ascorbic acid, 10 sensors were tested under in vitro conditions in phosphate buffer at 37°C. The sensors were tested at 0.6 mM and 1.5 mM of ketone in solution. After the sensor signal was stabilized, ascorbic acid was introduced to achieve an ascorbic acid concentration of 2 mg/dL that corresponds to a peak plasma concentration after ingesting 1000 mg of ascorbic acid.21,22 The change in sensor response after the addition of ascorbic acid was measured.
In vivo Studies
In vivo performance of the CKM was performed at a single clinical site in the United States (Sansum Diabetes Research Institute, Santa Barbara, California). The study enrolled a total of 12 volunteers to participate in the study. Study participants were required to be on a low carbohydrate diet (ketogenic diet) and willing to remain on that diet throughout the study. Study staff applied 2 sensors onto the back of both upper arms for each of the study participants (4 sensors per participant), who wore the sensors for up to 14 days. Three of these sensors were functional CKM sensors and one of the sensors was a non-functional sensor whose sensing chemistry did not contain β- HBDH enzyme to measure in vivo background current. The sensors were activated using the reader and sensors started measuring the signal 60 minutes after activation. All sensor results were masked to the study participants. The study participants were required to perform 8 daily fingerstick measurements using Precision Xtra ketone test strips, over the waking period, preferably upon waking, before each meal, an hour after each meal and at bedtime. The sensors stored the data for up to 14 days and were transferred to the reader by the study staff at the end of wear.
Data analysis
A background current signal model was developed using the data from the non-functional sensors from all study participants. Signals from each of the functional sensors were first corrected with this background signal. A retrospective calibration for each sensor was derived by correlating the sensor current to the reference values. A sensitivity value was determined for each capillary ketone measurement as a ratio of the sensor current (corrected for temperature) to the capillary ketone value, that is, sensitivity = current/capillary ketone concentration. The sensitivity assigned to each sensor was the median of the individual sensitivity measurements for that sensor. To simulate no calibration by the user, no further adjustments were made to evaluate the accuracy over 14 days.
The accuracy assessments were conducted by comparing the sensor ketone results from each of the active sensors worn by the study participants to the capillary ketone reference results obtained by the Precision Xtra ketone test strips. At concentrations below 1.5 mM, the accuracy against the reference was calculated as mM, and at or above 1.5 mM, it was calculated as percentage. A split-level rule was applied consistent with glucose (test strips and CGMs) and on-market ketone test strips. A concentration of 1.5 mM was used for split-level as it marks a high-risk ketoacidosis.23 The data presentation may need to be different depending on whether the intended use of the device is for medical indications or as a diet management product.
Results
The calibrated sensor response generated by the sensors at 37°C with sequential addition of ketone aliquots is presented in Figure 1. The average coefficient of variation of the sensor response across the ketone levels is 5.0%. The sensor responded to the change in the ketone concentration within 4 minutes (average response time is 228 seconds) of adding the ketone aliquots to the test solution. The sensors show a linear response against the ketone concentration (Figure 2) with a R2 = 0.9994.
Figure 1.
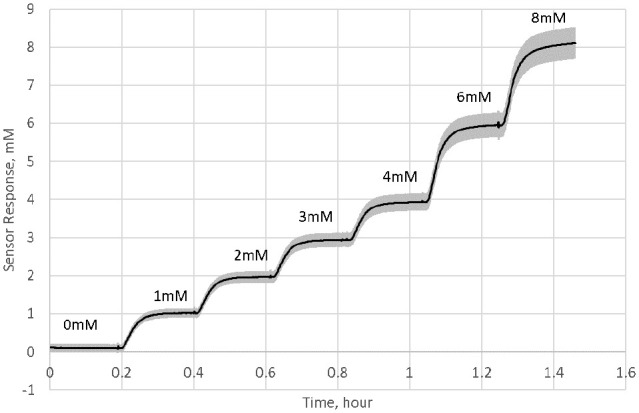
Change in the sensor response with sequential addition of ketone aliquots (solid line is the mean and shaded area is 1 standard deviation of the data from 16 sensors). Sensors were immersed in PBS at 37°C while aliquots of 1 M ketone were added to produce step responses: 1, 2, 3, 4, 6, and 8 mM.
Figure 2.
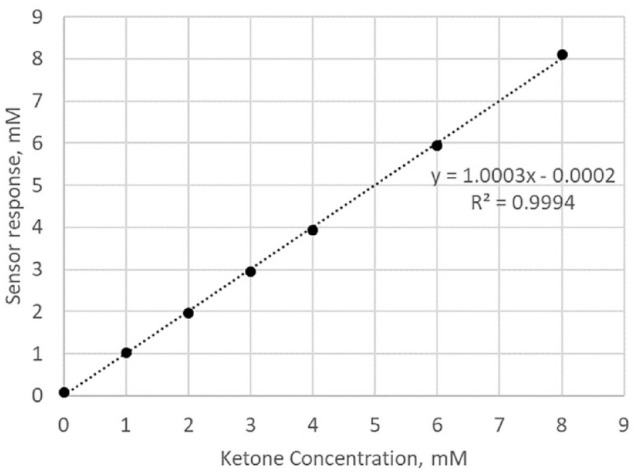
Plot of calibrated sensor response as a function of ketone concentration.
The operational stability of the sensor under in vitro conditions is presented in Figure 3. The sensor signal at 8 mM was stable over the 14 days with an average daily signal loss of 0.15% (total signal loss over 14 days is 2.1%). The interference from ascorbic acid when exposed to an ascorbic acid concentration of 2 mg/dL suggests that the sensor signal may change by no more than 0.2 mmol/L equivalent. This interference is independent of the concentration of ketone.
Figure 3.
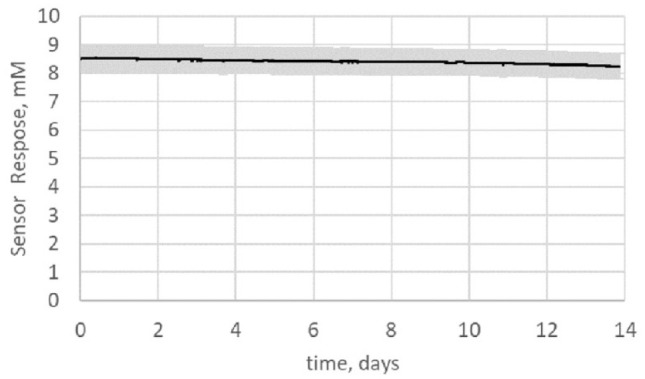
Change in sensor response (solid line is the mean and shaded area is 1 standard deviation of the data from 16 sensors) at 8 mM ketone held at 37°C for 14 days.
The clinical study included 11 female and 1 male participants with an average age of 32.3 (range: 20-51) years. One of the participants had T1D and the remaining participants were not PWD. None of the participants were taking SGLT2 inhibitor. One of the participants was of Hispanic race while all other participants identified themselves as white. Average BMI was 24.3 (range: 18.6-30) kg/m2 with 7 of the 12 participants having a BMI of <25 kg/m2. All participants self-reported as practicing a low carbohydrate diet and none had DKA.
Out of 36 ketone sensors and 12 background sensors tested in the study, 31 ketone sensors and 11 background sensors had evaluable data. The other 5 ketone sensors and 1 background sensor failed to collect any valid data, due to device issues, and were therefore excluded from data analysis. No other data were excluded from the analysis. There were no adverse events reported.
Figure 4 presents the response of the 3 functional sensors over the 14 days to ketone levels in the body for one of the study participants. All 3 sensors accurately track the capillary ketone reference through the entire 14 days of wear.
Figure 4.
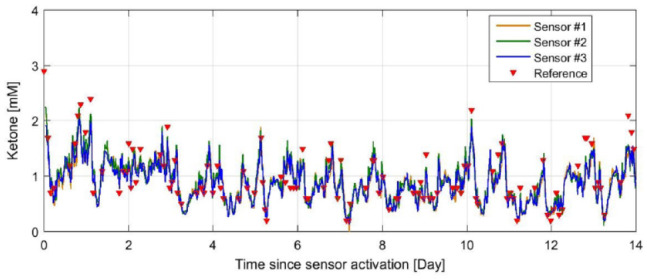
Response of 3 sensors simultaneously worn by 1 subject with changing concentration of ketone in the body over the 14 days (336 hours). The reference ketone measurements are overlaid with the sensor responses.
A total of 3132 paired datapoints were collected from the study from the 12 study participants over the 14 days. The reference measurement ranged from 0-5.1 mM, with a median value of 0.6 mM. Figure 5 shows the correlation between sensor ketone results and reference values based on a retrospective sensor calibration. The accuracy and bias results are summarized in Table 1. For reference ketone concentrations <1.5 mM, the overall MAD (mean absolute difference) is 0.129 mM with 83.4% of sensor results within ± 0.225 mM and 91.7% of the sensor results within ± 0.3 mM of the reference. For reference ketone concentrations ≥1.5 mM, the overall MARD (mean absolute relative difference) is 14.4% with 76.0% of the sensor results within 20% and 89.7% of the results within 30% of the reference. For the full range of concentrations, the values are 82.4% within 0.225 mM/20% and 91.4% within 0.3 mM/30% of the reference.
Figure 5.
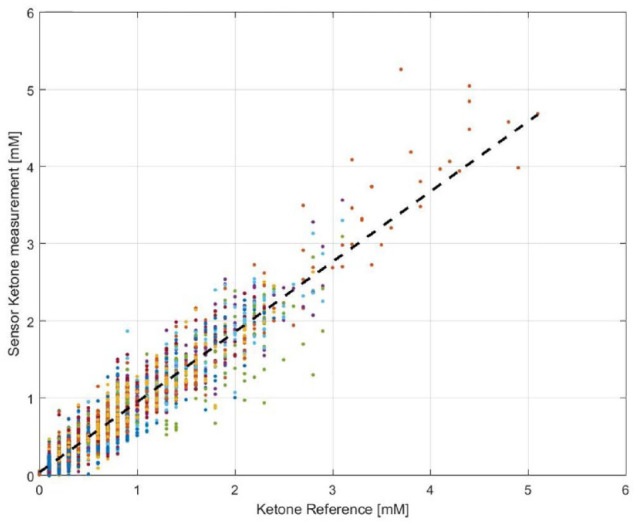
Plot of ISF ketone values measured by the sensors against capillary ketone strip reference measurements. Number of Paired data points is 3132.
Table 1.
Accuracy of the ISF Ketone Sensor Results Compared to the Capillary Ketone Strip Reference. Accuracy and Bias Results for Ketone Concentrations <1.5 mM are in mM.
| Concentration range | Percentage within 0.225 mM/20% | Percentage within 0.3 mM/30% | MAD/MARD Mean | Between subject SD/%CV | Within subject, SD/%CV | Number of paired data points |
|---|---|---|---|---|---|---|
| <1.5 mM | 83.4% | 91.7% | 0.129 mM | 0.051 mM | 0.029 mM | 2724 |
| >=1.5 mM | 76.0% | 89.7% | 14.4% | 5.40% | 7.46% | 408 |
| Combined | 82.4% | 91.4% | 3132 |
SD is the standard deviation and CV is the coefficient of variation.
Discussion
Measuring urine or blood ketone levels using a strip-based technology has its limitations.16 It provides episodic information that confirms an already ongoing ketosis or DKA event. Early identification of production of ketones may warn of impending ketoacidosis that could reduce the complications of DKA and perhaps even prevent it. Real-time continuous ketone monitoring could also help clinicians manage ketoacidosis. For individuals who are on low carbohydrate diets, the sensor may serve as a tool to monitor the effectiveness of their diet and indicate the effect of diet or exercise on the ketone levels.
Blood ketone test strips typically have a measurement range of 0-8 mM. The appropriate measurement range for a ketone sensor is to be determined based on its use scenario. The in vitro performance of the sensor has been demonstrated up to 8 mM, showing that the sensor responds linearly to the change in the concentration of ketone and with minimal variation between sensors. In an in vitro setting, the sensor responds to changes to the ketone concentration quickly, with a response time of less than 4 minutes across a measurement range.
Unlike glucose, the baseline ketone levels are typically very low and calibrating the sensor at these concentration levels may not be effective. Therefore, operational stability is critical for a ketone sensor. Achieving operational stability for over 14 days for a NAD+ dependent chemistry is even more challenging as NAD+ is a free molecule, difficult to be retained in the sensing chemistry. Figure 3 shows that the operational stability of the sensor is very good with 2.1% signal change over 14 days.
The first human study of these ketone sensors demonstrated that ISF ketone concentrations in the subcutaneous ketone can be measured with these sensors, which tracks the capillary ketone levels over a 14-day period. Typically, there is a difference between in vivo and in vitro sensitivity of the sensor because of differences in matrices, requiring calibration of the sensor for addressing this difference. A single retrospective calibration was used for the assessment of the accuracy of the sensor with no further adjustments to the calibration over the wear period. From Figures 3 and 4, it can be determined that the sensor is stable over the 14-day period.
The data from the clinical study showed that 82.4% of sensor values were within 0.225 mM/20% and 91.4% within 0.3 mM/30% of the reference. The ketone concentration range generated by our study participants from a low carbohydrate diet was limited. Studies are required to evaluate the performance of the sensor across a wider range and including patients with DKA in hospitalized patients, specifically a critical care setting, such as emergency rooms, intensive care units, operation rooms, and so on.
The accuracy of any CKM sensors needs to be established against an independent plasma ketone assay. For self-monitoring of glucose using a blood glucose meter or a continuous glucose monitor (CGM) sensor, there are performance standards and/or expectations.24-26 Currently, such performance standards do not exist for a ketone sensor. Additional data from intended use population are required to develop appropriate performance specifications for the clinical utility of the CKM sensor.
CGM sensors are either factory calibrated or calibrated by the user at the time of use to achieve the required accuracy. Calibration of the CKM sensor by the user at the time of use is not feasible since the baseline ketone level is typically very low (<0.6 mM) which renders in vivo finger stick calibration impractical and challenging. Therefore, a prospective factory calibration is essential for a CKM sensor for real time monitoring of ketones. In order to establish a factory calibration, 4 features should be present, (a) the sensor manufacturing process should have minimal variation between sensors, (b) sensors should be stable when stored, (c) sensor sensitivity should be stable during wear, and (d) sensors should demonstrate minimal variability within and between patients.27 Once the expected accuracy performance requirements for CKM are determined, which may be dependent on the intended use, appropriate specifications to these features can be set. The feasibility of sensors with a low between sensor variability (5%) that are stable over the wear period has been demonstrated. However, characterization of storage stability and within and between person sensitivity differences under conditions of ketosis and ketoacidosis is required to determine whether a prospective factory calibration is achievable.
The medical or clinical utility of a CKM will require considerations for most relevant data interface, such as presentation of the trend in ketone levels, which offers opportunity to use CKM technology to identify severity of DKA and when a medical intervention is required. In some applications, a stand-alone CKM may be adequate while for some others, the user may benefit from integration of the ketone functionality with other analytes such as glucose. This requires coexistence of 2 sensors or 2 chemistries in 1 sensor, and such a configuration should consider the dimensions of the resulting sensor system and its delivery to the subcutaneous tissue. CKM technology for use in DKA may require clinical studies targeting enrollment in this disease state. Additional work is required to address these needs. Nonetheless, this article represents a significant milestone in sensing technology, because we have shown that an inserted sensor can feasibly measure analytes other than glucose in ISF.
Conclusions
Expansion of sensing technology to analytes other than glucose will allow medical and lifestyle management of additional analytes of interest. Demonstration of feasibility of a ketone sensor is a significant step toward that goal. However, more work is necessary to evaluate the performance against independent plasma reference as well as demonstrate that these sensors will work effectively in conditions of diabetic ketoacidosis and in a wider dynamic range. A prospective calibrated system is required for real time monitoring of ketones. However, this first human study suggests that a continuous ketone sensor similar to continuous glucose sensors is achievable.
Acknowledgments
The authors acknowledge the study participants and the research staff at the study site.
Footnotes
Abbreviations: BHB, β-hydroxybutyrate; BMI, body mass index; CGM, continuous glucose monitoring; CKM, continuous ketone monitoring; DKA, diabetes ketoacidosis; EDKA, euglycemic diabetic ketoacidosis; β-HBDH, β-hydroxybutyrate dehydrogenase; ISF, interstitial fluid; MAD, mean absolute difference; MARD, mean absolute relative difference; NAD+, nicotinamide adenine dinucleotide; PWD, people with diabetes; SGLT2, sodium-glucose co-transporter-2; T1D, type 1 diabetes; T2D, type 2 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SA, HC, and JO are employees of Abbott Diabetes Care. KC conducts research sponsored by Medtronic, Dexcom, Abbott, Insulet, and Novo Nordisk, and has been a speaker for Dexcom and Abbott.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Abbott Diabetes Care.
ORCID iD: Shridhara Alva  https://orcid.org/0000-0003-3596-2034
https://orcid.org/0000-0003-3596-2034
References
- 1. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes., Diabetes Metab Res Rev. 1999;15(6):412-426. doi: 10.1002/(sici)1520-7560(199911/12)15:6 <412::aid-dmrr72>3.0.co;2-8. PMID: 10634967 [DOI] [PubMed] [Google Scholar]
- 2. Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship Front Psychol. 2015;6:1-9. doi: 10.3389/fpsyg.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76:65-70. [DOI] [PubMed] [Google Scholar]
- 4. McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28-35. doi: 10.1111/j.1471-4159.2012.07670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health. 2014;11:2092-2107. doi: 10.3390/ijerph110202092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Athinarayanan SJ, Hallberg SJ, McKenzie AL, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19: 208. doi: 10.1186/s12933-020-01178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westman EC, Yancy WS, Mavropoulos JC, et al. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. 2008;5:36. doi: 10.1186/1743-7075-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamel KS, Hlalperin ML. Acid-base problems in diabetic ketoacidosis. N Engl J Med. 2015;372:546-554. doi: 10.1056/NEJMra1207788 [DOI] [PubMed] [Google Scholar]
- 9. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 10. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schade DS, Eaton RP. Metabolic and clinical significance of ketosis. Spec Top Endocrinol Metab. 1982;4:1-27. [PubMed] [Google Scholar]
- 12. Laffel LMB, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2005; 23:278-284. [DOI] [PubMed] [Google Scholar]
- 13. Wallace TM, Meston NM, Gardner SG, Matthews DR. The hospital and home use of a 30-second hand-held blood ketone meter: guidelines for clinical practice. Diabet Med. 2001;18:640-645. [DOI] [PubMed] [Google Scholar]
- 14. Larsson CR, Januszewski AS, McGrath RT, et al. Suboptimal behaviour and knowledge regarding overnight glycaemia in adults with type 1 diabetes is common. Intern Med J. 2018;48:1080-1086. doi: 10.1111/imj.13798 [DOI] [PubMed] [Google Scholar]
- 15. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020; 43(5): 1146-1156. [DOI] [PubMed] [Google Scholar]
- 16. Lee MH, Paldus B, Krishnamurthy B, et al. The clinical case for the integration of a ketone sensor as part of a closed loop insulin pump system. J Diabetes Sci Technol. 2019;13:967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stocker D, Vlaminck L, Schauvliege S, Delbeke D. Continuous real-time ketone sensing in subcutaneous tissue with combined glucose/ketone sensor: proof-of-concept in pig model. Diabetes Technol Ther. 2020;122:A46-A47. [Google Scholar]
- 18. Teymourian H, Moonla C, Tehrani F, et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal Chem. 2020;92:2291-2300. [DOI] [PubMed] [Google Scholar]
- 19. Hoss U, Budiman ES, Liu H, Christiansen MP. Continuous glucose monitoring in the subcutaneous tissue over a 14-day sensor wear period,. J Diabetes Sci Technol. 2013;7(5):1210-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. FreeStyle Libre 2 user’s manual. accessed January 16, 2021. https://freestyleserver.com/Payloads/IFU/2020/q2/ART40703-001_rev-D-Web.pdf
- 21. CLSI. Supplemental Tables for Interference Testing in Clinical Chemistry, EP37. 1st ed. Clinical Laboratory Standards Institute; April 2018. [Google Scholar]
- 22. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rewers A. Current controversies in treatment and prevention of diabetes ketoacidosis. Adv Pediatr. 2010:57:247-267. [DOI] [PubMed] [Google Scholar]
- 24. ISO. In vitro Diagnostic Test Systems – Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus, ISO 15197. Geneva, Switzerland: International Organization of Standards; 2013. [Google Scholar]
- 25. FDA. Self-monitoring blood glucose test systems for over-the-counter use. Accessed January 16, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use.
- 26. CLSI. Performance Metrics for Continuous Interstitial Glucose Monitoring, POCT05. 2nd ed. Nov 2020. [Google Scholar]
- 27. Hoss U, Jeddi I, Schulz M, Budiman E, Bhogal C, McGrraugh G. Continuous glucose monitoring in subcutaneous tissue using factory-calibrated sensors: a pilot study. Diabetes Technol Ther. 2010;12:591-597. [DOI] [PubMed] [Google Scholar]


