Abstract
Aims:
Given the variability of previously reported results, this systematic review aims to determine the clinical effectiveness of convalescent plasma employed in the treatment of hospitalized patients diagnosed with COVID-19.
Methods:
We conducted a systematic review of controlled clinical trials assessing treatment with convalescent plasma for hospitalized patients diagnosed with SARS-CoV-2 infection. The outcomes were mortality, clinical improvement, and ventilation requirement.
Results:
A total of 51 studies were retrieved from the databases. Five articles were finally included in the data extraction and qualitative and quantitative synthesis of results. The overall risk of bias in the reviewed articles was established at low-risk only in two trials. The meta-analysis suggests that there is no benefit of convalescent plasma compared with standard care or placebo in reducing the overall mortality and the ventilation requirement. However, there could be a benefit for the clinical improvement in patients treated with plasma.
Conclusion:
Current results led to assume that the convalescent plasma transfusion cannot reduce the mortality or ventilation requirement in hospitalized patients diagnosed with SARS-CoV-2 infection. More controlled clinical trials conducted with methodologies that ensure a low risk of bias are still needed.
The reviews of this paper are available via the supplemental material section.
Keywords: clinical improvement, convalescent plasma, COVID-19, mortality, SARS-CoV-2
Introduction
The SARS-CoV-2 virus, first detected in Wuhan, China, has caused a global pandemic. 1 What is known about the microorganism is established by genomic analysis as the disease spreads.2,3 The pandemic still represents a global health threat; in mid-January 2021, the total number of COVID-19 cases reported by the World Health Organization (WHO) is close to 100 million, while deaths have exceeded two million. 4 One year after the onset of the disease, it is known that there are cases with different degrees of severity ranging from asymptomatic cases to critical patients, in whom respiratory failure, septic shock, or multi-organ failure occurs, requiring various hospital care and supportive treatment. 5 WHO points out that there are more than 200 vaccines under investigation; only some in phase III and IV are being distributed currently.6–9
The distribution of these vaccines is subject to each country’s production, acquisition, storage, and distribution capacities. As of January 2021, 90% of the vaccines produced are concentrated in nine countries. 10 These problems limit a large percentage of the world population to be vaccinated as soon as possible and therefore reach herd protection, this limitation being even more significant in low-income countries, which will have to wait a considerable time longer; this is because of the pre-order manufacturing contracts for vaccines to 13% of the population mainly in the European Union. 6 All this implies that people will continue to be infected and will continue to die from this infection in low-income countries. Therefore, to find a treatment that reduces the severity of the disease and reduces the incidence of fatality is still a significant public health concern.
On the other hand, besides the limitations of access to the vaccine, their efficacy has been established in a range from 50% to 95%.8,9 Therefore, in countries where there will not be prompt protection of the population, it is necessary to use treatments to recover hospitalized patients when the effectiveness of the vaccines is not as expected.
Among the repurposed treatments, the use of passive immunity has been suggested as an alternative since the beginning of the pandemic. 11 The use of convalescent plasma as a treatment against COVID-19 was approved in March 2020 by the Food and Drug Administration. 12 The treatment uses the administration of antibodies collected from people recently infected and recovered from COVID-19. The results of the use of plasma are variable, reporting efficacy if its use is in the first 16 days of illness, which was associated with an improvement in the first days after treatment and lower requirements for ventilatory support. On the other hand, some studies show no evidence of clinical effectiveness of plasma in severely or critically ill patients for preventing disease progression.13–19 Given the variability of previously reported results, the present work aims to determine the clinical effectiveness of convalescent plasma employed in the treatment of hospitalized patients with diagnosis of COVID-19.
Methods
A systematic review was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting systematic reviews. 20 The question in this review was: What is the clinical effectiveness of convalescent plasma employed in the treatment of hospitalized patients with diagnosis of COVID-19?
To conduct the review, the PICOS structure was followed according to these points:
Patients: adults hospitalized with a diagnosis of SARS-CoV-2 infection;
Intervention: treatment with convalescent plasma;
Comparison: placebo or standard care;
Outcomes: overall mortality, clinical improvement at 7 days, clinical improvement at 14 days, clinical improvement at 28 days, ventilation requirement, duration of hospitalization (days), virological clearance, and severe adverse events;
Studies (type of): clinical trials published in peer-reviewed journals.
The search was carried out in PubMed, Scopus, and Web of Science databases between 20 November 2020 and 9 January 2020. The references of the selected articles were also reviewed for an integral reading to include additional studies not indexed in these databases. The ClinicalTrials.gov website was also scanned to obtain potential published reports of registered trials. The search strategies included the following keywords: convalescent plasma, COVID-19, SARS-CoV2, hospitalized. See the Supplemental material file online for more details on the search strategies.
Studies that met the following criteria were included: (i) controlled clinical trials, (ii) studies that included hospitalized patients with SARS-CoV-2 infection, (iii) published in 2020 and 2021, (iv) published in English, Chinese, Spanish, or Portuguese. The exclusion criteria were: (i) not being a clinical controlled trial, (ii) not treating hospitalized patients, and (iii) not using convalescent plasma.
All references were managed with Mendeley® software. The selection of the articles began with the removal of duplicate articles and proceeded with the reading of the title and abstract, carried out independently by reviewers 1, 2, and 3. The final decision in cases of disagreement was based on the criteria of a fourth reviewer. In the second phase, the same reviewers read the full text of the studies to define which would be included for the extraction and synthesis of data. The data were stored in Microsoft Office Excel spreadsheets and organized in an instrument constructed by the authors considering: characteristics of the study (author, year, country), sample, study design, and characteristics of the results.
The risk of bias of the studies was evaluated using the ROB2 tool. 21 The included studies were independently assessed by reviewers 1 and 5 (see Supplemental file).
The qualitative synthesis was developed following the assessed outcomes: overall mortality, clinical improvement at 7 days, clinical improvement at 14 days, clinical improvement at 28 days, ventilation requirement, hospital stay (days), virological clearance, and severe adverse events.
Statistical analysis
Meta-analyses of inverse variance were conducted for three outcomes: clinical improvement at day 7, ventilation requirement, and overall mortality. Meta-analyses were conducted with Revman v5.4 using pooled fixed effects odds ratios. The significance and the magnitude of heterogeneity across studies were calculated using the Q and I2 statistics. Odds ratios (ORs) with 95% confidence intervals (CIs) were plotted for the association between convalescent plasma compared with standard care or placebo. Subgroup analyses were performed to examine differences according to the population (all adults and older adults) in the treatment with convalescent plasma for the ventilation requirement and the overall mortality in patients diagnosed with SARS-CoV-2 infection.
The review protocol was registered on the PROSPERO platform (CRD42020184436).
Results
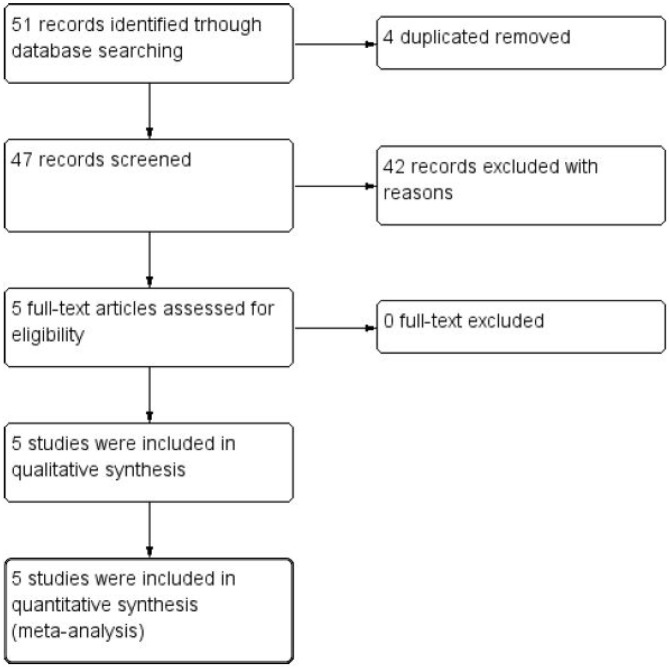
Following the described PICOS structure, this systematic review retrieved 51 studies from the databases. After the removal of four duplicates, 47 articles were read in title and abstract. Forty-two were eliminated, resulting in five articles for full-text reading. Four articles were finally included in the data extraction and qualitative and quantitative synthesis of results (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the inclusion process in the systematic review.
CI, confidence interval; IV, inverse variance.
The overall risk of bias in the reviewed articles was established at low-risk only in two randomized, double-blind clinical trials.17,22 The remaining three studies were established at high risk of bias due to issues in the randomization process.16,18,19 More details can be seen in the Supplemental file.
Patient samples ranged from 103 (the study with the fewest patients) to 464 (the study with the most patients); four clinical trials included adult patients,16,18,19,22 while the fifth study was focused only on older adult patients. 17 The retrieved results were: mortality, clinical improvement at 7, 14, and 28 days (defined by clinical scales), ventilation requirement, the mean duration of hospitalization (in days), virological clearance (by laboratory tests), progression to severe disease, and severe adverse events (Table 1).
Table 1.
Main characteristics of the included studies.
| Author | Study site | Design | Sample | Intervention | Neutralizing antibodies test | Control | Antiviral treatment | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. 19 | China | Randomized, open-label, multicenter, clinical trial | 52 intervention, 51 control | Convalescent plasma 4–13 ml/kg of recipient body weight. 96% received a single dose transfusion. IgG titer greater than 1:640 |
Tissue Culture Median Infectious Dose (TCID50) Reed–Muench to calculate neutralizing anti-group titers A serum neutralization titer of 1:80 is roughly equivalent to a titer of 1:1280 for S-RBD-specific IgG |
Standard care: symptomatic control | Yes, both groups | Clinical improvement at days 7, 14 and 28 | Mortality at day 28, hospital stay, virological clearance |
| Agarwal et al. 18 | India | Randomized, open-label, multicenter, clinical trial | 235 intervention, 229 control | Two doses of 200 ml convalescent plasma, transfused 24 h apart. IgG titer greater than 1:1280. |
Microneutralization assays in cell culture (TCID50) | Standard care following institutional protocol | Yes, only the control group. | Progression to severe disease and mortality at day 28 | Clinical improvement at day 7, O2 requirement, respiratory support, ventilation requirement, organ failure, virological clearance, levels of biomarkers, and vasopressor support |
| Abolghasemi 16 | Iran | Non-randomized, multicenter, clinical trial | 115 intervention, 74 control | One dose of 500 ml of convalescent plasma. The second dose of 500 ml was administered if the patient did not show any improvement after 24 h. Titer cut off index higher than 1.1 | No tests | Standard care | Yes, both groups | Mortality and hospital stay | Ventilation requirement and adverse events |
| Libster et al. 17 | Argentina | Randomized, double-blind, multicenter, clinical trial | 80 intervention, 80 control | 250 ml of convalescent plasma with IgG titer greater than 1:1000 | No tests | 250 ml of placebo (0.9% normal saline) | Not reported | Progression to severe disease | Life-threatening respiratory disease, ventilation requirement, admission to intensive care unit (ICU), critical systematic illness, multiple organ dysfunction, mortality, and adverse events |
| Simonovich et al. 22 | Argentina | Randomized, double blind, clinical trial | 228 intervention, 105 control | 500 ml of convalescent plasma with igG titer greater than 1:800 | No tests | 500 ml of placebo (0.9% normal saline) | Yes, both groups | Clinical status at day 30 | Clinical status at days 7 and 14, time (days) to discharge, time to discharge from ICU, time to death, time to full functional recovery, and adverse events |
The five studies reported using convalescent plasma at different dosages. Li et al. 19 reported a 4–13 ml/kg of recipient body weight dose, with the possibility of receiving a second dose (96% received a single dose transfusion). Agarwal et al. 18 reported two doses of 200 ml, transfused 24 h apart. Abolghasemi et al. 16 reported one dose of 500 ml, followed by a second dose of 500 ml if the patient did not improve after 24 h. The study developed by Libster et al. 17 in Argentina was the only one that reported 250 ml of convalescent plasma with IgG titer greater than 1:1000; while the trial led by Simonovich, 22 also in Argentina, reported one single dose of 500 ml. Also, it is important to highlight that three studies used antiviral drugs in both groups,16,19,22 one study used antiviral treatment in the control group, 18 and one study did not report the use of drugs in any group. 17
All studies quantified anti-COVID-19 antibody titers, but only Li et al. 19 and Agarwal et al. 18 reported tests for quantifying neutralizing antibodies using two cell culture methodologies. Also, Li et al. 19 reported that the correlation of serum neutralization titer of 1:80 is approximately equivalent to a titer of 1:1280 for S-RBD-specific IgG.
Outcomes assessed
The primary outcome assessed by this systematic review was the mortality in hospitalized patients diagnosed with SARS-CoV-2 infection. Two clinical trials assessed the mortality of hospitalized patients at day 28,18,19 and three studies reported mortality at any time from allocation16,17,22 (Table 1).
Three studies reported clinical improvement. Li et al. 19 has measured this outcome at days 7, 14, and 28 using the National Early Warning Score 2, 23 while Agarwal et al. 18 has measured this outcome at day 7 as the proportion of participants showing resolution of symptoms of fever, shortness of breath, or fatigue. Simonovich et al. 22 used an adapted version of the WHO Clinical Scale. 24 Abolghasemi et al. 16 and Libster et al. 17 did not assess clinical improvement (Table 1).
The progression to severe disease was assessed by Agarwal et al. and by Libster et al.17,18 The definitions of this outcome differed in both clinical trials: PaO2/FiO2 ratio <100 mmHg any time within 28 days of enrolment; 18 and respiratory rate of 30 breaths/min or more, SpO2 <93% at ambient air, or both 17 (Table 1).
Two studies18,19 reported the virological clearance using different criteria, and all studies reported adverse events identified in patients.16–19,22 The study published by Li et al. 19 was the only one that assessed subgroups: all patients, patients with severe disease, and patients with life-threatening disease. Other assessed outcomes are shown in Table 1.
Table 2 shows the main results from the four articles included in the qualitative synthesis. The reduction in overall mortality is supported by Li et al. 19 and Abolghasemi et al.; 16 but Agarwal et al., 18 Libster et al., 17 and Simonovich et al. 22 did not find statistically significant differences.
Table 2.
Reported outcomes in the included studies.
| Outcomes | Li et al. 19 | Agarwal et al. 18 | Abolghasemi et al. 16 | Libster et al. 17 | Simonovich et al. 22 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | |
| Overall mortality (%) | 8/51 (15.7) | 12/50 (24) | 34/235 (15) | 31/229 (14) | 17/115 (14.8) | 18/74 (24.3) | 2/80 (2) | 1/80 (1) | 25/228 (11) | 12/105 (11.4) |
| Clinical improvement at day 7 (%) | 5/52 (9.6) | 5/51 (9.8) | 140/176 (76) | 119/181 (66) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Clinical improvement at day 14 (%) | 17/52 (32.7) | 9/51 (17.6) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Clinical improvement at day 28 (%) | 27/52 (51.9) | 22/51 (43.1) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Progression to severe disease (%) | Not reported | Not reported | 17/235 (7.2) | 17/229 (7.4) | Not reported | Not reported | 13/80 (16) | 25/80 (31) | Not reported | Not reported |
| Hospital stay | Median 41 (IQR 31–indeterminate) | Median 53 (IQR 35–indeterminate) | Median 14 (IQR 10–19) | Median 13 (IQR 10–18) | Mean 9.54 (SD 5.07) | Mean 12.88 (SD 7.19) | Not reported | Not reported | Median 13 (IQR 8–30) | Median 12 (IQR 7–indeterminate) |
| Ventilation requirement (%) | Not reported | Not reported | 19/227 (8) | 19/224 (8) | 107/115 (93) | 59/74 (79.7) | 2/80 (2) | 4/80 (5) | 19/228 (8.3) | 10/105 (9.5) |
| Severe adverse events (%) | 1/51 (1.9) | 0/50 (0) | 2/235 (0.8) | 0/229 (0) | 0/115 (0) | 0/74 (0) | 0/80 (0) | 0/80 (0) | 54/228 (23.7) | 19/105 (18.1) |
IQR, interquartile range.
Li et al., 19 Simonovich et al., 22 and Agarwal et al. 18 concluded that the convalescent plasma transfusion did not benefit the intervention groups, while the studies published by Abolghasemi et al. 16 and Libster et al. 17 stated that convalescent plasma was clinically effective in COVID-19 patients. Since the conclusions reported by the included studies differed, we decided to conduct the meta-analysis to obtain global estimations for our interest outcomes.
Meta-analysis
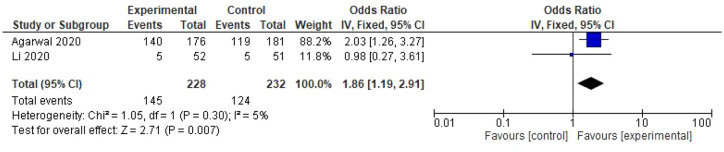
The result of two studies was integrated into the fixed-effects meta-analysis for comparing convalescent plasma versus standard care in the clinical improvement of patients diagnosed with SARS-CoV-2 infection.18,19 In this case, convalescent plasma has shown a benefit for patients (OR: 1.86; CI: 1.19–2.91) (Figure 2).
Figure 2.
Forest plot of convalescent plasma transfusion for hospitalized patients with SARS-CoV-2 infection. Comparison: convalescent plasma versus standard care. Outcome: clinical improvement at day 7.
CI, confidence interval; IV, inverse variance.
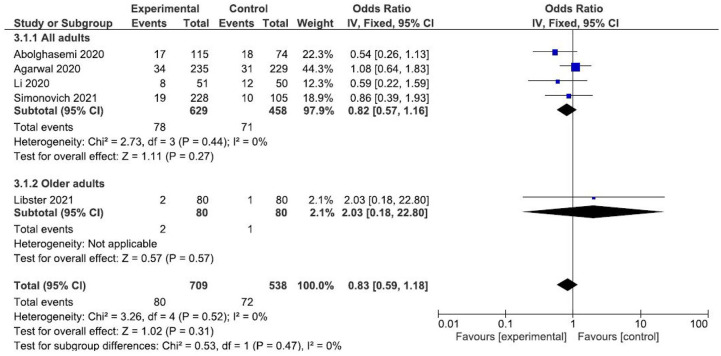
Four studies reporting ventilation requirement outcomes16–18,22 were compared to test the overall effect of convalescent plasma. The random-effects meta-analysis results show no association with ventilation requirement (OR: 1.23; CI: 0.63–2.42) (Figure 3).
Figure 3.
Forest plot of convalescent plasma transfusion for hospitalized patients with SARS-CoV-2 infection. Comparison: convalescent plasma versus standard care. Outcome: ventilation requirement.
CI, confidence interval; IV, inverse variance.
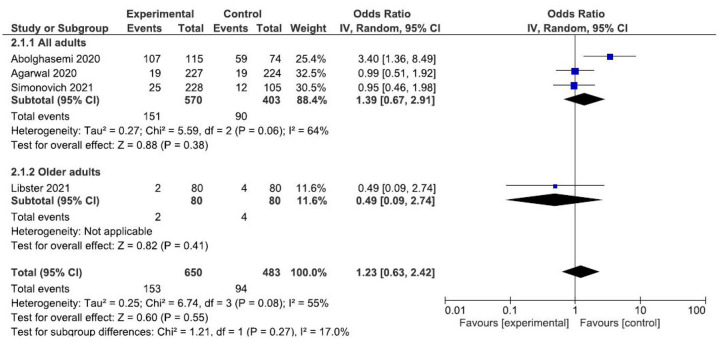
Finally, the results of four studies16,18,19,22 were meta-analyzed to establish comparisons on the overall mortality. The meta-analysis of fixed effects suggests no benefits using the convalescent plasma transfusion for reducing the risk of overall mortality (OR: 0.83; CI: 0.59–1.18) (Figure 4).
Figure 4.
Forest plot of convalescent plasma transfusion for hospitalized patients with SARS-CoV-2 infection. Comparison: convalescent plasma versus standard care. Outcome: overall mortality.
CI, confidence interval; IV, inverse variance.
Discussion
This systematic review was focused on adult hospitalized patients diagnosed with SARS-CoV-2 infection, treated with convalescent plasma transfusion. The studies included in this review were quite heterogeneous regarding the doses of plasma administered, the co-treatment with repositioned antiviral drugs in the experimental group and the control group, and the results obtained by each clinical trial. Considering only controlled clinical trials published in peer-reviewed journals, 1249 patients were included in hospitals from China, India, Iran, and Argentina.
Regarding the overall quality of the studies, three out of five clinical trials were considered at high risk of bias due to the lack of blinding; this is a common characteristic of many clinical studies that started to run under emergency conditions due to the persistent health crisis, with recurring waves in some countries in Latin America, and other countries. 25
Four published systematic reviews on convalescent plasma have shown that this treatment could reduce the mortality,26–29 but did not include only controlled trials and did not include the last studies produced in Argentina by Libster et al. 17 and Simonovich et al. 22 The current systematic review did not show any benefit on the mortality reduction, consistent with other three published systematic reviews,30–32 while other systematic reviews were focused on other infectious diseases such as Ebola, influenza, or SARS,33–35 or other target populations. 36
The use of convalescent plasma was associated with clinical improvement, which is consistent with other previously published studies;26,29,31 however, Li et al. 19 have stated that plasma treatment has not been effective in critically ill patients, which suggests that more stratified analyses are needed in primary studies. The plasma transfusion has not been effective for avoiding ventilation requirement, as stated previously by Chai. 31 All this could suggest that the clinical effects of an earlier transfusion of convalescent plasma should continue to be assessed in subsequent clinical trials, just like Libster et al. 17 suggests in mildly ill patients in the early stages of the infection. In the decade of the 1970s, one study on hemorrhagic fever in Argentina has shown more effectiveness of convalescent plasma in the first days of the clinical course. 37
This early-stage plasma therapy is also supported by a study conducted in the Netherlands, 38 which had to be halted prematurely. The authors showed that late-administered plasma therapy did not benefit patients because they already had high virus-neutralizing antibody titers on the day of study inclusion with titers comparable to the recovered donors’.
The use of neutralizing antibody tests 19 is strongly recommended to ensure the quality of the plasma transfused to patients, without forgetting that the host’s response depends on particular phenomena, such as original antigenic sin. This phenomenon shows that previous exposure to a similar virus triggers a strong but not specifically neutralizing immune response, so having high antibody titers or a strong immune response does not indicate that the virus is being neutralized.
Still, the optimal dosage and the best time point for the convalescent plasma transfusion need to be determined in well-designed clinical trials. 39 Additionally, the genetic variability of the virus and the patients should be analyzed since the treatment may not have the specific immunoglobulins to resolve the infection. 2
Regarding the influence of antiviral drugs used in the articles included in this systematic review, the study by Li et al. 19 reported the use of antiviral drugs, steroids, immunoglobulin, and interferon, since they have treated patients with severe or life-threatening disease. For its part, the Agarwal study reported the joint use of plasma with some of these drugs: hydroxychloroquine, remdesivir, lopinavir/ritonavir, oseltamivir, steroids, and tocilizumab. 18 The Abolghasemi et al. 16 study reported the concomitant use of lopinavir/ritonavir, hydroxychloroquine, and an anti-inflammatory agent together with plasma therapy. Previous studies report some benefits from using these drugs in adult patients;40,41 however, more studies are needed here to control this variable, although, in clinical practice, this is almost impossible when treating patients with severe disease. The only study that could control this variable was Libster et al. 17 because it included patients only with early-stage disease.
There is enough evidence proving that convalescent plasma administration does not have many severe adverse events in transfusion.16–19,29,33 In contrast, more research is needed on the synergistic effect that plasma could have with other repositioning drugs, as has been demonstrated, for example, with the use of remdesivir, as has been published in other studies.19,29,42
Among the limitations of this study, the rapid generation of new knowledge in times of the pandemic can potentially affect the timeliness of this review in a few months. The second limitation is the heterogeneity and high risk of bias in the studies. In this review, we chose not to issue recommendations with the GRADE methodology due to heterogeneity and the high risk of bias. Another limitation is that not all studies have used the same dosage of convalescent plasma in infected patients. The fourth limitation that must be considered is the use of antiviral drugs in the control groups or both groups of patients in three out of four clinical trials included in this review.
In times of recurring waves of the COVID-19 pandemic, the analysis of potential treatments proposed for hospitalized patients is still necessary since vaccines’ procurement and logistics are still seen within a complex scenario for many low-income countries. In many low-income countries, vaccination is likely to occur six to twelve months after high-income countries, in part due to logistical problems, as stated by the World Bank. 43 so the search for a clinically effective treatment is still a major concern globally.
In the ClinicalTrials.gov platform dozens of clinical trials are currently registered that assess the treatment with plasma, so the addition of new evidence in the coming months could change the direction of the analyses in this review.
Conclusion
The current evidence suggests that transfusion with convalescent plasma cannot reduce the mortality or ventilation requirement in hospitalized patients diagnosed with SARS-CoV-2 infection. More controlled clinical trials conducted with methodologies that ensure a low risk of bias with neutralizing antibody tests to ensure a good quality of plasma are still needed.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-6-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Footnotes
Author contributions: RAAZ contributed to the development of the research project.
RAAZ, MVE, SMC, AYNO, DGM, and RAMGV performed data collection, analyzed and interpreted the results.
RAAZ, SCM, and RAMGV wrote the article. All authors reviewed and approved the final version.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data accessibility statement: All data are available upon request to the corresponding author.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Abeldaño Zuñiga, Roberto Ariel  https://orcid.org/0000-0002-2627-278X
https://orcid.org/0000-0002-2627-278X
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Roberto Ariel Abeldaño Zuñiga, University of Sierra Sur, Miahuatlan de Porfirio Diaz, Oaxaca, 70800, Mexico.
Ruth Ana María González-Villoria, Post Graduate Department. University of Sierra Sur, Oaxaca, Mexico.
María Vanesa Elizondo, Institute of Biomedical Sciences, Catholic University of Cuyo, San Juan, Argentina.
Anel Yaneli Nicolás Osorio, Post Graduate Department. University of Sierra Sur, Oaxaca, Mexico.
David Gómez Martínez, Public Health Research Institute. University of Sierra Sur, Oaxaca, Mexico.
Silvia Mercedes Coca, Public Health Research Institute. University of Sierra Sur, Oaxaca, Mexico.
References
- 1. Hsu LY, Chia PY, Lim JF. The novel coronavirus (SARS-CoV-2) epidemic. Ann Acad Med Singap 2020; 49: 105–107. [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoque MN, Chaudhury A, Akanda MAM, et al. Genomic diversity and evolution, diagnosis, prevention, and therapeutics of the pandemic COVID-19 disease. PeerJ 2020; 8: e9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. WHO coronavirus disease (COVID-19) dashboard, https://covid19.who.int/ (2021, accessed 16 January 2021). [PubMed]
- 5. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mullard A. How COVID vaccines are being divvied up around the world. Nature. Epub ahead of print 30 November 2020. DOI: 10.1038/d41586-020-03370-6. [DOI] [PubMed] [Google Scholar]
- 7. Korang SK, Juul S, Nielsen EE, et al. Vaccines to prevent COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project). Syst Rev 2020; 9: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. Epub ahead of print 30 December 2020. DOI: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. Epub ahead of print 17 November 2020. DOI: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Our World in Data. COVID-19 vaccine doses administered per 100 people, https://ourworldindata.org/grapher/covid-vaccination-doses-per-capita (2021, accessed 16 January 2021).
- 11. Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016; 14: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ 2020; 368: m1256. [DOI] [PubMed] [Google Scholar]
- 13. Murphy M, Estcourt L, Grant-Casey J, et al. International survey of trials of convalescent plasma to treat COVID-19 infection. Transfus Med Rev 2020; 34: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu STH, Lin H-M, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med 2020; 26: 1708–1713. [DOI] [PubMed] [Google Scholar]
- 15. Erkurt MA, Sarici A, Berber İ, et al. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci 2020; 59: 102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abolghasemi H, Eshghi P, Cheraghali AM, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci 2020; 59: 102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial). BMJ 2020; 371: m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA 2020; 324: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 22. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021; 384: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Royal College of Physicians. National Early Warning Score (NEWS) 2. RCP London, https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (2017, accessed 8 September 2020).
- 24. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: e192–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. United Nations Office for the Coordination or Humanitarian Affairs. Epidemiological alert: recurring waves and outbreaks of COVID-19, https://reliefweb.int/report/world/epidemiological-alert-recurring-waves-and-outbreaks-covid-19-9-october-2020 (2020, accessed 14 January 2021).
- 26. Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol 2021; 93: 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meher BR, Padhy BM, Das S, et al. Effectiveness of convalescent plasma therapy in the treatment of moderate to severe COVID 19 patients: a systematic review and meta-analysis. J Assoc Physicians India 2020; 68: 35–43. [PubMed] [Google Scholar]
- 28. Shao S, Wang Y, Kang H, et al. Effect of convalescent blood products for patients with severe acute respiratory infections of viral etiology: a systematic review and meta-analysis. Int J Infect Dis 2021; 102: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wenjing L, Yuanzheng F, Li J-Y, et al. Safety and efficacy of convalescent plasma therapy in severely and critically ill patients with COVID-19: a systematic review with meta-analysis. Aging (Albany NY). Epub ahead of print 16 November 2020. DOI: 10.18632/aging.202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakhtawar N, Usman M, Khan MMU. Convalescent plasma therapy and its effects on COVID-19 patient outcomes: a systematic review of current literature. Cureus. Epub ahead of print 3 August 2020. DOI: 10.7759/cureus.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chai KL, Valk SJ, Piechotta V, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. Epub ahead of print 12 October 2020. DOI: 10.1002/14651858.CD013600.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Huo P, Dai R, et al. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int Immunopharmacol 2021; 91: 107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Devasenapathy N, Ye Z, Loeb M, et al. Efficacité et innocuité du plasma de convalescent en cas de forme grave de COVID-19, extrapolée de données relatives à d’autres formes graves d’infections respiratoires virales : revue systématique et méta-analyse. Can Med Assoc J 2020; 192: E1559–E1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu T, Xu A, Bai X, et al. Effect of convalescent plasma and immunoglobulin on patients with severe acute respiratory syndrome: a systematic review. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020; 32: 435–438. [DOI] [PubMed] [Google Scholar]
- 35. Sun M, Xu Y, He H, et al. A potentially effective treatment for COVID-19: a systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int J Infect Dis 2020; 98: 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaffanello M, Piacentini G, Nosetti L, et al. The use of convalescent plasma for pediatric patients with SARS-CoV-2: a systematic literature review. Transfus Apher Sci. Epub ahead of print 23 December 2020. DOI: 10.1016/j.transci.2020.103043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maiztegui J, Fernandez N, De Damilano A. Efficacy of immune plasma in treatment of Argentine Hemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 1979; 314: 1216–1217. [DOI] [PubMed] [Google Scholar]
- 38. Gharbharan A, Jordans C, Geurtsvankessel C, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv Prepr Serv, 2020. DOI: 10.1101/2020.08.26.20182444. [DOI] [Google Scholar]
- 39. Saverino D. Hyper-immune/convalescent plasma: an old option and a valid strategy for treatment of COVID-19? Minerva Med. Epub ahead of print 14 May 2020. DOI: 10.23736/S0026-4806.20.06616-1. [DOI] [PubMed] [Google Scholar]
- 40. Abeldaño Zuñiga RA, Coca SM, Abeldaño GF, et al. Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis. Ther Adv Respir Dis 2021; 15: 175346662110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laure G, Viet-Thi T, Elodie P, et al. 14-Day survival among older adults with severe SARS-Cov2 infection treated with corticosteroid: a cohort study. Clin Microbiol Infect. Epub ahead of print 2 April 2021. DOI: 10.1016/j.cmi.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The World Bank. Different scenarios for global growth, in five charts, https://blogs.worldbank.org/developmenttalk/different-scenarios-global-growth-five-charts (2021, accessed 16 January 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-6-tar-10.1177_17534666211028077 for Clinical effectiveness of convalescent plasma in hospitalized patients with COVID-19: a systematic review and meta-analysis by Roberto Ariel Abeldaño Zuñiga, Ruth Ana María González-Villoria, María Vanesa Elizondo, Anel Yaneli Nicolás Osorio, David Gómez Martínez and Silvia Mercedes Coca in Therapeutic Advances in Respiratory Disease