Abstract
Background
Nelfinavir (NFV), an HIV‐1 protease inhibitor, has been shown to sensitize cancer cells to chemoradiation (CRT). The objectives of this phase 1 trial were to evaluate safety and identify the recommended phase 2 dose of NFV added to concurrent CRT for locally advanced cervical cancer.
Methods
Two dose levels of NFV were evaluated: 875 mg orally twice daily (dose level 1 [DL1]) and 1250 mg twice daily (DL2). NFV was initiated 7 days before CRT and continued through CRT completion. Toxicity, radiographic responses, and pathologic responses were evaluated. Serial tumor biopsies (baseline, after NFV monotherapy, on NFV + CRT, and posttreatment) were evaluated by immunohistochemistry, NanoString, and reverse‐phase‐protein‐array analyses.
Results
NFV sensitized cervical cancer cells to radiation, increasing apoptosis and tumor suppression in vivo. Patients (n = 13) with International Federation of Gynecology and Obstetrics stage IIA through IVA squamous cell cervical carcinoma were enrolled, including 7 patients at DL1 and 6 patients at DL2. At DL1, expansion to 6 patients was required after a patient developed a dose‐limiting toxicity, whereas no dose‐limiting toxicities occurred at DL2. Therefore, DL2 was established as the recommended phase 2 dose. All patients at DL2 completed CRT, and 1 of 6 experienced grade 3 or 4 anemia, nausea, and diarrhea. One recurrence was noted at DL2, with disease outside the radiation field. Ten of 11 evaluable patients remained without evidence of disease at a median follow‐up of 50 months. NFV significantly decreased phosphorylated Akt levels in tumors. Cell cycle and cancer pathways also were reduced by NFV and CRT.
Conclusions
NFV with CRT is well tolerated. The response rate is promising compared with historic controls in this patient population and warrants further investigation.
Keywords: carcinoma, chemoradiotherapy, nelfinavir, squamous cell, uterine cervical neoplasms
Short abstract
In a phase 1 clinical trial, nelfinavir, an HIV protease inhibitor, was determined to be safe and tolerable when added to standard chemoradiation therapy for locally advanced cervical cancer. Cervical tumor pAkt and pS6 protein levels decrease with nelfinavir treatment, and cell cycle and cancer‐associated pathways are further reduced with nelfinavir added to chemoradiation.
Introduction
Cervical cancer is the second most common malignancy in women worldwide, and it remains a leading cause of cancer‐related deaths for women in developing countries. 1 , 2 Advanced‐stage cervical cancer disproportionately affects women of low socioeconomic backgrounds and of non‐white race. Despite treatment with current standard‐of‐care cisplatin‐based chemoradiation (CRT), up to 40% of these cancers will recur, leading to death from disease. 3 Human papillomavirus (HPV) is the etiologic agent of cervical cancer in 99.9% of cases worldwide. 4 , 5
Nelfinavir (NFV), an orally administered human immunodeficiency virus 1 (HIV‐1) protease inhibitor, has been shown to radiosensitize HPV‐related head and neck squamous cell carcinomas (HNSCCs) as well as non–HPV‐related pancreatic cancers in both in vivo and in vitro models. 6 , 7 , 8 In clinical trials, NFV increased the radiosensitivity of HNSCC and pancreatic cancers. 9 , 10 NFV has also been shown to induce autophagy, leading to apoptosis as monotherapy in HPV‐related cervical cancer cells and in‐ vivo. 11 NFV is an inhibitor of the PI3K/Akt signaling pathway, which has been implicated in both carcinogenesis and in resistance to radiation therapy in HPV‐related cancers, including cervical cancer. 6 , 12 , 13 , 14 Therefore, NFV is a rational targeted agent to combine with radiation for cervical cancer treatment.
NFV received approval from the US Food and Drug Administration (FDA) in 1997 for the treatment of adults and children with HIV infection and has over a decade of safety and pharmacokinetic data with minimal toxicity. 15 The most common side effect is mild diarrhea (grade ≤2) in up to 70% of patients. 10 , 16 NFV is now off‐patent, making it a potentially cost‐effective option for the treatment of cancer. In a phase 1 trial in locally advanced pancreatic cancer, NFV was added to the standard regimen of cisplatin, gemcitabine, and radiation; there was a 20% increase in the response rate (50% vs 30%) compared with historic controls, 10 and 1250 mg twice daily was defined as the recommended phase 2 dose (RP2D). NFV has also been combined with standard CRT followed by surgery in a phase 1 trial for locally advanced rectal cancer that defined 750 mg twice daily as the recommended dose. 17 A phase 1/2 trial evaluated NFV 1250 mg twice daily in combination with cisplatin‐based CRT for unresectable stage IIIA/IIIB non‐small‐cell lung cancer, and there was a significant increase in median overall survival to 41.1 months (95% CI, 19.0‐63.1 months) compared with 17 months in historic controls. 18 , 19 NFV has not yet been explored in clinical trials for the radiosensitization of locally advanced cervical cancer.
The current study was a 2‐institution phase 1 trial with the objectives of determining the safety, recommended RP2D, and genomic and proteomic effects of adding NFV to standard, concurrent CRT in locally advanced cervical cancer. Briefly, we demonstrated that NFV sensitizes cervical cancer cells and xenografts to CRT. We identified that the standard dose of NFV at 1250 mg twice daily was tolerable with combination CRT and is the RP2D for further studies in locally advanced cervical cancer. Biomarker studies indicated that phosphorylated Akt (pAkt) protein expression may be a biomarker for target engagement in preliminary studies. Cell cycle and apoptosis pathways were significantly affected by NFV in combination with CRT, but not by NFV alone.
Materials and Methods
Preclinical Studies
Cell cycle and apoptosis assay
HeLa and Caski cells were maintained in RPMI media. Cells were plated and treated with NFV at increasing concentrations (10, 15, 20, and 25 µM) or vehicle control (methanol) for 48 hours and subjected to fluorescence‐activated cell sorting (FACS) analysis using propidium iodide. Annexin V labeling with flow cytometric analysis was used to determine apoptosis after treatment for 72 hours. Results represent the mean ± SD of experiments performed in triplicate. Two‐way analyses of variance were used for statistical analysis, and the post‐hoc Tukey honestly significant difference test was used for between‐group analyses.
Clonogenic survival assay
Cultures in log growth were counted and plated as a single‐cell suspension in 60‐mm dishes. Cells were treated with 15 µM NFV irradiated with an X‐RAD 320iX Irradiator at a dose rate of 2.72 grays (Gy) per minute for 16 hours. Media containing NFV was removed 4 to 6 hours after radiation. Colonies were stained and counted 10 to 14 days after irradiation. The surviving fraction was calculated as follows: (number of colonies formed)/(number of cells plated × plating efficiency).
Tumor generation in mice and drug treatment
Female Ncr‐nu/nu mice were obtained from Charles River Laboratories and housed in the animal facilities of the University of Pennsylvania. All experiments were carried out in accordance with the University Institutional Animal Care and Use Committee guidelines. Tumors were initiated in the flanks of mice aged 5 to 7 weeks by subcutaneous injection of 1 × 106 HeLa cells suspended in 100 µL of Matrigel (BD Collaborative Research). From 5 to 10 mice were used per group. Tumors were palpable between 5 and 7 days post‐inoculation. Then, 10 mg/kg of NFV or vehicle control was administered intravenously for 3 doses: 2 days, 1 day, and 2 hours before irradiation. Tumor growth was recorded 3 times weekly using a digital caliper, and tumor volume was calculated using the formula (L × W2)/2, where L is the tumor length and W is the tumor width. Mice were killed when the tumor volume reached 1700 mm3 or if they showed any sign of significant distress. Differences in response were analyzed using a 2‐way analysis of variance to perform comparisons between treatment groups.
Clinical Trial
Eligibility
Patients with previously untreated, histologically confirmed, primary, locally advanced squamous cell cervical carcinoma (stage IIA, IIB, IIIA, IIIB, or IVA according to the 2009 International Federation of Gynecology and Obstetrics staging system) were eligible for the study. 20 Patients were required to have adequate bone marrow, renal, and hepatic function; an Eastern Cooperative Oncology Group performance status ≤2; and have seronegative HIV status. Patients who had positive para‐aortic lymph nodes on pretreatment imaging (either computed tomography [CT] or [18F]fluorodeoxyglucose positron emission tomography/CT) were excluded. Patients with a history of prior chemotherapy, pelvic or abdominal radiation, and those taking medications that are substrates of CYP3A4 and CYP2C19 were excluded. The institutional review boards at the University of Miami and the University of Pennsylvania approved this study, and all patients signed informed consent. The principal investigator (F.S.) for the trial changed institutions between the enrollment of the 2 dose level cohorts; therefore, the patients who received dose level 1 (DL1) were enrolled at the University of Miami, and those who received DL2 were enrolled at the University of Pennsylvania.
Trial design
The trial design is summarized in Figure 1. Patients began taking oral NFV twice daily at a starting dose 875 mg (dose level 1; DL1) 7 days before the initiation of CRT. NFV was continued at the prescribed dose level during the approximately 7‐week course of cisplatin and pelvic external‐beam radiation therapy (PEBRT) with endocavitary brachytherapy. The NFV dose was escalated to 1250 mg twice daily (dose level 2; DL2) according to the standard dose‐escalation design, as recommended by the National Cancer Institute and the Investigational Drug Steering Committee's Task Force on Clinical Trial Design. 21
Figure 1.

The phase 1 clinical trial design for nelfinavir (NFV) with chemoradiation in cervical cancer is illustrated. (A) Patients were enrolled at the time they were diagnosed with stage IB to IVA cervical cancer. Patients received 1 week of NFV pretreatment before the initiation of 7 to 9 weeks of standard‐of‐care chemoradiation with cisplatin and combined external‐beam radiotherapy (EBRT) and brachytherapy (XRT). Patients were evaluated for toxicity throughout treatment and at each follow‐up visit for 1 year. Imaging was performed at baseline and at 3, 6, and 12 months after treatment. Tumor biopsies were obtained (A) at baseline, (B) after 1 week of NFV pretreatment, (C1) at 4 weeks or (C2) 6 weeks after the initiation of chemoradiation, and (D) 3 months after treatment. Gy/fx indicates grays per fraction.
The intended PEBRT dose was from 45 to 50 Gy in 25 to 28 fractions to the planning tumor volume delivered once daily during the business week. Patients received low‐dose‐rate or high‐dose‐rate brachytherapy at the discretion of the radiation oncologist. Patients who were treated with low‐dose‐rate brachytherapy received 1 administration of brachytherapy, with a 40‐Gy dose delivered to anatomic point A, with the option of a second application at the discretion of the radiation oncology. Patients who were treated with high‐dose‐rate brachytherapy received from 24 to 28 Gy to anatomic point A in 4 or 5 applications. Patients who required extended‐field radiation therapy were excluded. All external‐beam and brachytherapy radiation plans were reviewed for quality assurance according to institutional practice. Standard chemotherapy, consisting of cisplatin 40 mg/m2, was administered once weekly for 6 cycles as concurrent therapy with radiation.
Toxicity evaluation
Dose‐limiting toxicities (DLTs) were defined using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.02, as any treatment‐related grade 3 or 4 non‐hematologic toxicity, grade 4 neutropenia lasting >7 days, grade 3 or 4 neutropenia associated with fever, grade 4 thrombocytopenia, grade 3 thrombocytopenia in the setting of bleeding that required transfusion, any toxicity that resulted in a delay of PEBRT for >1 week, or any toxicity resulting in the delay of NFV and/or cisplatin for >2 weeks. Patients were monitored for toxicities weekly during the 8 weeks of treatment and then every 3 months for 1 year. DLTs were evaluated starting the week of NFV monotherapy and weekly during CRT in combination with chemotherapy.
Measurement of response
All patients underwent CT or magnetic resonance imaging of the chest, abdomen, and pelvis before treatment initiation and at 3, 6, and 12 months post‐treatment for response assessment. Further imaging follow‐up was conducted every 6 to 12 months, according to institutional practice. All CT scans were scored using the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). 22 Changes in the greatest dimension (unidimensional measurement) of the tumor lesions and in the shortest dimension in the case of malignant lymph nodes were recorded using RECIST. Patients who had measurable disease at baseline, had received at least 1 cycle of chemotherapy, and had their disease re‐evaluated were considered evaluable for response. Measurable disease was defined as tumor that could be accurately measured in at least 1 dimension as ≥10 mm with a CT scan or clinical examination. Malignant lymph nodes were defined as lymph nodes ≥15 mm in the short axis when measured on a CT scan. A complete response (CR) was defined as the disappearance of all target lesions, a partial response (PR) was defined as a decrease ≥30% in the sum of the greatest dimensions of target lesions, progressive disease (PD) was defined as an increase ≥20% in the sum of the greatest dimensions of target lesions, and stable disease was defined as neither sufficient shrinkage to qualify for a PR nor a sufficient increase to qualify for PD. Recurrence‐free and overall survival were calculated using Kaplan‐Meier curves. Recurrence‐free survival was defined as the time from treatment initiation to recurrence or death from any cause.
Correlative Genomic and Proteomic Tumor Studies
Cervical biopsies were performed at 4 time points: before the initiation of treatment (time point A), after 1 week of NFV (time point B), at either week 4 (time point C1) or week 6 (time point C2) of combined NFV and CRT, and 3 months after the completion of treatment (time point D). Biopsies were originally obtained at week 6 (time point C2) of combined NFV and CRT; however, an analysis of the initial biopsies revealed minimal to no residual active tumor by week 6. Therefore, for the remainder of the trial, an additional biopsy was obtained at week 4 (time point C1). Biopsies were evaluated for the presence of tumor and expression of pAkt Ser473 (Leica Microsystems; catalog no. NCL‐L‐pAkt), pS6 Ser235/236 (Cell Signaling; catalog no. 4858L), p16INK4A (Roche Diagnostics; catalog no. 9517), CD3 (Leica Microsystems; catalog no. PA0553), CD8 (Agilent Dako; catalog no. M7103), and hypoxia‐inducible factor α (HIF1α) (Biocare Medical; catalog no. CME349B) by immunohistochemistry (IHC). Quantification of pAKT and pS6 activity was performed using ImageJ software and the methodology described by Nyugen et al. 23 HIF1α expression was graded as negative (−), weak positive (+), positive (++), or strong positive (+++) using the methodology described by Bai et al. 24 CD3 and CD8 quantification was assessed by counting the number of positive cells per 10 high‐power fields.
Tumor samples from fresh tumor biopsies that demonstrated >80% tumor cells using hematoxylin‐and‐eosin (H&E) staining also were evaluated by reverse‐phase protein array (RPPA) performed by the Proteomics Core at The University of Texas MD Anderson Cancer Center. In addition, RNA was extracted from the paraffin‐embedded biopsy samples using the Oncogenomics Core Facility at the University of Miami and from fresh tumors using the Oncogenomics Core at Wistar Institute for gene expression analysis. Gene expression was analyzed using a custom HPV NanoString panel and the commercially available NanoString PanCancer Immune Panel. Data from each of the 3 assays were analyzed separately. Analyses were performed on quantile‐normalized, log‐scaled NanoString data and normalized log‐scaled RPPA data. Batch effects between samples collected at different sites revealed by principal component analysis were corrected using the ComBat algorithm. 25 Pair‐wise group comparisons were performed using paired Student t tests, and estimation of the false‐discovery rate (FDR) to adjust for multiple testing was done using the Benjamini‐Hochberg algorithm, with significance defined as an FDR < 5% threshold unless stated otherwise. 26 Gene set enrichment analysis was performed with the DAVID algorithm using a combined set of features from all platforms as a reference set, and the top 10 biologic processes and top 5 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that passed an FDR < 5% threshold were reported unless stated otherwise. 27
Results
Preclinical Studies
It has been demonstrated that NFV targets the AKT pathway, sensitizing cancer cells to radiation. 6 , 7 , 12 At 72 hours, NFV treatment significantly induced G1 cell cycle arrest in HeLa squamous cervical carcinoma cells at 20 μM (77.9% in G1 phase; P = .04) and 25 μM (83.7% in G1 phase; P = .004) compared with controls (65.4% in G1 phase) and significantly decreased cells entering into S phase in both HeLa cells (4.7% in S phase; P = .03) and Caski cells (7.2% in S phase; P = .015) at 25 μM compared with controls (14.2% in S phase for both cell lines) (Fig. 2A). NFV treatment significantly induced apoptosis in HeLa cells at 20 μM (41.5% apoptotic cells; P < .0001) and 25 μM (68% apoptotic cells; P = .03) compared with controls (3.1% apoptotic cells) and also significantly induced apoptosis in Caski cells at 25 μM (62.6% apoptotic cells; P = .0009) compared with controls (3.7% apoptotic cells) after 72 hours of treatment (Fig. 2B). In addition, NFV treatment (15 μM) for 16 hours sensitized HeLa and SiHa cervical squamous cell carcinoma cells to x‐ray irradiation (Fig. 2C). Pretreatment with NFV significantly decreased the initial shoulder of the radiation survival curve, when sublethal DNA damage repair is accumulating but has not resulted in cell death. Subsequently, in athymic nude mice with HeLa tumor xenografts, pretreatment with NFV (10 mg/kg) before x‐irradiation resulted in significantly delayed tumor growth compared with radiation alone (Fig. 2D). These in vitro and in vivo studies provided the preclinical data to support the scientific rationale to evaluate whether NFV would further sensitize cervical cancer to radiation when added to CRT in patients with cervical cancer. A phase 1 study was initiated to first address safety and is presented here (ClinicalTrials.gov identifier NCT01485731).
Figure 2.
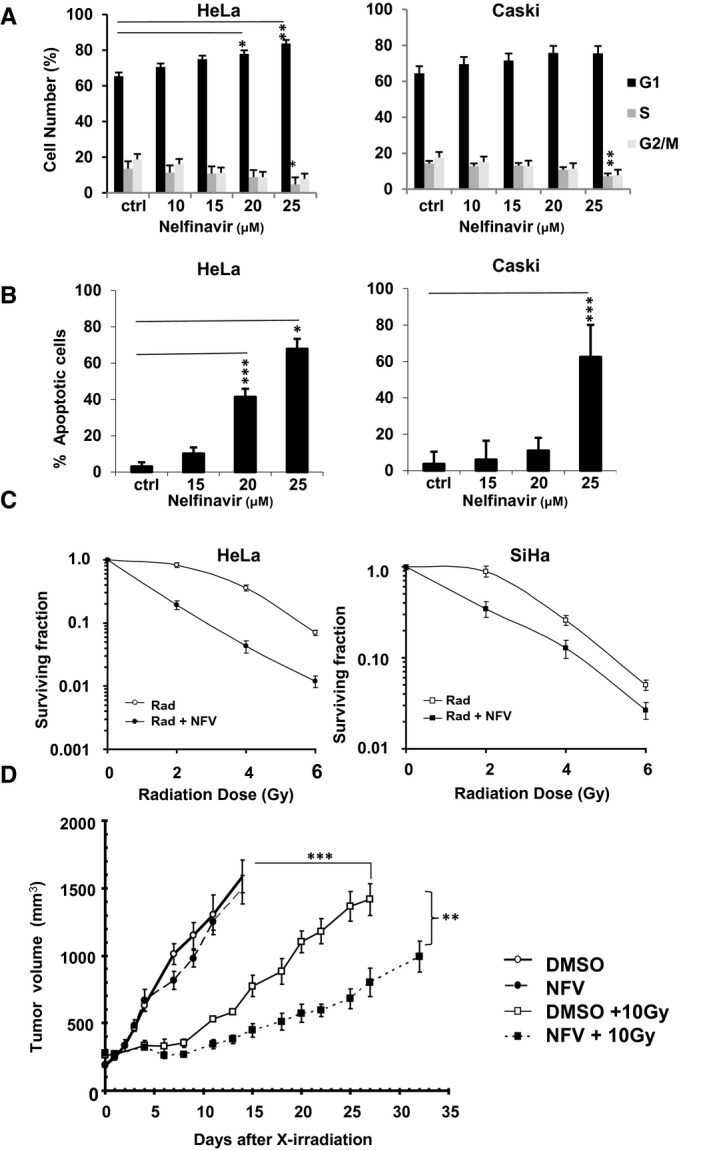
Nelfinavir (NFV) sensitizes cervical cancer cells and xenografts to radiation. (A) HeLa and Caski cells were treated with NFV (10, 15, 20, or 25 µM) for 72 hours and subjected to fluorescence‐activated cell‐sorting analysis with propidium iodide. The results represent the mean ± SD of experiments performed in triplicate, with between‐group comparison with controls (ctrl) performed using a 2‐way (ANOVA) with the post‐hoc Tukey honestly significant difference (HSD) test (*P < .05, **P < .01, ***P < .001). (B) HeLa and Caski cells were exposed to NFV (15, 20, or 25 µM) for 72 hours. Annexin V labeling with flow cytometric analysis was used to determine apoptosis. Results represent the mean ± SD of experiments performed in triplicate, with between‐group comparison with controls performed using a 2‐way ANOVA with the post‐hoc Tukey HSD test (*P < .05, **P < .01, ***P < .001). (C) HeLa or SiHa cells were grown in media containing either control solvent or NFV (15 µM) for 16 hours and then irradiated with 0, 2, 4, or 6 grays (Gy) of radiation. The surviving fraction is plotted on a log scale on the y‐axis versus the radiation dose (Rad) on the x‐axis. Each point on the survival curve represents the mean surviving fraction from at least 3 replicate dishes ± SD. (D) Tumor volume curves of mice implanted with HeLa cells are shown. HeLa xenografts were grown in nude mice, as detailed in the text (see Materials and Methods). There were 4 groups of 10 mice per group (with radiation, without radiation, with NFV, and without NFV). The NFV‐treated mice received 10 mg/kg NFV intravenously 48, 24, and 2 hours before irradiation. Tumor growth was tracked until their size reached 1500 mm3, at which time the mice were killed (n = 5‐10 mice per group). Data represent the mean as volume ± standard error of the mean using a 2‐way ANOVA (dimethyl sulfoxide [DMSO]/saline + 10 Gy vs NFV + 10 Gy, P < .01; DMSO/saline + 10 Gy vs NFV + DMSO/saline, P < .001).
Patient Characteristics
Thirteen patients with biopsy‐proven, locally advanced carcinoma of the cervix were enrolled from January 2012 to June 2016. Eleven patients completed 6 cycles of cisplatin (40 mg/kg) with whole‐pelvic external‐beam radiotherapy and brachytherapy. In total, 7 patients were accrued to DL1 because expansion to 6 patients was required after a second patient developed DLTs and 1 patient withdrew from the study. Six patients were enrolled at DL2. One patient at DL2 (patient 8) withdrew consent before initiating CRT in the setting of active cocaine use and paranoia. The remaining 11 patients were followed for toxicities and treatment response. One additional patient at DL2 (patient 12) withdrew from the study after 5 weeks because of an inability to tolerate treatment, specifically, the inability to swallow NFV tablets and multiple gastrointestinal complaints, none of which were individual criteria for removal from treatment. She was still evaluable for toxicity. Patient characteristics are listed in Table 1. The mean patient age was 50 years. All patients at DL1 had stage IIB squamous cell carcinoma of the cervix, whereas the patients at DL2 were distributed between stages IIA (1 patient), IIB (2 patients), IIIB (2 patients), and IVA (1 patient).
TABLE 1.
Clinical and Pathologic Patient Characteristics for Dose Level 1 and Dose Level 2
| Characteristics | No. of Patients (%) | ||
|---|---|---|---|
| DL1, n = 7 | DL 2, n = 6 | Overall, n = 13 | |
| Age: Median [range], Years | 50 [27‐57] | 50.5 [43‐65] | 50 [28‐65] |
| Race | |||
| Black | 3 (42.9) | 4 (66.7) | 7 (53.8) |
| Non‐Hispanic | 3 (42.9) | 3 (50.0) | 6 (46.2) |
| Hispanic | — | 1 (16.7) | 1 (7.7) |
| White | 4 (57.1) | 2 (33.3) | 6 (46.2) |
| Non‐Hispanic | — | 2 (33.3) | 2 (15.4) |
| Hispanic | 4 (57.1) | — | 4 (30.7) |
| Histology | |||
| Squamous cell | 7 (100.0) | 6 (100.0) | 13 (100.0) |
| Adenocarcinoma | — | — | — |
| Stage, FIGO 2009 | |||
| IIA | — | 1 (16.7) | 1 (7.7) |
| IIB | 7 (100.0) | 2 (33.3) | 9 (69.2) |
| IIIA | — | — | — |
| IIIB | — | 2 (33.3) | 2 (15.3) |
| IVA | — | 1 (16.7) | 1 (7.7) |
| Positive pelvic lymph nodes on CT/PET‐CT | 4 (57.1) | 3 (50.0) | 7 (53.8) |
Abbreviations: CT, computed tomography; DL1, dose level 1; DL2, dose level 2; FIGO, International Federation of Gynecology and Obstetrics; PET, positron emission tomography.
Toxicities
Twelve patients were evaluable for toxicity in DL1 and DL2 (Table 2). At DL1, 6 patients were evaluable for toxicity assessment. One patient experienced DLTs (grade 4 hypokalemia and hypomagnesemia and grade 3 diarrhea) because of noncompliance with management of nausea and diarrhea. Another patient at DL1 experienced grade 3 vomiting for 1 day because of noncompliance with antiemetics, which was attributed to chemotherapy administration and resolved once she used oral antiemetics as instructed. Grade 4 lymphopenia was observed in 1 patient and grade 3 lymphopenia was observed in 3 patients, but these toxicities did not result in treatment delays, and the patients recovered after completion of CRT. Another patient experienced grade 3 urinary obstruction and grade 3 thromboembolism, which we deemed were related not to NFV but to the underlying disease process. Grade 3 hypertension was observed in a patient who was not compliant with antihypertensive therapy and had poorly controlled hypertension before initiation of the trial. As stated above, 1 patient at DL1 withdrew study consent before the initiation of CRT and thus was not eligible for toxicity analysis. All other patients completed the treatment course with no significant delays. No patients required a dose reduction of NFV.
TABLE 2.
Incidence of Treatment‐Related Adverse Events During Treatment With Nelfinavir and Chemoradiation a
| Toxicity During NFV + CRT | Grade 1 | Grade 2 | G1/G2 | Grade 3 | Grade 4 | G3/G4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 | DL2 | DL1 | DL2 | DL1 | DL2 | DL1 | DL2 | DL1 | DL2 | DL1 | DL2 | |
| Acute hematologic toxicity | ||||||||||||
| Anemia | 2 | 1 | 4 | 2 | 6 | 3 | 1 | 1 | ||||
| Leukopenia | 1 | 1 | 4 | 1 | 5 | 3 | 3 | |||||
| Neutropenia | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | ||||
| Lymphopenia | 4 | 1 | 5 | |||||||||
| Thrombocytopenia | 2 | 2 | ||||||||||
| Acute nonhematologic toxicity | ||||||||||||
| Gastrointestinal | ||||||||||||
| Nausea | 3 | 3 | 3 | 1 | 6 | 4 | 1 | 1 | ||||
| Vomiting | 3 | 2 | 1 | 2 | 4 | 1 | 1 | |||||
| Diarrhea | 1 | 2 | 4 | 2 | 5 | 4 | 1 | 1 | 1 | 1 | ||
| Constipation | 2 | 2 | 1 | 2 | 3 | |||||||
| Anorexia | 1 | 1 | 3 | 4 | 4 | 5 | ||||||
| Flatulence | 4 | 5 | 4 | 5 | ||||||||
| Dyschezia | 2 | 2 | ||||||||||
| Hematochezia | 2 | 2 | ||||||||||
| Abdominal pain | 1 | 1 | 1 | |||||||||
| Metabolic | ||||||||||||
| Hyperglycemia | 5 | 4 | 1 | 5 | 5 | |||||||
| Hyponatremia | 2 | 5 | 2 | 5 | ||||||||
| Hypokalemia | 1 | 4 | 1 | 2 | 4 | 1 | 1 | |||||
| Hyperkalemia | 1 | 2 | 1 | 2 | 2 | |||||||
| Hypercalcemia/hypocalcemia | 2 | 2 | 1 | 1 | ||||||||
| Hypomagnesemia | 2 | 5 | 2 | 4 | 5 | 1 | 1 | |||||
| Hypoalbuminemia | 3 | 3 | ||||||||||
| Elevation of ALT/AST | 2 | 1 | 2 | 1 | ||||||||
| Creatinine elevation | 1 | 1 | 1 | 1 | 2 | 2 | ||||||
| Genitourinary | ||||||||||||
| Cystitis | 4 | 2 | 6 | |||||||||
| Vaginal bleeding/discharge | 3 | 1 | 2 | 5 | 1 | |||||||
| Dysuria | 4 | 4 | ||||||||||
| Hematuria | 4 | 4 | 1 | 1 | ||||||||
| Pelvic pain | 3 | 1 | 1 | 4 | 4 | 5 | ||||||
| Constitutional | ||||||||||||
| Fatigue | 2 | 1 | 2 | 2 | 3 | |||||||
| Flushing | 2 | 2 | ||||||||||
| Weight loss | 2 | 2 | ||||||||||
| Neuropsychological | ||||||||||||
| Tinnitus | 2 | 3 | 2 | 3 | ||||||||
| Vasomotor symptoms | 2 | 2 | ||||||||||
| Peripheral sensory neuropathy | 2 | 3 | 2 | 3 | ||||||||
| Depression/anxiety | 2 | 2 | ||||||||||
| Headache | 2 | 2 | ||||||||||
| Vision changes | 2 | 2 | ||||||||||
Abbreviations: ALT/AST, alanine and aspartate aminotransferase levels; CRT, chemoradiation; DL1, dose level 1; DL2, dose level 2; G1/G2, grade 1 and 2 toxicities combined; G3/G4, grade 3 and 4 toxicities combined; NFV, nelfinavir.
Toxicity was graded at each dose level according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.02.
Of 5 patients who were evaluable for toxicity at DL2, 1 experienced grade 3 nausea and ileus, which necessitated hospitalization for intravenous hydration, bowel rest, and optimization of bowel regimen. Her symptoms were subsequently well controlled on oral antiemetics and laxatives, and she was able to complete treatment without further delay. Another patient experienced grade 3 diarrhea that improved to grade 0 or 1 within 24 hours of starting antidiarrheal medication. This patient reported difficulty swallowing pills (including study drug) and continued to experience multiple low‐grade (grade 1‐2) gastrointestinal complaints (nausea, occasional vomiting, cramping pain, reflux, general malaise), and she ultimately withdrew from the study after 5 weeks because of an inability to tolerate treatment, although none of her symptoms individually met criteria for removal from treatment. She completed CRT without NFV and continued to be followed for toxicity and response according to the study protocol. All other patients completed the treatment course without significant delays. One patient required a dose reduction of cisplatin because of impaired renal function secondary to obstructive uropathy from her cervical lesion. She experienced grade 3 hematuria, which was attributed to tumor invasion into the bladder and to the presence of her diverting percutaneous nephrostomy tubes. No patients required a dose reduction of NFV. Although only 5 of 6 patients enrolled at DL2 were eligible for evaluation of response, enrollment to the study was terminated at this point because the RP2D was identified (primary endpoint) and secondary to the lack of funding to continue an additional expansion phase.
Response and Follow‐Up
Table 3 illustrates treatment by NFV dosing level, radiation dose, disease response at 3 months after completion of treatment, and disease status at the end of follow‐up for the 11 evaluable patients. The median overall follow‐up was 50 months (range, 14‐69 months). Response to treatment was assessed by radiologic (RECIST) and pathologic assessments 3 months after the completion of NFV and CRT. At DL1, 1 (16%) patient demonstrated a partial radiologic response, and 5 patients (83%) demonstrated complete radiologic response by RECIST criteria. Four patients at DL1 had a biopsy performed at time point D, and all 4 demonstrated a complete pathologic response. Patients 4 and 7 did not have a biopsy performed at time point D, and both demonstrated partial pathologic responses from biopsies performed during CRT (week 6, at time point C2). There were no disease recurrences at DL1 at a median follow‐up of 64 months (range, 50‐69 months).
TABLE 3.
Treatment and Response to Therapy
| Patient No | NFV Dose, mg | Stage: FIGO 2009 | NFV Completed | EBRT Dose, Gy | BT Type | BT Dose, Gy | Size of Target Lesion, cm | Response by RECIST | Pathologic Response After Treatment | Recurrence: Yes/No | Length of Follow‐Up, mo | Disease Status at Last Contact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 875 BID | IIB | Yes | 47.6 | LDR | 24 | 8.0 | PR | CR | No | 50 | NED |
| 2 | 875 BID | IIB | Yes | 45 | LDR | 24 | 6.0 | CR | CR | No | 51 | NED |
| 3 | 875 BID | IIB | Yes | 41.4 | LDR | 24 | 6.0 | CR | CR | No | 69 | NED |
| 4 | 875 BID | IIB | Yes | 45 | LDR | 28 | 7.0 | CR | — | No | 68 | NED |
| 5 | 875 BID | IIB | Yes | 45 | LDR | 27.9 | 8.0 | CR | CR | No | 67 | NED |
| 6 | 875 BID | IIB | No | — | — | — | — | — | — | — | — | — |
| 7 | 875 BID | IIB | Yes | 45 | LDR | 28 | NA | CR | — | No | 61 | NED |
| 8 | 1250 BID | IIB | No | — | — | — | — | — | — | No | — | — |
| 9 | 1250 BID | IIIB | Yes | 39.6 | HDR | 27.5 | 5.6 | CR | — | No | 33 | NED |
| 10 | 1250 BID | IIA | Yes | 45 + 7.2 | HDR | 27.5 | 3.4 | CR | CR | No | 33 | NED |
| 11 | 1250 BID | IIIB | Yes | 45 + 9 | HDR | 27.5 | 5.0 | CR | — | Yes | 14 | DOD |
| 12 | 1250 BID | IIB | No | 45 + 9 | HDR | 30 | 3.5 | CR | — | No | 25 | NED |
| 13 | 1250 BID | IVA | Yes | 45 | Syed | 50.4 | 6.5 | CR | CR | No | 33 | NED |
Abbreviations: —, not eligible/evaluable; BID, twice daily; BT, brachytherapy; CR, complete response; DOD, dead of disease; EBRT, external‐beam radiation therapy; FIGO, International Federation of Gynecology and Obstetrics; Gy, grays; HDR, high‐dose radiation; LDR, low‐dose radiation; NA, not applicable; NED, no evidence of disease; NFV, nelfinavir; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors.
At DL2, all patients who were evaluable for response demonstrated a CR according to RECIST criteria at 3 months postcompletion of treatment. Patients 10 and 11 demonstrated partial pathologic responses on biopsy while on treatment at time point C; however, no biopsy was performed at time point D for either patient. Patient 12 also demonstrated a partial pathologic response at time point C while on treatment, but no biopsy was performed at time point D because the patient had withdrawn consent for the trial at that point. Patient 13 demonstrated a complete pathologic response at time point C while on treatment but did not have a biopsy performed at time point D after the completion of therapy. Patient 11 had disease identified outside of the radiation field in the paraaortic and retrocrural lymph nodes at the first 3‐month scan after the completion of treatment. She was treated with carboplatin, paclitaxel, and bevacizumab, had disease progression on therapy, and ultimately died of disease 14 months after diagnosis. No other recurrences have been reported in the DL2 cohort at a median follow‐up of 33 months (range, 25‐33 months).
Overall, the median follow‐up for the combined DL1 and DL2 cohorts was 50 months (range, 14‐69 months). The overall and recurrence‐free survival rate was 91% for the combined DL1 and DL2 cohorts. The locoregional control rate was 100%, and no in‐field recurrences were observed.
Correlative Tumor Studies
Immunohistochemistry
For transcriptomic and proteomic biomarker studies to evaluate NFV treatment effects, patients underwent cervical tumor biopsies before treatment (time point A), after 1 week of NFV alone (time point B), at 4‐6 weeks while receiving NFV and CRT (time points C1 and C2), and at 3 months (time point D) after the completion of treatment (Fig. 2). Cervical tumor biopsies were evaluated by H&E and p16 staining to confirm cervical carcinoma and for signaling pathways known to be affected by NFV treatment, such as the PI3K/AKT pathway by pAKT and downstream effector pS6 staining, 6 , 7 HIF1α (a marker for hypoxia 24 , 28 ), and CD3/CD8 to evaluate T‐cell infiltration 29 , 30 by IHC (Fig. 3A,B; see Supporting Fig. 1). Cervical tumor H&E staining and staining for p16, pAKT, and pS6 at time points A and B for patient 13 are illustrated in Figure 3A as a representative example. H&E, p16, CD3, CD8, HIF1α, pAkt, and pS6 analyses at time points A, B, C and D are illustrated in Supporting Figure 1, and the staining is representative of the patterns observed across all patients.
Figure 3.
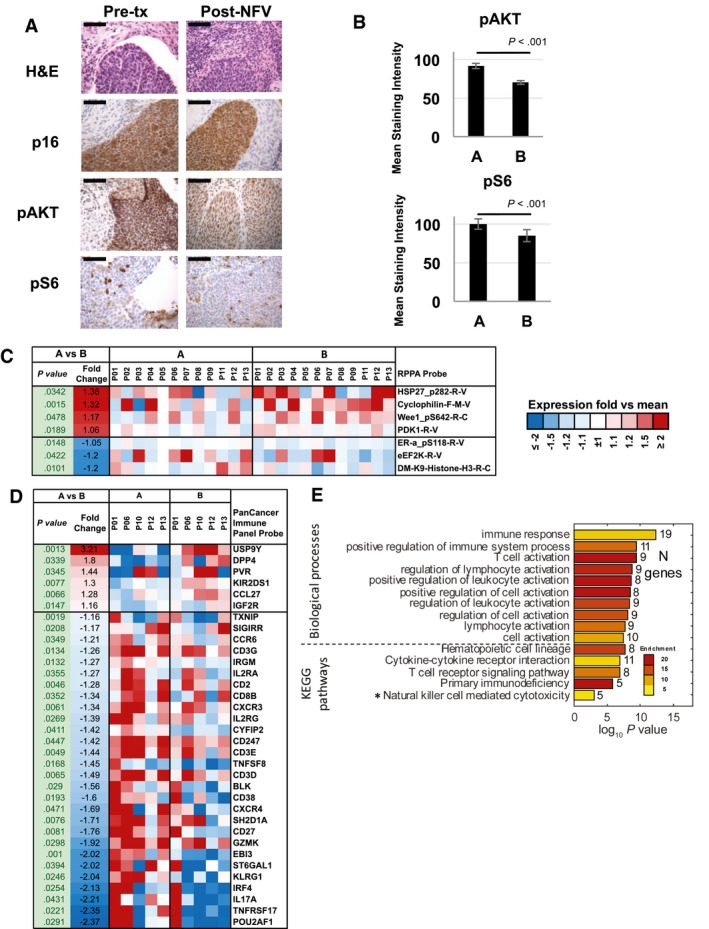
Proteomic and transcriptomic effects with nelfinavir (NFV) treatment alone at time point A versus time point B are illustrated. Tumor biopsies obtained (A) at baseline (pretreatment [Pre‐tx]) and (B) after 1 week of NFV pretreatment (Post‐NFV) were evaluated by H&E and p16 staining and with pAKT and pS6 immunohistochemistry (data from patient 13; original magnification ×40; scale bar = 1 mm). (B) pAKT and pS6 were quantified using ImageJ software for all patients. The mean staining intensity (MSI) was evaluated in 10 randomly selected fields within the tumor per slide. Bars represent the aggregate mean MSI for all samples at time point A versus time point B. (C) Protein from reverse‐phase protein array (RPPA) assay significantly changed (nominal P < .05) after NFV treatment alone. (D) Genes from the PanCancer Immune Panel significantly changed (nominal P < .05) after NFV treatment alone. (E) Biologic processes and pathways were significantly enriched (false‐discovery rate [FDR] < 5% [except *FDR = 6%]) among genes that were affected by NFV. KEGG indicates Kyoto Encyclopedia of Genes and Genomes.
In all patients, H&E staining revealed abundant tumor at baseline (time point A) and after 7 days (time point B) of treatment with NFV alone (Fig. 3A). Similarly, p16 staining was strong and diffuse at these 2 time points (A and B), correlating with the presence of tumor. Islands of tumor were variously observed in a background of stroma and inflammatory infiltrate after 4 or 6 weeks at both DL1 and DL2 with both H&E and p16 staining. For the patients who had a biopsy performed 3 months after treatment, no tumor was observed by H&E or p16 staining in any sample (see Supporting Fig. 1). IHC for pAKT performed on the DL1 samples was unsuccessful secondary to technical issues. At DL2, pAKT was strongly expressed at baseline (time point A) in tumors from all patients; this expression was quantified and is presented in aggregate in Figure 3B. After 7 days of treatment with NFV (time point B), strong staining of pAKT was again observed, but it was significantly decreased in intensity from the staining at time point A (Fig. 3B). Similarly, strong staining for pS6, a downstream target in the AKT pathway, was observed at baseline in the tumor. Staining of pS6 was present but significantly decreased at time point B (Fig. 3B). At time point C, pAKT and pS6 were observed in any remaining tumor cells and were more prominent in superficial squamous cell debris and infiltrating neutrophils and lymphocytes, but the levels decreased significantly overall from pretreatment levels. When a biopsy was taken at time point D, both pAKT and pS6 staining were observed in superficial squamous cell debris and leukocytes. Inflammatory infiltration by CD3 and CD8 T cells was observed in the tumor and the surrounding stroma at baseline (time point A) and persistently throughout treatment (see Supporting Fig. 1). No clear changes were observed with treatment. In addition, no clear changes were observed in HIF1α staining with treatment across all time points (see Supporting Fig. 1). In summary, NFV treatment alone appears to have significantly decreased pAKT and modestly decreased pS6 with further reduction observed with NFV combined with CRT.
Protein and gene expression analysis
To further evaluate treatment effects on tumor transcriptomic and protein expression, we used RPPA, the NanoString PanCancer Immune panel, and a NanoString custom panel for HPV‐targeted transcripts (Custom). First, we studied the effect of NFV on gene and protein expression between samples collected at baseline before treatment (time point A) and after 1 week of NFV treatment (time point B). Although, after correction for multiple testing, no proteins or genes reflected changes that passed the significance threshold, we identified 7 proteins and 41 genes with upregulation or downregulation that passed the nominal P < .05 significance cutoff (Fig. 3C,D). Several of these changes were in genes and proteins relating to immune function, including CD3G, CD3E, CD3D, CD2, CD8B, CD247, CD38, and CD27. Similarly, enrichment analysis of these genes and proteins revealed significant overrepresentation of changes in functions related to the regulation and activation of immune biologic processes (Fig. 3E), suggesting an effect of NFV treatment on the immune system and, specifically, on the function of T cells, although treatment did not appear to alter the already high levels of T‐cell infiltration by IHC. This may represent a change in the functional status of the lymphocytes present.
Next, we studied changes observed between the samples collected at baseline (time point A) and after 1 week of NFV treatment (time point B) and the samples collected while patients were receiving NFV with CRT at week 4 or week 6 (time points C1 and C2) and 3 months after treatment (time point D). We identified 7 proteins that were significantly upregulated later in the study, whereas 13 were downregulated (FDR < 5%) (Fig. 4A). In addition, 32 genes that were assayed using the NanoString Custom probes were significantly upregulated at later time points (FDR < 5%) (see Supporting Fig. 3), whereas 50 were significantly downregulated (FDR < 5%) (see Supporting Fig. 4). None of the NanoString PanCancer Immune probes showed significant differences in expression at an FDR < 5%. Results of the enrichment analysis (Fig. 4B) demonstrated significant alterations in genes involved in the regulation of cell cycle, cell death, and apoptosis and also demonstrated significant enrichment in genes identified in the KEGG Pathways in Cancer, a broad network of genes previously identified to have roles in tissue invasion and metastasis, sustained angiogenesis, evasion of apoptosis, proliferation, genomic instability and damage, cell immortality, chemotherapeutic resistance, and insensitivity to antigrowth signaling. 31
Figure 4.
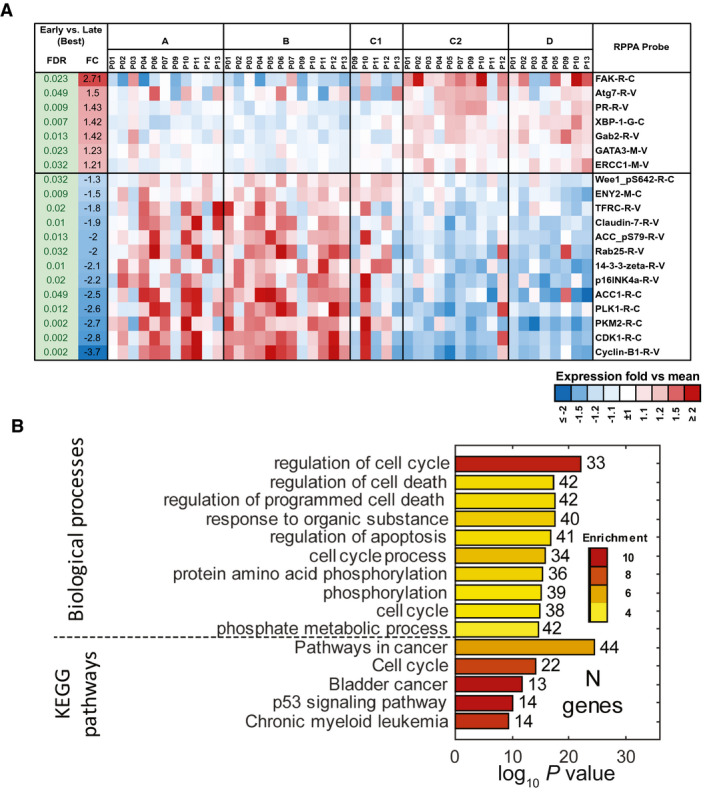
The effects of nelfinavir (NFV) in combination with chemoradiation on protein and messenger RNA expression are illustrated. (A) Significant changes in the expression of proteins before chemoradiation (baseline and post‐NFV alone) versus after the initiation of chemoradiation with NFV are revealed on reverse‐phase protein array (RPPA) analysis at time points A and B versus time points C and D (false‐discovery rate [FDR] < 5%). (B) Biologic processes and pathways were significantly enriched (FDR < 5%) among genes that were affected by chemotherapy and radiation. FC indicates fold change; KEGG, Kyoto Encyclopedia of Genes and Genomes.
In summary, there do not appear to be large effects from the initial 7 days of NFV treatment on transcript and protein expression in cervical tumor biopsies by RPPA and NanoString analyses compared with baseline, and the mild changes that are observed are primarily immune molecular functions. Larger effects are observed after the initiation of CRT, which likely dominates gene expression changes in samples collected later in the study. Inhibition of cancer and cell cycle pathways is observed at these later time points. These studies were limited by the small sample size.
Discussion
Despite the treatment of locally advanced cervical cancer with standard‐of‐care cisplatin and radiation, approximately 40% to 50% of women develop recurrent disease. 1 Our preclinical data demonstrating that NFV sensitizes cervical cancer cells to radiation both in vitro and in xenograft mouse models provided the scientific rationale for evaluating this regimen in the clinic. We report the safety and the RP2D for NFV added to standard‐of‐care CRT in patients with locally advanced squamous cell cervical carcinoma.
Overall, the addition of NFV to standard CRT was well tolerated. The most common toxicities reported were low‐grade hematologic (anemia, leukopenia, or neutropenia) and gastrointestinal (nausea, vomiting, or diarrhea) toxicities. Notably, hematologic and gastrointestinal toxicity are the most commonly reported adverse events among patients receiving standard‐of‐care, cisplatin‐based CRT, with 66% of patients experiencing some level of leukopenia (23% of which were grade 3 or 4) and 72% experiencing gastrointestinal events (12% of which were grade 3 or 4). 5 , 32 In the current study, high‐grade (grade 3 or 4) anemia was experienced by 9% of patients, high‐grade leukopenia was experienced by 27%, and high‐grade neutropenia was experienced by 9%. No patient experienced hematologic DLTs. Grade 3 or 4 gastrointestinal toxicity was limited: high‐grade nausea was experienced by 9% of patients, high‐grade vomiting was experienced by 9%, and high‐grade diarrhea was experienced by 18%. Adding an agent with gastrointestinal toxicity (diarrhea) to pelvic radiation, which already has notable gastrointestinal toxicity, was a concern. However, we observed that diarrhea was easily managed by having patients prepared to take antidiarrheal agents immediately upon the first sign of diarrhea. Patient compliance is critical, as demonstrated in this study. One patient did not follow instructions to take antidiarrheal medications and developed grade 4 electrolyte imbalances. Once she took the provided loperamide, her diarrhea resolved, and she completed study treatment without further problems. Interestingly, 42% of patients reported low‐grade constipation in addition to diarrhea. The only other grade 3 or 4 toxicity was grade 3 hematuria in 1 patient at DL2, which was determined to be related to her underlying disease and not to treatment. Overall, the addition of NFV to CRT did not result in a significant increase in hematologic or nonhematologic toxicities compared with those reported using CRT alone.
The RP2D of NFV was determined to be DL2 (1250 mg twice daily). This is similar to the RP2D determined for NFV in combination with cisplatin, gemcitabine, and radiation for locally advanced pancreatic cancer and for unresectable stage IIIA/IIIB nonsmall cell lung cancer. 10 , 18 Notably, NFV 750 mg twice daily was previously established by Buijsen et al in combination with capecitabine and radiotherapy in locally advanced rectal cancer, the only other trial to evaluate NFV in conjunction with pelvic CRT. 17
As discussed above, the addition of NFV to standard‐of‐care CRT demonstrated an improvement in disease response compared with historic controls in early phase trials for various disease sites. The addition of NFV to standard cisplatin, gemcitabine, and radiation resulted in a 20% increase in the response rate (50% vs 30%) compared with historic controls in locally advanced pancreatic cancer. 10 Buijsen and colleagues reported a pathologic CR rate of 27%, and an additional 36% of their patients demonstrated a major pathologic response compared with 10% to 33% historically who received standard‐of‐care CRT. 17 The addition of NFV to cisplatin‐based CRT for unresectable stage IIIA/IIIB non‐small cell lung cancer increased median overall survival to 41.1 months compared with 17 months in historic controls. 18 , 19 Across all disease stages in locally advanced cervical cancer, historical trials with cisplatin‐based CRT alone demonstrated a progression‐free survival rate of 67% at 24 months. 5 The efficacy of NFV added to standard‐of‐care CRT for locally advanced cervical cancer in our phase 1 trial demonstrated an overall survival and recurrence‐free survival rate of 91% at a median of 50 months. Only 1 patient experienced disease recurrence, which was noted at the first posttreatment imaging assessment while on study (a recurrence to para‐aortic lymph nodes, outside the radiation field). These findings are consistent with our preclinical data indicating that NFV is a radiosensitizer. However, given the small sample size, definitive conclusions about the added effect of NFV to standard CRT cannot be drawn. Further investigation of combination CRT with or without NFV in a phase 2/3 clinical trial is merited given these promising results.
It is critical to identify biomarkers for validating target engagement and, ideally, drug response to justify the use of targeted therapies in the clinic. Toward this goal, the accessibility of the cervix for in‐office biopsy enabled a unique evaluation of gene and protein expression at multiple points before and throughout treatment. Cervical tumor biopsies were evaluated by IHC as well as by RPPA and NanoString profiling. Because biopsies were taken at baseline (time point A), after 1 week of NFV treatment alone (time point B), during NFV and CRT at week 4 or 6 (time points C1 and C2), and 3 months after treatment completion (time point D), we were able to compare the effects of NFV treatment alone with baseline biopsies (time point A vs time point B) (Fig. 3; see Supporting Fig. 2) and to compare the profile of tumors before versus after the initiation of CRT (time points A and B vs time points C and D) (Fig. 4; see Supporting Figs. 3 and 4).
It has been proposed that the downregulation of the PI3K‐Akt pathway by NFV is mediated by the inhibition of proteasome activity, which results in endoplasmic reticulum stress, and that the ensuing unfolded protein response activates signaling, which results in the dephosphorylation of Akt by PP1/GADD34 complexed phosphatase. 33 As predicted, NFV pretreatment alone resulted in the significant downregulation of pAkt and its downstream target pS6 on IHC, and this downregulation continued to be observed throughout CRT. Although it was previously demonstrated that NFV downregulated Akt activation in peripheral blood mononuclear cells, to our knowledge, this is the first demonstration that NFV treatment indeed downregulates the Akt pathway in the target tumor in human patients. 10 No changes were noted in mRNA levels for Akt or S6 using NanoString profiling, likely reflecting that the signaling in this pathway is modulated through protein phosphorylation as opposed to transcriptional regulation. Future studies with NFV and CRT should consider including IHC staining for pAkt and pS6 as a pharmacodynamic biomarker for initial target engagement.
It also has been demonstrated that NFV downregulates HIF1α, which is induced by hypoxic conditions and functions as a key signaling factor to promote hypoxic adaptation and cell survival. 28 , 34 NFV has also been shown to increase oxygenation to tumor, which has been proposed as a potential mechanism for the radiosensitizing effects of NFV. 28 , 35 However, we did not observe changes in HIF1α with NFV or CRT. This likely reflects the significant instability of the HIF1α protein, which is rapidly degraded within minutes if the hypoxic environment is not maintained, potentially precluding an accurate assessment of protein levels in patient‐derived biopsies, given the inherent delays in fixing and processing clinically obtained fresh biopsies. 36
There was a significant infiltration of lymphocytes (CD3 and CD8) appreciated in the majority of cervical tumors at baseline on IHC. In contrast to other studies, which have demonstrated changes in levels of inflammatory infiltrates throughout treatment, our studies showed relatively stable levels of CD3 and CD8 T cells within the tumor and stroma. Given the limited sample size, we cannot draw definite conclusions, but a study by Lippens et al demonstrated that a stable or decreasing CD8 score was correlated with better cause‐specific survival in women with cervical carcinoma who received CRT followed by hysterectomy. 37 There were modest changes in markers of immune response, including T‐cell activation, T‐cell receptor signaling, and regulation of lymphocyte and leukocyte activation, such as KIR2DS1, KLRG1, CD3G, CD3E, CD3D, CD2, CD8B, CD247, CD38, and CD27. Functional activation of these immune populations was not evaluated but could be considered for future studies.
Overall, for the 1335 probes for genes and the >300 phosphorylated and total proteins assayed using NanoString profiling and RPPA, modest differences in expression between the various time points of the study were observable (Figs. 3 and 4). However, there did not appear to be a significant effect of initial NFV administration (time point B) compared with baseline (time point A). These probes evaluated a broad range of genes and proteins across a variety of cellular functions, including cellular metabolism, cell cycle regulation, cellular adhesion, and biosynthesis. It is possible that it takes longer than 1 week for NFV alone to have significant effects on most gene and protein expression that is not directly affected by the unfolded protein response or is not directly involved in the targeted PI3K/Akt pathway.
The effects of CRT tended to dominate gene and protein expression in samples collected later in the study (at time points C and D). This was a phase 1 study without a comparator group of patients receiving only CRT without NFV; therefore, we are unable to draw conclusions regarding whether NFV combined with CRT altered gene expression beyond the genetic and proteomic changes that would be observed with CRT alone. Furthermore, although biopsies were taken of areas that grossly appeared to be involved with tumor, our pathologic findings demonstrated lower than expected levels of tumor even at 4 to 6 weeks after the initiation of CRT (time points C1 and C2). We do note a significant decrease in the expression of p16 on RPPA after the initiation of CRT, consistent with the decreased tumor burden in the later samples identified by H&E and p16 staining on pathology. Given these findings, the gene and protein profile of samples collected later in the study may be more reflective of the remaining cervical stroma and inflammatory infiltrate rather than of changes in the tumor itself. In future studies, consideration should be given to a biopsy collected earlier in the course of CRT to better capture transcriptomic and proteomic changes in the tumor itself.
In summary, this phase 1 clinical trial demonstrates the safety and tolerability of NFV to standard‐of‐care, cisplatin‐based CRT in patients with locally advanced squamous cell cervical carcinoma. Clinical outcomes are promising compared with historic controls. A randomized phase 3 trial, with an NFV dose of 1250 mg twice daily, is now ongoing to evaluate the efficacy of NFV with CRT in cervical cancer (ClinicalTrials.gov identifier NCT03256916). Studies evaluating NFV in combination with CRT are also underway in other disease sites, including vulvar cancer (ClinicalTrials.gov identifier NCT03256916).
Funding Support
This study was supported in part by an American Society for Clinical Oncology (ASCO) Young Investigator Award (Nathalie D. McKenzie, principal investigator; Fiona Simpkins, mentor), an Abramson Cancer Center Radiation Oncology pilot grant (Fiona Simpkins, Lilie L. Lin, and Amit Maity), the National Institutes of Health (grant R01 CA174976; Amit Maity), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (Gordon B. Mills).
Conflict of Interest Disclosures
Gordon B. Mills reports grants, personal fees, nonfinancial support, and other support from Amphista, AstraZeneca, Chrysallis Biotechnology, Myriad Genetics, ImmunoMET, Ionis, Lilly, PDX Pharmaceuticals, Signalchem Lifesciences, Symphogen, Tarveda, Turbine, Zentalis Pharmaceuticals, Genentech, Glaxo‐Smith‐Kline, and Catena Pharmaceuticals, outside the submitted work. Lilie L. Lin reports grants and travel support from AstraZeneca and grants from Pfizer, outside the submitted work. The remaining authors made no disclosures.
Author Contributions
Arlene E. Garcia‐Soto: Wrote and edited the article. Nathalie D. McKenzie: Conceived of and designed the clinical trial. Margaret E. Whicker: Enrolled patients to the trial or performed data analysis and interpretation and wrote and edited the article. Joseph M. Pearson: Enrolled patients to the trial or performed data analysis and interpretation. Edward A. Jimenez: Performed or analyzed preclinical in vitro and in vivo studies. Lorraine Portelance: Enrolled patients to the trial or performed data analysis and interpretation. Jennifer J. Hu: Performed or analyzed preclinical in‐vitro and in‐vivo studies. Joseph A. Lucci III: Enrolled patients to the trial or performed data analysis and interpretation. Rehman Qureshi: Performed and analyzed the genomic and proteomic studies. Andrew Kossenkov: Performed and analyzed the genomic and proteomic studies. Lauren Schwartz: Performed the immunohistochemistry studies. Gordon B. Mills: Performed and analyzed the genomic and proteomic studies. Amit Maity: Performed or analyzed preclinical in vitro and in vivo studies and enrolled patients to the trial or performed data analysis and interpretation. Lilie L. Lin: Enrolled patients to the trial or performed data analysis and interpretation. Fiona Simpkins: Conceived of and designed the clinical trial, enrolled patients to the trial or performed data analysis and interpretation, and wrote and edited the article. All authors provided critical revisions of the original draft and approved the final version for publication.
Supporting information
Fig S1‐S4
Supplementary Material
Garcia‐Soto AE, McKenzie ND, Whicker ME, Pearson JM, Jimenez EA, Portelance L, Hu JJ, Lucci JA III, Qureshi R, Kossenkov A, Schwartz L, Mills GB, Maity A, Lin LL, Simpkins F. Phase 1 trial of nelfinavir added to standard cisplatin chemotherapy with concurrent pelvic radiation for locally advanced cervical cancer. Cancer. 2021. 10.1002/cncr.33449
The first 3 authors contributed equally to this work.
References
- 1. Brun JL, Stoven‐Camou D, Trouette R, Lopez M, Chene G, Hocke C. Survival and prognosis of women with invasive cervical cancer according to age. Gynecol Oncol. 2003;91:395‐401. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer, World Health Organization . Cancer Today. Age standardized (World) incidence rates, cervix uteri, all ages. Accessed September 1, 2020. https://gco.iarc.fr/today/data/factsheets/cancers/23‐Cervix‐uteri‐fact‐sheet.pdf
- 3. Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta‐analysis. Lancet. 2001;358:781‐786. doi: 10.1016/S0140-6736(01)05965-7 [DOI] [PubMed] [Google Scholar]
- 4. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12‐19. doi: [DOI] [PubMed] [Google Scholar]
- 5. Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144‐1153. doi: 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 6. Gupta AK, Lee JH, Wilke WW, et al. Radiation response in two HPV‐infected head‐and‐neck cancer cell lines in comparison to a non‐HPV‐infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928‐933. doi: 10.1016/j.ijrobp.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256‐8265. doi: 10.1158/0008-5472.CAN-05-1220 [DOI] [PubMed] [Google Scholar]
- 8. Al‐Assar O, Bittner MI, Lunardi S, Stratford MR, McKenna WG, Brunner TB. The radiosensitizing effects of nelfinavir on pancreatic cancer with and without pancreatic stellate cells. Radiother Oncol. 2016;119:300‐305. doi: 10.1016/j.radonc.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 9. Wilson JM, Fokas E, Dutton SJ, et al. ARCII: a phase II trial of the HIV protease inhibitor nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother Oncol. 2016;119:306‐311. doi: 10.1016/j.radonc.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699‐2706. doi: 10.1200/JCO.2007.15.2355 [DOI] [PubMed] [Google Scholar]
- 11. Davis MA, Delaney JR, Patel CB, Storgard R, Stupack DG. Nelfinavir is effective against human cervical cancer cells in vivo: a potential treatment modality in resource‐limited settings. Drug Des Devel Ther. 2016;10:1837‐1846. doi: 10.2147/DDDT.S102241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goda JS, Pachpor T, Basu T, Chopra S, Gota V. Targeting the AKT pathway: repositioning HIV protease inhibitors as radiosensitizers. Indian J Med Res. 2016;143:145‐159. doi: 10.4103/0971-5916.180201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Wu J, Ling MT, Zhao L, Zhao K‐N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol Cancer. 2015;14:87. doi: 10.1186/s12943-015-0361-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CM, Fuhrman CB, Planelles V, et al. Phosphatidylinositol 3‐kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin Cancer Res. 2006;12:250‐256. doi: 10.1158/1078-0432.CCR-05-1084 [DOI] [PubMed] [Google Scholar]
- 15. Kaldor SW, Kalish VJ, Davies JF 2nd, et al. Viracept (nelfinavir mesylate, AG1343): a potent, orally bioavailable inhibitor of HIV‐1 protease. J Med Chem. 1997;40:3979‐3985. doi: 10.1021/jm9704098 [DOI] [PubMed] [Google Scholar]
- 16. Tebas P, Powderly WG. Nelfinavir mesylate. Expert Opin Pharmacother. 2000;1:1429‐1440. doi: 10.1517/14656566.1.7.1429 [DOI] [PubMed] [Google Scholar]
- 17. Buijsen J, Lammering G, Jansen RLH, et al. Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiother Oncol. 2013;107:184‐188. doi: 10.1016/j.radonc.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 18. Rengan R, Mick R, Pryma D, et al. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non‐small cell lung cancer: a report of toxicities and clinical response. J Thorac Oncol. 2012;7:709‐715. doi: 10.1097/JTO.0b013e3182435aa6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rengan R, Mick R, Pryma DA, et al. Clinical outcomes of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non‐small cell lung cancer: a phase 1/2 trial. JAMA Oncol. 2019;5:1464‐1472. doi: 10.1001/jamaoncol.2019.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103‐104. doi: 10.1016/j.ijgo.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 21. Ivy SP, Siu LL, Garrett‐Mayer E, Rubinstein L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the Clinical Trial Design Task Force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res. 2010;16:1726‐1736. doi: 10.1158/1078-0432.CCR-09-1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen DH, Zhou T, Shu J, Mao JH.Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Accessed June 15, 2020. https://www.cancerincytes.org/quantifying‐chromogen‐intensity‐in‐immunohistochemistry‐
- 24. Bai H, Ge S, Lu J, Qian G, Xu R. Hypoxia inducible factor‐1α‐mediated activation of survivin in cervical cancer cells. J Obstet Gynaecol Res. 2013;39:555‐563. doi: 10.1111/j.1447-0756.2012.01995.x [DOI] [PubMed] [Google Scholar]
- 25. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118‐127. doi: 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289‐300. [Google Scholar]
- 27. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44‐57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 28. Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down‐regulates hypoxia‐inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66:9252‐9259. doi: 10.1158/0008-5472.CAN-06-1239 [DOI] [PubMed] [Google Scholar]
- 29. Nedergaard BS, Ladekarl M, Thomsen HF, Nyengaard JR, Nielsen K. Low density of CD3+, CD4+ and CD8+ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97:1135‐1138. doi: 10.1038/sj.bjc.6604001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor‐infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972‐2978. doi: 10.4049/jimmunol.167.5.2972 [DOI] [PubMed] [Google Scholar]
- 31. Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27‐30. doi: 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isohashi F, Takano T, Onuki M, et al. A multi‐institutional observational study on the effects of three‐dimensional radiotherapy and weekly 40‐mg/m2 cisplatin on postoperative uterine cervical cancer patients with high‐risk prognostic factors. Int J Clin Oncol. 2019;24:575‐582. doi: 10.1007/s10147-018-01380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta AK, Li B, Cerniglia GJ, Ahmed MS, Hahn SM, Maity A. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia. 2007;9:271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164‐167. [PubMed] [Google Scholar]
- 35. Qayum N, Muschel RJ, Im JH, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347‐6354. doi: 10.1158/0008-5472.CAN-09-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaluz S, Kaluzova M, Liao SY, Lerman M, Stanbridge EJ. Transcriptional control of the tumor‐ and hypoxia‐marker carbonic anhydrase 9: a one transcription factor (HIF‐1) show? Biochim Biophys Acta. 2009;1795:162‐172. doi: 10.1016/j.bbcan.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lippens L, Van Bockstal M, De Jaeghere EA, et al. Immunologic impact of chemoradiation in cervical cancer and how immune cell infiltration could lead toward personalized treatment. Int J Cancer. 2020;147:554‐564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Supplementary Material


