Abstract
Aims:
Psoriatic arthritis (PsA) is associated with accelerated atherosclerosis due to underlying inflammation. Whether inflammatory burden and drugs used to suppress inflammation over time are associated with cardiovascular (CV) events remained unclear. This study aims to examine the time-varying effect of C-reactive protein (CRP) levels and the use of drugs, including non-steroidal anti-inflammatory drugs (NSAIDs) and disease modifying anti-rheumatic drugs, on the risk of CV events independent of traditional CV risk factors in PsA patients.
Methods:
A retrospective cohort analysis was performed in patients with PsA who were recruited from 2008 to 2015 and followed until the end of 2019. The outcome was occurrence of a first CV event. Framingham risk score (FRS) was used to quantify the traditional CV risk. Cox proportional hazard models with time-varying CRP levels and drugs used were analysed to identify the risk factors for CV events in PsA patients.
Results:
Two hundred patients with PsA [median age: 47.5 (40.0–56.0); male: 119 (59.5%)] were recruited. After a mean follow-up of 8.8 ± 3.8 years, 30 (15%) patients developed a first CV event. The multivariable Cox regression model showed that time-varying CRP level [hazard ratio (HR) 1.02, 95% confidence interval (CI) 1.00–1.04] and NSAIDs exposure (HR 0.38, 95% CI 0.15–0.96) were significantly associated with CV events after adjusting for baseline FRS (HR 5.06, 95% CI 1.84–13.92).
Conclusion:
Increased inflammatory burden as reflected by elevated CRP level was associated with increased risk of CV events, while the risk was significantly reduced with NSAIDs use in PsA patients.
Keywords: C-reactive protein, cardiovascular disease, NSAID, psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory joint disease associated with elevated cardiovascular (CV) morbidity. 1 Compared with the general population, PsA patients experience 43% increased risk of CV diseases. 2 The risk of myocardial infarction (MI) also remained significantly increased after adjusting for traditional CV risk factors.1,3 The reported incidence rates for MI and stroke were 2.3 and 1.2 per 1000 patient-years in PsA patients respectively, although they were lowered among biological disease-modifying anti-rheumatic drugs (bDMARDs) users compared with non-bDMARDs or conventional synthetic DMARDs (csDMARDs) users according to a recent cohort. 3 Nonetheless, study suggested that the incidence of CV events was similar across PsA, rheumatoid arthritis (RA) and ankylosing spondylitis (AS), reflecting that systematic inflammation drives the excess CV risk. 4
Data are accumulating that inflammatory burden reflected by the level of inflammatory markers and disease activity measures is associated with an increased CV risk. Baseline elevated joint count, dactylitic digits count and erythrocyte sedimentation rate (ESR) level in women with PsA are independently associated with increased CV events. 5 We have also reported recently that baseline disease activity, measured by Disease Activity in Psoriatic Arthritis, independently predicts CV risk in addition to traditional CV risk factors. 6 However, one measurement at baseline may not accurately predict future CV risk as inflammatory burden and disease activity vary over time. Indeed, we have previously reported that cumulative inflammatory burden was associated with increased arterial stiffness in PsA patients even after adjustment for CV risk factors, emphasizing the important role of chronic inflammation in accelerating atherosclerosis in PsA patients. 7 Whether these time-dependent inflammatory states predict future CV risk would definitely be worth exploring.
Reduction of inflammation by drugs, on the other hand, may reduce the CV risks in PsA. Current bDMARDs use was associated with a reduction in CV events in a recent cohort study while no association was found between current methotrexate (MTX) use. 8 Nonetheless, data from another large cohort indicate that the risk of major adverse cardiovascular events (MACEs) is reduced in PsA patients with prescription of DMARDs compared with patients without DMARDs. 9 The association between non-steroidal anti-inflammatory drugs (NSAIDs) and CV events is also controversial. While the CV toxicity of NSAIDs is demonstrated in the general population and patients with osteoarthritis, the association between NSAIDs and CV events among patients with inflammatory joint diseases is not fully elucidated. 10 In RA, the CV toxicity is predominantly associated with rofecoxib, but not with celecoxib and non-selective NSAIDs. 11 In fact, CV risk related to NSAIDs use was significantly lower in inflammatory polyarthritis (IP) patients, with a 46% risk reduction of CV mortality demonstrated in a new-onset IP inception cohort. 12 The inverse relationship between NSAIDs and mortality was also observed in a cohort of AS patients followed for 30 years. Indeed, not using any NSAIDs was associated with over four-fold higher all-cause mortality [odds ratio (OR) = 4.35, 95% confidence interval (CI) (1.75, 10.77)] in this AS cohort in which circulatory disease contributed to 40% of mortality. 13 In a population-based study from Canada, risk for vascular mortality in AS was decreased with use of traditional NSAIDs [hazard ratio (HR) = 0.1, 95% CI (0.01–0.61); p = 0.01]. 14 Furthermore, CV toxicity was only observed with diclofenac use but not with naproxen use in a recent spondylarthritis cohort. 15 However, data on CV risk of NSAIDs among PsA patients are scarce and the effect of NSAIDs use in PsA patients is not clear.
Overall, the effects of inflammatory burden and drugs used for suppressing inflammation on CV events in PsA has not been fully studied. Here, we hypothesize that chronic inflammation in PsA is associated with increased CV risk independent of traditional CV risk factors. In this study, we aimed to (1) identify the time-varying effect of inflammation and (2) the use of NSAIDs and DMARDs on the risk of developing CV events in PsA patients after adjusting for baseline traditional CV risk factors.
Methods
Study design and patients
This was a retrospective cohort study with patients’ data drawn from the Clinical Data Analysis and Reporting System (CDARS), an electronic public healthcare database in Hong Kong. The database is managed and updated automatically on a daily basis. It captures clinical information of patients in all public hospitals and clinics. According to the International Classification of Diseases ninth edition diagnosis codes, patients with diagnosed PsA (696.0) were drawn from the Prince of Wales Hospital using the database. All patients were diagnosed by rheumatologists and they also fulfilled the CASPAR criteria. 16 Patients with other concomitant inflammatory or painful conditions, for example, gout, osteoarthritis and connective tissue diseases, were excluded. The recruitment was from 2008 to 2015. Patients with established cardiovascular diseases (CVDs) [MI, percutaneous transluminal coronary angioplasty, surgery for ischaemic heart disease (IHD), transient ischaemic attack (TIA) and stroke] at baseline were excluded. Follow-up began with the baseline clinic visit and continued until the end of 2019, an endpoint, or death, or loss to follow-up, whichever came first.
Clinical parameters
The patients were seen during scheduled visits every 6–12 months. During these visits, inflammatory markers were measured including ESR and C-reactive protein (CRP). Patients’ demographic details, traditional CV risk factors including hypertension, diabetes mellitus, body mass index, dyslipidaemia, smoking and drinking habits were retrieved at baseline from the CDARS. Framingham risk score (FRS) was calculated at baseline to quantify the traditional CV risk. 17 FRS >10% was considered as elevated CV risk. Drug history (bDMARDs, csDMARDs, NSAIDs and lipid-lowering drugs) and inflammatory markers were retrieved from CDARS at a yearly interval starting from baseline visit until the end of study. Use of drugs at baseline, ever use in the study and time-varying drug exposure during the follow-up assessments were recorded.
Endpoints
The primary outcome was the occurrence of a first CV event, including IHD, all stable and unstable angina, MI, TIA, coronary insufficiency, peripheral arterial disease, stroke, congestive heart failure (CHF) and death due to CVD.
Statistical analysis
Descriptive statistics were expressed as number with percentage, mean ± SD for normally distributed data and median with interquartile range (IQR) for non-normally distributed data. Clinical parameters at baseline between CVD positive group and CVD negative group and between NSAIDs users and non-users were assessed using χ2 test for categorical variables and Student’s t-test or Mann–Whitney U-test for continuous variables. These analyses were performed using SPSS V.22.0.
Cox proportional hazards model with time-varying covariate and Kaplan–Meier survival curves were used to analyse the association between clinical parameters and CV events. Inflammatory marker was added as a continuous variable (ESR/CRP) and as a dichotomous variable: pro-inflammatory response (yes/no). The cut-off value for the presence of pro-inflammatory response was defined as CRP >3 mg/l, which was suggested to be associated with elevated CV risk in the general population. 18 Inflammatory markers were measured regularly, every 6–12 months, and a yearly interval was maintained for the time-dependent covariate in the Cox proportional hazard regression analyses. These time segments corresponded with ESR/CRP measurements at yearly intervals. Patients with CRP >3 mg/l were compared with patients with CRP ⩽3 mg/l at each interval. In addition, drug use was treated as a time-varying exposure and the relationship between drug use and CV events was explored for each interval of follow-up. Patients who were identified as non-users of NSAIDs, MTX and bDMARDs were compared with those identified as NSAIDs, MTX and bDMARDs users respectively, at any interval of assessments. Different kinds of NSAIDs and bDMARDs use were conflated respectively to form a mutually exclusive group of either user or non-user. Intervals with missing data were dropped from the analysis. Univariable analysis was performed for each variable. Variables with a p-value less than 0.05 were included in the multivariable analysis. Forest plot was used to illustrate the final multivariable model. These analyses were performed in R version 4.0 (https://www.r-project.org/) using the counting process method in the package ‘survival’ and ‘survminer’. The survival distributions were compared using log-rank test.
Results
Patient characteristics and CVD risk at baseline
Two hundred patients with PsA [median age: 47.5 (40.0–56.0) years; male: 119 (59.5%)] were recruited. At baseline, the median disease duration was 4.3 (IQR 1.8–7.9) years. Seventy-one out of 200 (35.5%) patients were classified as having elevated risk for CV events by FRS >10%. Table 1 summarizes patients’ baseline characteristics.
Table 1.
Baseline demographic and clinical characteristics, cardiovascular risk factors and treatments received.
| Variables |
n = 200 Median (IQR) or n (%) |
|---|---|
| Male, n (%) | 119 (59.5) |
| Age, years | 47.5 (40.0–56.0) |
| BMI, kg/m2 | 25.2 (23.1–28.6) |
| Disease duration, years | 4.3 (1.8–7.9) |
| Diabetes, n (%) | 45 (22.5) |
| Hypertension, n (%) | 68 (34.0) |
| CRP, mg/l | 4.9 (1.7–12.6) |
| ESR, mm/h | 21 (10.0–38.0) |
| Total cholesterol, mmol/l | 4.9 (4.2–5.6) |
| HDL, mmol/l | 1.3 (1.1–1.6) |
| LDL, mmol/l | 2.9 (2.3–3.4) |
| Triglycerides, mmol/l | 1.2 (0.9–1.8) |
| Glucose, mmol/l | 5.1 (4.8–5.6) |
| Systolic blood pressure, mmHg | 125 (115–140) |
| Diastolic blood pressure, mmHg | 78 (70–85) |
| Ever smoker, n (%) | 59 (34.9) |
| Ever drinker, n (%) | 50 (33.6) |
| FRS | 8.4 (4.0–17.0) |
| Current treatment | |
| Lipid-lowering drugs, n (%) | 30 (15.0) |
| MTX, n (%) | 99 (49.5) |
| bDMARDs, n (%) | 17 (8.5) |
| NSAIDs, n (%) | 139 (69.2) |
| Steroid, n (%) | 2 (1.0) |
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FRS, Framingham risk score; HDL, high-density lipoprotein cholesterol; IQR, interquartile range; LDL, low-density lipoprotein cholesterol; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drug.
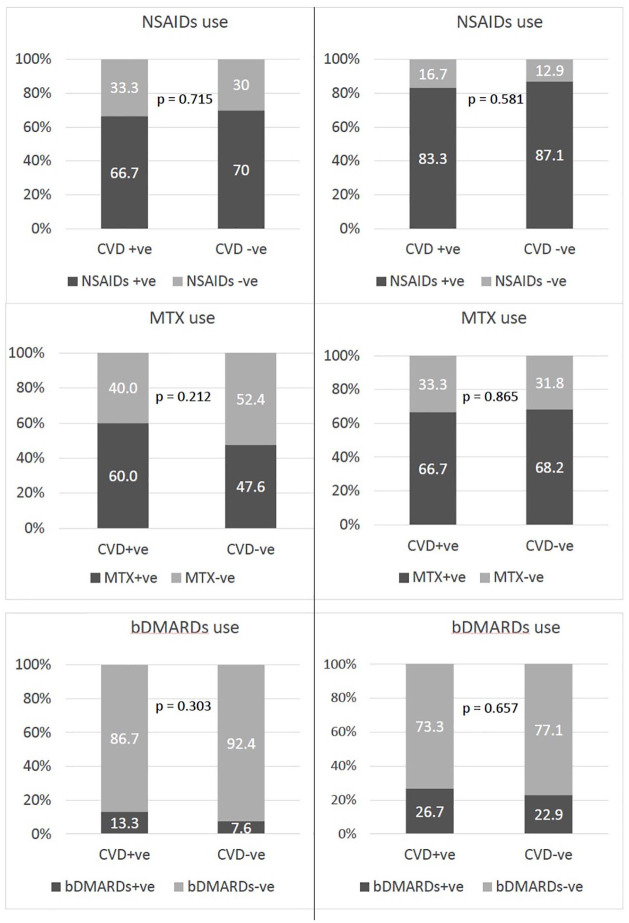
At baseline, 139 (69.5%), 99 (49.5%) and 17 (8.5%) patients were NSAIDs, MTX and bDMARDs users (Figure 1). The baseline clinical characteristics were similar between patients who were using NSAIDs or not (Table 2). A further 34 (total 173, 86.5%), 37 (total 136, 68%) and 30 (total 47, 23.5%) patients were identified as using NSAIDs, MTX and bDMARDs during the follow-up assessments. The mean duration of NSAIDs, MTX and bDMARDs exposure during the period of this study, estimated from the number of study assessments where these drugs’ use was reported, was 5.46 ± 3.49 years, 5.66 ± 3.72 years and 4.87 ± 3.19 years respectively. Of the 173 ever NSAIDs users, NSAIDs use was reported in 58.8% of all follow-up intervals. A total of 27 (13.5%) patients reported no NSAIDs exposure at any of the study assessments. During the study period, only 24 (12%) patients were treated with selective COX2 inhibitors at any time. This exposure was limited to 52 patient-years. During the study, diclofenac was used most frequently, identified in 60.7% of the NSAIDs exposures during the study, followed by naproxen (27.4%) and ibuprofen (5.9%). Other less frequently used drugs included sulindac, indomethacin, piroxicam and mefenamic acid. Switching between different NSAIDs was frequently observed during the follow-up period. Using χ2 tests, no significant differences were found between patients who developed CV events (CVD +ve group) and those who did not (CVD −ve group) in terms of drugs used at baseline and ever (Figure 1).
Figure 1.
Comparison of the prevalence of drugs used at baseline (left panel) and ever (right panel) among CVD +ve and CVD −ve patients.
bDMARDs +ve, patients who used biological disease-modifying anti-rheumatic drugs; bDMARDs -ve, patients who did not use biological disease-modifying anti-rheumatic drugs; CVD +ve, patients who developed cardiovascular events; CVD –ve, patients who did not develop cardiovascular events; MTX+ve, patients who used methotrexate; MTX-ve, patients who did not use methotrexate; NSAIDs+ve, patients who used non-steroidal anti-inflammatory drugs; NSAIDs-ve, patients who did not use non-steroidal anti-inflammatory drugs.
Table 2.
Comparison of the baseline demographic and clinical characteristics, cardiovascular risk factors and treatments according to cardiovascular outcomes and baseline NSAID use.
| Variables | CVD –ve n = 170 |
CVD +ve n = 30 |
p-value | NSAIDs –ve n = 61 |
NSAIDs +ve n = 139 |
p-value |
|---|---|---|---|---|---|---|
| Male, n (%) | 100 (58.6) | 19 (63.3) | 0.228 | 39 (63.9) | 80 (57.6) | 0.397 |
| Age, years | 46.5 (37.7–54.0) | 57.0 (45.3–65.8) | <0.001* | 49.0 (44.0–56.5) | 46.0 (38.0–54.8) | 0.176 |
| BMI, kg/m2 | 25.3 (22.8–29.1) | 25.2 (23.7–27.2) | 0.718 | 25.4 (23.7–28.2) | 25.1 (22.2–29.1) | 0.732 |
| Disease duration, years | 4.1 (1.7–7.0) | 6.0 (2.1–8.6) | 0.066 | 4.6 (1.5–8.7) | 4.3 (1.9–7.3) | 0.393 |
| Diabetes, n (%) | 30 (17.6) | 15 (50.0) | <0.001* | 10 (16.4) | 35 (25.2) | 0.171 |
| Hypertension, n (%) | 46 (27.1) | 22 (73.3) | <0.001* | 21 (34.4) | 47 (33.9) | 0.933 |
| CRP, mg/l | 4.2 (1.5–12.0) | 11.3 (2.4–19.6) | 0.035* | 5.5 (1.7–15.1) | 7.2 (1.4–15.8) | 0.770 |
| ESR, mm/h | 20 (9–35) | 31 (14–60) | 0.038* | 21 (7–33) | 21 (11–43) | 0.291 |
| Total cholesterol, mmol/l | 4.9 (4.2–5.6) | 5.1 (4.3–5.6) | 0.476 | 4.9 (4.4–5.6) | 4.9 (4.2–5.6) | 0.909 |
| HDL, mmol/l | 1.3 (1.1–1.5) | 1.4 (1.0–1.6) | 0.809 | 1.4 (1.2–1.6) | 1.2 (1.0–1.5) | 0.056 |
| LDL, mmol/l | 2.9 (2.4–3.3) | 2.8 (2.2–3.4) | 0.912 | 2.9 (2.4–3.3) | 2.9 (2.3–3.4) | 0.949 |
| Triglycerides, mmol/l | 1.2 (0.9–1.8) | 1.4 (1.1–2.0) | 0.156 | 1.2 (0.8–1.7) | 1.2 (1.0–1.5) | 0.746 |
| Glucose, mmol/l | 5.1 (4.8–5.5) | 5.2 (4.8–6.0) | 0.167 | 5.0 (4.6–5.4) | 5.1 (4.8–5.6) | 0.137 |
| Systolic blood pressure, mmHg | 124 (115–137) | 144 (129–160) | <0.001* | 123 (118–137) | 125 (115–141) | 0.889 |
| Diastolic blood pressure, mmHg | 78 (70–84) | 82 (72–90) | 0.199 | 78 (72–86) | 78 (70–85) | 0.697 |
| Ever smoker, n (%) | 50 (35.0) | 8 (30.8) | 0.678 | 20 (37.7) | 38 (32.8) | 0.527 |
| Ever drinker, n (%) | 42 (33.9) | 8 (32.0) | 0.857 | 13 (28.9) | 37 (35.6) | 0.427 |
| FRS | 7.5 (3.3–14.0) | 19.6 (13.4–43.0) | <0.001* | 7.9 (3.5–15.5) | 8.7 (4.2–17.1) | 0.885 |
| Current treatment | ||||||
| Lipid-lowering drug, n (%) | 25 (14.7) | 5 (16.7) | 0.782 | 9 (14.8) | 21 (15.1) | 0.949 |
| MTX, n (%) | 81 (47.6) | 18 (60.0) | 0.212 | 25 (41.0) | 74 (53.2) | 0.111 |
| bDMARDs, n (%) | 13 (7.6) | 4 (13.3) | 0.303 | 6 (9.8) | 11 (7.9) | 0.654 |
| NSAIDs, n (%) | 119 (70.0) | 20 (66.7) | 0.715 | NA | NA | NA |
| Steroid, n (%) | 2 (1.2) | 0 (0) | 1.000 | 1 (1.6) | 1 (0.7) | 0.518 |
Statistically significant at p ⩽ 0.05.
bDMARD, biological disease-modifying anti-rheumatic drug; BMI, body mass index; CRP, C-reactive protein; CVD +ve, patients who developed cardiovascular events during subsequent follow-up; CVD −ve, patients who did not develop cardiovascular events during subsequent follow-up; ESR, erythrocyte sedimentation rate; FRS, Framingham risk score; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MTX, methotrexate; NA, not available; NSAIDs+ve, patients who used non-steroidal anti-inflammatory drugs; NSAIDs-ve, patients who did not use non-steroidal anti-inflammatory drugs.
A total of 1753 person-years of follow-up were available for analysis. After a mean follow-up of 8.8 ± 3.8 years, 30 (15%) patients developed a first CV event (1.7 events per 100 patient-years), which included 15 (50.0%) IHD, six (20%) stroke, five (16.7%) MI, two (6.7%) TIA and two (6.7%) CHF.
Table 2 summarizes the differences in baseline characteristics between CVD +ve group and CVD −ve group. CVD +ve group were significantly older, with higher prevalence of diabetes and hypertension, elevated systolic blood pressure, CRP and ESR levels and higher FRS. Baseline use of NSAIDs was not associated with incident CV events.
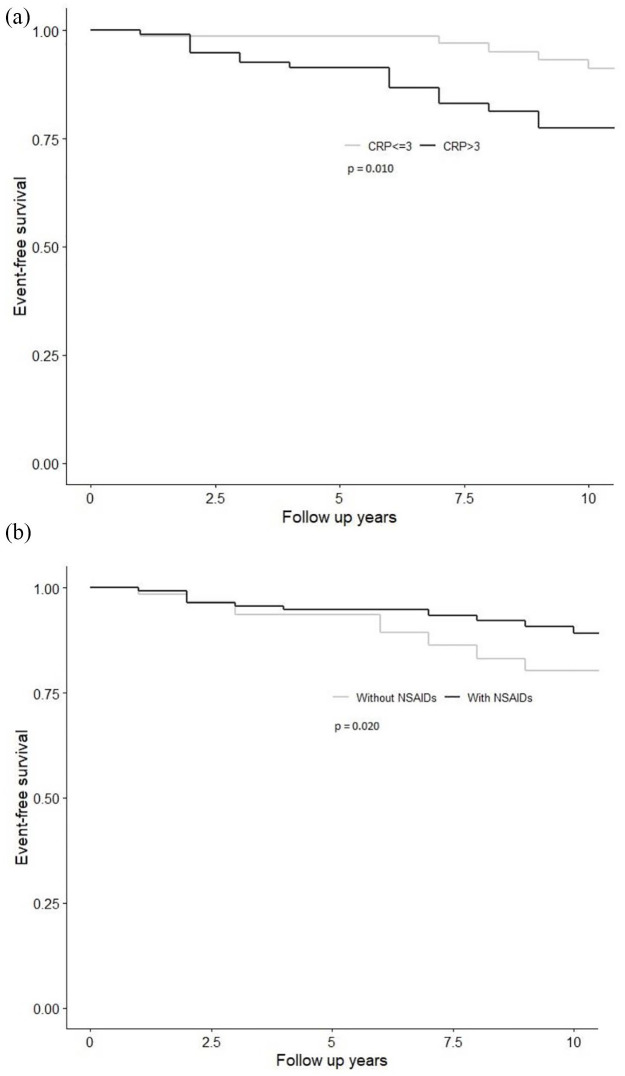
The univariable Cox regression analysis showed that higher FRS at baseline, increasing CRP and ESR levels during the follow-up period, and particularly if CRP was >3 mg/l, were associated with a higher risk of developing CV events. In contrast, more frequent use of NSAIDs during follow-up was associated with lower risk of developing CV events (Table 3). In the sub-group analysis, only non-selective NSAIDs use was associated with lower risk of CV events, while COX2 inhibitors did not have any association. Figure 2(a) demonstrates the Kaplan–Meier survival estimates for CV event-free survival in patients with and without CRP >3 mg/l. Time-varying NSAIDs exposure was explored univariately and an inverse relationship was observed with CV event [Figure 2(b)].
Table 3.
Univariable analysis with time-dependent Cox proportional hazard regression.
| Variables # | Total person-time intervals | Time-dependent HR (95% CI) | p-value |
|---|---|---|---|
| CRP | 1327 | 1.01 (1.00, 1.02) | 0.024* |
| CRP >3 mg/l | 1327 | 2.71 (1.12, 6.51) | 0.026* |
| ESR | 1272 | 1.01 (1.00, 1.02) | 0.045* |
| MTX | 1618 | 1.16 (0.56, 2.40) | 0.681 |
| NSAIDs | 1618 | 0.41 (0.19, 0.88) | 0.022* |
| COX2 selective inhibitor | 0.82 (0.11, 6.14) | 0.850 | |
| Non-COX2 selective inhibitor | 0.44 (0.21, 0.96) | 0.038* | |
| Diclofenac | 0.76 (0.35, 1.67) | 0.490 | |
| Naproxen | 0.19 (0.03, 1.47) | 0.114 | |
| Sulindac | 1.04 (0.14, 7.62) | 0.972 | |
| Ibuprofen | 0.00 (0.00, ∞) | 0.997 | |
| Mefanamic acid | 0.00 (0.00, ∞) | 0.997 | |
| Indomethacin | 0.00 (0.00, ∞) | 0.997 | |
| Piroxicam | 0.00 (0.00, ∞) | 0.997 | |
| bDMARDs | 1618 | 0.72 (0.22, 2.38) | 0.591 |
| Lipid-lowering drugs | 1618 | 1.22 (0.49, 3.05) | 0.675 |
| Anti-diabetic drugs | 1618 | 1.75 (0.77, 3.94) | 0.180 |
| Anti-hypertensive drugs | 1618 | 1.60 (0.69, 3.74) | 0.276 |
| FRS at baseline | N/A | 6.89 (2.59, 18.33) | <0.001* |
Variables are time-varying unless otherwise specified.
Statistically significant at p ⩽ 0.05.
bDMARD, biological disease-modifying anti-rheumatic drug; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FRS, Framingham risk score; HR, hazard ratio; MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug.
Figure 2.
Kaplan–Meier curves showing the cardiovascular event-free survival between patients with (a) CRP ⩽3 mg/l and CRP >3 mg/l; (b) treated with NSAIDs and without NSAIDs use during their follow-up intervals.
CRP, C-reactive protein; NSAID, non-steroidal anti-inflammatory drug.
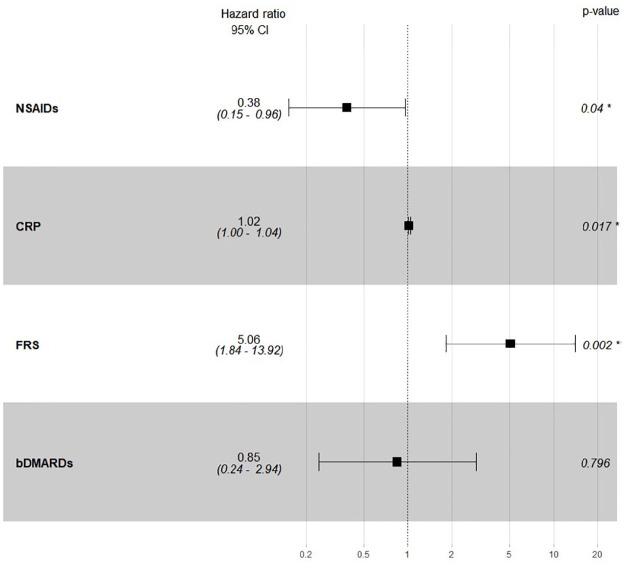
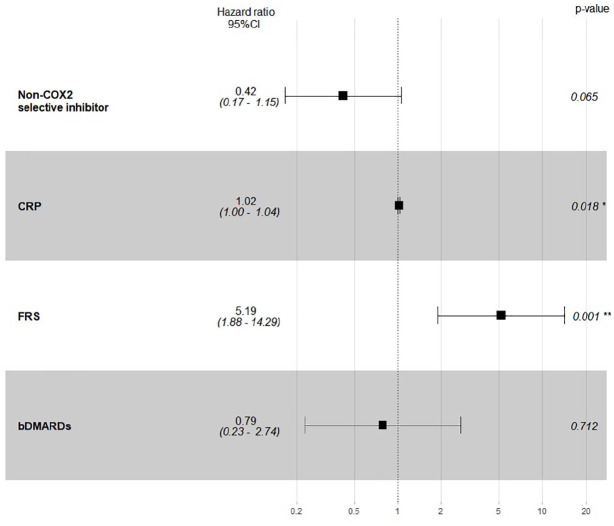
In the multivariable model (Figures 3 and 4), the association between time-varying covariates (ESR, CRP, the use of NSAIDs, non-selective NSAIDs and bDMARDs) for each interval of follow-up and CV events was adjusted for baseline FRS. Time-varying CRP level [HR = 1.02, 95% CI (1.00, 1.04)] and NSAIDs exposure [HR = 0.38, 95% CI (0.15, 0.96)] were significantly associated with CV events after adjusting for baseline FRS [HR = 5.06, 95% CI (1.84, 13.92)], while the use of non-selective NSAIDs was no longer associated with CV events after adjusting for other covariates. No significant interactions were found between time-varying CRP and NSAIDs nor ESR and NSAIDs. Time-varying CRP >3 mg/l and ESR were no longer significant after adjusting for FRS at baseline, bDMARDs and NSAIDs.
Figure 3.
Multivariable Cox regression for predicting cardiovascular events.
*Statistically significant at p ⩽ 0.05
bDMARD, biological disease-modifying anti-rheumatic drug; CI, confidence interval; CRP, C-reactive protein; FRS, Framingham risk score; NSAID, non-steroidal anti-inflammatory drug.
Figure 4.
Multivariable Cox regression for predicting cardiovascular events.
*Statistically significant at p ⩽ 0.05
bDMARD, biological disease-modifying anti-rheumatic drug; CI, confidence interval; CRP, C-reactive protein; FRS, Framingham risk score.
Secondary analyses were performed to explore whether increasing duration of NSAIDs exposure was associated with reduced CV events. The duration of NSAIDs exposure as covariate was entered into the logistic regression model predicting CV events (Supplemental material Table 1 online). Longer duration of NSAIDs use was not independently associated with a significant reduction in CV event risk (p = 0.074) during this study for each additional period of NSAID exposure after adjusting for traditional CV risks and CRP at baseline.
An additional test was performed to test the effect of NSAIDs dose on CV risk. The NSAIDs equivalent score recommended by the Assessment of Spondyloarthritis International Society (ASAS) was used to quantify and standardize the dose and strength of different NSAIDs. 19 Time-averaged NSAIDs equivalent score was calculated for predicting CV risk. There were no significant differences in the time-averaged NSAIDs score between CVD +ve and CVD −ve groups (Supplemental Table 2). Univariable Cox regression did not reveal significant association between the time-averaged NSAIDs score and CV events. (Supplemental Table 3).
A sensitivity analysis was performed to assess whether the final multivariable model can independently predict MACE only. MACE was defined as a composite cardiovascular event of myocardial infarction, stroke and coronary revascularization (n = 11). The univariable Cox regression analysis showed that higher FRS at baseline and increasing CRP levels during follow-up were associated with a higher risk of developing MACE (all p < 0.05; Supplemental Table 4). There was also a trend suggesting that more frequent use of NSAIDs during the follow-up period was associated with a lower risk of developing MACE (p = 0.068).
In the multivariable model (Supplemental Table 5), the association between time-varying covariates (CRP, the use of NSAIDs and non-selective NSAIDs) for each interval of follow-up and CV events was adjusted for baseline FRS. Time-varying CRP remained significantly associated with increased risk of MACE [HR = 1.03, 95% CI (1.01, 1.05)].
Discussion
This is the first study to demonstrate that chronic inflammatory burden, as reflected by the time-varying CRP level, can predict CV events among PsA patients independent of traditional CV risk factors. Similar to other inflammatory arthritis, the excess CV risk in PsA is partly driven by chronic inflammation, accelerating the progression of atherosclerosis, which eventually leads to the development of CV events. 4 While CRP may not be a perfect biomarker for assessing systemic inflammation in patients with psoriatic diseases, it can still quantify the elevated inflammatory burden in a subset of patients with elevated CRP, mainly observed in psoriatic patients with joint disease. 20 Indeed, a recent longitudinal study suggested that elevated CRP level at first visit was associated with poor outcomes, including radiographic damage and higher number of comorbidities in PsA patients. 21 Our study further suggests that persistently raised CRP levels in PsA could also predict long-term CV events. More importantly, our data illustrate that a subset of patients with more severe chronic inflammation is associated with higher risk of CV events. Although the usefulness of CRP in predicting CV events in PsA has not been widely reported, it is well observed in RA. In RA patients, 30% excess risk for developing CV events is attributable to RA disease characteristics and CRP alone contributed 1.5% risk after adjusting for other CV risk factors. 22 Whether normalization of inflammatory biomarker, including CRP, could reduce the risk of CV events in PsA would need to be assessed in a larger-scale prospective study.
The association between various drugs and CV risks in PsA is controversial. Our data have demonstrated that use of MTX and bDMARDs is not associated with CV events among PsA patients. MTX and bDMARDs have been found to be associated with reduced risk of developing CV events among patients with RA in previous studies.8,23,24 The cardio-protective effect of MTX [relative risk (RR) = 0.72, 95% CI (0.57, 0.91)] and bDMARDs [RR = 0.70, 95% CI (0.57, 0.91)] has been extensively suggested among RA patients but not in PsA patients according to a systematic review of observational studies. 11 DMARDs could effectively suppress inflammation and disease activity thereby protecting the cardiovascular system from inflammatory damage, which is suggested in RA patients but not in PsA patients. Furthermore, a recent cohort study found that current MTX use was not associated with CV events while bDMARDs use was associated with reduced CV events among PsA patients. 8 A possible reason for the inconsistent effect on CV risk between MTX and bDMARDs could be that bDMARDs suppress inflammation and disease activity more effectively than MTX does. 25 Indeed, failure to prevent atherosclerotic events by using MTX was demonstrated by a recent large-scale trial in 4786 patients with previous myocardial infarction or multivessel coronary disease. 26 Effectiveness in reducing inflammation by using MTX in the trial was also questioned as there was no significant reduction in the levels of interleukin-1β, interleukin-6 and CRP after MTX treatment. Nonetheless, the inflammatory burden of these patients was minimal (low baseline CRP concentration) in contrast to the elevated inflammatory burden among PsA patients. In this study, no association for either MTX and bDMARDs use with CV events was found. In a clinical trial setting, we have previously demonstrated that using a treat-to-target strategy, the use of bDMARDs and patients who achieved sustained minimal disease activity were independently associated with prevention of subclinical atherosclerosis progression over a period of 2 years. 27 The reason for the disagreement in the current study may due to a limitation in the access of bDMARDs in the usual care cohort as these drugs are not reimbursed by the Hong Kong Government. The low prevalence (<30%) and non-sustained use of bDMARDs in our cohort may under-estimate the cardiovascular protective effects of these agents.
Our study is the first to suggest a cardio-protective effect of NSAIDs use among PsA patients. This is the first study to examine the relevancy between NSAIDs dose (as quantify by the ASAS recommended NSAIDs equivalent score) and CV risk among PsA patients. Previous studies have indicated the CV toxicity of NSAIDs related to COX2 inhibition in the general population. 28 Notably, selective COX2 inhibitors (rofecoxib and celecoxib) and diclofenac (non-COX2 selective inhibitor) were associated with elevated CV risk.29,30 In contrast, naproxen was not associated with excess CV risk according to a large-scale meta-analysis of over 200 trials. 29 In the PRECISION trial, no significant difference in the CV risk was found between celecoxib and two other non-COX2 selective inhibitors (naproxen and ibuprofen). 31 However, the trial population consisted of around 90% osteoarthritis (OA) patients and only around 10% RA patients, which may not be representative in an inflammatory arthritis population. In RA patients, NSAIDs were associated with an overall increase in CV risk found in another meta-analysis. 11 However, the risk was no longer significant in the subgroup analysis of non-selective NSAIDs alone, suggesting that the excess CV risk was driven by COX2 inhibitors. 11 Similar to this finding, a cohort study with over 17,000 RA patients found that only rofecoxib and diclofenac were independently associated with increased CV risk while other NSAIDs were not. 32 Hence these findings indicated that NSAIDs use in inflammatory arthritis, depending on their selectivity of COX2 inhibition, may not be as CV toxic as they were in OA patients and in the general population. Indeed, not using any NSAIDs was associated with over four-fold higher all-cause mortality [OR = 4.35, 95% CI (1.75, 10.77)] in a AS cohort study in which CVD, valvular disease, atrioventricular conduction disease and cardiomyopathy contributed to 40% of mortality. 13 This finding may possibly suggest an enhanced survival by NSAIDs use in AS patients through dampening inflammation and cardiovascular burden. Whether NSAIDs use in PsA patients is cardioprotective would need further confirmation.
Our study has several strengths. To date, this is the first cohort study to investigate the effect of long-term NSAIDs use on CV outcome among PsA patients. We have also demonstrated that the chronic inflammation reflected by time-varying CRP is associated with increased CV risk in PsA patients. Our study also has a few limitations. First, sample size was small with only 30 out of 200 patients developing a first CV event. Nonetheless, this is the only study that provided almost 10 years of longitudinal data. In the sensitivity analysis, NSAIDs exposure lost statistical significance probably because of the small number of patients with MACE (n = 11). The ASAS recommended NSAIDs equivalent score did not consider two NSAIDs, namely sulindac and mefenamic acid, which are prescribed in our cohort. This further reduce the NSAIDs exposure available for statistical test. Second, systemic steroids were usually not prescribed in our cohort because of the risk of flare of psoriasis on steroid tapering. 33 Therefore, we would not be able to address the effect of systemic steroid on CV risk. Third, retrospective design of the current study may result in high risk of indication bias for the NSAIDs protective effect, including the subtype of NSAID. As NSAIDs (especially COX2 inhibitors) have been associated with increased CV risk, physicians probably avoided their use in patients with an elevated risk of CVD. Last, the observational study could show only association so the result should not be interpreted as evidence of prevention of CV events by NSAIDs. Whether NSAIDs can prevent CV events in patients with PsA would need to be confirmed in a prospective study.
In conclusion, this study demonstrates that sustained inflammation reflected by elevated CRP levels is associated with greater risk of CV events in PsA patients. In contrast, the use of NSAIDs may reduce the risk of CV events in PsA patients. Studies are needed to reveal the effect of therapeutic interventions and optimal control of inflammation in preventing the enhanced CV events in PsA patients.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211027712 for Association of C-reactive protein and non-steroidal anti-inflammatory drugs with cardiovascular events in patients with psoriatic arthritis: a time-dependent Cox regression analysis by Steven H. Lam, Ho So, Isaac T. Cheng, Edmund K. Li, Priscilla Wong, Tena K. Li, Alex Pui-Wai Lee and Lai-Shan Tam in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211027712 for Association of C-reactive protein and non-steroidal anti-inflammatory drugs with cardiovascular events in patients with psoriatic arthritis: a time-dependent Cox regression analysis by Steven H. Lam, Ho So, Isaac T. Cheng, Edmund K. Li, Priscilla Wong, Tena K. Li, Alex Pui-Wai Lee and Lai-Shan Tam in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to show our gratitude to all medical staff, research assistants and participating patients. Patient and public involvement: patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Author contributions: All authors critically revised the manuscript for important intellectual content. Specific roles included: study design (LS Tam, Steven Lam, Ho So), data collection (LS Tam, Edmund Li, Priscilla Wong, Martin Li, Tena Li, Isaac Cheng, Steven Lam). Data analysis (Steven Lam), drafting of manuscript (Steven Lam, LS Tam, Ho So).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: Ethical approval: The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee, No. 2019.247. Written informed consents were waived
ORCID iDs: Steven H. Lam  https://orcid.org/0000-0003-2147-0995
https://orcid.org/0000-0003-2147-0995
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Steven H. Lam, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
Ho So, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong.
Isaac T. Cheng, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
Edmund K. Li, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
Priscilla Wong, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong.
Tena K. Li, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
Alex Pui-Wai Lee, Department of Medicine & Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong.
Lai-Shan Tam, Department of Medicine and Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong.
References
- 1. Schieir O, Tosevski C, Glazier RH, et al. Incident myocardial infarction associated with major types of arthritis in the general population: a systematic review and meta-analysis. Ann Rheum Dis 2017; 76: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 2. Polachek A, Touma Z, Anderson M, et al. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2017; 69: 67–74. [DOI] [PubMed] [Google Scholar]
- 3. Persson R, Hagberg KW, Qian Y, et al. The risks of major cardiac events among patients with psoriatic arthritis treated with apremilast, biologics, DMARDs or corticosteroids. Rheumatology (Oxford) 2021; 60: 1926–1931. [DOI] [PubMed] [Google Scholar]
- 4. Lauper K, Courvoisier DS, Chevallier P, et al. Incidence and prevalence of major adverse cardiovascular events in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis. Arthritis Care Res (Hoboken) 2018; 70: 1756–1763. [DOI] [PubMed] [Google Scholar]
- 5. Eder L, Wu Y, Chandran V, et al. Incidence and predictors for cardiovascular events in patients with psoriatic arthritis. Ann Rheum Dis 2016; 75: 1680–1686. [DOI] [PubMed] [Google Scholar]
- 6. Lam SHM, Cheng IT, Li EK, et al. DAPSA, carotid plaque and cardiovascular events in psoriatic arthritis: a longitudinal study. Ann Rheum Dis 2020; 79: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 7. Shen J, Shang Q, Li EK, et al. Cumulative inflammatory burden is independently associated with increased arterial stiffness in patients with psoriatic arthritis: a prospective study. Arthritis Res Ther 2015; 17: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JL, Sinnathurai P, Buchbinder R, et al. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther 2018; 20: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2015; 74: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braun J, Baraliakos X, Westhoff T. Nonsteroidal anti-inflammatory drugs and cardiovascular risk – a matter of indication. Semin Arthritis Rheum 2020; 50: 285–288. [DOI] [PubMed] [Google Scholar]
- 11. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodson NJ, Brookhart AM, Symmons DP, et al. Non-steroidal anti-inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis 2009; 68: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis 2011; 70: 1921–1925. [DOI] [PubMed] [Google Scholar]
- 14. Haroon NN, Paterson JM, Li P, et al. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med 2015; 163: 409–416. [DOI] [PubMed] [Google Scholar]
- 15. Dubreuil M, Louie-Gao Q, Peloquin CE, et al. Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis. Ann Rheum Dis 2018; 77: 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 17. D’Agostino RB, Sr Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008; 117: 743–753. [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol 2016; 67: 712–723. [DOI] [PubMed] [Google Scholar]
- 19. Dougados M, Simon P, Braun J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011; 70: 249–251. [DOI] [PubMed] [Google Scholar]
- 20. Sokolova MV, Simon D, Nas K, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther 2020; 22: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haroon M, Gallaghar P, Ahmad M, et al. Elevated CRP even at the first visit to a rheumatologist is associated with long-term poor outcomes in patients with psoriatic arthritis. Clin Rheumatol 2020; 39: 2951–2961. [DOI] [PubMed] [Google Scholar]
- 22. Crowson CS, Rollefstad S, Ikdahl E, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018; 77: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Halm VP, Nurmohamed MT, Twisk JW, et al. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther 2006; 8: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, et al. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis 2016; 75: 2087–2094. [DOI] [PubMed] [Google Scholar]
- 25. Nam JL, Takase-Minegishi K, Ramiro S, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum 2017; 76: 1113–1136. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng IT, Shang Q, Li EK, et al. Effect of achieving minimal disease activity on the progression of subclinical atherosclerosis and arterial stiffness: a prospective cohort study in psoriatic arthritis. Arthritis Rheumatol 2019; 71: 271–280. [DOI] [PubMed] [Google Scholar]
- 28. Weir MR, Sperling RS, Reicin A, et al. Selective COX-2 inhibition and cardiovascular effects: a review of the rofecoxib development program. Am Heart J 2003; 146: 591–604. [DOI] [PubMed] [Google Scholar]
- 29. Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fosbøl EL, Gislason GH, Jacobsen S, et al. Risk of myocardial infarction and death associated with the use of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther 2009; 85: 190–197. [DOI] [PubMed] [Google Scholar]
- 31. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016; 375: 2519–2529. [DOI] [PubMed] [Google Scholar]
- 32. Lindhardsen J, Gislason GH, Jacobsen S, et al. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 2014; 73: 1515–1521. [DOI] [PubMed] [Google Scholar]
- 33. Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol 2013; 27: 1022–1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211027712 for Association of C-reactive protein and non-steroidal anti-inflammatory drugs with cardiovascular events in patients with psoriatic arthritis: a time-dependent Cox regression analysis by Steven H. Lam, Ho So, Isaac T. Cheng, Edmund K. Li, Priscilla Wong, Tena K. Li, Alex Pui-Wai Lee and Lai-Shan Tam in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-2-tab-10.1177_1759720X211027712 for Association of C-reactive protein and non-steroidal anti-inflammatory drugs with cardiovascular events in patients with psoriatic arthritis: a time-dependent Cox regression analysis by Steven H. Lam, Ho So, Isaac T. Cheng, Edmund K. Li, Priscilla Wong, Tena K. Li, Alex Pui-Wai Lee and Lai-Shan Tam in Therapeutic Advances in Musculoskeletal Disease