Abstract
Background/aims
Direct‐acting antivirals (DAAs) are highly effective in treating chronic hepatitis C virus (HCV)‐infected patients. The real‐world treatment outcome in Taiwanese patients on a nationwide basis is elusive.
Methods
The Taiwan HCV Registry (TACR) programme is a nationwide registry platform including 48 study sites, which is organized and supervised by the Taiwan Association for the Study of the Liver. The primary endpoint was sustained virological response (SVR12, undetectable HCV RNA 12 weeks after end‐of‐treatment).
Results
A total of 13 951 registered patients with SVR12 data available were analysed (mean age, 63.0 years; female, 55.9%; HCV genotype‐1 [GT1], 57.9%; cirrhosis, 38.4%; preexisting hepatocellular carcinoma [HCC], 10.6%; and hepatitis B virus coinfection, 7.7%). The overall SVR12 rate was 98.3%, with 98.7%, 98.0%, 98.4% and 97.4% in treatment‐naïve noncirrhotic, treatment‐naïve cirrhotic, treatment‐experienced noncirrhotic and treatment‐experienced cirrhotic patients, respectively. The SVR12 rate was > 95% across all subgroups except treatment‐experienced cirrhotic patients who received sofosbuvir/ribavirin (88.7%), treatment‐naïve noncirrhotic patients (94.8%) and treatment‐experienced cirrhotic (94.8%) patients who received daclatasvir/asunaprevir. The most important factor associated with treatment failure was DAA adherence < 60% ( adjusted odds ratio [aOR]/95% confidence interval [CI]: 117.1/52.4‐261.3, P < .001), followed by GT3/GT2 (aOR/CI: 5.78/2.25‐14.9, P = .0003 and aOR/CI: 1.55/1.05‐2.29, P = .03, compared with GT1), active hepatocellular carcinoma (aOR/CI: 4.29/2.57‐7.16, P < .001), the use of sofosbuvir/ribavirin (aOR/CI: 2.51/1.67‐3.77, P < .001) and daclatasvir/asunaprevir (aOR/CI: 3.29/1.94‐5.58, P < .001), decompensated liver cirrhosis (aOR/CI: 2.50/1.20‐5.22, P = .02) and high HCV viral loads (aOR/CI: 2.16/1.57‐2.97, P < .001).
Conclusions
DAAs are highly effective in treating Taiwanese HCV patients in the real‐world setting. Maintaining DAA adherence and selecting highly efficacious regimens are keys to ensure treatment success.
Keywords: CHC, DAA, HCV, real world, registry, Taiwan
Abbreviations
- ASV
asunaprevir
- CHC
chronic hepatitis C
- DAA
direct‐acting antiviral agents
- DCV
daclatasvir
- EBR
elbasvir
- GLE
glecaprevir
- GZR
grazoprevir
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LDV
ledipasvir
- PIB
Pibrentasvir
- PrOD
paritaprevir/ritonavir/ombitasvir/dasabuvir
- RBV
ribavirin
- SAE
serious adverse event
- SOF
sofosbuvir
- SVR
sustained virological response
- VEL
velpatasvir
Lay summary.
Directly acting antivirals (DAAs) are highly effective in treating chronic hepatitis C virus (HCV) infected patients. The real‐world treatment outcome in Taiwanese patients on a nationwide basis has never been addressed. Taiwan HCV Registry (TACR) programme is a nationwide registry platform organized and supervised by the Taiwan Association for the Study of the Liver. By July 2020, 13 951 patients (including 4421 cirrhotic patients and 1473 HCC patients, respectively) from 48 study sites participate in the programme. The study also comprised the largest cohort with hepatitis B dual infection (n = 1068). The primary endpoint was sustained virological response (SVR12, undetectable HCV RNA 12 weeks after end‐of‐treatment). The overall SVR rate was 98.3%, with 98.7%, 98.0%, 98.4% and 97.4% in treatment‐naïve noncirrhotic, treatment‐naïve cirrhotic, treatment‐experienced noncirrhotic and treatment‐experienced cirrhotic patients, respectively. We denoted that the most important factor independently associated with treatment failure was DAA adherence < 60%. The SVR rate was 98.5% in the 1068 HBV co‐infected patients, which was similar to those with HCV monoinfection (98.3%, P = .61). The highly effective treatment outcome of DAAs could be explicitly translated to Taiwanese patients with different characteristics at the nationwide level.
1. INTRODUCTION
Approximately 71 million individuals are chronically infected with hepatitis C virus (HCV), which accounts for a major disease burden worldwide. 1 HCV eradication by antivirals reduces liver‐related complications, improves quality of life and prolongs lifespan in chronic hepatitis C (CHC) patients. With the introduction of all‐oral direct‐acting antivirals (DAAs) in 2014, high treatment efficacy and satisfactory tolerability can ultimately be attained across all patient groups. 2 , 3 The breakthrough landscape would bring about the goal of achieving HCV elimination in the foreseeable future.
HCV is endemic in Taiwan, with an estimated prevalence of 3.28% (1.8 − 5.5%) in the general population and > 10% in several HCV hyperendemic areas. 4 , 5 There exists a considerable gap between clinical efficacy and community effectiveness in terms of HCV treatment, which leaves > 70% of CHC patients being untreated in the interferon era. 4 , 6 The Taiwan National Health Insurance (NHI) programme started to reimburse DAAs in 2017, and more than 75 000 CHC patients received DAAs until the end of 2019. 7 Although several real‐world data regarding DAA treatment have been reported in Taiwan, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 all were on a single‐centre or small‐scale basis, which failed to address potential causes of virological failure due to limited sample size. Nationwide data depicting the treatment efficacy and tolerability of CHC patients receiving DAA in Taiwan are still lacking, especially data from special populations. Herein, we conducted a real‐world multicentre cohort study using the nationwide HCV registry database in Taiwan. All patients from each participating site had well‐characterized demographic and virological characteristics. The primary objective of the current study was to explore the real‐world efficacy and factors associated with DAA failure in Taiwanese CHC patients at the national level.
2. METHODS
The Taiwan Association for the Study of the Liver (TASL) HCV Registry (TACR) is a nationwide registry programme organized by the TASL that set up and manages the database and biobank of HCV patients who receive DAA therapy in Taiwan. By July 2020, 48 study sites, including 21 medical centres, 22 regional hospitals and five primary clinics, were participating in the registry. Individual patient records were reviewed, and data were extracted and validated at each participating study centre using a standardized case report form and a unified coding dictionary. Eligible patients were those who (1) were aged > 20 years, (2) had detectable HCV RNA and (3) received DAA‐containing regimes for at least one dosage of any DAA and had treatment outcomes available. All the patients had precisely defined patient (including demographics, laboratory results, comorbidities and cirrhotic status) and virological characteristics (including HCV genotypes, viral loads and treatment outcomes) before and after antiviral treatment. The treatment regimens and strategies conformed to the regulations of the Health and Welfare Department of Taiwan 17 and regional guidelines. 18 , 19 The study was approved by the institutional review board at each study site, which conformed to the guidelines of the International Conference on Harmonization for Good Clinical Practice. All patients provided written informed consent.
The primary endpoint was sustained virological response (SVR12, defined as undetectable HCV RNA [<12 or < 25 IU/mL depending on individual laboratory testing]) throughout 12 weeks of the post‐treatment follow‐up in patients with treatment outcome available. Liver cirrhosis was defined by any of the following: liver histology, 20 transient elastography (FibroScan®; Echosens, Paris, France) (>12 kPa), 21 acoustic radiation force impulse (>1.98 m/s), 22 fibrosis‐4 index (>6.5) 23 or the presence of clinical, radiological, endoscopic or laboratory evidence of cirrhosis and/or portal hypertension. Hepatocellular carcinoma (HCC) was confirmed by histological or clinical diagnosis based on the guidelines of the American Association for the Study of Liver Diseases 24 or the Asian Pacific Association for the Study of the Liver. 25 Patients with inactive HCC were defined as those with HCC who were subjected to local ablation (alcohol injection, radiofrequency or microwave), surgical resection or liver transplantation and who were without imaging evidence of recurrence within 3 months prior to DAA initiation. 8 Hepatitis B virus (HBV) DNA levels were checked at baseline, Week 4 of treatment, end‐of‐treatment and 3 months after the‐end‐of‐treatment if feasible. HBV virological reactivation was defined as a > 1‐log increase in HBV DNA from baseline in a patient with pretreatment detectable HBV DNA, or HBV DNA > 100 IU/mL in a patient with pretreatment undetectable HBV DNA. HBV clinical reactivation was defined as an alanine aminotransferase increase of > 2‐fold from nadir and > 100 U/L or > 2‐fold increase from baseline, concomitant with HBV reactivation. The use of prophylactic or rescued nucleotide/nucleoside analogues (NAs) for HBV activation is at the investigators’ discretion.
2.1. Statistical analyses
Frequency was compared between groups using the x 2 test with the Yates correction or Fisher's exact test. Group means (presented as the mean standard deviation) were compared using analysis of variance and Student's t test or the nonparametric Mann–Whitney U test when appropriate. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) Equation 26 Variables selected for analysing DAA treatment failure were those commonly presented patient characteristics and demography. To explore the potential impact of drug compliance on DAA failure in the real world setting, the factor of treatment adherence was also put into analysis. DAA adherence was defined as the percentage of actual dosage being taken divided by the anticipated DAA dosage throughout the treatment course in each subject. Stepwise logistic regression analysis was performed to determine factors associated with SVR12 by analysing the covariates with a P value < 0.1 in the univariate analysis. Collinear test was applied to assess whether if the independent factors were highly correlated. The statistical analyses were performed using the SPSS 12.0 statistical package (SPSS). All statistical analyses were based on two‐sided hypothesis tests with a significance level of P < .05.
3. RESULTS
3.1. Patients
A total of 14 213 CHC patients were registered in TACR platform during the study period. Of which, 13 951 (98.2%) patients with treatment outcome available were enrolled in the current study. The mean age was 63.0 years, and females accounted for 55.9% of the population. The dominant viral genotype was HCV genotype 1 (GT1, 57.9%), followed by GT2 (36.1%). A total of 5370 (38.4%) patients had liver cirrhosis. Among them, 242 (1.7%) patients had liver decompensation; 1473 (10.6%) patients had preexisting HCC (active, 2.4%; inactive, 8.2%) before DAA treatment; 1068 (7.7%) and 154 (1.1%) patients were dually infected with HBV and human immunodeficiency virus (HIV), respectively; 243 (1.7%) patients had a history of intravenous drug abuse, and 41 (0.3%) patients had a history of liver transplantation; and 10 978 (78.7%) patients were treatment naïve (Table 1). Regarding the DAA regimen prescribed, the most commonly used was sofosbuvir (SOF)/ledipasvir (LDV) + ribavirin (RBV) (29.4%), followed by paritaprevir/ritonavir/ombitasvir/dasabuvir (PrOD) + RBV (18.1%), elbasvir (EBR)/grazoprevir (GZR) (15.1%), glecaprevir (GLE)/pibrentasvir (PIB) (14.3%) and SOF/RBV (12.0%). The majority (99.7%) of the patients had DAA adherence > 80% (Table 2).
TABLE 1.
Baseline characteristics and clinical features of the 13 951 HCV patients
| Variables | Mean ± SD or N (%) |
|---|---|
| Age, y | 63.0 ± 11.8 |
| >65 y, n (%) | 6113 (43.8) |
| >80 y, n (%) | 813 (5.8) |
| Female gender | 7802 (55.9) |
| BMI, kg/m2 | 24.8 ± 4.0 |
| AST, IU/L | 65.5 ± 53.6 |
| ALT, IU/L | 78.2 ± 75.6 |
| Platelet count, ×103 U/L | 168.8 ± 69.0 |
| Albumin, g/dL | 4.2 ± 0.4 |
| Total bilirubin, mg/dL | 0.85 ± 0.50 |
| Creatinine, mg/dL | 1.13 ± 1.53 |
| FIB‐4 | 3.77 ± 3.76 |
| eGFR, mL/min/1.73 m2 | 88.6 ± 32.8 |
| Comorbidity | |
| Diabetes | 3052 (21.9) |
| Hypertension | 4713 (33.8) |
| Dyslipidemia | 1597 (11.5) |
| Cardiovascular disease | 1433 (10.3) |
| Persons who inject drugs | 243 (1.7) |
| Major thalassemia | 34 (0.2) |
| Hemophilia | 8 (0.1) |
| Human immunodeficiency virus co‐infection | 154 (1.1) |
| Virology | |
| HCV genotype, 1 a /2 b /1 + 2/3/4/5/6/unclassified | 8084 (57.9)/5031 (36.1)/108 (0.8)/101 (0.7)/13 (0.1)/3 (0.02)/544 (3.9)/67 (0.5) |
| HCV RNA, log10 IU/mL | 5.92 ± 0.97 |
| >800,000 IU/mL | 8,527 (61.1) |
| Liver‐related disease | |
| Hepatitis B virus dual infection | 1,068 (7.7) |
| Liver cirrhosis | 5,370 (38.4) |
| Compensated cirrhosis | 4,421 (31.7) |
| Decompensated cirrhosis | 242 (1.7) |
| Child‐Pugh B | 234 (1.68) |
| Child‐Pugh C | 8 (0.06) |
| Unknown | 707 (5.1) |
| Hepatocellular carcinoma | 1473 (10.6) |
| Inactive | 1145 (8.2) |
| Active | 328 (2.4) |
| Liver transplantation | 41 (0.3) |
| Anti‐HCV treatment history | |
| Naive | 10 978 (78.7) |
| Experienced c | 2973 (21.3) |
| Interferon | 2877 (98.6) |
| DAA | 42 (1.4) |
Values expressed as mean ± standard deviation or sample size and proportion (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DAA, directly‐acting antivirals; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); FIB‐4; fibrosis‐4 index; HCV, hepatitis C virus.
1a (n = 706), 1b (n = 7036), unsubtyped (n = 342).
2a (n = 504), 2b (n = 118), unsubtyped (n = 4409).
Regimens not available in 54 (0.4%) patients.
TABLE 2.
Allocation of current DAA regimens and DAA adherence of the 13 951 HCV patients
| DAA regimen | N (%) |
|---|---|
| DCV/ASV | 556 (4.0) |
| <24 wks | 40 (0.3) |
| 24 wks | 516 (3.7) |
| PrOD ± RBV | 2524 (18.1) |
| <12 wks | 7 (0.05) |
| 12 wks | 2382 (17.1) |
| 24 wks | 135 (1.0) |
| EBR/GZR | 2099 (15.0) |
| <12 wks | 16 (0.1) |
| 12 wks | 2080 (14.9) |
| 16 wks | 3 (0.02) |
| SOF/LDV ± RBV | 4101 (29.4) |
| <12 wks | 15 (0.1) |
| 12 wks | 4071 (29.2) |
| 12‐24 wks | 3 (0.02) |
| 24 wks | 12 (0.09) |
| SOF/RBV | 1670 (12.0) |
| <12 wks | 5 (0.04) |
| 12 wks | 1652 (11.8) |
| 16 wks | 13 (0.09) |
| SOF/DCV ± RBV | 523 (3.7) |
| <12 wks | 2 (0.01) |
| 12 wks | 509 (3.6) |
| 12‐24 wks | 7 (0.05) |
| 24 wks | 5 (0.04) |
| GLE/PIB | 1989 (14.3) |
| <8 wks | 1 (0.007) |
| 8 wks | 1618 (11.6) |
| 12 wks | 352 (2.5) |
| 16 wks | 18 (0.1) |
| SOF/VEL | 390 (2.8) |
| <12 wks | 1 (0.007) |
| 12 wks | 383 (2.7) |
| 12‐24 wks | 1 (0.007) |
| 24 wks | 5 (0.04) |
| SOF/VEL/VOX | 20 (0.1) |
| <12 wks | 2 (0.01) |
| 12 wks | 18 (0.1) |
| Others | 79 (0.6) |
| DAA adherence | |
| >80% | 13 903 (99.7) |
| 60%‐80% | 14 (0.1) |
| 40%‐60% | 8 (0.1) |
| 20%‐40% | 14 (0.1) |
| <20% | 12 (0.1) |
Values expressed as mean ± standard deviation or sample size and proportion (%).
Abbreviations: ASV, asunaprevir; DAA, directly‐acting antivirals; DCV, daclatasvir; EBR, elbasvir; eGFR, estimated glomerular filtration rate (mL/min/1.73 m2); GLE, glecaprevir; GZR, grazoprevir; LDV, ledipasvir; PIB, pibrentasvir; PrOD, paritaprevir/ritonavir/ombitasvir/dasabuvir; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
Among the 262 patients who did not have SVR12 data, 39 patients died, and 223 patients lost follow‐up during the study period. Compared with the 13 951 patients, those without treatment outcome available were older; had lower platelet counts and albumin levels, higher bilirubin, creatinine levels and FIB‐4; and had higher proportions of comorbidities (including diabetes, dyslipidemia and cardiovascular disease), liver cirrhosis and HCC history (Table S1).
3.2. Treatment responses
The overall SVR was 98.3% (13 715/13 951). The proportion of SVR12 was 98.7% (7128/7223), 98.0% (3678/3755), 98.4% (1336/1358) and 97.4% (1573/1615) in treatment‐naïve noncirrhotic, treatment‐naïve cirrhotic, treatment‐experienced noncirrhotic and treatment‐experienced cirrhotic patients, respectively (Figure 1A). While patients were stratified according to HCV genotype, the proportion of SVR12 was 98.6% (7972/8084) in GT1, 97.9% (4924/5031) in GT2, 98.2% (106/108) in mixed GT1/GT2, 95.1% (96/101) in GT3, 76.9% (10/13) in GT4, 100% (3/3) in GT5, 98.7% (537/544) in GT6 and 100% (67/67) in the unclassified genotype (Figure 1B). The treatment responses in patients with commonly infected HCV genotypes stratified by treatment experience and cirrhotic status are shown in Figure S1. A substantially lower proportion of SVR12 was noted in GT2 treatment‐experienced cirrhotic patients (93.2%, 219/235) and GT3 treatment‐naïve cirrhotic patients (86.7%, 13/15). By treatment settings, the proportions of SVR were similar among the 21 medical centres (98.4%, 9036/9185), 22 regional hospitals (98.2%, 4637/4723) and the five primary clinics (97.7%, 42/43).
FIGURE 1.
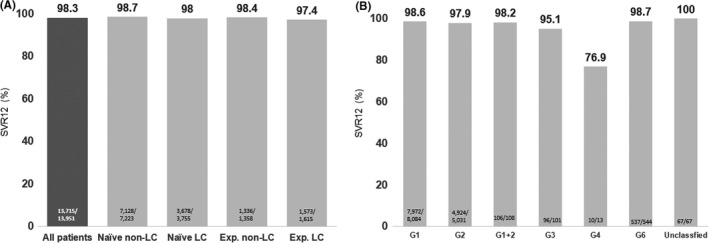
A, Sustained virological response (SVR) rate stratified by cirrhotic status and prior treatment experience. B, SVR rate stratified by hepatitis C virus (HCV) genotype
3.3. The proportion of SVR12 in patients with commonly infected genotypes stratified by treatment regimens, treatment experience and cirrhotic status
Among patients with GT1 infection, the proportion of SVR12 was 95.7% (531/555) with daclatasvir (DCV)/asunaprevir (ASV), 98.8% (2493/2524) with PrOD, 98.8% (2064/2090) with EBR/GZR, 100% (22/22) with SOF/DCV, 98.6% (2075/2104) with SOF/LDV, 98.8% (169/171) with SOF/velpatasvir (VEL) and 100% (567/567) with GLE/PIB. Among patients with GT2 infection, SVR12 was 96.3% (1596/1657) with SOF/RBV, 99.2% (481/485) with SOF/DCV, 98.3% (1472/1497) with SOF/LDV, 99.0% (204/206) with SOF/VEL and 98.7% (1143/1158) with GLE/PIB. Among patients with GT3 infection, SVR12 was 90% (9/10) with SOF/VEL and 95.4% (82/86) with GLE/PIB. Among patients with GT6 infection, SVR12 was 98.8% (412/417) with SOF/LDV, 100% (14/14) with SOF/VEL and 99.1% (107/108) with GLE/PIB (Figure 2).
FIGURE 2.
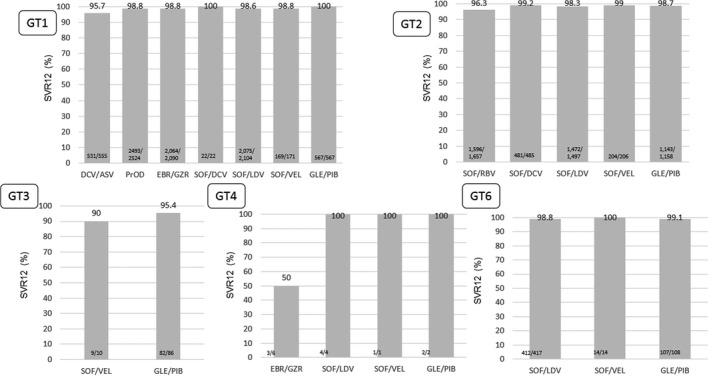
Sustained virological response (SVR) rate stratified by hepatitis C virus (HCV) genotype and treatment regimen
When the patients were further divided according to commonly used regimens and stratified by treatment experience and cirrhotic status, the proportion of SVR12 was > 95% among all subgroups with a respectable sample size except treatment‐experienced cirrhotic GT2 patients who received SOF/RBV (88.7%, 110/124), treatment‐naïve noncirrhotic patients (94.8%, 110/116) and treatment‐experienced cirrhotic (94.8%, 164/173) GT1 patients who received DCV/ASV (Table 3).
TABLE 3.
SVR12 rate of the major HCV genotypes stratified by DAA regimens, treatment experience and cirrhotic status
| n/N (%) | All | G1 | G2 | G3 | G6 | Unclassified |
|---|---|---|---|---|---|---|
| SOF + RBV | 1609/1670 (96.4) | 5/5 (100.0) | 1596/1657 (96.3) | 2/2 (100.0) | – | 6/6 (100.0) |
| Naïve non‐LC | 689/706 (97.6) | 1/1 (100.0) | 686/703 (97.6) | – | – | 2/2 (100.0) |
| Naïve LC | 724/752 (96.3) | 3/3 (100.0) | 718/746 (99.2) | – | 3/3 (100.0) | |
| Exp. non‐LC | 83/85 (97.7) | ‐ | 82/84 (97.6) | 1/1 (100.0) | – | |
| Exp. LC | 113/127 (89.0) | 1/1 (100.0) | 110/124 (88.7) | 1/1 (100.0) | – | 1/1 (100.0) |
| DCV/ASV | 532/556 (95.7) | 531/555 (95.7) | – | – | – | 1/1 (100.0) |
| Naïve non‐LC | 110/116(94.8) | 110/116 (94.8) | – | – | – | – |
| Naïve LC | 139/142(97.9) | 139/142 (97.9) | – | – | – | – |
| Exp. non‐LC | 119/125 (95.2) | 118/124 (95.2) | – | – | – | 1/1 (100.0) |
| Exp. LC | 164/173(94.8) | 164/173 (94.8) | – | – | – | – |
| PrOD + RBV | 2493/2524 (98.8) | 2493/2524 (98.8) | – | – | – | – |
| Naïve non‐LC | 520/525 (99.1) | 520/525 (99.1) | – | – | – | – |
| Naïve LC | 613/621 (98.7) | 613/621 (98.7) | – | – | – | – |
| Exp. non‐LC | 513/521 (98.5) | 513/521 (98.5) | – | – | – | – |
| Exp. LC | 847/857 (98.8) | 847/857 (98.8) | – | – | – | – |
| EBR/GZR | 2070/2099 (98.6) | 2064/2090 (98.8) | 1/1 (100.0) | – | 2/2 (100.0) | |
| Naïve non‐LC | 1256/1268 (99.1) | 1253/1264 (99.1) | 1/1 (100.0) | – | 1/1 (100.0) | |
| Naïve LC | 562/574 (97.9) | 561/572 (98.1) | – | – | – | – |
| Exp. non‐LC | 132/135 (97.8) | 131/134 (97.8) | – | – | – | 1/1 (100.0) |
| Exp. LC | 120/122 (98.4) | 119/120 (99.2) | – | – | – | – |
| SOF + DCV ± RBV | 518/523 (99.0) | 22/22 (100.0) | 481/485 (99.2) | 2/2 (100.0) | 1/2 (50.0) | 5/5 (100.0) |
| Naïve non‐LC | 196/198 (99.0) | 7/7 (100.0) | 183/184 (99.5) | 2/2 (100.0) | 1/2 (50.0) | |
| Naïve LC | 229/231 (99.1) | 8/8 (100.0) | 213/215 (99.1) | – | – | 5/5 (100.0) |
| Exp. non‐LC | 42/42 (100.0) | 6/6 (100.0) | 35/35 (100.0) | – | – | |
| Exp. LC | 51/52(98.1) | 1/1 (100.0) | 50/51 (98.0) | – | – | |
| SOF/LDV ± RBV | 4040/4101 (98.5) | 2075/2104 (98.6) | 1472/1497 (98.3) | – | 412/417 (98.8) | 28/28 (100.0) |
| Naïve non‐LC | 2569/2606 (98.6) | 1037/1049 (98.9) | 1225/1245 (98.4) | – | 259/262 (98.9) | 17/17 (100.0) |
| Naïve LC | 1023/1043 (98.1) | 713/727 (98.1) | 174/178 (97.8) | – | 114/116 (98.3) | 2/2 (100.0) |
| Exp. non‐LC | 257/258 (99.6) | 174/174 (100.0) | 55/56 (98.2) | – | 21/21 (100.0) | 5/5 (100.0) |
| Exp. LC | 191/194 (98.5) | 151/154 (98.1) | 18/18 (100.0) | – | 18/18 (100.0) | 4/4 (100.0) |
| SOF/VEL | 405/410 (98.8) | 169/171 (98.8) | 204/206 (99.0) | 9/10 (90.0) | 14/14 (100.0) | 1/1 (100.0) |
| Naïve non‐LC | 241/241 (100.0) | 103/103 (100.0) | 119/119 (100.0) | 5/5 (100.0) | 10/10 (100.0) | 1/1 (100.0) |
| Naïve LC | 96/97 (99.0) | 33/33 (100.0) | 51/51 (100.0) | 3/4 (75.0) | 4/4(100.0) | – |
| Exp. non‐LC | 40/42 (95.2) | 21/21 (100.0) | 19/21 (90.5) | – | 0/0 (0.0) | – |
| Exp. LC | 28/30 (93.3) | 12/14 (85.7) | 15/15(100.0) | 1/1 (100.0) | 0/0 (0.0) | – |
| GLE/PIB | 1969/1989 (99.0) | 567/567 (100.0) | 1143/1158 (98.7) | 82/86 (95.4) | 107/108 (99.1) | 23/23 (100.0) |
| Naïve non‐LC | 1491/1507 (98.9) | 421/421 (100.0) | 882/895 (98.6) | 61/64 (95.3) | 77/77 (100.0) | 19/19 (100.0) |
| Naïve LC | 276/279 (98.9) | 55/55 (100.0) | 183/184 (99.5) | 10/11 (90.9) | 15/16 (93.8) | 3/3 (100.0) |
| Exp. non‐LC | 144/144 (100.0) | 73/73 (100.0) | 52/52 (100.0) | 4/4 (100.0) | 12/12 (100.0) | 1/1 (100.0) |
| Exp. LC | 58/59 (98.3) | 18/18 (100.0) | 26/27 (96.3) | 7/7 (100.0) | 3/3 (100.0) | – |
Abbreviations: ASV, asunaprevir; DCV, daclatasvir; EBR, elbasvir; Exp, experienced; G1, genotype 1; GLE, glecaprevir; GZR, grazoprevir; LC, liver cirrhosis; LDV, ledipasvir; PIB, pibrentasvir; PrOD, paritaprevir/ritonavir/ombitasvir/dasabuvir; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virological response; VEL, velpatasvir; VOX, voxilaprevir.
3.4. Factors associated with DAA treatment failure
As displayed in Table 4 and per univariate analysis, patients with higher baseline HCV RNA levels, HCV GT 2/3, prior treatment failure, DAA adherence < 60% and liver cirrhosis and preexisting HCC and patients who received DCV/ASV or SOF/RBV were more likely to experience DAA failure. While other DAA regimens were taken together except DCV/ASV and SOF/RBV (Model 1), the most important factor independently associated with treatment failure was DAA adherence < 60% (adjusted odds ratio [aOR]/95% confidence interval [CI]: 117.1/52.4‐261.3, P < .001), followed by GT 3 and GT2 (aOR/CI: 5.78/2.25‐14.9, P = .0003 and aOR/CI: 1.55/1.05‐2.29, P = .03, compared with GT1), active HCC (aOR/CI: 4.29/2.57‐7.16, P < .001), the use of SOF/RBV (aOR/CI: 2.51/1.67‐3.77, P < .001) and DCV/ASV (aOR/CI: 3.29/1.94‐5.58, P < .001) compared with other regimens, decompensated liver cirrhosis (aOR/CI: 2.50/1.20‐5.22, P = .02) and high HCV viral loads (HCV RNA > 6 000 000 IU/mL, aOR/CI: 2.16/1.57‐2.97, P < .001). The results remained consistent while the effect of DAA regimen on treatment outcome was judged separately (Model 2). There was no collinearity among the independent factors (Table S2).
TABLE 4.
Factors associated with DAA failure
| DAA failure | Crude OR | Adjusted Model 1 | Adjusted Model 2 | ||||
|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, y | |||||||
| 20‐39 | 9/596 (1.5) | 1 | |||||
| 40‐59 | 76/4442 (1.7) | 1.14 (0.57‐2.28) | .72 | ||||
| 60‐79 | 131/7883 (1.7) | 1.10 (0.56‐2.18) | .78 | ||||
| ≥80 | 20/1027 (2.0) | 1.30 (0.59‐2.86) | .52 | ||||
| Gender | |||||||
| Male | 115/6149 (1.9) | 1.21 (0.94‐1.57) | .15 | ||||
| Female | 121/7802 (1.5) | 1 | |||||
| Prior treatment | 1 | 1 | |||||
| No | 172/10 978 (1.6) | 1 | |||||
| Yes | 64/2973 (2.2) | 1.38 (1.03‐1.85) | .03 | 1.25 (0.89‐1.72) | .21 | 1.42 (1.00‐2.03) | .052 |
| DAA regiments | |||||||
| DCV/ASV | 24/556 (4.2) | 4.67 (1.77‐12.3) | .002 | 3.29 (1.94‐5.58) | <.001 | 5.99 (1.96‐18.38) | .002 |
| SOF + RBV | 61/1670 (3.7) | 3.93 (1.57‐9.83) | .004 | 2.51 (1.67‐3.77) | <.001 | 3.78 (1.50‐9.56) | .005 |
| Others | 151/11 646 (1.3) | 1 | – | – | |||
| SOF + LDV | 61/4101 (1.5) | 1.56 (0.63‐3.91) | .34 | – | 2.05 (0.78‐5.36) | .15 | |
| EBR/GZR | 29/2099 (1.4) | 1.45 (0.56‐3.77) | .44 | – | 2.96 (0.99‐8.80) | .052 | |
| PrOD | 31/2524 (1.2) | 1.29 (0.50‐3.33) | .60 | – | 1.33 (0.44‐4.02) | .62 | |
| SOF/VEL | 5/410 (1.2) | 1.28 (0.37‐4.45) | .70 | – | 1.40 (0.39‐5.00) | .61 | |
| GLE/PIB | 20/1989 (1.1) | 1.05 (0.39‐2.82) | .92 | – | 1.18 (0.42‐3.29) | .75 | |
| SOF/DCV | 5/523 (1.0) | 1 | – | 1 | |||
| HCV RNA, IU/mL | |||||||
| <6,000,000 | 176/11 731(1.5) | 1 | 1 | 1 | |||
| > 6,000,000 | 60/2179 (2.8) | 1.87 (1.39‐2.51) | <.0001 | 2.16 (1.57‐2.97) | <.001 | 2.14 (1.55‐2.95) | <.001 |
| HCV genotype | |||||||
| 1 | 114/8192 (1.4) | 1 | 1 | 1 | |||
| 2 | 107/5031 (2.1) | 1.54 (1.18‐2.01) | .002 | 1.55 (1.05‐2.29) | .03 | 2.01 (1.21‐3.35) | .007 |
| 3 | 5/101 (5.0) | 3.69 (1.47‐9.24) | .005 | 5.78 (2.25‐14.9) | .0003 | 9.50 (3.22‐27.99) | <.001 |
| others | 10/627 (1.6) | 1.15 (0.60‐2.20) | .68 | 1.66 (0.84‐3.27) | .14 | 1.79 (0.86‐3.72) | .12 |
| DAA adherence | |||||||
| >80% | 215/13 903 (1.5) | 1 | 1 | 1 | |||
| 60%‐80% | 1/14 (7.1) | 4.90 (0.64‐37.06) | .12 | 6.03 (0.74‐49.1) | .09 | 6.49 (0.79‐53.62) | .08 |
| <60% | 20/34 (58.8) | 90.95 (45.34‐182.45) | <.0001 | 117.1 (52.4‐261.3) | <.001 | 135.74 (59.16‐311.44) | .0009 |
| HBV dual infection | |||||||
| No | 220/12 883 (1.7) | 1.14 (0.69‐1.91) | .61 | ||||
| Yes | 16/1068 (1.5) | 1 | |||||
| HIV coinfection | |||||||
| No | 209/12 397 (1.7) | 1 | |||||
| Yes | 4/154 (2.6) | 1.56 (0.57‐4.24) | .39 | ||||
| CKD | |||||||
| No | 196/11 653 (1.7) | 1 | |||||
| Yes | 40/2292 (1.7) | 1.04 (0.74‐1.46) | .83 | ||||
| PWID | |||||||
| Yes | 3/243 (1.2) | 1 | |||||
| No | 233/13 708 (1.7) | 1.38 (0.44‐4.35) | .58 | ||||
| LC | |||||||
| No LC | 117/8581 (1.4) | 1 | 1 | 1 | |||
| CLC | 94/4421 (2.1) | 1.57 (1.20‐2.07) | .0012 | 1.20 (0.89‐1.61) | .24 | 1.26 (0.93‐1.70) | .14 |
| DLC | 10/242 (4.1) | 3.12 (1.61‐6.03) | .0007 | 2.50 (1.20‐5.22) | .02 | 2.53 (1.19‐5.39) | .02 |
| HCC | |||||||
| No | 185/12 478 (1.5) | 1 | 1 | 1 | |||
| Yes, inactive | 28/1145 (2.4) | 1.67 (1.11‐2.49) | .01 | 1.44 (0.90‐2.30) | .13 | 1.47 (0.92‐2.34) | .11 |
| Yes, active | 23/328 (7.0) | 5.01 (3.20‐7.84) | <.0001 | 4.29 (2.57‐7.16) | <.001 | 4.37 (2.62‐7.31) | <.001 |
Abbreviations: ASV, asunaprevir; CKD, chronic kidney disease; CLC, compensated liver cirrhosis; DAA, direct‐acting antiviral; DCV, daclatasvir; DLC, decompensated liver cirrhosis; EBR, elbasvir; GLE, glecaprevir; GZR, grazoprevir; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; LC, liver cirrhosis; LDV, ledipasvir; OR, odds ratio; PIB, pibrentasvir; PrOD, paritaprevir/ritonavir/ombitasvir/dasabuvir; PWID, persons who inject drugs; SOF, sofosbuvir; VEL, velpatasvir.
3.5. Subgroup analysis
The proportion of SVR12 did not differ between patients with or without HIV coinfection (97.4% [n = 154] vs. 98.3 [n = 12,397], P = .34). The proportion of SVR12 was 98.5% in the 1068 HBV‐coinfected patients, which was similar to that of patients with HCV monoinfection (98.3%, P = .61). The treatment efficacy also did not differ between the two groups, while patients were stratified by prior treatment experience, liver disease severity and DAA regimens (Table S3).
The proportion of SVR12 was significantly lower in the 1473 HCC patients than in the 12 478 non‐HCC patients (96.5% vs. 98.5%, P < .001). When HCC patients were divided into those with inactive or active HCC, a significantly lower proportion of SVR12 was noted both in patients with inactive HCC (97.6%, P = .01) and those with active HCC (93.0%, P < .001). The significantly lower SVR rate in patients with active HCC was particularly noted in cirrhotic patients who were either treatment‐naïve (91.6%) or treatment‐experienced (92.2%) (Figure 3).
FIGURE 3.
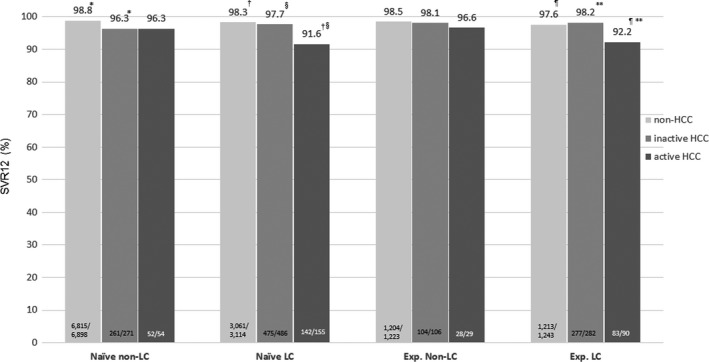
Sustained virological response (SVR) rate in patients with and without hepatocellular carcinoma (HCC) stratified by cirrhotic status and prior treatment experience. * P = .003, †§ P < .001, ** P = .01
Of the 137 HBV dual‐infected patients with available HBV data and NA information, 36 patients (26.3%) received prophylactic NAs. Among the 101 patients who did not received prophylactic NAs, the rate of HBV virological activation and clinical activation was 20.8% (n = 21) and 3.0% (n = 3), respectively. All the three patients with clinical HBV activation received rescued NAs.
3.6. Serious adverse events
Two hundred forty‐eight (1.8%) patients had serious adverse events (SAEs) during DAA treatment. The most common SAE was liver‐related disease (0.5%, n = 65), followed by gastrointestinal disorder (0.1%, n = 19) and infection (0.1%, n = 12) (Table S4).
4. DISCUSSION
The current study confirmed that DAAs were effective in treating Taiwanese CHC patients in a real‐world setting at the national level. The satisfactory treatment outcome could be thoroughly generalized to patients with different viral genotypes and liver disease severity except for certain subpopulations and less potent regimens. Apart from other study cohorts, the current study population possessed several unique characteristics; more than one‐third of the patients had liver cirrhosis, and the cohort represented the largest sample size with HBV dual infection. Until now, reports regarding DAA treatment in CHC patients were performed only in single centres or with limited patient numbers in Taiwan, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 which precluded the identification of reasons for failing to achieve SVR. By adopting the nationwide, multicentre database, we identified certain factors associated with virological failure in Taiwanese patients, including poor DAA adherence; possessing high baseline viral loads, active HCC and hepatic decompensation; and the use of DCV/ASV and SOF/RBV.
The current study consisted of a high proportion of patients with advanced liver fibrosis because the Taiwan Health Insurance Administration started DAA reimbursement based on prioritization by patients’ disease severity]. 27 Only patients with advanced fibrosis were reimbursed in 2017 and 2018 and not until 2019 did the government start to reimburse DAAs without any restriction. 28 As noted, this study reinforced the effectiveness of DAAs in several subpopulations, including patients with HBV or HIV infection. The favourable treatment outcome in CHC patients dually infected with HBV or HIV has been proven in previous clinical trials or case cohorts. 29 , 30 , 31 Unlike studies from the West where the majority patients are with HCV monoinfection, the treatment efficacy in patients with HBV dual infection has never been validated on a large population basis. Both HBV and HCV are rampant in Taiwan. We found that the SVR rate was similarly high in HBV dual‐infected patients compared with HCV‐monoinfected patients. Equal treatment response was observed when patients were stratified by treatment experience and cirrhotic status of which the SVR rate ranged from 97.2% to 98.9%. Compared with previous reports, a lower rate of HBV activation in the cohort may attribute to a higher proportion of patients who received prophylactic NAs. 31 , 32 The issue of HBV reactivation with longer follow‐up period and long‐term outcome in the subpopulation opens a window for future exploration.
SOF/LDV was designated and allocated to patients with HCV genotypes 1, 4, 5 and 6. Based on one Phase 2 and two Phase 3 studies with a proportion of SVR12 of 96%‐100% in Asia, 29 , 33 , 34 , 35 SOF/LDV was approved for HCV genotype 2 infection in certain regions, including Taiwan. Large real‐world data regarding the use of SOF/LDV in patients with HCV‐2 infection have seldom been reported. In the current study, we demonstrated that a proportion of SVR12 of 98.3% could be achieved in Taiwanese patients, indicating the clinical feasibility of the regimen in the real‐world setting. SOF/LDV remains one of the treatment choices in some Asian countries such as Japan, Korea and China. The favourable treatment outcome in Taiwanese patients would share the real‐world evidence in terms of the clinical utility in this regard. SOF/RBV for HCV‐2 has been recommended as the treatment choice by prior regional guidelines. 36 However, the treatment response was proven to be suboptimal in Asian treatment‐experienced cirrhotic patients, 37 as in the current study. Twenty‐four‐week DCV/ASV has been reimbursed for GT1b patients without nonstructure 5A resistance‐associated substitutions in Taiwan since 2017. 28 A relatively low SVR rate of 94.8% was noted in the TACR cohort. It has been proven that adding ribavirin to DCV/ASV could shorten the treatment duration to 12 weeks without compromising the efficacy for GT1b patients without nonstructure 5A resistance‐associated substitutions (SVR rate 97.1%). 38 Nevertheless, both regimens are currently waived due to the availability of other more potent interferon‐free, ribavirin‐free DAAs with shorter treatment durations. In general, the treatment efficacy in the Asian study was similar to those being reported in the West with the use of more potent DAAs. 39 , 40
The issue regarding the treatment efficacy of DAAs in HCC patients has been extensively explored. It is believed that patients with active HCC are prone to encounter treatment failure. 41 , 42 The current study was in line with previous observations that indicated a lower SVR rate in patients with active HCC. Notably, we identified that inferior efficacy in patients was noted in cirrhotic patients but not in noncirrhotic HCC patients. The clinical dilemma would be whether HCV eradication provides survival benefits for patients with active HCC, in particular for those who were already decompensated. 41 , 43
Apart from the clinical trial data, lack of compliance with treatment or follow‐up programmes has been an unfavourable factor for treatment failure in the real‐world setting. 44 Although the patient number was small, we identified that DAA compliance < 60% was the most important factor associated with virological failure. Like the majority of other registry systems, the current study included patients with available SVR12 data. Patients who discontinued treatment without known treatment outcomes were not included. The impact of poor drug compliance on virological failure might be underestimated and should not be overlooked. Pretreatment communication of understanding treatment goals as well as the provision of patient education regarding drug adherence and post‐treatment follow‐up 3 is the key to treatment success in daily practice.
There were some limitations in the current study. This is a retrospective–prospective study. The registration is performed retrospectively for patients who started DAA treatment before 1 September 2018. However, the data analysed were obtained prospectively by the regulation of Taiwan Health Insurance Administration for DAA therapy. Secondly, 1.8% registered patients did not have SVR12 data available and were excluded from analysis. The subpopulation may include patients with relatively poor prognostic characters, indicating a potentially suboptimal treatment outcome among these patients. Finally, the treatment outcomes for certain populations may be inconclusive due to limited patient numbers, such as those with HCV‐3 or hepatic decompensation. However, this is the first and largest real‐world‐based registry including patients from medical centres, regional hospitals and local clinics in Taiwan. The patient characteristics and treatment outcome explicitly reflect the real‐world situation of Taiwanese patients.
In conclusion, DAAs were highly effective and safe in treating CHC patients across viral genotypes, fibrosis status and special subgroups in Taiwan. Using highly potent DAA regimens and maintaining DAA adherence are mandatory to ensure treatment efficacy. Further research should be extended to address the long‐term hepatic and extrahepatic outcomes of the cohort.
ETHICS APPROVAL STATEMENT
The study was approved by the institutional review board at each study site, which conformed to the guidelines of the International Conference on Harmonization for Good Clinical Practice.
CONFLICT OF INTEREST
Ming‐Lung Yu, Research support from Abbvie, Abbott, BMS, Gilead, Merck and Roche. Consultant for Abbvie, Abbott, Ascletis, BMS, Gilead, J&J, Merck, Novartis, Pharmaessential and Roche. Speaker for Abbvie, Abbott, Ascletis, BMS, Gilead, Merck, Pharmaessential and Roche. Chung‐Feng Huang, Speaker for Abbvie, BMS, Gilead, Merck and Roche. Chen‐Hua Liu, Advisory board for Abbvie, Golead Sciences and Merck Sharp & Dohme. Speaker for Abbott, Abbvie, Golead Sciences and Merck Sharp & Dohme. Research grant from Abbvie, Golead Sciences and Merck Sharp & Dohme.
PATIENT CONSENT STATEMENT
All patients provided written informed consent.
Supporting information
Figure S1
Table S1‐S4
ACKNOWLEDGEMENTS
The authors would like to thank the Taiwan Association for the Study of Liver (TASL), the TASL Foundation and Taiwan Liver Research Foundation for grant support and the TACR study group for data collection. We also thank the Center for Medical informatics and Statistics of Kaohsiung Medical University for providing administrative and funding support.
Chen C‐Y, Huang C‐F, Cheng P‐N, et al. Factors associated with treatment failure of direct‐acting antivirals for chronic hepatitis C: A real‐world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021;41:1265–1277. 10.1111/liv.14849
Chi‐Yi Chen and Chung‐Feng Huang contributed equally.
Handling Editor: Benjamin Maasoumy
Funding information
The study was also supported by grants from Kaohsiung Medical University (MOST 109‐2314‐B‐037‐044) and Kaohsiung Medical University Hospital (KMUH‐DK(整)109005 ~ 1, KMUH‐DK(世)109002, KMUH108‐8R05, KMUH108‐8R09, MOHW109‐TDU‐B‐212‐114006 and MOST 109‐2314‐B‐037‐044).
Contributor Information
Han‐Chieh Lin, Email: hclin@vghtpe.gov.tw.
Ming‐Lung Yu, Email: fish6069@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. The Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in a modelling study. The lancet Gastroenterology & hepatology. 2015;2017(2):161‐176. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C ‐ Final update of the series. J Hepatology. 2020;73(5):1170‐1218. [DOI] [PubMed] [Google Scholar]
- 3. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. http://www.hcvguidelines.org/. Accessed on Dec 15, 2019
- 4. Yu ML, Yeh ML, Tsai PC, et al. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan. Medicine. 2015;94:e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang JF, Lin CI, Huang JF, et al. Viral hepatitis infections in southern Taiwan: a multicenter community‐based study. Kaohsiung J Med Sci. 2010;26:461‐469. [DOI] [PubMed] [Google Scholar]
- 6. Wu GH, Pwu RF, Chen SC. Achieving hepatitis C elimination in Taiwan‐Overcoming barriers by setting feasible strategies. J Formos Med Assoc. 2018;117:1044‐1045. [DOI] [PubMed] [Google Scholar]
- 7. https://www.nhi.gov.tw/Content_List.aspx?n=A4EFF6CD1C4891CA&topn=5FE8C9FEAE863B46. Access on 2020/02/17
- 8. Huang CF, Yeh ML, Huang CI, et al. Equal treatment efficacy of direct‐acting antivirals in patients with chronic hepatitis C and hepatocellular carcinoma? BMJ Open. 2019;9:e026703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen CH, Chen CH, Lin CL, Lin CY. Real‐world safety and efficacy of paritaprevir/ritonavir/ombitasvir plus dasabuvir +/‐ ribavirin in patients with hepatitis C virus genotype 1 and advanced hepatic fibrosis or compensated cirrhosis: a multicenter pooled analysis. Sci Rep. 2019;9:7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu CH, Liu CJ, Su TH, et al. Real‐world effectiveness and safety of sofosbuvir and ledipasvir with or without ribavirin for patients with hepatitis C virus genotype 1 infection in Taiwan. PLoS One. 2018;13:e0209299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu CH, Liu CJ, Su TH, et al. Real‐world effectiveness and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with or without ribavirin for patients with chronic hepatitis C virus genotype 1b infection in Taiwan. J Gastroenterol Hepatol. 2018;33:710‐717. [DOI] [PubMed] [Google Scholar]
- 12. Tsai TC, Deng ST, Hsu CW. The efficacy and safety of elbasvir/grazoprevir treatment in HCV genotype 1 patients in Taiwan. J Med Virol. 2020;92:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong CM, Liu CH, Su TH, et al. Real‐world effectiveness of direct‐acting antiviral agents for chronic hepatitis C in Taiwan: real‐world data. J Microbiol Immunol Infect. 2020;53(4):569‐577. [DOI] [PubMed] [Google Scholar]
- 14. Cheng PN, Chiu YC, Chien SC, Chiu HC. Real‐world effectiveness and safety of sofosbuvir plus daclatasvir with or without ribavirin for genotype 2 chronic hepatitis C in Taiwan. J Formos Med Assoc. 2019;118:907‐913. [DOI] [PubMed] [Google Scholar]
- 15. Chen JJ, Lee PL, Chiu HC, Tung HD. Real‐world effectiveness and safety of ledipasvir/sofosbuvir for genotype 6 chronic hepatitis C patients in Taiwan. J Gastroenterol Hepatol. 2020;35(3):467‐472. [DOI] [PubMed] [Google Scholar]
- 16. Liu CH, Liu CJ, Hung CC. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection: real‐world effectiveness and safety in Taiwan. Liver Int. 2020;40:758‐768. [DOI] [PubMed] [Google Scholar]
- 17. https://www.nhi.gov.tw/Content_List.aspx?n = A4EFF6CD1C4891CA&topn = 3FC7D09599D25979. Access on 2020/07/08
- 18. Yu ML, Chen PJ, Dai CY, et al. 2020 Taiwan consensus statement on the management of hepatitis C: part (I) general population. J Formos Med Assoc. 2020;119:1019‐1040. [DOI] [PubMed] [Google Scholar]
- 19. Yu ML, Chen PJ, Dai CY, et al. 2020 Taiwan consensus statement on the management of hepatitis C: Part (II) special populations. J Formos Med Assoc. 2020;119(7):1135‐1157. [DOI] [PubMed] [Google Scholar]
- 20. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372‐374. [DOI] [PubMed] [Google Scholar]
- 21. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343‐350. [DOI] [PubMed] [Google Scholar]
- 22. Lin YH, Yeh ML, Huang CI, et al. The performance of acoustic radiation force impulse imaging in predicting liver fibrosis in chronic liver diseases. Kaohsiung J Med Sci. 2016;32:362‐366. [DOI] [PubMed] [Google Scholar]
- 23. Wang CC, Liu CH, Lin CL, et al. Fibrosis index based on four factors better predicts advanced fibrosis or cirrhosis than aspartate aminotransferase/platelet ratio index in chronic hepatitis C patients. J Formos Med Assoc. 2015;114:923‐928. [DOI] [PubMed] [Google Scholar]
- 24. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, MD). 2018;67:358‐380. [DOI] [PubMed] [Google Scholar]
- 25. Omata M, Cheng AL, Kokudo N, et al. Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 27. Yu ML, Huang CF, Yeh ML, et al. Time‐degenerative factors and the risk of hepatocellular carcinoma after antiviral therapy among hepatitis C virus patients: a model for prioritization of treatment. Clin Cancer Res. 2017;23:1690‐1697. [DOI] [PubMed] [Google Scholar]
- 28. https://www.nhi.gov.tw/Content_List.aspx?n = A4EFF6CD1C4891CA&topn = 3FC7D09599D25979. Accessed on 2020.09.23
- 29. Liu CJ, Chuang WL, Sheen IS, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology. 2018;154:989‐997. [DOI] [PubMed] [Google Scholar]
- 30. Chen CP, Cheng CY, Zou H, et al. Evaluation of cost‐effectiveness of peginterferon plus ribavirin for chronic hepatitis C treatment and direct‐acting antiviral agents among HIV‐infected patients in the prison and community settings. J Microbiology Immunol Infect. 2019;52:556‐562. [DOI] [PubMed] [Google Scholar]
- 31. Yeh ML, Huang CF, Huang CI, et al. Hepatitis B‐related outcomes following direct‐acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co‐infection. J Hepatol. 2020;73:62‐71. [DOI] [PubMed] [Google Scholar]
- 32. Mucke MM, Backus LI, Mucke VT, et al. Hepatitis B virus reactivation during direct‐acting antiviral therapy for hepatitis C: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2018;3:172‐180. [DOI] [PubMed] [Google Scholar]
- 33. Asahina Y, Itoh Y, Ueno Y. Ledipasvir‐sofosbuvir for treating Japanese patients with chronic hepatitis C virus genotype 2 infection. Liver Int. 2018;38:1552‐1561. [DOI] [PubMed] [Google Scholar]
- 34. Gane EJ, Hyland RH, Yang Y, et al. Efficacy of ledipasvir plus sofosbuvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2 infection. Gastroenterology. 2017;152:1366‐1371. [DOI] [PubMed] [Google Scholar]
- 35. Asahina Y, Liu CJ, Gane E, Itoh Y, Kawada N, Ueno Y. Twelve weeks of ledipasvir/sofosbuvir all‐oral regimen for patients with chronic hepatitis C genotype 2 infection: integrated analysis of three clinical trials. Hepatol Res. 2020;50:1109‐1117. [DOI] [PubMed] [Google Scholar]
- 36. Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang CF, Iio E, Jun DW, et al. Direct‐acting antivirals in East Asian hepatitis C patients: real‐world experience from the REAL‐C Consortium. Hepatol Int. 2019;13:587‐598. [DOI] [PubMed] [Google Scholar]
- 38. Yu ML, Hung CH, Huang YH, et al. Efficacy and safety of 12 weeks of daclatasvir, asunaprevir plus ribavirin for HCV genotype‐1b infection without NS5A resistance‐associated substitutions. J Formos Med Assoc. 2019;118:556‐564. [DOI] [PubMed] [Google Scholar]
- 39. Berg T, Naumann U, Stoehr A, et al. Real‐world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German Hepatitis C‐Registry. Aliment Pharmacol Ther. 2019;49:1052‐1059. [DOI] [PubMed] [Google Scholar]
- 40. Mangia A, Milligan S, Khalili M, et al. Global real‐world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40:1841‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang CF, Yu ML. Unmet needs of chronic hepatitis C in the era of direct‐acting antiviral therapy. Clin Mol Hepatol. 2020;26:251‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogawa E, Toyoda H, Iio E, et al. HCV cure rates are reduced in patients with active but not inactive hepatocellular carcinoma‐ a practice implication. Clin Infect Dis. 2020;31:2840‐2848. [DOI] [PubMed] [Google Scholar]
- 43. Dang H, Yeo YH, Yasuda S, Huang CF. Cure with interferon‐free direct‐acting antiviral is associated with increased survival in patients with hepatitis C Virus‐related hepatocellular carcinoma from both East and West. Hepatology. 2020;71:1910‐1922. [DOI] [PubMed] [Google Scholar]
- 44. Marshall MC, Herrera JL. Lack of patient compliance in real‐world Practice negatively affects sustained viral response rates to direct acting agent therapy for Hepatitis C. Dig Dis Sci. 2018;63:3228‐3232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S4
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


