Abstract
The toxic unit and additive index approaches were used to understand how 2 pesticides, 3‐trifluoromethyl‐4‐nitrophenol (TFM) and 2,5‐dichloro‐4‐nitrosalicylanilide (niclosamide; Nic), interact in mixtures. Our first objective was to determine whether the interaction was strictly additive or greater than additive at doses comparable to those used to control invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes, and our second was to compare the utility of the toxic unit and additive index models for determining how TFM and Nic interacted. Typically, TFM is mixed with Nic (1–2%, w/v) to increase its potency and reduce TFM use. However, there is little information on how the 2 chemicals interact. Using a well‐studied, resident nontarget fish, the rainbow trout (Oncorhynchus mykiss), we conducted toxicity tests with TFM, Nic, and TFM:Nic (100:1, w/v; TFM/1% Nic) mixtures over 12 h to determine if the interaction was strictly additive, less than additive (antagonistic), or greater than additive (synergistic). The toxic unit and additive index approaches indicated synergistic interactions at environmentally relevant concentrations, suggesting that both are valid approaches for predicting how TFM and Nic interact. The toxic unit approach was simpler to conceptualize and to calculate, and we recommend that it be used when describing how TFM and Nic, and other similar organic compounds, interact with each other in aquatic ecosystems. Environ Toxicol Chem 2021;40:1419–1430. © 2021 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Lampricide, Mixtures, Toxic unit, Additive index, TFM, Niclosamide
INTRODUCTION
The piscicides 3‐trifluoromethyl‐4‐nitrophenol (TFM) and 2,5‐dichloro‐4‐nitrosalicylanilide (niclosamide; Nic; Figure 1) have been used for 5 to 6 decades to control invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes (Wilkie et al. 2019). The invasion of the Great Lakes by sea lamprey devastated culturally and economically important fisheries because of parasitism/predation by parasitic juvenile lamprey in the early to mid‐1900s, after sea lamprey gained access to Lake Erie and the upper Great Lakes from Lake Ontario, following modifications to the Welland canal (Siefkes 2017). Applied to rivers infested with larval sea lamprey, TFM and Nic, commonly referred to as “lampricides,” have reduced the number of parasitic juvenile lampreys in the Great Lakes by almost 90%, resulting in the rehabilitation of its fisheries (Siefkes 2017; Wilkie et al. 2019).
Figure 1.
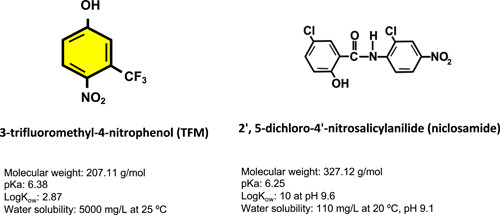
Chemical structures of 3‐trifluoromethyl‐4‐nitrophenol (TFM) and 2,5‐dichloro‐4‐nitrosalicylanilide (niclosamide). Chemical information obtained from https://pubchem.ncbi.nlm.nih.gov/compound/4477. K OW = octanol–water partition coefficient.
Because it specifically targets larval sea lamprey with minimal effects on nontarget fishes, TFM is considered an ideal lampricide (Dawson et al. 1977; Boogaard et al. 2003). This specificity is due to the limited ability of larval sea lamprey to detoxify TFM via glucuronidation, which is used by nontarget species, such as the rainbow trout, to produce TFM‐glucuronide, leading to effective excretion via the bile (Lech and Costrini 1972; Lech 1974). The levels of TFM used during a treatment vary with water pH and alkalinity, based on the pH/alkalinity model, with total (ionized + un‐ionized) concentrations ranging from 1.3 mg/L TFM in low‐alkalinity (30 mg/L CaCO3) waters to 3.6 mg/L TFM in high‐alkalinity (260 mg/L CaCO3) waters, at a constant pH of 8.0 (Bills et al. 2003; Hepditch et al. 2019). With a pK a of 6.38, TFM is a weak acidic phenol, and it completely dissociates in water (McConville et al. 2016). Niclomide is more toxic than TFM, less costly to manufacture, and nonspecific; therefore, it is added at 1% of TFM, to reduce the amount of TFM used (Bills and Marking 1976; Dawson et al. 1977; Marking and Bills 1985; Boogaard et al. 2003). There is evidence that Nic toxicity is also impacted by water chemistry (Dawson 2003). With a pK a of 6.25, Nic is a weak acid, but it is difficult to keep in solution because of its high octanol–water partition coefficient (log K OW) of 10 at pH 9.6 (Tomlin 1994). Because of the similar pK a to that of TFM, the equilibrium between the ionized and un‐ionized forms of Nic is pH‐dependent, much like TFM (Dawson 2003). Niclosamide adsorbs more strongly than TFM to organic matter, tending to accumulate more in sediment‐rich organic content (McConville et al. 2016). Both lampricides may interact with ions (i.e., calcium and magnesium) in water, but it appears that photodegradation and bacterial degradation are the biggest influences on the persistence of the chemicals in the environment following a treatment (McConville et al. 2016).
Although TFM is the preferred lampricide, it is often mixed with small amounts of Nic (1–2%, w/v), which increases its toxicity, without loss of its specificity to the lamprey, thereby reducing the amount of TFM required (Boogaard et al. 2003; Wilkie et al. 2019). Our understanding of how TFM and Nic interact with one another to increase toxicity remains uncertain. This is especially important for nontarget fish species that share their habitat with sea lamprey, which may be at higher risk during TFM/Nic applications (Siefkes 2017; Wilkie et al. 2019). In addition, a better understanding of the interaction of the current lampricides may provide insights into the development of novel, more specific methods of sea lamprey control (i.e., next‐generation lampricides), which is one of the current goals of the Great Lakes Fishery Commission (Wilkie et al. 2019).
The interactions between 2 or more toxicants can be evaluated using several approaches. Two such approaches, based on the concept of concentration additivity or the concentration addition model, are the toxic unit model (Norwood et al. 2003; Playle 2004) and the additive index model (Marking and Dawson 1975). According to the toxic unit model (Figure 2A), 1 toxic unit can be set to any convenient endpoint for the system being studied. For instance, if 1 toxic unit equals the individual 12‐h LC50 (concentration required to kill 50% of the animals over 12 h) of each compound, the expected mortality following exposure to 0.5 toxic unit of each chemical in a mixture would be defined as "strictly additive” if their combined effects equal 1.0 toxic unit, which is 50% mortality. If the combined effect of 0.5 toxic unit was <1.0 (<50% mortality), the effect would be less than additive (“antagonistic”); and if the effect was >1.0 (>50% mortality), the interaction would be greater than additive (“synergistic”; Norwood et al. 2003; Playle 2004).
Figure 2.
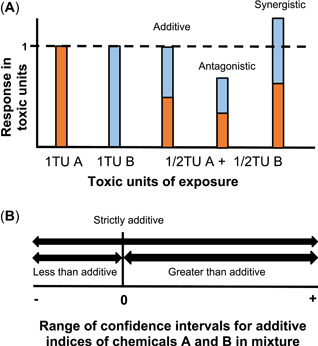
Theoretical models for assessing the interaction of chemicals in mixture. The combined effects of 2 chemicals in fish can be quantified by expressing the concentration of toxicants A and B in mixture either as (A) toxic units (TU) or (B) linearly, using the additive index. For the toxic unit approach (A), if 1 toxic unit (dashed horizontal line) is defined as a one‐half unit of response, then 1toxic unit A + 1toxic unit B = 1 unit of response if the interaction is strictly additive; if the interaction is less than additive (antagonistic), then 1toxic unit A + 1toxic unit B < 1 unit of response; if the interaction is greater than additive (synergistic), then 1toxic unit A + 1toxic unit B > 1 unit of response. The additive index (B) represents a linear scaling of the combined effects of chemicals A and B. If the range of the effects spans 0, then the interaction of the 2 toxicants is strictly additive; if the range <0, then the interaction is less than additive (antagonistic); and if the range of the additive index is >0, then the interaction is greater than additive (synergistic). Figure adapted from Newman (2015).
With the additive index approach (Figure 2B), additive indices quantify the combined activity of mixtures (Marking and Dawson 1975; Marking 1977; Marking and Bills 1985). An additive index, which is based on the toxic unit concept, is derived by summing the toxicity contributions (such as the LC10, LC50, LC99, which are the lethal concentrations required to kill, 10, 50, and 99% of the population, respectively) of each constituent compound and converting the sum to a linear, quantitative value (Marking 1977; Newman 2015). Typically, LC50 values are used to accurately derive additive indices, and the corresponding confidence interval (CI) range is used to determine the interaction between the chemicals. If the numerical value of the additive index's CI range overlaps zero, then the interaction is strictly additive. If the CI does not span zero, then negative ranges represent a less than additive interaction (“antagonistic”), whereas positive ranges represent greater than additive interactions (“synergistic”; Marking and Dawson 1975; Marking 1977; Marking and Bills 1985; Newman 2015).
A number of early studies on the effects of lampricide mixtures in nontarget fish species indicated that TFM–Nic interactions were additive or less than additive, depending on the concentrations used, the duration of exposure, and the species of fish tested (Howell et al. 1964; Bills and Marking 1976; Marking and Bills 1985; Dawson et al. 1977), whereas others suggested that the interaction was greater than additive or synergistic (Marking and Dawson 1975). Accordingly, the goal of the present study was to resolve if TFM and Nic interacted in a less than, strictly, or greater than additive manner, using both the toxic unit and additive index approaches. This interaction was explored in rainbow trout (Oncorhynchus mykiss), a representative nontarget fish. Rainbow trout share their habitat with larval sea lamprey and are likely to be exposed to TFM/Nic mixtures. In addition, this species has been used as a model organism in previous studies exploring the effects of lampricides in nontarget species (Marking and Bills 1985), making it an ideal nontarget fish model for understanding the interaction of the 2 lampricides in mixture.
To this end, rainbow trout juveniles were exposed to a range of TFM, Nic, and TFM/1% Nic mixture concentrations to determine the 12‐h LC10, LC25, LC50, and LC99. These LC values were used to understand the nature of the interaction of the 2 chemicals by taking 2 approaches: toxic unit and additive index. The additive index approach has been used previously to determine how TFM and Nic interact in both target and nontarget species, but the results of those studies were inconclusive, predicting both additive and synergistic interactions (Bills and Marking 1976; Dawson 1977 Marking and Bills 1985). No studies to date, however, have explored whether the toxic unit approach is effective at predicting the interaction of the 2 lampricides. The simplicity of the toxic unit model makes it a useful tool for predicting interactions of various multimixture agents, including insecticide applications for the control of agricultural pests, or assessing metal toxicity (Playle 2004).
MATERIAL AND METHODS
Experimental animals and holding
Rainbow trout (mass = 13.6 ± 0.2 g, length = 11.1 ± 0.1 cm, n = 326) were purchased from Silver Creek Aquaculture and held in 200‐L polyethylene tanks continuously receiving Wilfrid Laurier University well water (water temperature ~12 °C, pH ~8.1, dissolved oxygen ~80–95% saturation, alkalinity = 210 mg/L as CaCO3, hardness = 460 mg/L as CaCO3). Fish were acclimated to their holding tanks for 7 d prior to experiments, during which they were held under a 12:12‐h light:dark cycle and fed 3 times/wk with commercial pellets (size 3.0) at 2% body weight (Corey Feed Mills). Fish were not fed 48 h before experiments, to minimize fouling of the water and ammonia accumulation in the experimental tanks. All experiments and fish husbandry were approved by the Wilfrid Laurier University Animal Care Committee (no. R14000) and followed the Canadian Council of Animal Care guidelines.
Experimental protocols
Acute toxicity of TFM and Nic individually
Field formulation TFM (35% active ingredient; Clariant SFC) and Nic (Bayluscide® Emulsifiable Concentrate; 16.9% active ingredient in ethanol solution) were used for each toxicity test, provided courtesy of the Sea Lamprey Control Centre, Fisheries and Oceans Canada. Working stocks of niclosamide used for the toxicity experiments were diluted 10 times in 95% ethanol prior to addition to each tank. The respective 12‐h LC50 values of TFM and Nic were calculated as described in section Determination of the toxicity of lampricides, alone and in mixture. All concentrations of TFM and Nic are reported as total (ionized + un‐ionized) lampricide.
All experiments were conducted in Wilfrid Laurier University well water (water temperature ~12 °C, pH ~8.1, dissolved oxygen ~80–95% saturation, alkalinity = 210 mg/L as CaCO3, hardness = 460 mg/L as CaCO3). Acute toxicity experiments for TFM and Nic were conducted over 12‐h static exposures of rainbow trout fingerlings (n = 18 per test concentration) to the respective nominal concentrations of TFM and Nic, in triplicate: TFM 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, and 25.0 mg/L; Nic 0.05, 0.1, 0.25, 0.5, and 1.0 mg/L, plus controls containing neither lampricide. To mimic field lampricide treatments, TFM was added to the treatment containers directly from the 35% active ingredient stock. Niclosamide (16.9% active ingredient) was first diluted in 95% ethanol to a concentration of 50 µg/L; from that, appropriate volumes of stock were added to each container, to achieve the desired concentrations. The controls used for TFM alone exposures did not receive the vehicle (i.e., isopropanol) because previous work in our laboratory found no difference between vehicle and no‐vehicle controls. The controls used for Nic and TFM/Nic mixtures received 888 µL ethanol as a vehicle, which represents the volume of ethanol added to the highest concentration of Nic (1.0 mg/L). All controls survived the length of the treatment. Prior to experiments, appropriate amounts of TFM, Nic, or vehicle (where appropriate) were added to test containers filled with 15 L of aerated water, followed by collection of water samples (20 mL prior to the addition of fish in the system) for measurement of TFM or Nic concentration, then left to mix and equilibrate overnight. Both TFM and Nic are hydrolytically stable in solutions, with half‐lives of 1444 to 4620 d at pH 5 to 9 for TFM and 8.88 to 382 d for Nic in the laboratory (Schultz and Harman 1978; Hubert 2003) and 16.6 to 32.9 h for TFM and 8.88 to 382 d for Nic in the field, depending on water pH, ultraviolet, and microbial degradation (McConville et al. 2016). The next morning, the trout were transferred to test containers (n = 6 per container), followed by water sample collection (7 mL once the fish were in the system) at 5 min and 1, 3, 6, and 12 h of exposure, and then used for immediate determination of TFM concentration or frozen at –20 °C and saved for later measurement of Nic concentration. Water temperature, dissolved oxygen, and pH were monitored prior to and following the 12‐h lampricide exposure, to not stress the fish during the actual exposure. Survival was monitored hourly for the first 8 h of the experiment and at 10 and 12 h of exposure. Unresponsive fish were immediately removed from the container, and surviving fish were anesthetized with 1.5 g/L tricainemethanesulfonate (Syndel Canada) buffered with 3.0 g NaHCO3 at the conclusion of the toxicity tests. The 12‐h exposure period approximates the total duration of an actual TFM or TFM/Nic mixture application (McDonald and Kolar 2007).
Toxicity of binary mixtures of TFM and Nic
To determine the nature of the interactions between TFM and Nic, fingerling trout were exposed to TFM:Nic (100:1, w/v; TFM/1% Nic) mixtures over a range of concentrations, to mimic those that could be encountered under field conditions. The nominal concentrations of TFM/Nic in the water were 2.5/0.025, 5.0/0.05, 7.5/0.075, 10.0/0.1, 15.0/0.15, and 25.0/0.25 mg/L. The TFM/Nic mixture experiment was conducted exactly as described for TFM and Nic in the previous section, in triplicate. The lampricides were added to 15 L of well water the night before experiments. Water samples for the determination of lampricide levels during the exposure were collected as previously described, with one exception: following the 12‐h exposure, the containers were left as they were overnight, and a 24‐h water sample was collected in the morning. When samples were pooled for Nic measurements, that sample was included. For consistency, TFM was also measured in the same 24‐h sample, and the resulting concentration was used to calculate the average TFM number for the exposures.
Analytical techniques
Water TFM concentrations were quantified by spectrophotometry using a SpectraMax 190 microwell plate spectrophotometer (Molecular Devices) at a wavelength of 395 nm, using certified standards provided courtesy of Fisheries and Ocean Canada (IOP:012.3) and adapted to the 96‐well plates. Water Nic levels were quantified using high performance liquid chromatography (HPLC; Waters) configured with a 2489 detector and a 515 pump. All water samples (prior to the start of the exposure and those collected at various time points during the exposure) that were collected from one tank were pooled to measure Nic. The instrument was fitted with a 77251i injector, and the analysis was conducted at 55 °C using a CHM column heater. Standards were prepared using Nic powder (Sigma‐Aldrich) dissolved in ethanol, following Fisheries and Oceans Canada standard operating procedures (IOP:015.4). Samples and standards were injected manually (100 μL) using a Hamilton glass syringe. The mobile phase used was 2 mmol/L sodium acetate (Sigma‐Aldrich) made in HPLC‐grade methanol. Data were collected using Empower II Software.
Data analysis and predictive models
Determination of the toxicity of lampricides, alone and in mixture
Replicate tanks were treated as individual exposures for the determination of the dose–response relationships. Probit analyses on mortality data obtained for each individual lampricide and mixtures were used to determine dose–response relationships for the lethal concentrations. Toxicity data were expressed as the 12‐h LC50 or LC99. Generation of the dose–response curves and of the LC values was done using the ecotox R package, Ver 1.4.2 (refer to Supplemental Data for the code).
Using the toxic unit approach to determine lampricide interactions
Using the data generated from each respective acute toxicity experiment, the corresponding 12‐h LC50 values that were determined for TFM and Nic alone (see above, Acute toxicity of TFM and Nic individually) were set to equal 1 toxic unit each. These values were subsequently used to quantify how TFM and Nic interacted in TFM/1% Nic mixtures. This was done by first calculating the total toxic unit (TU) of exposure,
| (1) |
where x and y denote the proportion of each toxic unit present in the mixture and TUTFM and TUNic denote the respective individual 12‐h LC50 of TFM and Nic previously determined (see above, Acute toxicity of TFM and Nic individually). The expected percentage mortality was calculated by multiplying each toxic unit of exposure by 0.5, which corresponds to 50% mortality, and combining the 2 values:
| (2) |
A line of strict additivity was generated by plotting the total toxic unit of exposure against the (expected) percentage mortality, as depicted in Figure 1. Thus, exposure to 1 toxic unit of TFM plus 1 toxic unit of Nic would result in an expected mortality of 100%, whereas exposure to 0.5 toxic unit of each would result in an expected mortality of 50%, and so on.
Finally, to define the type of TFM and Nic interaction at each combination, the total toxic unit exposure was plotted against the observed percentage mortality, with points falling on the line of strict additivity representing strictly additive interactions, those falling above indicating greater than additive (synergistic) interactions, with any points falling below the line denoting less than additive (antagonistic) interactions (Figure 1A).
Using the additive index to determine lampricide interactions
The additive index and its CIs was determined by separately calculating the respective 12‐h LC10, LC25, LC50, and LC99 and the 95% CIs of the individual exposures (see above, Acute toxicity of TFM and Nic individually). This was followed by calculation of the TFM LC (LCTFM‐mix) or the Nic LC (LCNic‐mix) when the fish were exposed to different mixtures of the chemicals. These values were used to define the additive toxicity index and its ranges (defined in the present study as CIs), as described by Marking and Dawson (1975) and Marking (1977), in which the sum of the toxic activity (S) of the compounds alone and in mixture was calculated as follows:
| (3) |
In Equation 3, LCTFM‐mix and LCNic‐mix are the corresponding LCs (i.e., LC10, LC25, LC50, LC99) of the TFM or Nic in mixture, whereas LCTFM‐i and LCNic‐i are the respective LCs of TFM or Nic measured independently.
The additive index and its ranges were calculated based on whether or not S was >1 or <1:
| (4) |
| (5) |
When the range of the CIs spans zero, the interaction is considered to be strictly additive (Figure 1B). If a calculated CI range is >0, the interaction is considered greater than additive, whereas a CI range <0 indicates a less than additive interaction. Additive indices quantify the combined effects of mixtures, while the CIs of the LC values for which the additive indices were calculated to define the significance of the indices.
Survival curves following exposure to lampricides, alone and in mixture
Survival curves ±95% CIs were generated using survival data collected over a 12‐h exposure of rainbow trout fingerlings to TFM and Nic, alone and in TFM/1% Nic mixtures. Survival curves and 95% CIs were generated from the 12‐h exposures of TFM, Nic, and the TFM/Nic binary mixture. Log‐rank (Mantel‐Cox) tests were used to determine if the curves were significantly different from each other (p < 0.05). Pairwise comparisons between different survival curves were made using the Bonferroni‐corrected threshold value of p < 0.0018 for TFM alone, p < 0.0033 for Nic alone, and p < 0.0024 for TFM/1% Nic mixture (refer to Supplemental Data, Tables S2–S4 for the statistical analysis). The p value for the comparisons was determined by dividing the overall level of significance (p < 0.05) by the total number of comparisons (K = 28 for TFM [Supplemental Data, Table S2], K = 15 for Nic [Supplemental Data, Table S3], K = 21 for TFM/1% Nic mixture [Supplemental Data, Table S4]). Next, pairwise comparisons were made between the survival curves of each lampricide alone and the curve for the same lampricide concentration in mixture (refer to Supplemental Data, Tables S5 and S6; Bonferroni‐corrected threshold value of p < 0.0006 for TFM alone vs TFM in mixture with Nic and p < 0.0083 for Nic vs Nic in mixture with TFM). Similarly, the p value for the comparisons was determined by dividing the overall level of significance (p < 0.05) by the total number of comparisons (K = 78 for TFM vs the corresponding TFM concentrations in mixture [Supplemental Data, Table S5], K = 6 for Nic vs the corresponding Nic concentrations in mixture [Supplemental Data, Table S6]). Survival curve statistical analyses were conducted using Prism 8.0 (GraphPad).
RESULTS
TFM and Nic toxicity alone and in mixtures
The measured levels of TFM and Nic in the acute toxicity experiments are presented in Figure 3, and concentrations in the controls were below detection. All LC values were calculated based on the measured levels of lampricides in water (please refer to Supplemental Data, Table S1). For TFM‐only exposures, concentrations for each of the replicate exposures are representative of the average TFM concentrations measured at 3, 6, and 12 h, except for the 25 mg/L exposures, where the average TFM was calculated from samples collected at 1 and 3 h because all the animals died after that time (Supplemental Data, Table S1). For the TFM/Nic mixture experiments, the TFM values in each container represent averages of the numbers recorded at 3, 6, 9, 12, and 24 h following the addition of the fish to the containers. The remainder of the water samples were all pooled to measure Nic levels, where appropriate.
Figure 3.
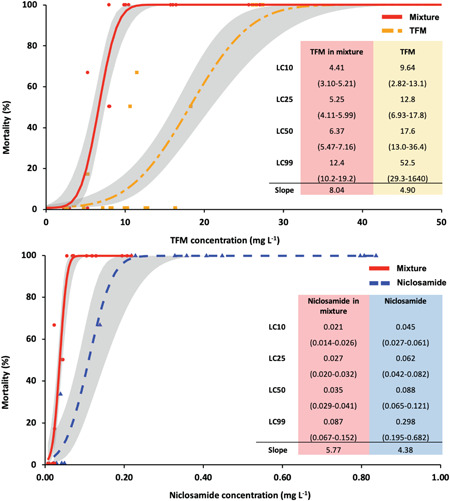
Dose–response curves for the acute toxicity experiments. Dose–response relationship for juvenile rainbow trout (Oncorhynchus mykiss) exposed to lampricides alone and in combination. (A) Dose response for 3‐trifluoromethyl‐4‐nitrophenol (TFM), either alone (orange) or in the presence of 1% niclosamide (Nic; red). (B) Dose response for Nic, either alone (blue) or in the presence of 100% TFM (red). n = 6 fish/tank, in triplicate, for each exposure concentration. Concentrations of TFM and Nic are plotted against percentage of survival. Insets denote the lethal concentration values calculated for each condition, along with the slopes of the dose–response curves.
The corresponding 12‐h LC50 for TFM alone was 18.2 mg/L (95% CI 18.0–18.4 mg/L; Figure 3A) in rainbow trout, which was approximately 165‐fold greater than the 12‐h LC50 of 0.11 mg/L (95% CI 0.09–0.13 mg/L) observed for Nic (Figure 3B). The measured concentrations of the TFM/1% Nic mixtures experiment were 2.5 (±0.2)/0.01 (±0.004), 5.2 (±0.03)/0.02 (±0.003), 7.9 (±0.03)/0.05 (±0.004), 10.1 (±0.2)/0.07 (±0.001), 16.0 (±0.2)/0.12 (±0.01), and 26.6 (±0.5)/0.21 (±0.01) mg/L, which represent the mean measured TFM and Nic concentrations in the water at all the time points where samples were collected for a given treatment. When trout were exposed to a TFM/1% Nic mixture, the 12‐h LC50 for TFM was approximately 60% lower at 6.64 mg/L (95% CI 6.57–6.71 mg/L) than for TFM alone. Similarly, the respective 12‐h LC10, LC25, and LC99 values for TFM in the TFM/Nic mixture were lower than when the fish were exposed to TFM alone (Figure 3C). The 12‐h LC50 for Nic within the TFM/Nic mixture was 0.038 mg/L (95% CI 0.035–0.041 mg/L), approximately 60% lower than when the fish were exposed to Nic alone. The 12‐h LC10, LC25, and LC99 values for Nic in the TFM/Nic mixture were also lower, 0.020, 0.028, and 0.071 mg/L, respectively (Figure 3D).
The dose–response curves were steeper when the fish were exposed to TFM/Nic mixtures compared to the curves generated for each lampricide alone (Figure 3). The slope for the TFM‐alone dose–response curve was 4.90, whereas in the presence of 1% Nic, the slope was 8.04 (Figure 3A). For Nic alone, the slope of the dose–response curve was 4.38, whereas in the presence of TFM, the slope was 5.76 (Figure 3B).
Survival following acute exposure to individual lampricides and mixtures
To better understand how the presence of one lampricide influences the toxicity of the other in mixture, we analyzed the survival profiles of the fish during the 12‐h exposures. Exposure of rainbow trout fingerlings to TFM, Nic, and TFM/1% Nic mixtures yielded different survival profiles over 12 h (Figure 4; p < 0.0001). There were no mortalities in the control groups. Following a 12‐h exposure to TFM alone (Figure 4A), all fish survived exposure to 2.5 and 7.5 mg/L TFM, and approximately 89 and approximately 94% of the fish survived exposure to 5 and 10 mg/L, respectively. In the fish exposed to 12.5 mg/L TFM, survival was approximately 78%, whereas in those exposed to 15 mg/L TFM, approximately 67% of the fish survived. No fish survived exposure to 25 mg/L TFM. Pairwise comparisons between the survival curves at different exposure concentrations yielded significant differences for the 25 mg/L TFM group, where approximately 50% of the fish died in the first 2 h and survival was significantly reduced compared to all the other lower concentrations (p = 0.0001; Supplemental Data, Table S4).
Figure 4.
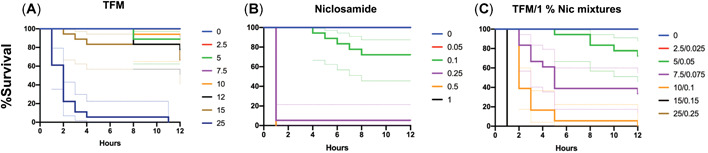
Effects of lampricide exposure on rainbow trout (Oncorhynchus mykiss) survival. Percentage of survival of rainbow trout fingerlings exposed to (A) 3‐trifluoromethyl‐4‐nitrophenol (TFM), (B) 2,5‐dichloro‐4‐nitrosalicylanilide (niclosamide; Nic), and (C) TFM:Nic (100:1, w/v; TFM/1% Nic) mixture. The nominal exposure concentrations (n = 6 fish/tank, in triplicate) for the lampricides were TFM 0.0, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, and 25.0 mg/L; Nic 0.0, 0.05, 0.1, 0.25, 0.5, and 1.0 mg/L; TFM/1% Nic 0.0/0.0, 2.5/0.025, 5.0/0.05, 7.5/0.075, 10.0/0.1, 15.0/0.15, and 25.0/0.25 mg/L. No mortalities were observed in controls. Colored solid lines represent the nominal exposure concentrations, whereas the dotted lines represent the 95% confidence intervals.
In the Nic‐alone exposures (Figure 4B), all fish survived the 12‐h exposure to 0.05 mg/L Nic. In the individuals exposed to 0.1 mg/L, survivorship was approximately 67% at the end of the 12‐h experiment, whereas no fish survived exposure to 0.25, 0.5, and 1.0 mg/L Nic. Survival curves for 0.25, 0.5, and 1.0 mg/L Nic were significantly different from those of the 0.05 and 0.1 mg/L groups (p < 0.0001; level of significance set at p = 0.0033; Supplemental Data, Table S5).
For the TFM/1% Nic mixture (Figure 4C, D), all fish exposed to 2.5/0.025 mg/L TFM/Nic survived the 12‐h exposure. In the 5/0.05 mg/L TFM/Nic group, approximately 72% of the fish survived the exposure, whereas in the 7.5/0.075 mg/L TFM/Nic group, survivorship was 33% at the end of the 12 h. No fish survived exposure to 10/0.1, 15/0.15, and 25/0.25 mg/L TFM/Nic. Significant differences (p < 0.0001) in survival curves (level of significance set at p = 0.0023; Supplemental Data, Table S6) were noted between 7.5/0.075, 10.0/0.1, and 15/0.15 mg/L TFM/Nic and all the respective lower exposure concentrations (2.5/0.025 and 5.0/0.05), except between 7.5/0.075 and 2.5/0.025 which showed similar survival curves. The survival curve for 25/0.25 mg/L was significantly different from those for 2.5/0.025, 5.0/0.05, 7.5/0.075, and 10.0/0.1; but it was not different from that of 15/0.15 mg/L.
When fish were exposed to 7.5, 10.0, 15.0, and 25.0 mg/L TFM alone, their survival was significantly different (p < 0.0006) than for those fish exposed to the same levels of TFM but with 1% Nic mixture (Supplemental Data, Table S7). With respect to niclosamide (alone vs mixture exposure), significant differences in survival (p < 0.0083) were noted between fish that were exposed to 0.1 mg/L Nic alone and those exposed to the same level of Nic in combination with TFM (comparing identical Nic concentrations between Figure 4B and C; Supplemental Data, Table S8). There was no significant difference in survival between fish exposed to 0.05 and 0.25 mg/L Nic, alone and in mixture with TFM.
Characterization of TFM–Nic interactions using the toxic unit approach
The 12‐h LC50s for TFM (18.2 mg/L) and Nic (0.11 mg/L) were assigned as 1 toxic unit. Based on these values, 1 toxic unit of TFM would correspond to a TFM concentration of 18.2 mg/L and 1 toxic unit of Nic to 0.11 mg/L, with each predicted to cause 50% mortality.
To determine if the responses of the trout to the TFM/Nic mixtures were strictly additive (falling on the line of strict additivity), less than or greater than additive, the total toxic unit of exposure was calculated based on the actual TFM and Nic exposure concentrations to which the fish were exposed and the observed mortality. Exposure to the lowest TFM/Nic exposure concentrations, of 2.54 and 0.013 mg/L, corresponded to 0.14 and 0.12 toxic unit, adding up to 0.26 total toxic unit of exposure, but resulted in no mortality (Figure 5A). As the respective concentrations of TFM and Nic increased to 0.28 and 0.18 toxic unit, equal to 0.46 total toxic unit of exposure, the observed mortality was 28%, near the expected 26% mortality if the interaction was strictly additive. As the concentrations of TFM and niclosamide in the water increased further, to 0.43 and 0.44 toxic unit of exposure, equivalent to 0.87 total toxic unit of exposure, mortality increased to 68%, above the expected 46% mortality if the interaction was strictly additive. At 0.55 and 0.63 toxic unit of exposure, equivalent to 1.18 total toxic unit of exposure, there was 100% mortality, well above the expected 62% mortality predicted from the line of strict additivity (Figure 5A). Not surprisingly, as the concentration of TFM plus Nic approached 2 toxic unit of exposure (0.88 toxic unit TFM plus 1.07 toxic unit Nic), 100% mortality was observed (Figure 5A), as well as at 2.56 total toxic units of exposure (1.46 toxic unit TFM:2.1 toxic unit Nic; data not shown).
Figure 5.
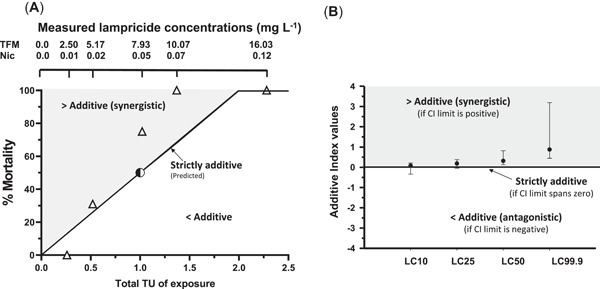
Interactions between 3‐trifluoromethyl‐4‐nitrophenol (TFM) and niclosamide (Nic) using the toxic unit (TU) and additive index approaches. (A) Plot displaying predicted percentage mortality (line of equivalence) based on strictly additive interactions in rainbow trout (Oncorhynchus mykiss) exposed to increasing concentrations of TFM plus Nic mixtures, expressed as the combined total toxic units of exposure (1 toxic unit = 12‐h median lethal concentration [LC50] of TFM or Nic) of each. Partially shaded circle depicts the expected mortality when the fish were exposed to their respective 12‐h LC50 for TFM or Nic alone, which are equivalent to 1.0 toxic unit. Open triangles denote the observed mortality when the fish were exposed to increasing concentrations of TFM and Nic, expressed as total toxic units of exposure. Note that for each combination of TFM and Nic tested, the greater than additive interactions increased with total toxic unit of exposure. (B) Plot displaying the additive indices calculated from LC values derived from individual and mixture (TFM:Nic, 100:1, w/v) exposures. The shaded area above the lines of strict additivity represents greater than additive (synergistic interactions) between the 2 agents, whereas the open area below the line represents less than additive interactions. CI = confidence interval.
Characterization of TFM–Nic interactions using the additive index approach
Toxicity values of the individual lampricides and their mixtures were used to quantify the interaction between TFM and Nic (Figure 3). The additive index was calculated after determining the 12‐h LC10, LC25, LC50, and LC99 for each lampricide, alone and in mixture, along with their respective CIs (Figure 5B). Using the CIs associated with each LC value, the range of the additive indices was calculated to characterize the interactions between TFM and Nic. The additive index, when the trout were exposed to the 12‐h LC10, was approximately 0.24, with a range of (+)0.04 to (+)0.041. The additive index for the 12‐h LC25 combination was determined to be approximately 0.36, with a range of (+)0.31 to (+)0.37, whereas at the 12‐h LC50, the additive index was approximately 0.42, with a range of (+)0.27 to (+)0.46. Lastly, at the 12‐h LC99, the additive index was 0.52, with a range of (+)0.47 to (+)0.55. Because all additive indices were >0 (or above the line of additivity in Figure 4B), the additive index predicted greater than additive interaction between TFM and Nic.
DISCUSSION
TFM and Nic interact in a greater than additive (synergistic) manner
Both the concentration addition (toxic unit) and additive index models revealed that the interactions between TFM and Nic, applied in proportions (~99% TFM:1% Nic) likely to be used in the field, are greater than additive (synergistic). On its own, Nic was significantly more toxic than TFM alone, with coapplication of the TFM/1% Nic mixture increasing the toxicity of both lampricides by approximately 2‐fold. These synergistic effects were demonstrated using both the toxic unit and the additive index approaches, indicating that either of the 2 models can be used to describe TFM–Nic interactions. Conceptually, the concentration addition model, which employs the relatively simple toxic unit concept, is easier to implement and to interpret. It should be noted that at low concentrations (0.14/0.13 toxic units of TFM/Nic), the toxic unit approach could not be used because we observed no mortality, whereas the additive index approach suggested that the effects are synergistic at all concentrations tested.
In agreement with earlier studies (Boogaard et al. 2003; McDonald and Kolar 2007; O'Connor et al. 2017), the 12‐h LC50 of TFM was approximately 165‐fold greater than that of Nic, and therefore much less toxic. However, Nic lacks the specificity of TFM, which is considered an ideal lampricide because it specifically targets larval lampreys as a result of their limited ability to detoxify it compared to nontarget fishes such as the rainbow trout (Boogaard et al. 2003; McDonald and Kolar 2007; Wilkie et al. 2019). Using the phase II detoxification pathways glucuronidation and sulfation, TFM is mainly detoxified by trout and other nontarget fishes, such as bullhead (Ameiurus nebulosus) and bluegill (Lepomis macrochirus), and then eliminated (Lech and Statham 1975; Birceanu et al. 2014; Bussy et al. 2018a, 2018b). Thus, TFM application on its own has minimal physiological and ecological impacts on nontarget fishes during routine treatments in the field, when applied at the appropriate concentration. Recent work has also shown that both sea lamprey and some nontarget fishes are able to detoxify TFM via phase I biotransformation, leading to a reduced form of TFM, TFMa (Bussy et al. 2018a, 2018b). Whether Nic is handled by fishes in the same way remains to be resolved.
Unlike TFM, Nic not only is much more toxic but also lacks specificity to larval sea lamprey, which exhibit comparable LC50 values to nontarget fishes (Boogaard et al. 2003; Hubert et al. 2005). When exposed to 0.05 mg/L of Nic, an environmentally realistic concentration that was also used in the present study, rainbow trout, channel catfish (Ictalurus punctatus), and largemouth bass (Micropterus salmoides) rapidly took up the pesticide (Dawson et al. 1982), which was distributed throughout the body, with the highest concentrations in the bile and liver (Lech and Statham 1975). Like TFM, Nic was detoxified via glucuronidation and sulfation in rainbow trout and catfish (Hubert et al. 2005). The rate of sulfation was much greater because the concentrations of Nic‐sulfate ester were 18‐fold greater than those of Nic‐glucuronide in the muscle of rainbow trout. Beyond this, little work has been done on the pharmacokinetics of Nic in sea lamprey and nontarget fishes and how these processes are influenced by the simultaneous presence of TFM in water. An intriguing possibility is that competitive or noncompetitive inhibition of sulfatase and/or glucuronyl transferase binding sites by either TFM or Nic could impair detoxification, leading to greater accumulation of TFM or Nic in the fish and greater toxicity. Indeed, the metabolism of numerous drugs is inhibited in this fashion, such as acetaminophen, in which glucuronidation is impaired in the presence of medications such as morphine and tetracycline (Bolanowska and Gessner 1978). Further mechanistic investigations on the detoxification and elimination of TFM and Nic, particularly as they pertain to phase II processes such as glucuronidation and sulfation and perhaps phase I enzymes that could potentially be involved, would certainly reveal more about the nature of their interactions.
Another intriguing possibility leading to the observed interactions between TFM and Nic in the present study is that the presence of one lampricide in water influences the ionization of the other, therefore impacting its toxicity. Indeed, previous research has found that the presence of metal ions (such as copper, chromium, manganese, and cobalt) in water inhibited the photodegradation of organophosphorous pesticides sensitized by humic acid (Kamiya and Kameyama 2001), suggesting that the addition of ionizing compounds to the water can impact chemical bioavailability. In the present study, we have shown that the presence of one lampricide in water alters the dose response of the other, increasing its toxicity (Figures 3 and 4), but whether this is due to a shift of the ionization equilibrium induced by the presence of both chemicals in solution has yet to be determined.
Because both TFM and Nic target the mitochondria, it is also possible that their combined effects on oxidative phosphorylation lead to greater impairment of adenosine triphosphate (ATP) production at a given dose of TFM, which ultimately increases the acute toxicity of TFM in lamprey and nontarget fishes alike. Both TFM and Nic are believed to uncouple oxidative phosphorylation, the oxygen‐dependent process required to generate ATP, the primary energy currency in the body (see Wilkie et al. 2019 for review). This results in a mismatch between ATP supply and demand in the body, which forces the fish to increase their reliance on anaerobic pathways of ATP production such as high‐energy phosphagens (e.g., phosphocreatine) and anaerobic glycolysis to meet their ATP demands for survival. Depending on the dose of TFM or Nic, anaerobic ATP production pathways may be sufficient to supplement the lower rates of ATP production by the mitochondria. At higher concentrations and/or as the lampricide accumulates in the tissues, the dose‐dependent effects of TFM and Nic on mitochondrial function will worsen (Birceanu et al. 2011; O. Birceanu et al., unpublished data). Ultimately, the amounts of these limited anaerobic resources become depleted, and the animal dies.
Perturbation of mitochondrial oxidative phosphorylation by TFM appears to be related to the depolarization of mitochondrial membrane potential, which results in the degeneration of the H+ electrochemical gradient across the inner mitochondrial membrane that is essential for the movement of H+ through the ATP synthase complex, the site where ADP is phosphorylated to ATP (Birceanu et al. 2011). Although less work has been done on the mode of action of Nic in fishes, there is strong evidence in other model organisms that it acts in a similar manner (Skulachev 1998; Moridani 2003; Ozaki et al. 2008; Solaini et al. 2011; Jurgeit et al. 2012). The greater toxicity of Nic compared to TFM may therefore be because the mitochondria are simply much more sensitive to this chemical. Indeed, comparisons to 2,4‐dinitrophenol, a classic uncoupler of oxidative phosphorylation, have indicated that mitochondria are much more sensitive to Nic (Park et al. 2011; Jurgeit et al. 2012). The reasons for greater mitochondrial sensitivity to Nic have not been resolved, but it could be related to differences in chemical properties such as lipid solubility or the presence of additional targets such as the protein complexes found on the inner mitochondrial membrane that are responsible for generating the H+ electrochemical gradient needed to drive ATP synthesis. Ongoing studies using isolated mitochondria, transcriptomics, and/or metabolomic approaches should shed additional light on the target(s) of not only Nic but also TFM, to provide a better mechanistic explanation about how these compounds interact with one another.
Another explanation of the greater than additive interactions between TFM and Nic is the possibility that one or both constituents have additional modes of action that have not yet been explored in fishes. Indeed, Nic has been shown to reduce cancer cell growth by preventing cell proliferation (Park et al. 2011), whereas a variety of other salicylanilides have been shown to interfere with pH regulation and the biochemical pathways involved in glycolysis, therefore impairing glycolysis and anaerobic energy production (Köhler 2001). These additional modes of action, when combined with Nic's protonophore properties, could be an explanation as to why this lampricide is much more toxic than TFM to both larval sea lamprey and nontarget fish species. When used in combination with TFM, the secondary mechanisms of toxicity of Nic plus the enhanced uncoupling effects of the 2 chemicals lead to increased toxicity of each chemical in mixtures.
The toxic unit and additive index approaches can be used to define TFM–Nic interactions
The concentration addition model assumes that the constituents of a chemical mixture exert their toxicity in a similar manner. Therefore, the concentration of the toxic constituents of the mixtures are added to predict toxicity (Norwood et al. 2003). Because TFM and Nic appear to similarly affect mitochondrial function (Wilkie et al. 2019), the concentration addition model can be used to determine if they interact in a less than, strictly, or greater than additive manner. Using the toxic unit approach, it is clear from our analysis that the greater mortality observed when TFM and Nic were applied together was greater than additive or synergistic (Figure 5A). This interpretation was supported by the additive index model (Figure 5B), in which the respective ranges for the LC10, LC25, LC50, and LC99 of the lampricides and mixtures were >0, which is an indication of synergism, proposed previously (Howell et al. 1964). These results differ from those of Marking and Bills (1985), who determined that the interaction between the lampricides in rainbow trout, white sucker, and fathead minnow were strictly additive when fish were exposed to their respective 96‐h LC50s of TFM and Nic, alone and in combination. This apparent contradiction is likely because Marking and Bills (1985) determined the acute toxicity of TFM and Nic over 96 h, which greatly exceeds a typical period of lampricide application, which typically is approximately 12 h (Wilkie et al. 2019). Moreover, the concentrations they used to make their calculations using the additive index model were far below those used in the present study. In other words, the Marking and Bills (1985) study would have been unlikely to exhibit any toxicity in trout after the first 12 h of exposure. Indeed, the respective concentrations of TFM and Nic were 90 and 50% lower in mixtures in the Marking and Bills (1985) study than in the present study. These differences highlight the need to consider exposure duration when attempting to describe the interactions between the constituents of chemical mixtures and the need to ensure that the concentration of the mixture is sufficient to cause death or some other suitable, quantifiable endpoint (e.g., changes in growth, loss of equilibrium, reproductive success). In the absence of a measurable response to the chemical mixture, conclusions regarding the presence of lower, strictly, or greater than additive interactions can be misleading.
We have also considered the possibility that having added ethanol and isopropanol as vehicles for Nic and TFM, respectively, may have impacted the response of the fish used in the present study. However, the highest percentage of ethanol and isopropanol used in the present study was <0.64 × 10–4%, in both individual lampricide exposures and mixtures. In previous studies, the no‐observed‐effect concentration for ethanol in zebrafish embryos was 0.5%, with a lowest‐observed‐effect concentration of 1.0% (Chromcova et al. 2012). In trout microsomes, ethanol at levels <0.5% had no effect on in vitro activity of CYP450 (Sakalli et al. 2015). Therefore, we believe that the low levels of vehicles that were used in the present study, either alone or in combination, had no effect on the responses we recorded in rainbow trout.
We have considered the possibility that impurities in the manufacturing process of the 2 lampricides may be present and, therefore, may have impacted our results, particularly because previous work has shown that TFM applications were associated with induction of mixed‐function oxygenase activity (Munkittrick et al. 1994). However, the formulations of TFM and Nic have been changed in the last 15 yr to increase purity and reduce the potential for the formation of impurities (J. Luoma and T. Hubert, Upper Midwest Environmental Sciences Center, US Geological Survey, personal communication). In addition, as part of the Environmental Protection Agency's reregistration process (Product Properties Test Guide OPPTS 830.1670), any impurities detected in the formulations of pesticides must be identified, and if toxicologically significant, further information must be provided. None were provided for the formulations of TFM and Nic that were used in the present study.
To further support the finding that TFM and Nic interact in a synergistic manner, as proposed by both the toxic unit and additive index models, we also tested whether a similar prediction can be made using an alternative model to concentration addition: the response addition (or effects addition) model (Norwood et al. 2003), where the survival rates of the animals exposed to the individual components of the mixtures are multiplied to predict their combined effects. Advantages of the response addition model are that it does not assume an identical mode of action of the chemicals present in the mixture (whereas both the toxic unit and additive index do) and that it is easy to employ (Norwood et al. 2003; Clemow and Wilkie 2015). Using the data generated in the present study, we can use the response addition model to predict the toxicity of TFM and Nic in mixtures. The 12‐h LC50 (50% or 0.5 survival) of TFM and Nic in mixtures is 6.64 and 0.06 mg/L, respectively. Survival of rainbow trout when exposed to 6.64 mg/L TFM only and 0.06 mg/L Nic only is 97% (0.97) and 83% (0.83), respectively. By multiplying the 2 values, the predicted survival rate in the mixture is approximately 0.80, or 80%. However, the observed survival rate was 50% (0.5), or 30% less than the predicted, therefore suggesting that the interaction of the 2 lampricides was greater than additive, in agreement with our conclusions using both the toxic unit approach and the additive index model.
Relevance for field applications and risk assessment
Using the toxic unit concept and the additive index, with the response addition model as a third method for assessing lampricide interactions, we have demonstrated that TFM and Nic interact in a greater than additive, or synergistic, manner in trout subjected to concentrations typically encountered in the field. Knowing that lethality caused by TFM/Nic mixtures is due to greater than additive or synergistic interactions may put fisheries managers, regulators, and sea lamprey control agents in a better position to assess how nontarget species respond to mixtures of TFM and Nic, particularly in waters where TFM/Nic mixtures have not been used previously. Such information will also help them to determine if mitigation measures are called for and what measures might be adopted to mitigate potential nontarget toxicity, such as altering the timing of treatments or application concentrations. We recognize that extrapolation of the present results on TFM/Nic interactions in rainbow trout to other fishes or invertebrates, including species at risk, is tenuous. Accordingly, we suggest that the experimental framework adopted in the present study could be used to learn more about how other nontarget species, not to mention sea lamprey, respond to TFM/Nic mixtures and the underlying associated mechanisms. Such information will be of great importance because the use of TFM and Nic to control sea lamprey populations will continue in the Great Lakes basin for the immediately foreseeable future and likely be of value when reregistration of these lampricides is required (Wilkie et al. 2019).
To conclude, the toxic unit and additive index approaches are equally effective methods that can be used to assess how fishes respond to TFM and Nic mixtures, and they yield similar results. Conceptually, the use of the toxic unit approach that underlies the concentration addition model is less cumbersome and conceptually clearer, and therefore easier to implement when assessing the possible impact of the 2 lampricides on fishes. We therefore recommend that this model be used to predict and explain how TFM/Nic mixtures are likely to affect lampreys and nontarget fishes more effectively.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at https://doi.org/10.1002/etc.4994.
This article has earned an Open Data/Materials badge for making publicly available the digitally‐shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.6084/m9.figshare.12691790. Learn more about the Open Practices badges from the Center for Open Science: https://osf.io/tvyxz/wiki.
Supporting information
This article includes online‐only Supplemental Data.
Supporting information.
Acknowledgment
We thank B. Scotland for his assistance operating the HPLC; F. Neave, B. J. Morrison, and the remaining DFO SLCC staff for their expertise in sea lamprey control operations; B.L. Hlina for his assistance with the statistical analysis; and T. Hubert and J. Luoma for assistance with revisions. We are also grateful to the Wilfrid Laurier University Animal Care Facility Staff for assistance with fish care. The present study was funded by a Great Lakes Fishery Commission contract, awarded to M.P. Wilkie (2014_WIL_54028). The present study was conducted at Wilfrid Laurier University, which exists on the traditional territory of the Neutral, Anishnawbe, and Haudenosaunee peoples.
REFERENCES
- Bills TD, Boogaard MA, Johnson DA, Brege DC, Scholefield RJ, Westman RW, Stephens BE. 2003. Development of a pH/alkalinity treatment model for applications of the lampricide TFM to streams tributary to the Great Lakes. J Great Lakes Res 29:510–520. [Google Scholar]
- Bills TD, Marking LL. 1976. Toxicity of 3‐trifluoromethyl‐4‐nitrophenol (TFM), 2′5‐dichloro‐4′‐nitrosalicylanilide (Bayer 73) and a 98:2 mixture to fingerlings of seven fish species and to eggs and fry of coho salmon. Investigations in Fish Control No. 69. US Fish and Wildlife Service, Washington, DC.
- Birceanu O, McClelland GB, Wang YS, Brown JCL, Wilkie MP. 2011. The lampricide 3‐trifluoromethyl‐4‐nitrophenol (TFM) uncouples mitochondrial oxidative phosphorylation in both sea lamprey (Petromyzon marinus) and TFM‐tolerant rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 153:342–349. [DOI] [PubMed] [Google Scholar]
- Birceanu O, Sorensen LA, Henry M, McClelland GB, Wang YS, Wilkie MP. 2014. The effects of the lampricide 3‐trifluoromethyl‐4‐nitrophenol (TFM) on fuel stores and ion balance in a non‐target fish, the rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 160:30–41. [DOI] [PubMed] [Google Scholar]
- Bolanowska W, Gessner J. 1978. Drug interactions: Inhibition of acetaminophen glucuronidation by drugs. Pharmacol Exp Ther 206:233–238. [PubMed] [Google Scholar]
- Boogaard MA, Bills TD, Johnson DA. 2003. Acute toxicity of TFM and a TFM/niclosamide mixture to selected species of fish, including lake sturgeon (Acipenser fulvescens) and mudpuppies (Necturus maculosus), in laboratory and field exposures. J Great Lakes Res 29:529–541. [Google Scholar]
- Bussy U, Chung‐Davidson YW, Buchinger T, Li K, Smith SA, Jones AD, Li W. 2018a. Metabolism of a sea lamprey pesticide by fish liver enzymes part A: Identification and synthesis of TFM metabolites. Anal Bioanal Chem 410:1749–1761. [DOI] [PubMed] [Google Scholar]
- Bussy U, Chung‐Davidson YW, Buchinger T, Li K, Smith SA, Jones AD, Li W. 2018b. Metabolism of a sea lamprey pesticide by fish liver enzymes part B: Method development and application in quantification of TFM metabolites formed in vivo. Anal Bioanal Chem 410:1763–1774. [DOI] [PubMed] [Google Scholar]
- Chromcova L, Stepanova S, Plhalova L, Praskova E, Svobodova S. 2012. Effect of four selected carrier solvents on embryonal stages of Danio rerio . Neuro Endocrinol Lett 33:60–65. [PubMed] [Google Scholar]
- Clemow YH, Wilkie MP. 2015. Effects of Pb plus Cd mixtures on toxicity, and internal electrolyte and osmotic balance in the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 161:176–188. [DOI] [PubMed] [Google Scholar]
- Dawson VK. 2003. Environmental fate and effects of the lampricide Bayluscide: A review. J Great Lakes Res 29:475–492. [Google Scholar]
- Dawson VK, Cumming KB, Gilderhuis PA. 1977. Efficacy of 3‐trifluoromethyl‐4‐nitrophenol (TFM), 2′,5‐dichloro‐4′‐nitrosalicylanilide (Bayer 73), and a 98:2 mixture as lampricides in laboratory studies. Investigations in Fish Control No. 77. US Fish and Wildlife Service, Washington, DC.
- Dawson VK, Sills JB, Luhning CW. 1982. Accumulation and loss of 2′5‐dichloro‐4′‐nitro salicylanilide Bayer 73 by fish—Laboratory studies. Investigations in Fish Control No. 90. US Fish and Wildlife Service, Washington, DC.
- Hepditch SLJ, Tessier LR, Wilson JM, Birceanu O, O'Connor LM, Wilkie MP. 2019. Mitigation of lampricide toxicity to juvenile lake sturgeon: The importance of water alkalinity and life stage. Conserv Physiol 7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JH, Everett LK Jr, Smith AJ, Hanson LH. 1964. Synergism of 5,2′‐dichloro‐4′‐nitro‐salicylanilide and 3‐trifluoromethyl‐4‐nitrophenol in a selective lamprey larvicide. Technical Report No. 8. Great Lakes Fishery Commission, Ann Arbor, MI, USA.
- Hubert TD. 2003. Environmental fate and effects of the lampricide TFM: A review. J Great Lakes Res 29:456–474. [Google Scholar]
- Hubert TD, Bernardy JA, Vue C, Dawson VK, Boogaard MA, Schreier TM, Gingerich WH. 2005. Residues of the lampricides 3‐trifluoromethyl‐4‐nitrophenol and niclosamide in muscle tissue of rainbow trout. J Agric Food Chem 53:5342–5346. [DOI] [PubMed] [Google Scholar]
- Jurgeit A, McDowell R, Moese S, Meldrum E, Schwendener R, Greber UF. 2012. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog 8:e1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya M, Kameyama K. 2001. Effects of selected metal ions on photodegradation of organophosphorus pesticides sensitized by humic acids. Chemosphere 45:231–235. [DOI] [PubMed] [Google Scholar]
- Köhler P. 2001. The biochemical basis of anthelmintic action and resistance. Int J Parasitol 31:336–345. [DOI] [PubMed] [Google Scholar]
- Lech JJ. 1974. Glucuronide formation in rainbow trout—Effect of salicylamide on the acute toxicity, conjugation and excretion of 3‐trifluoromethyl‐4‐nitrophenol. Biochem Pharmacol 23:2403–2410. [DOI] [PubMed] [Google Scholar]
- Lech JJ, Costrini NV. 1972. In vitro and in vivo metabolism of 3‐trifluoromethyl‐4‐nitrophenol (TFM) in rainbow trout. Comp Gen Pharmacol 3:160–166. [DOI] [PubMed] [Google Scholar]
- Lech JJ, Statham CN. 1975. Role of glucuronide formation in the selective toxicity of 3‐trifluoromethyl‐4‐nitrophenol (TFM) for the sea lamprey: Comparative aspects of TFM uptake and conjugation in sea lamprey and rainbow trout. Toxicol Appl Pharmacol 31:150–158. [DOI] [PubMed] [Google Scholar]
- Marking LL. 1977. Method of assessing additive toxicity of chemical mixtures. Special Technical Publication 634. American Society for Testing and Materials, Philadelphia, PA.
- Marking LL, Bills TD. 1985. Effects of contaminants on toxicity of the lampricides TFM and Bayer 73 to three species of fish. J Great Lakes Res 11:171–178. [Google Scholar]
- Marking LL, Dawson VK. 1975. Method for assessment of toxicity or efficacy of mixtures of chemicals. Investigations in Fish Control No. 67. US Fish and Wildlife Service, Washington, DC.
- McConville MB, Hubert TD, Remucal CK. 2016. Direct photolysis rates and transformation pathways of the lampricides TFM and niclosamide in simulated sunlight. Environ Sci Technol 50:9998–10006. [DOI] [PubMed] [Google Scholar]
- McDonald DG, Kolar CS. 2007. Research to guide the use of lampricides for controlling sea lamprey. J Great Lakes Res 33:20–34. [Google Scholar]
- Moridani M. 2003. Quantitative structure toxicity relationships for phenols in isolated rat hepatocytes. Chem Biol Interact 145:213–223. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, Servos MR, Parrot JL, Martin V, Carey JH, Flett PA, Vanderkraak GJ. 1994. Identification of lampricide formulations as a potent inducer of MFO activity in fish. J Great Lakes Res 20:355–365. [Google Scholar]
- Newman MC. 2015. Fundamentals of Ecotoxicology: The Science of Pollution, 4th ed. CRC, Boca Raton, FL, USA. [Google Scholar]
- Norwood WP, Borgmann U, Dixon DG, Wallace A. 2003. Effects of metal mixtures on aquatic biota: A review of observations and methods. Hum Ecol Risk Assess 9:795–811. [Google Scholar]
- O'Connor LM, Pratt TC, Steeves TB, Stephens B, Boogaard M, Kaye C. 2017. In situ assessment of lampricide toxicity to age‐0 lake sturgeon. J Great Lakes Res 43:189–198. [Google Scholar]
- Ozaki S, Kano K, Shirai O. 2008. Electrochemical elucidation on the mechanism of uncoupling caused by hydrophobic weak acids. Phys Chem Chem Phys 10:4449–4455. [DOI] [PubMed] [Google Scholar]
- Park SJ, Shin JH, Kang H, Hwang JJ, Cho DH. 2011. Niclosamide induces mitochondria fragmentation and promotes both apoptotic and autophagic cell death. BMP Rep 44:517–522. [DOI] [PubMed] [Google Scholar]
- Playle RC. 2004. Using multiple metal–gill binding models and the toxic unit concept to help reconcile multiple‐metal toxicity results. Aquat Toxicol 67:359–370. [DOI] [PubMed] [Google Scholar]
- Sakalli S, Burkina V, Zlabek V, Zamaratskaia G. 2015. Effects of acetone, acetonitrile, ethanol, methanol and DMSO on cytochrome P450 in rainbow trout (Oncorhynchus mykiss) hepatic microsomes. Toxicol Mech Methods 25:501–506. [PubMed] [Google Scholar]
- Schultz DP, Harman PD. 1978. Uptake, distribution, and elimination of the lampricide 2',5‐dichloro‐4'‐nitro[14C]salicylanilide (Bayer 2353) and its 2‐aminoethanol salt (Bayer 73) by largemouth bass. J Agric Food Chem 26:1226–1230. [DOI] [PubMed] [Google Scholar]
- Siefkes MJ. 2017. Use of physiological knowledge to control the invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conserv Physiol 5:cox031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP. 1998. Uncoupling: New approaches to an old problem of bioenergetics. Biochim Biophys Acta 1363:100–124. [DOI] [PubMed] [Google Scholar]
- Solaini G, Sgarbi G, Baracca A. 2011. Oxidative phosphorylation in cancer cells. Biochim Biophys Acta 1807:534–542. [DOI] [PubMed] [Google Scholar]
- Tomlin C. 1994. The Pesticide Manual: World Compendium: Incorporating the Agrochemicals Handbook , 10th ed. The British Crop Protection Council, Surrey, UK. [Google Scholar]
- Wilkie MP, Hubert TD, Boogaard MA, Birceanu O. 2019. Control of invasive sea lampreys using the piscicides TFM and niclosamide: Toxicology, successes & future prospects. Aquat Toxicol 211:235–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting information.


