Abstract
We describe an emergency department (ED)-based, Latino patient focused, unblinded, randomized controlled trial to empirically test if automated bilingual computerized alcohol screening and brief intervention (AB-CASI), a digital health tool, is superior to standard care (SC) on measures of alcohol consumption, alcohol-related negative behaviors and consequences, and 30-day treatment engagement. The trial design addresses the full spectrum of unhealthy drinking from high-risk drinking to severe alcohol use disorder (AUD). In an effort to surmount known ED-based alcohol screening, brief intervention, and referral to treatment process barriers, while addressing racial/ethnic alcohol-related health disparities among Latino groups, this trial will purposively use a digital health tool and seek enrollment of English and/or Spanish speaking self-identified adult Latino ED patients. Participants will be randomized (1:1) to AB-CASI or SC, stratified by AUD severity and preferred language (English vs. Spanish). The primary outcome will be the number of binge drinking days assessed using the 28-day timeline followback method at 12 months post-randomization. Secondary outcomes will include mean number of drinks/week and number of episodes of driving impaired, riding with an impaired driver, injuries, arrests, and tardiness and days absent from work/school. A sample size of 820 is necessary to provide 80% power to detect a 1.14 difference between AB-CASI and SC in the primary outcome. Showing efficacy of this promising bilingual ED-based brief intervention tool in Latino patients has the potential to widely and efficiently expand prevention efforts and facilitate meaningful contact with specialized treatment services.
Keywords: Alcohol Screening, Behavioral Intervention, Emergency Department, Digital Health, Health Disparities, Latino
INTRODUCTION
U.S. Latinos and Alcohol-Related Disease Burden
For U.S. Latinos, disparities in consequences of drinking and limited treatment services carry a heavy disease burden. This is expected to worsen as the Latino population doubles by 2060 becoming nearly one-third of the U.S. population [1–3]. Studies document greater burden of disease from both social and health perspectives among Latino drinkers compared to other groups [4–7]. The first national alcohol survey to emphasize race and ethnicity was conducted in 1984 [8]. This and subsequent studies show complexity and importance of racial/ethnic variations in drinking and related consequences [9–12]. Latino men have a high prevalence of daily heavy drinking with prolonged duration and are more likely to binge drink [6, 13–18]. Moreover, although non-Latino Whites are more likely than Latinos to become dependent in their lifetime, once alcohol dependent, Latinos have higher prevalence than non-Latino Whites of recurrent or persistent dependence [7, 14, 19, 20]. Latinos also face higher rates of negative consequences, such as impaired driving and related lifetime arrests [21, 22]. While research focused on important distinctions between/within Latino subgroups (e.g., Mexican, Puerto Rican) and AUD prevalence has increased, it is still very limited.
U.S. Emergency Departments and Alcohol Use Disorders
National Hospital Ambulatory Medical Care Survey data show that alcohol remains a major contributor to ED visits [23]. In 2017, U.S. EDs had more than 138 million visits (43.3/100 persons; over 22 million visits by Latinos). Nearly 40 million were injury-related. Furthermore, for the chronic alcohol-related condition category alone, there were over 4.2 million ED alcohol-misuse/abuse/dependence visits. White et al., show that between 2006–2014, U.S. ED alcohol-related visits increased by over 61% [24]. Acute and chronic alcohol-related visits also increased by over 51% and 75% respectively. Similarly, another study shows that hours spent in care for alcohol-related ED visits nearly doubled from 5.6 million (2001) to 11.6 million (2011), a 108.5% increase while overall ED hours concurrently only increased 54.0% with a corresponding 232.2% increase in ED resource utilization [25]. A more recent study reveals that between 1999–2017, among those 16 y/o and older, the number of annual alcohol-related deaths doubled (35,914 to 72,558) and the alcohol-related death rate increased by 50% [26].
EDs Offer Opportunity to Address Alcohol-Related Disparities
ED visits provide unique opportunity to identify, intervene, initiate treatment, and address alcohol-related health disparities among those with AUDs, particularly in more vulnerable populations [27]. However, previous research identifies barriers (e.g., time burden, language, intervention fidelity) to effectively deploying and consistently applying ED screening, brief intervention and referral to treatment (SBIRT) [28–30]. Automated bilingual (English and Spanish) computerized alcohol screening and intervention (AB-CASI) can help eliminate time and resource challenges while providing patient anonymity that lends itself to self-disclosure of sensitive topics. It can bolster intervention fidelity and integrity which is important given variations that exist in accuracy and reliability of measures across different racial/ethnic groups while providing tailored intervention feedback [31–34]. Here we describe the construction of our AB-CASI clinical trial and highlight design decisions and considerations.
METHODS
With a health disparity focus, this randomized controlled trial will accomplish three aims: 1) To compare the efficacy of AB-CASI to Standard Care (SC) in the reduction of alcohol consumption in unhealthy (i.e., high-risk) drinkers [35]; 2) To compare the efficacy of AB-CASI to SC in the reduction of alcohol-related negative health behaviors and consequences; 3) To compare the efficacy of AB-CASI to SC in 30-day treatment engagement. Further, given the paucity of ED-based alcohol SBIRT research conducted in Latino subgroups, the trial will explore variation of AB-CASI on alcohol consumption, alcohol-related negative health behaviors and consequences, and 30-day treatment engagement across Latino subpopulations (Puerto-Rican, Mexican-American, Cuban-American, South/Central American) as well as other potential modifiers (age, birthplace, gender, preferred language, dependence, reason for ED visit, and smoking status). Participants will be consented and enrolled in English or Spanish according to their language preference.
At the time of development of this trial, the DSM-IV was widely in use. From the outset, this investigation encompassed the inclusion of the whole spectrum of unhealthy drinking which at that time, definitionally, included those drinking above the low-risk limits (i.e., women and all those older than 65 y/o who have > 3 drinks/occasion or > 7 drinks/week; men who have > 4 drinks/occasion or > 14 drinks/week) through dependence [35]. In May of 2013, the DSM-5 was released and what was previously categorized in the DSM-IV as two different alcohol disorders (i.e., alcohol abuse and alcohol dependence) became one disorder (i.e., AUD) with three levels of severity; mild, moderate, and severe. Currently, the NIAAA points to the U.S. Department of Health and Human Services and U.S. Department of Agriculture’s 2015–2020 Dietary Guidelines for Americans to reference high-risk drinking (i.e., for women ≥4 drinks on any day or ≥8 drinks per week; for men ≥5 drinks on any day or ≥15 drinks per week; binge drinking - drinking ≥4 drinks for women and ≥5 drinks for men within about 2 hours) [36, 37]. Here forward, we use the most current alcohol disorder nomenclature to minimize confusion.
Data are being collected using electronic case report forms in the Oncore Clinical Trials Management System (Forte Research, Madison, WI). The protocol for this study was reviewed and approved by Human Research Protection Program and Institutional Review Board at Yale University and the trial was registered with Clinicaltrials.gov with identifier NCT02247388.
Design
This study is a randomized, controlled, unblinded, parallel group trial to evaluate efficacy of AB-CASI compared to SC to reduce alcohol consumption in adult Latino ED drinkers that are high-risk drinker through severe AUD. Table 1 lists key RCT planning design decision and respective rationale.
Table 1.
Design decisions and rationale
| Decision | Rationale |
|---|---|
| Enrollment of self-identified Latino ED patients | Address alcohol-related health disparities in a highly vulnerable group in a ED-SBIRT context |
| Bilingual (English and Spanish) ED-SBIRT | Address known major language barrier limitation in ED-SBIRT |
| Prescreen [Health Screen] | Ensure enrollment of consistent above low-risk limit drinkers |
| 5 baseline assessments | Capture alcohol use quantity and frequency, severity of AUD, negative and injury-related outcomes, type and amount of treatment services received |
| Randomization | Facilitate testing efficacy of AB-CASI intervention |
| Stratification [dependence and language] | Explore treatment modification |
| 1-mo, 6-mo, 12-mo follow-up assessment | Longitudinal assessment testing efficacy in outcomes of interest |
Participants and Setting
The study is being conducted in a large tertiary care center urban ED, American College of Surgeons verified Level 2 trauma center in the northeastern U.S. At the time when trial was started, the city in which the ED is located was known to have 44% of households speaking another language other than English at home and Latinos made up 38% of the more than 144,000 total residents. The population of the hospital’s primary catchment area was 400,000 and included a diverse ethnic and cultural mix. The annual census of the ED was over 77,000 visits of which 35% were Latino, 32% White, 31% Black, and Asian/American Indian/Hawaiian Pacific Islander/Other 2%. Table 2 shows the study inclusion and exclusion criteria.
Table 2.
Inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| Self-identified adult Latino ED patients | Current enrollment in alcohol or substance abuse treatment program |
| English and/or Spanish speaking | At the time of enrollment known to be pregnant |
| High-risk drinking [35, 37] | Current ED visit for acute psychosis (i.e. suicidal or homicidal ideation) |
| Condition that precludes interview or AB-CASI use i.e., life threatening injury/illness including sexual assault, poor decisional capacity due to cognitive impairment | |
| Police custody | |
| Inability to provide two contact numbers for follow-up |
Measures
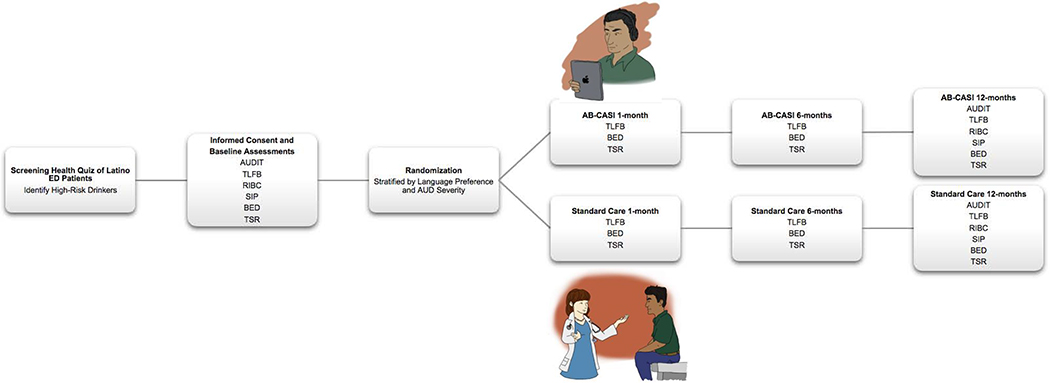
The study assessments and their respective timing (Figure 1.) were organized in a manner that would allow for rigorous testing of efficacy of the AB-CASI intervention against a SC condition in comparing alcohol consumption, negative health behaviors and consequences, and 30-day treatment engagement. Although participants are not blinded to the intervention, interviewers conducting the assessments are blinded to intervention assignment.
Figure 1. Schedule of Assessments.
Alcohol Use Disorders Identification Test ((AUDIT); Timeline Followback (TLFB); Revised Injury Behavior Checklist (RIBC); Short Inventory of Problems (SIP); Brief Event Data (BED); Treatment Services Review (TSR)
Screening Health Quiz (Pre-enrollment screening)
Administered in English or Spanish, the Health Quiz contains questions regarding alcohol, tobacco, exercise and seatbelt use [38, 39]. The embedded questionnaire approach has been noted by the World Health Organization to improve the reliability of self-reported behavior. The alcohol questions embedded in the questionnaire ask three standard quantity and frequency questions [35]; 1) On average, how many days per week do you drink alcohol?; 2) On a typical day when you drink, how many drinks do you have?; 3) How many times in the past month have you had “X” or more drinks on any occasion?, where “X” is 5 for men and 4 for women. Patients who are consistently high-risk drinking are identified and approached for consent and inclusion in the trial.
Alcohol Use Disorders Identification Test ((AUDIT) Baseline)
The AUDIT is used at baseline to identify patients with alcohol dependence (AUDIT score ≥ 20) for the purpose of study sample stratification [40]. This metric has good operating characteristics in an emergency department setting [32]. The 10 AUDIT questions cover drinking behavior, adverse psychological reactions, alcohol-related problems, quantity and frequency of consumption.
Timeline Followback ((TLFB) Baseline, 1-mo, 6-mo, 12-mo)
The validity of self-report data on alcohol consumption has been documented previously in the literature. This evidence supports our use of the 28-day TLFB by telephone as a good method for capturing quantity and frequency of alcohol consumption and frequency of binging episodes [41–43].
Revised Injury Behavior Checklist ((RIBC) Baseline, 12-mo)
The RIBC will facilitate assessment of the patient’s injury history, if they needed medical treatment for an injury, or were drinking within hours of an injury event. Originally developed for an adolescent population by Starfield [44], it was revised for use with an injured adult population by Longabaugh [45]. Its construct validity was established by relating it to the AUDIT variables in both college and ED populations.
Short Inventory of Problems ((SIP) Baseline, 1-mo, 6-mo, 12-mo)
As a validated shortened version of the Drinker Inventory of Consequences (DrInC) [46, 47]. The SIP contains 15-items and measures several domains (i.e., physical, social, intra/interpersonal) of negative consequences due to drinking.
Brief Event Data ((BED) Baseline, 1-mo, 6-mo, 12-mo)
The BED contains questions related to motor vehicle events, legal problems, and employment-related events. It provides information that will enable evaluation of alcohol- related negative consequences (driving impaired, riding with impaired driver, injuries, arrests, tardiness, days absent from school or work). Items from this assessment are from the Non-Study Medical Services Form [48, 49].
Treatment Services Review ((TSR) Baseline, 1-mo, 6-mo, 12-mo)
The TSR is a brief structured interview that will be administered to collect information on the type and amount of services received by participants [50]. This includes ED visits, hospitalizations, primary medical care visits and self-help sources of support (e.g. Alcoholics Anonymous). All patients who self-report treatment at a specialized treatment facility will have their data verified by the agency. Consent to contact the treatment agency is part of the original consent.
AB-CASI Intervention
The theoretical underpinnings of the AB-CASI intervention are rooted in development and use of the Brief Negotiation Interview (BNI). Over the last 20 years, members of our study team and colleagues, have successfully developed, refined, and empirically tested the BNI in large clinical trials [51–53]. Originally developed with colleagues at Boston University in conjunction with Rollnick [54], the BNI has been improved over time. The purpose of the BNI is to assist patients to reduce or abstain from unhealthy alcohol use, or to engage in formal treatment. Combining techniques founded in motivational interviewing as well as the stages of change model, this alcohol SBIRT intervention takes approximately 10-minutes to complete and has been effectively used in other clinical intervention trials focused on alcohol and drugs [55–58].
AB-CASI is a bilingual (English or Spanish) digital health tool that was developed for automated bilingual ED-SBIRT [59, 60]. The version of the AB-CASI tool used runs on iPads® and is taken to the patient’s bedside by a trained bilingual research assistant. Questions and messages are displayed on the iPad® screen and spoken in English or Spanish through headphones for patient privacy. Recognizing that some Latino patients may be conversationally competent in English but prefer to consume more personal health information in Spanish, patients are able to select the tool interface language based upon their comfort and preference (i.e., in both text and audio) [60]. Demographic information is collected followed by automated administration of the AUDIT. With pre-programmed logic branching, patients who screen as high-risk drinkers receive an automated BNI, including automated personalized feedback, readiness to change evaluation, reasons for cutting down, goal setting, a printed personalized alcohol use reduction plan, and counseling referral information (Figure 2.). Patients found to be dependent by the AUDIT also receive an automated BNI that includes respectful and reflective questioning, personalized non-judgmental feedback, and supportive communication intentionally focused on treatment engagement. AB-CASI is able to reliably identify high-risk drinkers and AUD in the ED, requires little time, and is highly accepted by patients [59, 61].
Figure 2.
Automated Bilingual Computerized Alcohol Screening and Intervention (AB-CASI): Example screen views in English and Spanish
Standard Care (SC) Condition
Patients randomized to SC do not receive the AB-CASI intervention. However, they receive SC as provided by the treating emergency care provider. All SC patients receive an informational sheet with primary care follow-up recommended. Consultation with social workers are at the discretion of the treating emergency care provider. All requirements for screening and referral are performed according to the American College of Surgeons (ACS) Level 2 trauma designation [62].
Follow-up Telephone Assessments
Formal telephone assessments by blinded, bilingual research assistants are planned at 1-, 6-, and 12-months. The 1-month follow-up will focus on collecting the extent and frequency of early treatment engagement. During this assessment, participants are asked if they received any physician-initiated advice regarding their use of alcohol during their visit to the ED. The 6-month follow-up is designed to collect early effects of the intervention on alcohol consumption and to allow enough time to collect sufficient number of negative behaviors and consequences as well as all treatment engagement episodes. The 12-month follow-up assessment is designed to detect long-term effects on alcohol consumption, alcohol-related negative behaviors and consequences, and delayed referral to treatment engagement. Several successful brief intervention studies have used these time intervals and we have chosen the same intervals to facilitate later comparison of previously published studies [45, 56, 63]. Because of the possibility of a “sleeper effect” or delayed emergence of treatment efficacy [64] [65], it is imperative to conduct the assessments to the 12-month interval and evaluate the effect of the intervention at each follow-up. The use of ancillary services, the occurrence of other injuries and/or illnesses, or advice from other persons may reinforce the BNI.
Data Analytic Plan
This study was designed as a single-site, randomized parallel group design to assess the superiority of AB-CASI compared to SC in reducing alcohol consumption, alcohol related negative health behaviors and consequences and increase 30-day treatment engagement in Latino unhealthy drinkers, from high-risk drinking to severe AUD. All analyses will consider participants according to their randomized assignment regardless of adherence to protocol.
Analysis of the Primary Outcome
The primary objective of the analysis is to demonstrate that AB-CASI will reduce number of binge episodes more than SC at 12 months. A generalized linear mixed model (GLMM) with a Negative Binomial distribution will be used to estimate differences in the number of binge drinking episodes in the past 28 days at 12 months. More specifically the mixed model will include fixed effects for intervention (AB-CASI vs. SC), time (1, 6, 12 months), and the interaction of intervention with time. Additional fixed effects will be included for baseline covariates (baseline number of drinks per week, baseline number of binge episodes, gender, English vs. Spanish preferred language and dependent status). This analysis will assume that missing data occurs at random (i.e. MAR, not informative). The inclusion of baseline, 1– 6- and 12-months outcome data in the model will assist in meeting this assumption. Furthermore, we will evaluate patterns of missing data as well as determine baseline characteristics that are predictive of dropout. If identified, these characteristics will be included in the model to meet the MAR assumption. Modification of the intervention effect by preferred language will be evaluated at the 0.10 significance level by including two and three-way interactions of language with intervention and time. If not significant, these interactions will be excluded and intervention effects pooled across preferred language strata. Similar procedures will be used to assess modification by dependence status. Linear contrasts (at the 0.05 two-sided significance level) will be used to estimate intervention group differences and 95% confidence intervals at the 1-, 6- and 12-month time points.
Analysis of Secondary Outcomes
We will determine whether number of drinks per week, negative behaviors and consequences (episodes of impaired driving, riding with an impaired driver, injuries, arrests, tardiness, days absent from work/school and SIP) during the 12-month follow-up will be improved in subjects receiving AB-CASI compared to those receiving SC. Similar repeated measures mixed model analysis as that specified for the primary outcome will be implemented for each of the secondary outcomes. Comparison of all secondary outcomes between study groups will be evaluated at the two-sided 0.01 significance level to control inflated type I error from multiple significance testing.
Analysis of Tertiary Outcome
We will determine the effect of the AB-CASI compared to SC on 30-day treatment engagement. Mantel-Haenszel chi-square analysis will be used to compare the likelihood of 30-day treatment engagement in AB-CASI to SC while adjusting for preferred language and dependent status. Significance will be judged at the two-sided 0.05 significance level. Heterogeneity of treatment effect will be evaluated by the Breslow-Day test. Participants dropping out or lost-to-follow-up will be considered to be not engaged in treatment for the primary analysis.
Heterogeneity of Treatment Effects (HTE)
In addition to the stratification factors (AUD severity, preferred language), HTE on the primary outcome will be assessed for subgroups based on factors assessed at baseline (Latino ancestry, age, birthplace, gender, reason for ED visit and smoking status). These subgroup analyses will be conducted within the Generalized Linear Mixed Model framework in an evaluation similar to that proposed for investigating modification by the stratification factors of dependence status and preferred language as described above. Significant interactions will be followed by the estimation and summarization of intervention effects within subgroups at both 1-, 6- and 12-month time points.
Sample Size
Estimation of sample size is based on randomizing and following a sufficient number of unhealthy drinkers to evaluate the primary hypothesis that AB-CASI will result in greater 12-month reductions in the primary outcome, the number of episodes of binge drinking over the past 28-days, compared to SC. Fleming et al [63]. demonstrated that the number of binge episodes in the past 30-days was reduced by 1.14 in the intervention compared to control conditions. D’Onofrio et al [66]. reported similar findings in an RCT conducted in hazardous and harmful drinkers. Given the following: 1) power of 80%, 2) a two-sided 0.05 significance level, 3) a standard deviation for number of binge episodes in the past 28 days of 5.2, and 4) a 1:1 intervention allocation, a sample size of 327 subjects per group will be required to detect a 1.14 difference between AB-CASI and SC in the number of binge episodes in the past 28 days at 12 months. A total of 820 unhealthy drinkers will be enrolled and randomized to accommodate up to 20% dropout. To maximize the ability to explore modification by preferred language, we will enroll an equal number of preferred English and Spanish speaking participants.
SUMMARY
The described first-of-a-kind ED-RCT, has been intentionally designed to address alcohol-related health disparities in adult U.S. Latino ED patients using AB-CASI. In this trial, we will test the efficacy of the AB-CASI intervention against a SC condition and compare alcohol consumption, negative health behaviors and consequences, and 30-day treatment engagement. Moreover, we will explore variations in intervention outcomes between Latino subpopulations. This study harnesses bilingual digital health tool providing a tablet-delivered brief negotiation interview (BNI). The intervention is conducted in a busy clinical setting that offers unique and important access to this vulnerable population that can benefit from directed disease prevention and health promotion efforts to close alcohol-related disparity gaps. Of particular note, this trial provides the opportunity to expand the evidence that well-known ED-SBIRT barriers (e.g., practitioner time burden, cost of intervention personnel, maintaining intervention fidelity, providing intervention in other languages) can be effectively surmounted. This could potentially reinvigorate and bolster prevention efforts to further advance national ED activities and programs addressing AUDs and ED-SBIRT practice known to currently lag behind national guidelines [67].
The design and efforts in this clinical trial are particularly unique for five reasons. First, the study is closely aligned with the NIAAA’s Strategic Plan to Address Health Disparities and its most recent overall Strategic Plan (2017–2021) committing to advance the science in health disparities and developing interventions that benefit the health of at-risk populations [68, 69]. Second, the design of the AB-CASI intervention has been recognized and singled out as a promising approach to address alcohol-related health disparities among racial/ethnic minorities specifically in the area of screening and brief intervention in unique settings that call for the use of innovative methods [70]. Third, with few exceptions [71], nearly all U.S. ED-SIBRT studies have not enrolled Spanish speaking participants. Further, none have used an automated bilingual intervention approach that facilitates disclosure of sensitive information. As a result, opportunities have been missed to capitalize on such trials in order to advance the knowledge of AUD and more specifically alcohol-related health disparities in patient-oriented outcomes among the largest minority population in the U.S. Fourth, the described trial, unlike many preceding ED-SBIRT RCTs, sets out to address and enroll participant from the full spectrum of unhealthy drinkers from high-risk drinking to severe AUD. Every day emergency care providers treat patients that present to the ED as a result of unhealthy drinking. As such, an evaluation of a broader ED-SBIRT intervention approach, that is, enrolling the full spectrum of AUD, is more congruent with the what emergency care providers routinely encounter; AUD patients with severity that results in major adverse events, such as acute and chronic physical and/or psychological harm.
If found to be effective, the AB-CASI intervention could provide more definitive evidence that not only can many previously identified ED-SBIRT barriers by overcome, but also that ED-SBIRT in the described manner could be scaled up and pragmatically implemented to significantly enhance and improve current alcohol brief intervention efforts in the ED. Moreover, because the ED is healthcare safety net for more vulnerable populations that are at greater risk for the development of alcohol-related injury, AUD, and requiring referral for specialized treatment services, an AB-CASI approach could improve the early identification AUD patient and help facilitate their referral to treatment services. On an even broader scale, if AB-CASI if found to be effective, its logical to consider that this approach could afford valuable opportunities for intervention adaptation. That is, it may also lend itself to systematic implementation of AB-CASI in a number of other languages as well as use in primary care where more recent literature suggests the need for greater scope of not only intentional screening but also monitoring of reductions in alcohol use as well as medical treatment of AUDs, both to the benefit of patients [72, 73].
Acknowledgements
We would like to thank Vanessa Zuniga, Jessica Navarro, Renny Parrilla and Yesenia Torres for their tireless work and commitment to this important clinical trial. We thank the Yale Center for Clinical Investigation (YCCI) and their support and use of the OnCore Clinical Trials Management System (Forte Research, Madison, WI).
Funding
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, Office of The Director, National Institutes of Health (OD), Office of Behavioral and Social Sciences Research (OBSSR) under Award Number R01AA022083
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chartier KG, Caetano R, Trends in alcohol services utilization from 1991–1992 to 2001–2002: ethnic group differences in the U.S. population, Alcohol Clin Exp Res 35(8) (2011) 1485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keyes KM, Liu XC, Cerda M, The role of race/ethnicity in alcohol-attributable injury in the United States, Epidemiol Rev 34(1) (2012) 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].U.S. Census Bureau, Hispanic Heritage Month 2017, 2017.
- [4].Mulia N, Ye Y, Greenfield TK, Zemore SE, Disparities in alcohol-related problems among white, black, and Hispanic Americans, Alcohol Clin Exp Res 33(4) (2009) 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Caetano R, Ramisetty-Mikler S, Rodriguez LA, The Hispanic Americans baseline alcohol survey (HABLAS): DUI rates, birthplace, and acculturation across Hispanic national groups, Journal of Studies on Alcohol & Drugs 69(2) (2008) 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Witbrodt J, Mulia N, Zemore SE, Kerr WC, Racial/ethnic disparities in alcohol-related problems: differences by gender and level of heavy drinking, Alcohol Clin Exp Res 38(6) (2014) 1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chartier K, Caetano R, Ethnicity and Health Disparities in Alcohol Research, Alcohol Res Health 33(1–2) (2010) 152–160. [PMC free article] [PubMed] [Google Scholar]
- [8].Alcohol Research Group and National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Alcohol Survey: 1984, U.S. Department of Health and Human Services; Bethesda, MD. [Google Scholar]
- [9].Galvan FH, Caetano R, Alcohol use and related problems among ethnic minorities in the United States, Alcohol Res Health 27(1) (2003) 87–94. [PMC free article] [PubMed] [Google Scholar]
- [10].Caetano R, Clark CL, Trends in alcohol-related problems among whites, blacks, and Hispanics: 1984–1995, Alcohol Clin Exp Res 22(2) (1998) 534–8. [PubMed] [Google Scholar]
- [11].Caetano R, Clark CL, Trends in alcohol consumption patterns among whites, blacks and Hispanics: 1984 and 1995, J Stud Alcohol 59(6) (1998) 659–68. [DOI] [PubMed] [Google Scholar]
- [12].Chartier KG, Scott DM, Wall TL, Covault J, Karriker-Jaffe KJ, Mills BA, Luczak SE, Caetano R, Arroyo JA, Framing ethnic variations in alcohol outcomes from biological pathways to neighborhood context, Alcohol Clin Exp Res 38(3) (2014) 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Institute on Alcohol Abuse and Alcoholism, Alcohol use and alcohol use disorders in the United States: Main findings from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), National Institutes of Health, Bethesda, MD, 2006. [Google Scholar]
- [14].Caetano R, Prevalence, incidence and stability of drinking problems among whites, blacks and Hispanics: 1984–1992, J Stud Alcohol 58(6) (1997) 565–72. [DOI] [PubMed] [Google Scholar]
- [15].Chartier KG, Vaeth PAC, Caetano R, Focus on: Ethnicity and the Social and Health Harms From drinking, Alcohol Research: Current Reviews 35(2) (2013) 229–237. [PMC free article] [PubMed] [Google Scholar]
- [16].Mulia N, Tam TW, Bond J, Zemore SE, Li L, Racial/ethnic differences in life-course heavy drinking from adolescence to midlife, J Ethn Subst Abuse 17(2) (2018) 167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caetano R, Vaeth PA, Chartier KG, Mills BA, Epidemiology of drinking, alcohol use disorders, and related problems in US ethnic minority groups, Handb Clin Neurol 125 (2014) 629–48. [DOI] [PubMed] [Google Scholar]
- [18].Levitt E, Ainuz B, Pourmoussa A, Acuna J, De La Rosa M, Zevallos J, Wang W, Rodriguez P, Castro G, Sanchez M, Pre- and Post-Immigration Correlates of Alcohol Misuse among Young Adult Recent Latino Immigrants: An Ecodevelopmental Approach, Int J Environ Res Public Health 16(22) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hasin DS, Stinson FS, Ogburn E, Grant BF, Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions, Arch Gen Psychiatry 64(7) (2007) 830–42. [DOI] [PubMed] [Google Scholar]
- [20].Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ, Recovery from DSM-IV alcohol dependence: United States, 2001–2002, Addiction 100(3) (2005) 281–92. [DOI] [PubMed] [Google Scholar]
- [21].Caetano R, Alcohol-related health disparities and treatment-related epidemiological findings among whites, blacks, and Hispanics in the United States, Alcohol Clin Exp Res 27(8) (2003) 1337–9. [DOI] [PubMed] [Google Scholar]
- [22].Caetano R, Clark CL, Hispanics, Blacks and White driving under the influence of alcohol: results from the 1995 National Alcohol Survey, Accid Anal Prev 32(1) (2000) 57–64. [DOI] [PubMed] [Google Scholar]
- [23].Rui P, Kang K, National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables, 2019. https://www.cdc.gov/nchs/ahcd/web_tables.htm. (Accessed March10 2020).
- [24].White AM, Slater ME, Ng G, Hingson R, Breslow R, Trends in Alcohol-Related Emergency Department Visits in the United States: Results from the Nationwide Emergency Department Sample, 2006 to 2014, Alcohol Clin Exp Res 42(2) (2018) 352–359. [DOI] [PubMed] [Google Scholar]
- [25].Mullins PM, Mazer-Amirshahi M, Pines JM, Alcohol-Related Visits to US Emergency Departments, 2001–2011, Alcohol and alcoholism 52(1) (2017) 119–125. [DOI] [PubMed] [Google Scholar]
- [26].White AM, Castle IP, Hingson RW, Powell PA, Using Death Certificates to Explore Changes in Alcohol-Related Mortality in the United States, 1999 to 2017, Alcohol Clin Exp Res 44(1) (2020) 178–187. [DOI] [PubMed] [Google Scholar]
- [27].Havard A, Shakeshaft A, Sanson-Fisher R, Systematic review and meta-analysis of strategies targeting alcohol problems in emergency departments: Interventions reduce alcohol-related injuries, Addiction 103(3) (2008) 368–376. [DOI] [PubMed] [Google Scholar]
- [28].D’Onofrio G, Becker B, Woolard RH, The impact of alcohol, tobacco, and other drug use and abuse in the emergency department, Emerg Med Clin North Am 24(4) (2006) 925–67. [DOI] [PubMed] [Google Scholar]
- [29].Cherpitel CJ, Screening and brief intervention for alcohol problems in the emergency room: is there a role for nursing?, Journal of Addictions Nursing 17(2) (2006) 79–82. [Google Scholar]
- [30].Vaca FE, Winn D, The basics of alcohol screening, brief intervention and referral to treatment in the emergency department, West J Emerg Med 8(3) (2007) 88–92. [PMC free article] [PubMed] [Google Scholar]
- [31].Cunningham RM, Bernstein SL, Walton M, Broderick K, Vaca FE, Woolard R, Bernstein E, Blow F, D’Onofrio G, Alcohol, tobacco, and other drugs: future directions for screening and intervention in the emergency department, Acad Emerg Med 16(11) (2009) 1078–88. [DOI] [PubMed] [Google Scholar]
- [32].Cherpitel CJ, Screening for Alcohol Problems in the Emergency Department, Annals of Emergency Medicine 26(2) (1995) 158–166. [DOI] [PubMed] [Google Scholar]
- [33].De Leeuw E, Hox J, Kef S, Computer-Assisted Self-Interviewing Tailored for Special Populations and Topics, Field Methods 15(3) (2003) 223–251. [Google Scholar]
- [34].Tourangeau R, Smith TW, Asking Sensitive Questions: The Impact of Data Collection Mode, Question Format, and Question Context, The Public Opinion Quarterly 60(2) (1996) 275–304. [Google Scholar]
- [35].National Institute on Alcohol Abuse and Alcoholism, Helping Patients Who Drink Too Much : A Clinician’s Guide Updated 2005 Edition US Department of Health and Human Services, National Institutes of Health; 2007. (Reprinted). [Google Scholar]
- [36].National Institute for Alcohol Abuse and Alcoholism, Rethinking Drinking, Alcohol and Your Health. https://www.rethinkingdrinking.niaaa.nih.gov/How-much-is-too-much/Is-your-drinking-pattern-risky/Drinking-Levels.aspx. (Accessed April 10 2020).
- [37].U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015–2020 Dietary Guidelines for Americans, Appendix 9. Alcohol, pp 101–103, 2015. [Google Scholar]
- [38].Fleming MF, Barry KL, A three-sample test of a masked alcohol screening questionnaire, Alcohol and alcoholism 26(1) (1991) 81–91. [PubMed] [Google Scholar]
- [39].Fleming MF, Bruno M, Barry K, Fost N, Informed consent, deception, and the use of disguised alcohol questionnaires, Am J Drug Alcohol Abuse 15(3) (1989) 309–19. [DOI] [PubMed] [Google Scholar]
- [40].Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care, World Health Organization, Department of Mental Health and Substance Dependence, Geneva, Switzerland, 2001. [Google Scholar]
- [41].Breslin C, Sobell LC, Sobell MB, Buchan G, Kwan E, Aftercare telephone contacts with problem drinkers can serve a clinical and research function, Addiction 91(9) (1996) 1359–64. [DOI] [PubMed] [Google Scholar]
- [42].Sobell LC, Appendix: Instrument Fact Sheets, Alcohol Timeline Followback (TLFB), in: Allen JP, Willson VB (Eds.), Assessing Alcohol Problems: A Guide for Clinicians and Researchers, National Institutes of Health, Bethesda, MD, 2003, pp. 301–305. [Google Scholar]
- [43].Cohen BB, Vinson DC, Retrospective self-report of alcohol consumption: test-retest reliability by telephone, Alcohol Clin Exp Res 19(5) (1995) 1156–61. [DOI] [PubMed] [Google Scholar]
- [44].Starfield B, Injury behavior checklist (adapted version) Subscale, Adolescent Health Status Instrument John Hopkins University, Baltimore, MD, 1991. [Google Scholar]
- [45].Longabaugh R, Woolard RE, Nirenberg TD, Minugh AP, Becker B, Clifford PR, Carty K, Licsw F Sparadeo, A. Gogineni, Evaluating the effects of a brief motivational intervention for injured drinkers in the emergency department, J Stud Alcohol 62(6) (2001) 806–16. [DOI] [PubMed] [Google Scholar]
- [46].Miller WR, Tonigan JS, Longabaugh R, The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. Test manual (Project MATCH Monograph Series Vol 4), National Institute on Alcohol Abuse and Alcoholism, Rockville, MD: 1995. [Google Scholar]
- [47].Marra LB, Field CA, Caetano R, von Sternberg K, Construct validity of the short inventory of problems among Spanish speaking Hispanics, Addict Behav 39(1) (2014) 205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE, Cost-effectiveness of extended buprenorphine-naloxone treatment for opioid-dependent youth: data from a randomized trial, Addiction 105(9) (2010) 1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Murphy SM, Campbell AN, Ghitza UE, Kyle TL, Bailey GL, Nunes EV, Polsky D, Cost-effectiveness of an internet-delivered treatment for substance abuse: Data from a multisite randomized controlled trial, Drug and alcohol dependence 161 (2016) 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McLellan TA, Zanis D, Incmikoski R, Treatment Service Review (TSR) The Center for Studies in Addiction, Department of Psychiatry: Philadelphia VA Medical Center & The University of Pennsylvania, Philadelphia, 1989. [Google Scholar]
- [51].Prochaska JO, DiClemente CC, Norcross JC, In search of how people change: Applications of addictive behaviors, Am Psychologist 47(9) (1992) 1102–14. [DOI] [PubMed] [Google Scholar]
- [52].D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA, Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial, JAMA 313(16) (2015) 1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ryan S, Pantalon MV, Camenga D, Martel S, D’Onofrio G, Evaluation of a Pediatric Resident Skills-Based Screening, Brief Intervention and Referral to Treatment (SBIRT) Curriculum for Substance Use, J Adolesc Health 63(3) (2018) 327–334. [DOI] [PubMed] [Google Scholar]
- [54].D’Onofrio G, Bernstein E, Rollnick S, Motivating patients for change: a brief strategy for negotiation, in: Bernstein E, Bernstein J (Eds.), Case studies in Emergency Medicine and the health of the Public Jones and Bartlett, Boston, 1996, pp. 295–303. [Google Scholar]
- [55].D’onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, Busch SH, Bernstein SL, O’Connor PG, A brief intervention reduces hazardous and harmful drinking in emergency department patients, Annals of Emergency Medicine 60(2) (2012) 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Neumann T, Neuner B, Weiss-Gerlach E, Tonnesen H, Gentilello LM, Wernecke KD, Schmidt K, Schroder T, Wauer H, Heinz A, Mann K, Muller JM, Haas N, Kox WJ, Spies CD, The effect of computerized tailored brief advice on at-risk drinking in subcritically injured trauma patients, J Trauma 61(4) (2006) 805–14. [DOI] [PubMed] [Google Scholar]
- [57].D’Onofrio G, Degutis LC, Integrating Project ASSERT: a screening, intervention, and referral to treatment program for unhealthy alcohol and drug use into an urban emergency department, Acad Emerg Med 17(8) (2010) 903–11. [DOI] [PubMed] [Google Scholar]
- [58].D’Onofrio G, Pantalon MV, Degutis LC, Fiellin DA, Busch SH, Chawarski MC, Owens PH, O’Connor PG, Brief intervention for hazardous and harmful drinkers in the emergency department, Ann Emerg Med 51(6) (2008) 742–750 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vaca F, Winn D, Anderson C, Kim D, Arcila M, Feasibility of emergency department bilingual computerized alcohol screening, brief intervention, and referral to treatment, Subst Abus 31(4) (2010) 264–9. [DOI] [PubMed] [Google Scholar]
- [60].Abujarad F, Vaca FE, mHealth Tool for Alcohol Use Disorders Among Latinos in Emergency Department, Proc Int Symp Hum Factors Ergon Healthc 4(1) (2015) 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vaca FE, Winn D, Anderson CL, Kim D, Arcila M, Six-month follow-up of computerized alcohol screening, brief intervention, and referral to treatment in the emergency department, Subst Abus 32(3) (2011) 144–52. [DOI] [PubMed] [Google Scholar]
- [62].American College of Surgeons - Committee on Trauma, Screening and Brief Intervention Training for Trauma Care Providers, 2007. https://www.facs.org/~/media/files/quality%20programs/trauma/publications/sbirtguide.ashx. (Accessed March 28 2020).
- [63].Fleming MF, Barry KL, Manwell LB, Johnson K, London R, Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices, Jama 277(13) (1997) 1039–45. [PubMed] [Google Scholar]
- [64].O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B, Six-month follow-up of naltrexone and psychotherapy for alcohol dependence, Arch Gen Psychiatry 53(3) (1996) 217–24. [DOI] [PubMed] [Google Scholar]
- [65].Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F, One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects, Arch Gen Psychiatry 51(12) (1994) 989–97. [DOI] [PubMed] [Google Scholar]
- [66].D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, Busch SH, Bernstein SL, O’Connor PG, A Brief Intervention Reduces Hazardous and Harmful Drinking in Emergency Department Patients, Ann Emerg Med (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cunningham RM, Harrison SR, McKay MP, Mello MJ, Sochor M, Shandro JR, Walton MA, D’onofrio GD, National survey of emergency department alcohol screening and intervention practices, Annals of Emergency Medicine 55(6) (2010) 556–562. [DOI] [PubMed] [Google Scholar]
- [68].National Institute on Alcohol Abuse and Alcoholism, NIAAA’s strategic plan to address health disparities. http://pubs.niaaa.nih.gov/publications/HealthDisparities/Strategic.html. (Accessed March 28 2020).
- [69].National Institute on Alcohol Abuse and Alcoholism, National Institute on Alcohol Abuse and Alcoholism, Strategic Plan 2017–2021, 2017. https://www.niaaa.nih.gov/sites/default/files/StrategicPlan_NIAAA_optimized_2017-2020.pdf. (Accessed March 28 2020).
- [70].Zemore SE, Karriker-Jaffe KJ, Mulia N, Kerr WC, Ehlers CL, Cook WK, Martinez P, Lui C, Greenfield TK, The Future of Research on Alcohol-Related Disparities Across U.S. Racial/Ethnic Groups: A Plan of Attack, J Stud Alcohol Drugs 79(1) (2018) 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Field CA, Caetano R, Harris TR, Frankowski R, Roudsari B, Ethnic differences in drinking outcomes following a brief alcohol intervention in the trauma care setting, Addiction 105(1) (2010) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Carvalho AF, Heilig M, Perez A, Probst C, Rehm J, Alcohol use disorders, Lancet 394(10200) (2019) 781–792. [DOI] [PubMed] [Google Scholar]
- [73].Witkiewitz K, Kranzler HR, Hallgren KA, O’Malley SS, Falk DE, Litten RZ, Hasin DS, Mann KF, Anton RF, Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder, Alcohol Clin Exp Res 42(12) (2018) 2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]