Abstract
Purpose/Objective(s):
The additional personnel and imaging procedures required for Adaptive Radiation Therapy (ART) pose a challenge for a broad implementation. We hypothesize that a change in transit fluence during the treatment course is correlated with the change of quality of life and thus can be used as a replanning trigger.
Materials/Methods:
Twenty-one head and neck cancer (HNC) patients filled out an MD Anderson Dysphagia Inventory (MDADI) questionnaire, before-and-after the radiotherapy treatment course. The transit fluence was measured by the Watchdog (WD) in-vivo portal dosimetry system. The patients were monitored with daily WD and weekly CBCTs. The region of interest (ROI) of each patient was defined as the outer contour of the patient between approximate spine levels C1 to C4, essentially the neck and mandible inside the beam’s eye view. The nth day integrated transit fluence change, Δϕn, and the volume change, ΔVROI, of the ROI of each patient was calculated from the corresponding WD and CBCT measurements. The correlation between MDADI scores and age, gender, planning mean dose to salivary glands <Dsg>, weight change ΔW, ΔVROI, and Δϕn, were analyzed using the ranked-Pearson correlation.
Results:
No statistically significant correlation was found for age, gender and ΔW. <Dsg> was found to have clinically important correlation with functional MDADI (ρ = −0.39, P = 0.081). ΔVROI was found to have statistically significant correlation of 0.44, 0.47 and 0.44 with global, physical and functional MDADI (P-value < 0.05). Δϕn was found to have statistically significant ranked-correlation (−0.46, −0.46 and −0.45) with physical, functional and total MDADI (P-value < 0.05).
Conclusion:
A transit fluence based decision support metric (DSM) is statistically correlated with the dysphagia risk. It can not only be used as an early signal in assisting clinicians in the ART patient selection for replanning, but also lowers the resource barrier of ART implementation.
Keywords: xerostomia, dysphagia, head and neck cancer, adaptive radiation therapy, in-vivo dosimetry, decision support informetric
Introduction
Adaptive radiotherapy (ART), the adaption of the treatment plan to account for significant anatomical changes 1 -5 occurring during the course of radiation therapy, is a viable clinical strategy to both maintain the PTV coverage and to control the degradation risk of quality of life for head and neck cancer (HNC) patients. The degradation is a result of radiation induced conditions such as dysphagia and xerostomia 6 -8 due to unplanned dose increases to the salivary glands. To capture longitudinal anatomical changes, regular 3-dimensional imaging procedures, 1 -3,5,9,10 such as cone beam computed tomography (CBCT) and magnetic resonance imaging (MRI), are performed and reviewed during the course of treatment. Although this technique has demonstrated the effectiveness in reducing dose to the parotid glands (PG), 1,3,5 the high operational cost, such as the additional time required for imaging procedures and physician assessment, is preventing ART from being more broadly implemented. 5 As the scans are usually taken on only weekly, the best time window to adapt potentially may be missed. Furthermore, significant variability in the anatomic longitudinal change during a treatment course is observed among patients. 11,12 Both real-time and offline electronic portal image detector (EPID) based in-vivo dosimetry systems that utilize exit radiation, or transit fluence, from patients during treatments have been investigated and implemented. 13 -16 Our recent study has established the feasibility of using transit fluence to monitor volume change in HNC patients 17 without additional patient exposure or significant workload increase to the therapists. However, no prior study has investigated the relationship between the patients’ self-reported outcome and corresponding transit fluence change as of the time of this study. This study focuses on the investigation of the effectiveness of using transit fluence change as a replanning trigger in ART. To evaluate the effectiveness, we hypothesize that the change of transit fluence is associated with the changes in dose deposition in critical structures, such as the salivary glands and pharyngeal constrictor muscles (PCM), and the dose change is associated with the risk of dysphagia.
Method
Transit Fluence Monitoring
Transit fluence is the sum of the attenuated primary fluence and the scatter fluence of any treatment field leaving a patient. In this study, the transit fluence was measured with the Watchdog (WD) transit dosimetry system 16 utilizing the on board electronic portal imager (EPID) in portal dosimetry mode. About 3000 to 4000 cine images per treatment were captured by WD. Every pixel of all the images for a given treatment session were summed across all treatment arcs to give an integrated transit fluence. Twenty-four patients, who were on a retrospective protocol, were randomly selected and monitored with WD daily and CBCT weekly. All patients were treated with normal fractionated 70 Gy treatments. About 76% of the patients also received chemotherapy. All treatments were delivered with a 6 MV photon beam using a Volumetric Modulated Arc Therapy (VMAT) technique with millennium 120 (M120) multi-leaf collimators (MLC). Patients were immobilized with custom thermoplastic facemasks. A pair of kV-kV images was used to register the bony landmark to the digitally reconstructed radiographs from the planning computed tomography (CT) on daily treatment setup. The residual daily setup variation is typically small but contributes to the uncertainty of the measurements. The region of interest (ROI) of each patient was defined by the outer contour of the patient between approximate spine levels C1 and C4, essentially the neck and the mandible inside the beam’s eye view. The average integrated transit fluence intersecting the projected ROI of each patient at each fraction, ϕ, was calculated from the corresponding WD measurement. The CBCT, performed on the same day, was used to determine the volume of the ROI. The changes on the nth day relative to the day 0 baseline in the transit fluence, Δϕn, and the volume of the ROI, ΔVROI, were defined as the ϕn − ϕo and VROI, n − VROI,0 respectively. It has been reported that a change of 10% of neck separation, 18 approximately equivalent to 5% volume change, is associated with the increase risk of grade 2 xerostomia which is a major contributing factor to dysphagia. 19 In this study, a 5% volume change, as determined by the CBCT, was thus used as the ART replanning trigger point, Trig(ΔVROI, n). When this Trig(ΔVROI, n) was reached, it would initiate the replanning process which typically include planning review by the clinicians to determine the necessity of a new adapted plan. The logistic regression was performed between the ART replanning trigger point and Δϕn. − min(Δϕn) where min(Δϕn) is the minimum fluence change in the patient cohort. The fitted parameter, β1 and the intercept, β0, were used to determine the Δϕn replanning trigger point, Trig(Δϕn).
The correlation and the area under the curve (AUC) of the receiver operating characteristic (ROC) curve of the logistic regression with a 5% volume change trigger between Δϕn and ΔVROI were computed and compared with our previous study 17 to assess the reproducibility. Additional volumetric triggers, 0.0%, −2.5% and −7.5%, were also used to calculate assess the stability of the AUC.
Quality of Life (QoL)
Twenty-one of the 24 patients filled out an MD Anderson Dysphagia Inventory (MDADI) questionnaire, before and after the radiation treatment course. Each set of questions was sub-divided into 4 subsets 20 : global, emotional, physical and functional. In brief, these are respectively the patient’s self-reported overall assessment of their daily routine, their affective response, their self-perception, and the impact on daily activities of their swallowing disorder. Each was graded according to MDADI protocol. The score of each subset is defined as the sum of the scores of the questions in the corresponding subset. The total MDADI score is the sum of all the subset question scores. To reduce the impact of outliers, the association between each of the 4 subsets of MDADI and each relevant factor were analyzed using ranked Pearson correlation. These factors are age, gender, planned mean dose to a structure composed of parotids and sub-mandibular glands <Dsg>, weight change ΔW, ΔVROI, and Δϕn . The P-value of the ranked Pearson correlation factors, ρ, were used to assess the significance of the association. P-values of less 0.05 and 0.10 were used for the thresholds for the classification of the statistically significant and clinically important association 21 respectively. A lower post-treatment MDADI score means a decrease of QoL resulting from dysphagia 20,22 and a change of 10 points or greater means a clinically significant deterioration in QoL. 23,24
Results
Transit Fluence Monitoring
The change in the ROI transit fluence was found to be strongly correlated with ROI volume. Nine of the 24 patients exhibited larger than 5.0% ROI volumetric reduction at some point in their treatment courses (Range 6% to 11%). For these patients a corresponding increase in transit fluence was observed (range 2% to 7%). The time observed for the volume to decrease by 5.0% or more was variable, ranging from 10 to 29 days from the start of treatment. For patients with a ROI volume change of less than 5%, the increase in transit fluence was also smaller (less than 3.5%). Excluding the baseline points, a total of 110 pairs of ΔVROI and Δϕn were obtained. The correlation was found to be −0.84 with the P-value less than 0.001. Figure 1 shows the scatter plot of ΔVROI and Δϕn of all the patients The R2 of the linear regression between ΔVROI and Δϕn is 0.70 with a slope of the regression of −1.39. The χ2 statistic of the model is 17.4 (P-value < 0.001). The β0 and β1 of the logistic regression are −10.04 and 99.38 respectively. The corresponding P-values of β0 and β1 are 0.001 and 0.003 respectively indicating that both factors are statistically significant. The logarithmic odds ratio (OR) of Δϕn was found to be 87.0 with a 95% confident interval of [27.4, 146.6]. With 5% volume change threshold, the Trig(Δϕn) was found to be 4.6%. Using Trig (ΔVROI, n) as the ground truth and Trig(Δϕn), the receiver operating curve (ROC) of Δϕn was calculated. The AUC of the ROC is 0.91 (Figure 2). Volumetric threshold of 0.0%, −2.5% and −7.5% were also plotted in Figure 2 for comparison. The AUC were found to be 0.88, 0.89 and 0.95 for 0.0%, −2.5% and −7.5% volume threshold. This indicates a statistically significant association between the transit fluence change and the corresponding volumetric change based replanning trigger. The observed AUC did also not vary significantly within the threshold level of this study.
Figure 1.
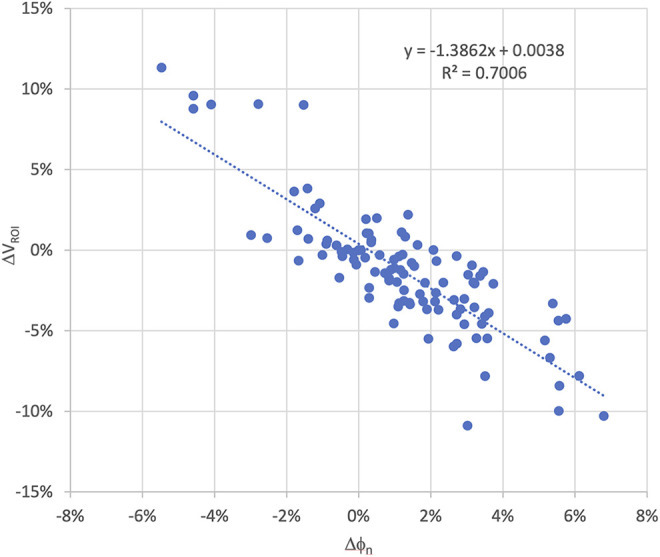
Scatter plot of the transit fluence variation and CBCT volumetric changes of all 24 the patients to assess the correlation between fluence and volume change during the course of treatment.
Figure 2.
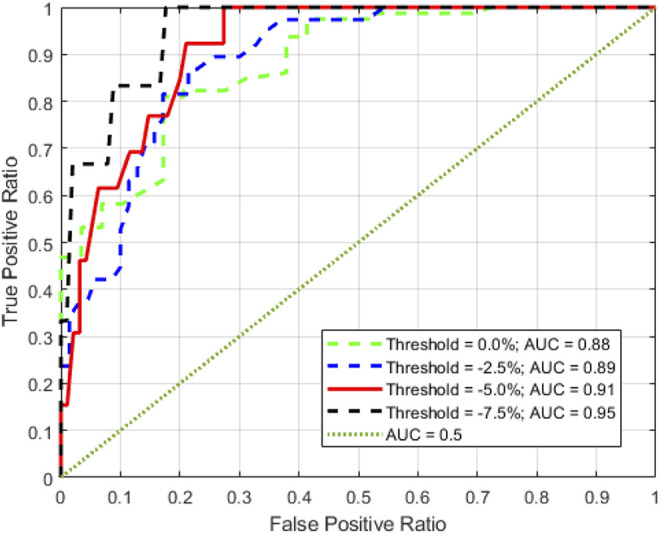
The solid line represents the ROC curve (AUC = 0.91) of Δϕn using 24 patients and −5.0% volumetric change. The green, blue and black dashed lines represent the volumetric change threshold of 0.0%, −2.5% and −7.5%, respectively; the dotted line represents the reference ROC curve with AUC of 0.50.
Quality of Life
Twenty-one of the 24 patients in this study answered the pre- and post-treatment MDADI questionnaire. The average and standard deviation of the total score were found to be 62.8 and 6.8 respectively. The gender mix was found to be 29% female and 71% male. The median age was 63 years old. The maximum and minimum were 86 and 32 years old respectively. The median weight and ROI volume were found to be 80.3 kg (range 57.3 to 118.3 kg) and 1530.4 cm3 (range 658.7 to 1942.8 cm3). The average weight change was found to be −6.0 kg (range +5.7 kg to −12.8 kg).
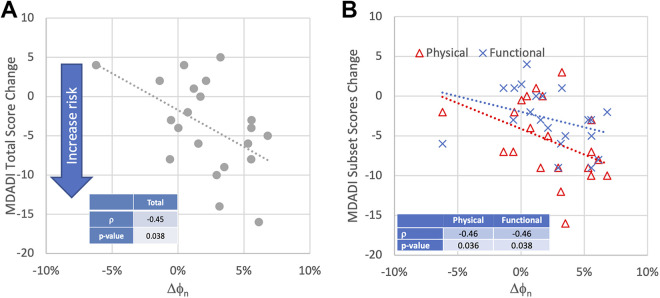
Table 1 shows the summary of the ranked correlation coefficients of age for global, emotion, physical, functional and total ranked correlation factors with the parameters and the corresponding P-values. Age, gender and weight change were found to have no statistically significant correlation with MDADI. The relationship between <Dsg> and total MDADI were also found to be insignificant, although a clinically important association was found between the functional subset and <Dsg>. The most important correlates with change in MDADI were found to be ΔVROI and Δϕn. Three of the 4 MDADI subsets, global, physical and functional, were found to be statistically significant correlated with ΔVROI. The MDADI total was also found to have a relational trend with ΔVROI with possible clinically importance (P-value = 0.073). Global, emotion, physical and functional subsets ranked correlation with Δϕn were found to be −0.35, 0.23, −0.46 and −0.46, respectively. The total MDADI change ranked correlation with Δϕn was found to be −0.45 (Figure 3A). Figure 3B shows the relationship between 2 of the MDADI subset changes, physical and functional, and the Δϕn. The significant ranked correlations were found to be between 0.036 and 0.038 with P-values less than 0.05 indicating a statistically significant correlation between transit fluence change and HNC patient self-reported outcome. Three out of the 21 patients were found to have a decrease of more than 10 points in their total scores, indicating a clinically significant deterioration of QoL. The associated Δϕn were found to be in range between 2.9% to 6.1%.
Table 1.
Ranked Correlation Between MDADI and Factors.
| Global | Emotion | Physical | Functional | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value | |
| Age | 0.22 | 0.33 | 0.14 | 0.56 | 0.08 | 0.74 | −0.08 | 0.73 | 0.30 | 0.18 |
| Gender | −0.30 | 0.18 | −0.23 | 0.32 | 0.12 | −0.60 | −0.17 | 0.47 | 0.09 | 0.71 |
| <Dsg> | −0.04 | 0.88 | 0.28 | 0.22 | −0.21 | 0.36 | −0.39 | 0.08 | −0.13 | 0.59 |
| Δweight | 0.29 | 0.20 | 0.06 | 0.80 | 0.03 | 0.89 | 0.18 | 0.43 | 0.11 | 0.65 |
| ΔVROI | 0.44 | 0.05 | −0.34 | 0.13 | 0.47 | 0.03 | 0.44 | 0.05 | 0.40 | 0.07 |
| Δϕn | −0.35 | 0.12 | 0.23 | 0.32 | −0.46 | 0.04 | −0.46 | 0.04 | −0.45 | 0.04 |
Figure 3.
The relationship between transit fluence change, Δϕn, and (A) total MDAD score change; (B) functional and physical MDADI subset scores change. The negative score change indicates the increase risk of quality of life degradation.
Discussion
With the increased patient number since the previous study, 17 Δϕn continues to show statistically significant correlation with ΔVROI. Here the correlation coefficient was found to be slightly higher indicating a stronger relationship between Δϕn and ΔVROI. In the cases with ΔVROI less than 5%, amounting to about 63% of all cases, both CBCT and transit fluence change show only minor volumetric change. This is an indication that ART is likely not necessary for these patients. The observed wide variation in the replanning trigger point timing indicates that a pre-determined replanning time is not an optimal strategy, as also suggested by van Dijk et al 12 and Marzi et al. 11 Together with the AUC of the ROC, this data set indicates that Δϕn can give useful information to physicians to support their ART decisions in terms of adaptation timing and patient selection without incurring a large increase in clinical workload.
With the increased number of patients in the current study, about 71% of the transit fluence change can be attributed to the ROI volume change indicating a closer relationship between transit fluence and volume change from the previous study. 17 However, the day-to-day variability in patient setup and machine performance will contribute some noise to the transit fluence signal. As OR > 0 is associated with higher odds of outcome, 21 the high OR of Δϕn indicates that a higher fluence transmission is associated with higher probability 21 of replanning. The study also demonstrates that the Δϕn signal has good accuracy with an AUC of 0.91. The AUC of a typical 25 ultrasound and a CT are 0.81 and 0.93 respectively. As volumetric change is a good predictor for the degradation of QoL, change in transit fluence has the potential to predict the volumetric changes during RT and act as a DSM for ART.
Similar to other studies, 26,27 age and gender were found to have no significant correlation with the change of QoL. Surprisingly, weight change during treatment was also found to have no significant association in this study. <Dsg> and normal tissue complication probability 28,29 (NTCP) are very effective in predicting high grade xerostomia prior to treatment. 8,26,30,31 Early dysphagia was also found to be significantly related to early xerostomia. 32,33 Interestingly, our study found <Dsg> is not an effective predictor of QoL. Van Dijk et al 34 and You et al 18 have also found that <Dsg> is not an effective predictor for xerostomia during treatment. As the planned dose of these patients were already within accepted planning guidelines 8 to minimize xerostomia, <Dsg> was found to be a weak predictor of xerostomia risk in line with Galbry’s study. 35 These factors may contribute to the weak association between <Dsg> and the outcome in this study. As this study does not measure xerostomia directly, a more specific patient self-reporting measure, such as European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—H&N35 (EORTC QLQ-H&N35), 36 and more sophisticated dosimetric metrics, such as dose gradient or parotid dose change, may be more useful. 37 Such investigation falls outside of the scope of the current study.
For ART, volumetric changes determined from parotid gland contours 12,22,27,38 and neck separation 18 are found to be stronger and earlier predictors of QoL degradation than dosimetric metrics. 12 Volumetric changes are also believed to be more strongly associated with stem cell sterilization. 39 The association between ΔVROI, i and the MDADI found in this study is consistent with these published findings. Interestingly, significant correlation was also found between Δϕn and the MDADI total score change. Looking at the subset decomposition, both DSM’s were also found to be associated with the physical and functional subsets of MDADI. This implies that using either DSM as an early ART replanning signal can potentially improve outcome in HNC treatment. Given the relatively less computational demanding implementation of Δϕn, it may hold an advantage over the more computationally intensive salivary specific version in the case of clinical and automation implementation. In terms of trigger level, the replanning trigger of 5%, based on the xerostomia study, may have been too relaxed as 2 of the 3 patients, who exhibited more than 10 points drop in MDADI, had less than 5.0% increase in the Δϕn. A lower Δϕn trigger, such as 2.5%, may be a more appropriate replanning trigger point without the significant decrease in the AUC of the ROC.
Although the current study is based on relatively small sample of 24 patients to demonstrate the feasibility of the transit fluence based ART replanning trigger DSM, the correlation with patient reported outcomes, associated with QoL degradation, was very encouraging. A larger data set will be beneficial to further verify the relationship between clinical outcome and the DSM. Automation of this analysis process can expedite the current labor-intensive ART workflow and broaden its clinical implementation. Dysphagia has also been reported to correlate significantly with the dose to PCM. 40,41 The relationship between the transit fluence change and the increase in dose deposition to the PCM will be further studied.
Conclusion
To the best of our knowledge, this is the first study to demonstrate the statistically significant correlation between the change in transit fluence and the change in quality of life which is associated with dysphagia. It can not only be used as an early signal in assisting clinicians in the ART patient selection for replanning, but also lowers the resource barrier of ART implementation.
Footnotes
Authors’ Note: Statistical analysis was performed by Lim SB, PhD. Our study was approved by Memorial Sloan Kettering Cancer Center Institution Review Board (approval no. 18-257). This is a retrospective study and does not require informed consent. Patient data will not be shared with outside parties.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
ORCID iD: Seng Boh Lim, PhD  https://orcid.org/0000-0002-4054-8789
https://orcid.org/0000-0002-4054-8789
References
- 1. Brouwer CL, Steenbakkers R, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285–294. [DOI] [PubMed] [Google Scholar]
- 2. Brown E, Owen R, Harden F, et al. Predicting the need for adaptive radiotherapy in head and neck cancer. Radiother Oncol. 2015;116(1):57–63. [DOI] [PubMed] [Google Scholar]
- 3. Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol (London, England). 2015;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guidi G, Maffei N, Meduri B, et al. A machine learning tool for re-planning and adaptive RT: a multicenter cohort investigation. Phys Med. 2016;32(12):1659–1666. [DOI] [PubMed] [Google Scholar]
- 5. Castadot P, Lee JA, Geets X, Gregoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol. 2010;20(2):84–93. [DOI] [PubMed] [Google Scholar]
- 6. Cooper JS, Fu K, Marks J, Silverman S. Late effects of radiation-therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31(5):1141–1164. [DOI] [PubMed] [Google Scholar]
- 7. Dijkema T, Raaijmakers CPJ, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined Michigan and Utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78(2):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deasy JO, Moiseenko V, Marks L, Chao KSC, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3):S58–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Physics. 2009;75(3):924–932. [DOI] [PubMed] [Google Scholar]
- 10. Zhang P, Simon A, Rigaud B, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120(1):41–47. [DOI] [PubMed] [Google Scholar]
- 11. Marzi S, Pinnaro P, D’Alessio D, et al. Anatomical and dose changes of gross tumour volume and parotid glands for head and neck cancer patients during intensity-modulated radiotherapy: effect on the probability of xerostomia incidence. Clin Oncol. 2012;24(3):E54–E62. [DOI] [PubMed] [Google Scholar]
- 12. van Dijk LV, Brouwer CL, van der Laan HP, et al. Geometric image biomarker changes of the parotid gland are associated with late xerostomia. Int J Radiat Oncol Biol Phys. 2017;99(5):1101–1110. [DOI] [PubMed] [Google Scholar]
- 13. Fuangrod T, Greer PB, Woodruff HC, et al. Investigation of a real-time EPID-based patient dose monitoring safety system using site-specific control limits. Radiat Oncol. 2016;11(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rozendaal RA, Mijnheer B, Hamming-Vrieze O, Mans A, van Herk M. Impact of daily anatomical changes on EPID-based in vivo dosimetry of VMAT treatments of head-and-neck cancer. Radiother Oncol. 2015;116(1):70–74. [DOI] [PubMed] [Google Scholar]
- 15. Rozendaal RA, Mijnheer BJ, van Herk M, Mans A. In vivo portal dosimetry for head-and-neck VMAT and lung IMRT: linking gamma-analysis with differences in dose-volume histograms of the PTV. Radiother Oncol. 2014;112(3):396–401. [DOI] [PubMed] [Google Scholar]
- 16. Woodruff HC, Fuangrod T, Van Uytven E, et al. First experience with real-time EPID-based delivery verification during IMRT and VMAT Sessions. Int J Radiat Oncol Biol Phys. 2015;93(3):516–522. [DOI] [PubMed] [Google Scholar]
- 17. Lim SB, Tsai CJ, Yu Y, et al. Investigation of a novel decision support metric for head and neck adaptive radiation therapy using a real-time in vivo portal dosimetry system. Technol Cancer Res Treat. 2019;18:1533033819873629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. You SH, Kim SY, Lee CG, et al. Is there a clinical benefit to adaptive planning during tomotherapy in patients with head and neck cancer at risk for xerostomia? Am J Clin Oncol-Cancer Clin Trials. 2012;35(3):261–266. [DOI] [PubMed] [Google Scholar]
- 19. Marcott S, Dewan K, Kwan M, Baik F, Lee YJ, Sirjani D. Where dysphagia begins: polypharmacy and xerostomia. Fed Pract. 2020;37(5):234–241. [PMC free article] [PubMed] [Google Scholar]
- 20. Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Ach Otolaryngol Head Neck Surg. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 21. Bland M. An Introduction to Medical Statistics. 3rd ed. Oxford University Press; 2000. [Google Scholar]
- 22. Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutcheson KA, Barrow MP, Lisec A, Barringer DA, Gries K, Lewin JS. What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? Laryngoscope. 2016;126(5):1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin A, Murray L, Sethugavalar B, et al. Changes in patient-reported swallow function in the long term after chemoradiotherapy for oropharyngeal carcinoma. Clin Oncol. 2018;30(12):756–763. [DOI] [PubMed] [Google Scholar]
- 25. Swets JA. Measuring the accuracy of diagnostic systems. Science (New York, NY). 1988;240(4857):1285–1293. [DOI] [PubMed] [Google Scholar]
- 26. Hawkins PG, Lee JY, Mao YP, et al. Sparing all salivary glands with IMRT for head and neck cancer: longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother Oncol. 2018;126(1):68–74. [DOI] [PubMed] [Google Scholar]
- 27. Sanguineti G, Ricchetti F, Wu B, McNutt T, Fiorino C. Parotid gland shrinkage during IMRT predicts the time to Xerostomia resolution. Radiat Oncol. 2015;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16(6):1623–1630. [DOI] [PubMed] [Google Scholar]
- 29. Lyman JT. Complication probability as assessed from dose volume histograms. Radiat Res. 1985;104(2):S13–S19. [PubMed] [Google Scholar]
- 30. Chao KSC, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49(4):907–916. [DOI] [PubMed] [Google Scholar]
- 31. Lee SW, Kang KW, Wu HG. Prospective investigation and literature review of tolerance dose on salivary glands using quantitative salivary gland scintigraphy in the intensity-modulated radiotherapy era. Head Neck. 2016;38:E1746–E1755. [DOI] [PubMed] [Google Scholar]
- 32. King SN, Dunlap NE, Tennant PA, Pitts T. Pathophysiology of radiation-induced dysphagia in head and neck cancer. Dysphagia. 2016;31(3):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Laan HP, Bijl HP, Steenbakkers RJ, et al. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiother Oncol. 2015;115(1):56–62. [DOI] [PubMed] [Google Scholar]
- 34. van Dijk LV, Brouwer CL, van der Schaaf A, et al. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother Oncol. 2017;122(2):185–191. [DOI] [PubMed] [Google Scholar]
- 35. Gabrys HS, Buettner F, Sterzing F, Hauswald H, Bangert M. Parotid gland mean dose as a xerostomia predictor in low-dose domains. Acta Oncol. 2017;56(9):1197–1203. [DOI] [PubMed] [Google Scholar]
- 36. Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17(3):1008–1019. [DOI] [PubMed] [Google Scholar]
- 37. Guo Y, Jiang W, Lakshminarayanan P, et al. Spatial radiation dose influence on xerostomia recovery and its comparison to acute incidence in patients with head and neck cancer. Adv Radiat Oncol. 2020;5(2):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belli ML, Scalco E, Sanguineti G, et al. Early changes of parotid density and volume predict modifications at the end of therapy and intensity of acute xerostomia. Strahlenther Onkol. 2014;190(11):1001–1007. [DOI] [PubMed] [Google Scholar]
- 39. Vissink A, van Luijk P, Langendijk JA, Coppes RP. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Diseases. 2015;21(1):E1–E10. [DOI] [PubMed] [Google Scholar]
- 40. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64–73. [DOI] [PubMed] [Google Scholar]
- 41. Tsai CJ, Jackson A, Setton J, et al. Modeling dose response for late dysphagia in patients with head and neck cancer in the modern era of definitive chemoradiation. JCO Clin Cancer Inform. 2017;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]