Abstract
Aims
We sought to analyse quality of life (QoL) measures derived from two questionnaires widely used in clinical trials, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the EuroQoL 5 dimensions (EQ‐5D), and to compare their prognostic value in men and women with heart failure and reduced ejection fraction (HFrEF).
Methods and results
From the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT‐CHF) we compared KCCQ and EQ‐5D at baseline and after 9 months in 1276 men and 373 women with new‐onset or worsening symptoms of HFrEF, who were sub‐optimally treated and in whom there was an anticipated up‐titration of guideline‐derived medical therapies. Women had significantly worse baseline QoL (median) as compared with men, both when assessed with KCCQ overall score (KCCQ‐OS, 44 vs. 53, P < 0.001) and EQ‐5D utility score (0.62 vs. 0.73, P < 0.001). QoL improved equally in women and men at follow‐up. All summary measures of QoL were independently associated with all‐cause mortality, with KCCQ‐OS showing the most remarkable association with mortality up to 1 year compared to the EQ‐5D scores (C‐statistic 0.650 for KCCQ‐OS vs. 0.633 and 0.599 for EQ‐5D utility score and EQ‐5D visual analogue scale, respectively). QoL was associated with all outcomes analysed, both in men and women (all P for interaction with sex >0.2).
Conclusion
Amongst patients with HFrEF, women reported significantly worse QoL than men. QoL was independently associated with subsequent outcome, similarly in men and women. The KCCQ in general, and the KCCQ‐OS in particular, showed the strongest independent association with outcome.
Keywords: Heart failure, Quality of life, Sex, Women, Outcome
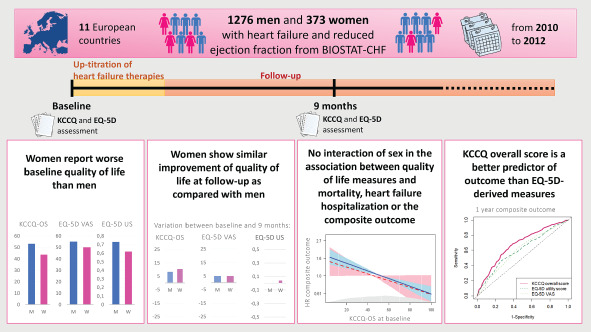
Sex differences in quality of life, in its relationship with outcomes, and predictive ability of different quality of life measures towards outcome. BIOSTAT‐CHF, BIOlogy Study to TAilored Treatment in Chronic Heart Failure; EQ‐5D, EuroQoL 5 dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire; OS, overall score; US, utility score; VAS, visual analogue scale.
Introduction
Patients with heart failure (HF) suffer from debilitating physical symptoms, frequently associated with depressive symptoms, anxiety, and cognitive disorders that further affect their daily function and quality of life (QoL). 1 Notably, patients with HF generally have a poor QoL, which is much lower compared to healthy individuals and even to patients with other chronic illnesses. 1
Previous analyses of trials and registries in HF highlighted several differences between men and women with regard to clinical features and event rates. 2 Of particular interest is the observed sex difference in QoL, with women experiencing poorer QoL and greater perceived disability as compared with men. 3 , 4 Lower QoL in HF with reduced ejection fraction (HFrEF) is associated with increased hospitalizations and mortality. 5 , 6 , 7 However, sex differences in the relationship of QoL to outcomes require further investigation.
Tools aimed at assessing patients' perception of their health status are widely used in research, and potentially approvable endpoints in medication development. 8 Various QoL surveys have been used for patients with HF, both generic and disease‐specific. 9 The EuroQol 5 dimensions (EQ‐5D) questionnaire is a widely used, standardized instrument for measuring generic health status, 10 whereas the Kansas City Cardiomyopathy Questionnaire (KCCQ) was specifically designed and validated for health‐related QoL assessment in patients with HF. 11 Head‐to‐head comparisons on the discriminative power and prognostic value of these two QoL assessment methods are limited.
We therefore sought to analyse sex differences in QoL as assessed with two widely used QoL questionnaires, and to assess whether there are sex differences in QoL variations after up‐titration of HF therapies. Moreover, we analysed the relationship between different measures of QoL and outcomes, testing for the interaction of sex in this association. Finally, we compared the predictive ability of different QoL measures towards outcome.
Methods
Patient population
We studied patients from the index cohort of the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT‐CHF), whose design has been described in detail elsewhere. 12 Briefly, BIOSTAT‐CHF was a multicentre, multinational, prospective, observational study. The index cohort included 2516 patients enrolled from 11 European countries between December 2010 and December 2012. To be enrolled in the study patients had to comply with protocol specified criteria. 12 The main inclusion criteria were signs and/or symptoms of new‐onset or worsening HF and sub‐optimal (≤50% target dose; online supplementary Table S1 ) treatment with angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) and/or beta‐blockers, with anticipated initiation or up‐titration of these drugs according to the target doses recommended by the European Society of Cardiology (ESC) guidelines 13 , 14 (online supplementary Table S1 ). Patients could be enrolled either as inpatients or from outpatient clinics. Investigators were encouraged to optimize treatment with ACEi/ARB and beta‐blockers during the first 3 months of the study.
All patients provided written informed consent to participate in the study and BIOSTAT‐CHF complied with the Declaration of Helsinki. The study was approved by the ethics committees of the participating centres.
Study procedures and quality of life assessment
The first study assessment was performed at baseline, then a second visit was planned after 9 months. Medical history, current medication, and physical examination were recorded at both visits, and blood and urine samples were collected for subsequent analyses. QoL was assessed with EQ‐5D and KCCQ, both at baseline and 9‐month visits; details on the questionnaires are described in the online supplementary Methods . Six‐minute walk test (6MWT) was also performed at both visits. 8
In our analyses, only patients with HFrEF, thus with a left ventricular ejection fraction <40%, were included (n = 1819). Patients who did not complete both KCCQ and EQ‐5D questionnaires at baseline were also excluded from the main analyses (n = 170). For the limited set of analyses considering the variation of QoL parameters at follow‐up, we also excluded patients who did not complete both KCCQ and EQ‐5D questionnaires at 9 months (n = 407). A flow diagram is displayed in online supplementary Figure S1 .
Follow‐up and outcomes
After the second study visit, patients were prospectively followed by ambulatory visits or telephone calls at 6‐month intervals until the end of the study, in April 2015. The protocol of BIOSTAT‐CHF used clear endpoint definitions, a structured case report form, and source data of all sites were closely monitored. All deaths and hospitalizations were recorded. The adjudication of HF hospitalization was performed by the treating physician. After the trial has ended, all medical reports of the deadly event were read and adjudicated by an independent committee of cardiologists. Median follow‐up was 21 months [interquartile range (IQR) 11–32 months]. 12
Statistical analysis
Normally distributed continuous variables are presented as means ± standard deviation and non‐normally distributed variables as median (IQR). Categorical variables are reported as numbers with percentages. Baseline clinical parameters were compared between men and women, and between patients with quartiles of KCCQ overall score (KCCQ‐OS). Group comparisons were made using ANOVA, Student's t‐tests, Chi‐square tests and Mann–Whitney U tests as appropriate. QoL variation between different timepoints was compared using paired samples t‐tests. Shift analysis was also performed to check for sex differences in QoL variation across the entire QoL spectrum. Baseline and 3‐month HF therapies were also compared using shift analysis (details in online supplementary Methods ).
Univariable and multivariable Cox proportional hazard models were used to evaluate the impact of QoL overall measures [KCCQ‐OS; EQ‐5D utility score (US), US; EQ‐5D visual analogue scale (VAS)] on mortality, HF hospitalization and the composite outcome (death and/or HF hospitalization). Hazard ratios are expressed as mean and 95% confidence interval (CI). To account for potential confounding factors, the previously published multivariable risk models of BIOSTAT‐CHF for mortality, HF hospitalization or the composite outcome at 1 year were used, as appropriate, for adjustment in the multivariable Cox and competing‐risk regression models. 15 The covariates in each model are displayed in the online supplementary Methods . QoL measures were modelled as continuous variables: 5 point‐change units for KCCQ‐OS and EQ‐5D VAS, and 0.1 point‐change units for EQ‐5D US were considered because of clinical meaning and comparable magnitude. 16 Univariable and multivariable Cox proportional hazard models were obtained for the total study population and for men and women separately. Additionally, for all relationships between QoL measures and outcome, interaction with sex was tested, and effect plots stratified by sex were obtained for immediate results visualization.
To compare the impact of each QoL measure on outcomes, between univariable Cox models the change in C‐statistics was computed, time‐dependent receiver operating characteristic (ROC) curves were plotted to study the strength of the association of each QoL measure to outcome over time, and net reclassification improvement (NRI) was calculated (details in online supplementary Methods ). We finally tested the additive ability of each QoL measure to reclassify risk of each outcome beyond the BIOSTAT‐CHF risk models, by examining categorical NRI.
Statistical analyses were performed using R, A Language and Environment for Statistical Computing, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and IBM SPSS Statistics for Windows, version 23.0.0.3 (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics
A total of 1649 patients were studied, 373 (23%) of whom were women. Baseline characteristics of men and women are displayed in Table 1 and online supplementary Table S2 . At baseline, women were older (71 vs. 66 years, P < 0.001), had slightly higher left ventricular ejection fraction (29% vs. 27%, P < 0.001), and were less likely to have an ischaemic HF aetiology (49% vs. 64%, P < 0.001). Clinical signs and symptoms of HF and vitals were fairly similar between men and women. Notably, there was no significant sex difference in New York Heart Association (NYHA) class (P = 0.688), median HF duration (4 vs. 2 months, P = 0.434), and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) (P = 0.076). Women were less likely to have most comorbidities, especially atherothrombotic disease in general. Baseline HF medications were similar in men and women, except for mineralocorticoid receptor antagonists (MRAs) and digoxin, that were more often prescribed in men (59% vs. 49%, P < 0.001% and 21% vs. 13%, P = 0.002, respectively). There was no significant sex difference in the fraction of target dose of ACEi/ARB or beta‐blockers both at baseline and after the up‐titration phase (online supplementary Figure S5 and S6 ).
Table 1.
Baseline characteristics stratified by sex
| Men | Women | P‐value | |
|---|---|---|---|
| No. of subjects | 1276 | 373 | |
| Demographics | |||
| Age, years | 66 ± 12 | 71 ± 12 | <0.001 |
| Race, n (%) | 0.013 | ||
| Caucasian | 1261 (98.8) | 367 (98.4) | |
| Other | 15 (1.1) | 6 (1.6) | |
| BMI, kg/m2 | 27.80 (5.19) | 27.11 (5.58) | 0.027 |
| Weight, kg | 85 ± 18 | 72 ± 16 | <0.001 |
| Height, cm | 174 ± 8 | 162 ± 7 | <0.001 |
| Clinical profile | |||
| NYHA class, n (%) | 0.688 | ||
| I | 31 (2.5) | 6 (1.6) | |
| II | 470 (37.5) | 131 (35.8) | |
| III | 611 (48.8) | 188 (51.4) | |
| IV | 140 (11.2) | 41 (11.2) | |
| LVEF, % | 26.8 ± 7 | 28.9 ± 6.3 | <0.001 |
| Systolic blood pressure, mmHg | 122.4 ± 20.0 | 126.5 ± 23.4 | 0.001 |
| Diastolic blood pressure, mmHg | 75.6 ± 12.2 | 74.8 ± 13.5 | 0.280 |
| Heart rate, bpm | 79.6 ± 18.8 | 80.7 ± 18.3 | 0.352 |
| Type of visit (%) | 0.695 | ||
| Outpatient | 482 (37.8) | 139 (37.2) | |
| Inpatient | 794 (62.2) | 234 (62.7) | |
| HF history | |||
| Months since first diagnosis | 3.70 [0.17–42.85] | 1.90 [0.10–10.40] | 0.434 |
| HF aetiology, n (%) | |||
| Ischaemic | 734 (63.6) | 158 (49.4) | <0.001 |
| Valvular | 498 (39) | 145 (38.9) | 0.320 |
| Past HF hospitalization | 431 (33.8) | 117 (31.4) | 0.420 |
| Medical history, n (%) | |||
| Hypertension | 744 (58.3) | 239 (64.1) | 0.053 |
| Atrial fibrillation | 558 (43.7) | 131 (35.1) | 0.004 |
| Myocardial infarction | 530 (41.5) | 105 (28.2) | <0.001 |
| Diabetes mellitus | 412 (32.3) | 103 (27.6) | 0.099 |
| COPD | 230 (18.0) | 44 (11.8) | 0.006 |
| Peripheral artery disease | 135 (10.6) | 22 (5.9) | 0.009 |
| Stroke | 109 (8.5) | 30 (8.0) | 0.842 |
| Medication, n (%) | |||
| ACEi or ARBs | 962 (75.4) | 268 (71.8) | 0.189 |
| Beta‐blockers | 1088 (85.3) | 305 (81.8) | 0.119 |
| MRAs | 752 (58.9) | 181 (48.5) | <0.001 |
| Laboratory | |||
| Haemoglobin, g/dL | 13.80 [12.30–14.90] | 12.80 [11.75–13.72] | <0.001 |
| Creatinine, mg/dL | 1.20 [1.00–1.50] | 1.00 [0.81–1.23] | <0.001 |
| Urea, mmol/L | 12.14 [8.00–19.99] | 9.70 [7.20–16.00] | <0.001 |
| Sodium, mmol/L | 140.00 [137.00–142.00] | 140.00 [138.00–142.00] | 0.064 |
| Potassium, mmol/L | 4.30 [4.00–4.60] | 4.20 [3.80–4.60] | 0.002 |
| NT‐proBNP, ng/L | 4148.00 [2288.00–8220.00] | 4706.50 [2471.00–9992.00] | 0.076 |
Continuous variables are presented as means ± standard deviation when normally distributed, or median [interquartile range] for non‐normally distributed variables. Categorical variables are shown as n (%).
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Sex differences in quality of life
Distributions of overall QoL measures at baseline and their change after 9 months are presented in Figure 1 . Women reported lower QoL than men as assessed with KCCQ‐OS (44 vs. 53, P < 0.001) and EQ‐5D US (0.6 vs. 0.7, P < 0.001), with a similar tendency for EQ‐5D VAS (50 vs. 55, P = 0.062). Similarly, women showed worse exercise capacity as assessed with 6MWT (180 vs. 281 m, P < 0.001; online supplementary Table S2 ). Baseline KCCQ domain scores and answers to EQ‐5D questions in men and women are displayed in Figure 2 . Upon enrolment, women reported a significantly higher burden of limitation as captured by six (out of seven) KCCQ domains and all the five dimensions in the EQ‐5D (Figure 2 B ). Notably, answers to KCCQ items show that the sex difference in perceived physical limitation increased as the considered activities became more demanding, with fatigue being the most limiting symptom in women as compared with men, and all social activities being more affected by HF symptoms in women (online supplementary Figure S2 ).
Figure 1.

Sex differences in the distribution of baseline quality of life measures (A–C), and their difference between baseline and 9 months (D–F), boxplots showing median values and interquartile ranges. EQ‐5D, EuroQoL 5 dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Figure 2.
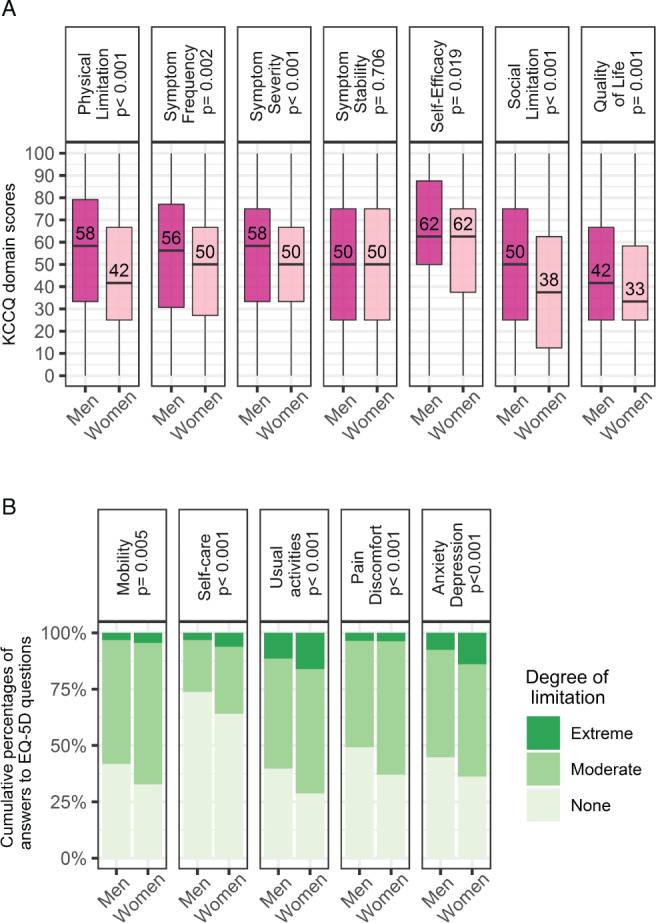
Sex differences in the baseline distribution of (A) the Kansas City Cardiomyopathy Questionnaire (KCCQ) domain scores (boxplot, medians and interquartile ranges), and (B) the answers to EuroQol 5 dimensions (EQ‐5D) items (cumulative percentages).
After 9 months, all QoL summary measures significantly improved in the total population, and in men and women separately (all P < 0.001; online supplementary Table S5 ). Likewise, 6MWT distance improved in the total population, and in men and women separately (all P < 0.001; online supplementary Table S5 ). The absolute change in QoL measures, more pronounced in KCCQ‐OS and EQ‐5D VAS, was similar in men and women (Figure 1 ). The magnitude of change in any QoL score at 9 months was neither dependent on sex (all P > 0.1) nor on the fraction of the target dose of ACEi/ARB or beta‐blocker achieved after the up‐titration period (all P > 0.05), with large QoL variability among each group (online supplementary Tables S3 and S4, and Figure S7 ). In particular, KCCQ single answers and domain scores, and EQ‐5D items at 9 months showed an improvement in QoL which was consistent across all the considered areas, and was slightly more pronounced in symptoms and physical limitation, similarly in men and women (online supplementary Table S5, and Figures S3 and S4 ).
Quality of life and outcome
Table 2 shows the association of baseline QoL measures with outcomes. Univariable Cox regression showed that all QoL measures were significantly associated with all‐cause mortality and the composite outcome in the entire study population and also in both sexes separately. After adjustment, all QoL measures were independently associated with mortality and the composite outcome in the total population and in men, with a similar tendency in women (Table 2 ). When considering HF hospitalization, in univariable Cox regression all QoL measures were significantly associated with both outcomes in the total population and in men, whereas in women KCCQ‐OS was the only QoL measure significantly associated with this outcome (Table 2 ). In multivariable analyses, only KCCQ‐OS and EQ‐5D VAS were independently associated with HF hospitalization in the entire population and in men (Table 2 ). As for the other analysed outcomes, hazard ratios showed a similar tendency in women as compared with men. For all outcomes, there was no significant QoL‐by‐sex interaction (all P > 0.2). Additionally, effect plots showed similar predicted probability in men and women for all considered outcomes across the levels of QoL measures (online supplementary Figures S8–S10 ).
Table 2.
Association of baseline quality of life measures with mortality, heart failure hospitalization, and mortality and/or heart failure hospitalization
| Population | n | Events (%) | Unadjusted HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| KCCQ‐OS | ||||||
| Total | 1649 | 371 (22.5) | 0.89 (0.87–0.92) | <0.001 | 0.95 (0.92–0.97) | <0.001 |
| Men | 1276 | 112 (23.4) | 0.90 (0.87–0.92) | <0.001 | 0.94 (0.91–0.97) | <0.001 |
| Women | 373 | 33 (19.3) | 0.86 (0.81–0.92) | <0.001 | 0.94 (0.88–1.00) | 0.078 |
| EQ‐5D VAS | ||||||
| Total | 1649 | 371 (22.5) | 0.94 (0.91–0.96) | <0.001 | 0.97 (0.93–0.99) | 0.009 |
| Men | 1276 | 112 (23.4) | 0.94 (0.92–0.96) | <0.001 | 0.97 (0.94–1.00) | 0.024 |
| Women | 373 | 33 (19.3) | 0.92 (0.87–0.97) | 0.002 | 0.96 (0.91–1.02) | 0.167 |
| EQ‐5D US | ||||||
| Total | 1649 | 371 (22.5) | 0.88 (0.86–0.91) | <0.001 | 0.94 (0.90–0.96) | <0.001 |
| Men | 1276 | 112 (23.4) | 0.87 (0.85–0.9) | <0.001 | 0.92 (0.89–0.96) | <0.001 |
| Women | 373 | 33 (19.3) | 0.88 (0.82–0.94) | <0.001 | 0.96 (0.89–1.02) | 0.229 |
| Heart failure hospitalization | ||||||
| KCCQ‐OS | ||||||
| Total | 1649 | 374 (22.7) | 0.9 (0.88–0.93) | <0.001 | 0.94 (0.92–0.97) | <0.001 |
| Men | 1276 | 293 (23) | 0.90 (0.88–0.92) | <0.001 | 0.94 (0.91–0.96) | <0.001 |
| Women | 373 | 81 (21.7) | 0.92 (0.87–0.97) | 0.003 | 0.96 (0.90–1.01) | 0.145 |
| EQ‐5D VAS | ||||||
| Total | 1649 | 374 (22.7) | 0.95 (0.93–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.016 |
| Men | 1276 | 293 (23) | 0.95 (0.92–0.97) | <0.001 | 0.97 (0.94–1.00) | 0.022 |
| Women | 373 | 81 (21.7) | 0.96 (0.91–1.00) | 0.094 | 0.97 (0.92–1.03) | 0.415 |
| EQ‐5D US | ||||||
| Total | 1649 | 374 (22.7) | 0.93 (0.90–0.96) | <0.001 | 0.97 (0.94–1.00) | 0.079 |
| Men | 1276 | 293 (23) | 0.92 (0.89–0.96) | <0.001 | 0.96 (0.93–1.00) | 0.063 |
| Women | 373 | 81 (21.7) | 0.95 (0.89–1.00) | 0.16 | 0.99 (0.92–1.06) | 0.772 |
| Mortality and/or heart failure hospitalization | ||||||
| KCCQ‐OS | ||||||
| Total | 1649 | 604 (36.6) | 0.90 (0.88–0.93) | <0.001 | 0.95 (0.93–0.97) | <0.001 |
| Men | 1276 | 799 (37.4) | 0.90 (0.88–0.92) | <0.001 | 0.95 (0.93–0.97) | <0.001 |
| Women | 373 | 127 (34) | 0.89 (0.85–0.94) | <0.001 | 0.95 (0.91–1.00) | 0.060 |
| EQ‐5D VAS | ||||||
| Total | 1649 | 604 (36.6) | 0.95 (0.93–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.004 |
| Men | 1276 | 799 (37.4) | 0.95 (0.93–0.96) | <0.001 | 0.97 (0.95–0.99) | 0.012 |
| Women | 373 | 127 (34) | 0.94 (0.9–0.98) | 0.002 | 0.97 (0.93–1.01) | 0.127 |
| EQ‐5D US | ||||||
| Total | 1649 | 604 (36.6) | 0.93 (0.9–0.96) | <0.001 | 0.96 (0.94–0.99) | 0.003 |
| Men | 1276 | 799 (37.4) | 0.9 (0.88–0.93) | <0.001 | 0.96 (0.93–0.99) | 0.003 |
| Women | 373 | 127 (34) | 0.92 (0.87–0.97) | 0.002 | 0.97 (0.92–1.03) | 0.308 |
CI, confidence interval; EQ‐5D, EuroQoL 5 dimensions; HR, hazard ratio; KCCQ‐OS, Kansas City Cardiomyopathy Questionnaire overall score; US, utility score; VAS, visual analogue scale.
The 6MWT showed a significant association with all‐cause mortality in the total population, and in men and women separately at univariable Cox regression; whilst only in the total population and in men after adjustment for the BIOSTAT‐CHF risk model (online supplementary Table S6 ). When the other outcomes were considered, 6MWT showed a modest association with outcome only at univariable analysis (online supplementary Table S6 ). Interestingly, a significant interaction of sex in the association between 6MWT and all‐cause mortality was observed, with women showing lower risk of death than men for similar 6MWT values (P for interaction: unadjusted 0.02, adjusted 0.046).
Univariable predictive power of KCCQ‐OS for all‐cause mortality was significantly better than the one of EQ‐5D VAS, but not EQ‐5D US, when models were compared using C‐statistic (0.650 vs. 0.599 and 0.633, respectively, P < 0.001 and P = 0.185) and NRI (0.268, 95% CI 0.123–0.383 and 0.089, 95% CI −0.023 to 0.224, respectively; online supplementary Table S8 ). Conversely, univariable predictive power of KCCQ‐OS towards HF hospitalization and the composite outcome was significantly better than those of both EQ‐5D‐derived measures, both when compared using C‐statistic (all P < 0.001; Table 3 ) and NRI (online supplementary Table S8 ). Using time‐dependent ROC curves, KCCQ‐OS resulted the strongest univariable predictor of mortality up to 1 year (Figure 3 A , and online supplementary Table S7A ), and of HF hospitalization and the composite outcome up to 2 years (Figure 3 B and 3C, and online supplementary Table S7B and S7C ). For all outcomes, adding any QoL measure did not significantly improve NRI compared to the corresponding BIOSTAT‐CHF risk model (online supplementary Table S8 ).
Table 3.
C‐statistic for different baseline quality of life measure‐based models for predicting mortality, heart failure hospitalization, and mortality and/or heart failure hospitalization
| C‐statistic | SE | P for comparison with KCCQ‐OS | P for comparison with EQ‐5D US | |
|---|---|---|---|---|
| Mortality | ||||
| KCCQ‐OS | 0.650 | 0.016 | – | – |
| EQ‐5D US | 0.633 | 0.016 | 0.185 | – |
| EQ‐5D VAS | 0.599 | 0.016 | <0.001 | 0.025 |
| Heart failure hospitalization | ||||
| KCCQ‐OS | 0.629 | 0.015 | – | – |
| EQ‐5D US | 0.562 | 0.015 | <0.001 | – |
| EQ‐5D VAS | 0.574 | 0.015 | <0.001 | 0.447 |
| Mortality and/or heart failure hospitalization | ||||
| KCCQ‐OS | 0.636 | 0.012 | – | – |
| EQ‐5D US | 0.587 | 0.012 | <0.001 | – |
| EQ‐5D VAS | 0.582 | 0.012 | <0.001 | 0.674 |
EQ‐5D, EuroQoL 5 dimensions; KCCQ‐OS, Kansas City Cardiomyopathy Questionnaire overall score; SE, standard error; US, utility score; VAS, visual analogue scale.
Figure 3.
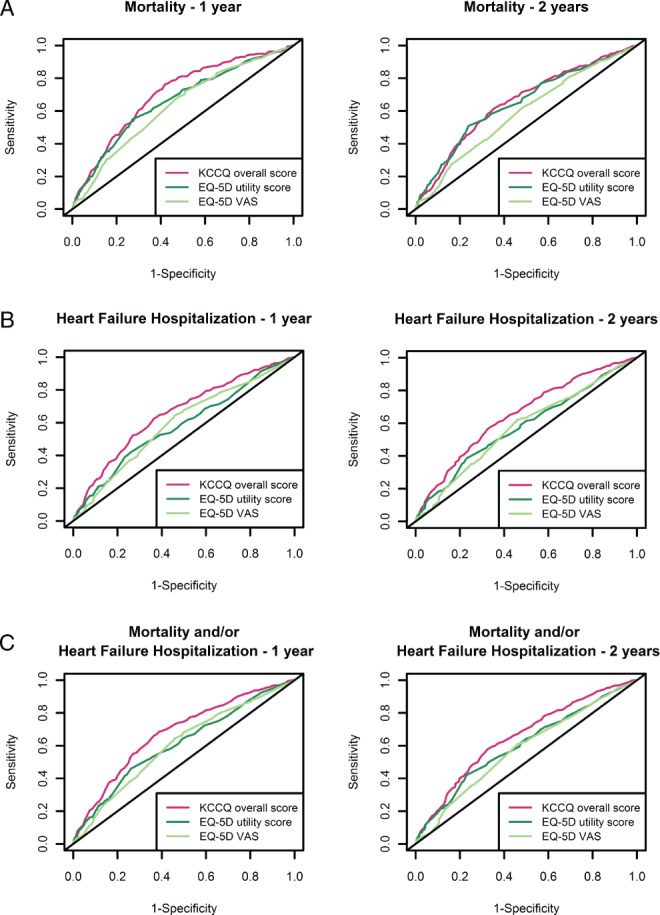
Time‐dependent receiver operating characteristic curves for different quality of life measure‐based models for predicting (A) mortality, (B) heart failure hospitalization, and (C) the composite outcome. EQ‐5D, EuroQoL 5 dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire; VAS, visual analogue scale.
Discussion
Assessment of QoL is an important tool that integrates physical examination with a comprehensive, reliable and reproducible characterization of HF patients' experience with their own illness. 9 , 17 QoL measures are useful for HF surveillance and prognostication, and constitute potential outcomes to support labelling claims for new drugs and devices. 1 , 17 Acknowledgement of sex differences in QoL and of their clinical impact is therefore important to correctly interpret QoL data. In our analysis of 1649 patients with HFrEF from 11 European countries, we confirmed that women with HFrEF report worse QoL than men; however, QoL equally improved in men and women during follow‐up. QoL measures were independent predictors of mortality, HF hospitalization, and the combined outcome with no significant interaction of sex. Despite KCCQ‐OS showed the best predictive value for all outcomes, there was no significant added prognostic value of QoL as assessed with this score to the predictive ability of the BIOSTAT‐CHF risk models 15 (Graphical Abstract).
The larger impact of HFrEF on QoL in women confirms previous findings, 3 , 4 and is concordant with the worse performance of women in 6MWT, an objective measure of exercise capacity. We observed a large (9 point) sex difference in KCCQ‐OS, with EQ‐5D also capturing the worse QoL perceived by women, particularly when scored with EQ‐5D US. Among KCCQ domains, the most remarkable sex differences were a 17 point difference in the physical limitation score and a 12 point difference in the social limitation score. The answers to EQ‐5D questions also revealed that HF impacted more on women's health status also psychologically, with more than 60% of women reporting moderate to severe anxiety or depression at baseline. These differences were observed even though physician‐assessed signs and symptoms of HF were fairly similar in men and women, and most comorbidities were more prevalent in men. Other markers of HF severity, including NT‐proBNP and HF hospitalizations in the year before enrolment, were also not significantly different between men and women, though women were older than men in our study population. This greater perceived physical and psychological disability of women despite similar physician‐assessed signs and symptoms of HF highlights the importance of QoL assessment for a comprehensive HF patient characterization, while future studies may address these differences in evaluating potential personalized therapeutic strategies for women with HFrEF.
At 9‐month follow‐up visit, both men and women showed a similar improvement in QoL, especially marked as assessed with KCCQ, regardless of sex or the fraction of target dose achieved, and consistent across all KCCQ domains and EQ‐5D items. Several are the potential explanations for this overall QoL trend over time. First, patients in BIOSTAT‐CHF were enrolled either with de novo or worsening HF symptoms, 12 thus maximizing the chances of capturing a QoL improvement at follow‐up. Second, patients enrolled in BIOSTAT‐CHF might have benefited from the close follow‐up entailed by the participation in a clinical study. However, QoL change over time was also evaluated in several of the pivotal trials of ACEi, 18 , 19 ARB 20 , 21 and beta‐blockers 22 , 23 in HFrEF, showing an overall improvement in QoL with these drugs as compared to placebo in stable, symptomatic HFrEF patients. The QoL metrics used in these studies were heterogeneous and mainly HF‐specific, and none of these analyses evaluated sex differences in QoL improvement after treatment. Our study, conducted on a large prospective HF cohort including both inpatients and outpatients, with the specific aim of up‐titrating ACEi/ARB and beta‐blockers according to recent ESC guidelines, shows that sex influences the patients' instantaneous QoL perception, but not QoL variations over time. This finding remains consistent both when QoL is evaluated with EQ‐5D, a general health status survey that allows the estimation of a health utility score particularly useful for economic analyses, and with KCCQ, one of the questionnaires that has the strongest overall clinical evidence supporting its use in HF. 17 Even though its prospective cohort design does not allow causation inference on the underlying reasons for the observed QoL improvement, our study confirms that QoL benefit is equally present in men and women when managed according to recent ESC guidelines.
In the current study, all three baseline QoL measures were associated with mortality, HF hospitalization, and the combined outcome at univariable analysis. In multivariable analyses, KCCQ‐OS and EQ‐5D VAS were independently associated with all outcomes, while EQ‐5D US only with mortality and the composite outcome. No significant interaction of sex was observed in any of these associations. Even though QoL surveys are still not routinely administered in clinical practice, 9 they are frequently used as surrogate endpoints in clinical trials, especially in phase 2 trials, and their use in phase 3 trials beside morbidity and mortality is currently encouraged by regulatory authorities. 8 The association with outcome of patient‐reported health status assessed with KCCQ 6 , 7 and EQ‐5D 5 measures in HFrEF was already established. However, this is the first study to our knowledge to specifically analyse sex differences in the association between QoL and outcome. Sex differences in HF have been overlooked for a long time, and clinical trials leading to drug approval in HFrEF enrolled predominantly men. 2 Mainly based on male‐derived data, conclusions of clinical trials in HFrEF may thus be male‐biased. Notably, we recently showed that women with HFrEF might even need lower doses of ACEi or ARBs and beta‐blockers than men to achieve lowest hazards of death or HF hospitalization. 24 The observation that QoL measures carry the same prognostic meaning in women and men bears great importance for interpreting data from past trials, especially those of drugs approved for symptom relief indication without a clear survival benefit (e.g. digoxin, ivabradine), and to inform QoL survey use in future clinical trials. On the other hand, the different prognostic meaning of 6MWT, an objective measure of functional capacity, towards mortality in men and women, is a hypothesis‐generating finding and warrants further investigation in other databases.
Although KCCQ and EQ‐5D evaluate different aspects of HF patients' health status, KCCQ‐OS showed the strongest association with all outcomes, also in the long term (1–2 years), though it conveyed no significant added prognostic value to the predictive ability of the previously validated set of clinical and laboratory variables in the BIOSTAT‐CHF risk models. 15 However, this finding encourages the use of KCCQ both as surrogate endpoint in clinical trials, as it reliably reflects patients' health‐related QoL and is independently associated with long‐term clinical outcomes, but also in the clinical setting, periodically during HF patients' follow‐up.
Overall, the findings from this study highlight the importance of evaluating patients' subjective QoL perception in the clinical setting, both in men and women, as it carries readily available prognostic information. Furthermore, these findings are especially important in the research setting, as they confirm the association of QoL with outcome in a broad HF population managed according to recent ESC guidelines, without any relevant interaction with sex in its prognostic meaning despite the sex differences observed in the overall QoL measures and the particular QoL domains. Finally, the head‐to‐head comparison of the features of KCCQ‐ and EQ‐5D‐derived measures provides important information for planning future clinical trials.
Limitations
Our study has several potential limitations. First, this was a post‐hoc analysis even though carried out on a prospective HF cohort. Secondly, patients enrolled in BIOSTAT‐CHF were predominantly Caucasian, thus limiting the generalizability of our findings to other ethnicities. In third place, we included in our analysis only patients that completed both KCCQ and EQ‐5D questionnaires at baseline, thus introducing a potential selection bias on a population subset with less severe HF at baseline. Moreover, we focused on HFrEF while many women with HF have preserved left ventricular ejection fraction, and our study population included more men than women, though this is very common in studies on HFrEF. Finally, we compared a single generic and a single HF‐specific QoL questionnaire, as more QoL surveys within each category were not available in our cohort.
Conclusions
While women with HFrEF had similar physician‐assessed symptoms, they reported worse QoL than men. However, men and women showed a similar improvement in QoL after 9‐month follow‐up. Baseline QoL was independently associated with subsequent mortality and HF hospitalization, similarly in men and women, with KCCQ‐OS showing the strongest association with outcome.
Funding
This work was supported by a grant from the European Commission [FP7‐242209‐BIOSTAT‐CHF].
Conflict of interest: B.T.S. reports grants from the Dutch Heart Foundation (2019 T094), during the conduct of this study. J.P.F. is a consultant for Boehringer Ingelheim. M.M. received personal honoraria for participation to trial committees, advisory boards or speeches at sponsored symposia from Abbott Vascular, Amgen, AstraZeneca, Bayer, Vifor Pharma, Servier, WindTree Therapeutics. All other authors have nothing to disclose.
Supporting information
Table S1. Recommended doses of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers (ARB) and beta‐blockers in European Society of Cardiology guidelines for patients with left ventricular ejection fraction <40%.
Table S2. Baseline characteristics stratified by sex.
Table S3. Variation in quality of life summary scores between baseline and 9 months in the total population (a), and in men (b) and women (c) separately, who received 0, 1–49%, 50–99%, or 100% or more of guideline‐recommended beta‐blocker dose.
Table S4. Variation in quality of life summary scores between baseline and 9 months in the total population (a), and in men (b) and women (c) separately, who received 0, 1–49%, 50–99%, or 100% or more of guideline‐recommended angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker dose.
Table S5. Variation of parameters of functional capacity between baseline and 9 months in the total study population and in men and women separately, median (interquartile range).
Table S6. Unadjusted and adjusted association of 6‐min walk test distance at baseline with outcome.
Table S7. Comparison between time‐dependent receiver operating characteristic curves' area under the curve for different quality of life measures‐based models (unadjusted) for predicting mortality (a), heart failure hospitalization (b), and the composite mortality and/or heart failure hospitalization.
Table S8. Reclassification among people who experienced or did not experience each studied outcome at 1 year, comparing different quality of life measures (A,C,E) or the use of quality of life measures on top of the BIOSTAT risk model for each explored outcome (B,D,F). (A,B) All‐cause death; (C,D) heart failure hospitalization; (E,F) All‐cause death and/or heart failure hospitalization.
Figure S1. Study flow diagram.
Figure S2. Sex differences in the answers to the items of the Kansas City Cardiomyopathy Questionnaire at baseline.
Figure S3. Sex differences in the answers to the items of the Kansas City Cardiomyopathy Questionnaire at 9 months.
Figure S4. Sex differences in the answers to the items of the EuroQol 5 dimensions questionnaire at 9 months.
Figure S5. Shift analysis showing sex differences in fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers at baseline.
Figure S6. Shift analysis showing sex differences in achieved fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers at 3 months.
Figure S7. Shift analysis showing sex differences between the quantiles of Kansas City Cardiomyopathy Questionnaire change (Delta KCCQ‐OS) from baseline to 9 months, at varying levels of achieved fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers.
Figure S8. Effect plots for all‐cause mortality by (a) Kansas City Cardiomyopathy Questionnaire overall score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding median quality of life measure as reference.
Figure S9. Effect plots for heart failure hospitalization by (a) Kansas City Cardiomyopathy Questionnaire Overall Score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding median quality of life measure as reference.
Figure S10. Effect plots for all‐cause mortality and/or heart failure hospitalization by (a) Kansas City Cardiomyopathy Questionnaire overall score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding quality of life measure as reference.
References
- 1. von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, Herrmann‐Lingen C, Garfias Macedo T, Koziolek M, Noutsias M, Schulze PC, Wachter R, Hasenfuß G, Laufs U. Improving exercise capacity and quality of life using non‐invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail 2021;23:92–113. [DOI] [PubMed] [Google Scholar]
- 2. Lam CS, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 3. Dewan P, Rørth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, Mogensen UM, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray JJ. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 4. Lesman‐Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age‐ and gender‐matched community controls. J Card Fail 2009;15:17–23. [DOI] [PubMed] [Google Scholar]
- 5. Ambrosy AP, Cerbin LP, DeVore AD, Greene SJ, Kraus WE, O'Connor CM, Piña IL, Whellan DJ, Wojdyla D, Wu A, Mentz RJ. Aerobic exercise training and general health status in ambulatory heart failure patients with a reduced ejection fraction – findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF‐ACTION) trial. Am Heart J 2017;186:130–138. [DOI] [PubMed] [Google Scholar]
- 6. Ekman I, Chassany O, Komajda M, Böhm M, Borer JS, Ford I, Tavazzi L, Swedberg K. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J 2011;32:2395–2404. [DOI] [PubMed] [Google Scholar]
- 7. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol 2017;2:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiuzat M, Lowy N, Stockbridge N, Sbolli M, Latta F, Lindenfeld J, Lewis EF, Abraham WT, Teerlink J, Walsh M, Heidenreich P, Bozkurt B, Starling RC, Solomon S, Felker GM, Butler J, Yancy C, Stevenson LW, O'Connor C, Unger E, Temple R, McMurray J. Endpoints in heart failure drug development: history and future. JACC Heart Fail 2020;8:429–440. [DOI] [PubMed] [Google Scholar]
- 9. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation 2013;127:2233–2249. [DOI] [PubMed] [Google Scholar]
- 10. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ‐5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 12. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 14. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008;10:933–989. [DOI] [PubMed] [Google Scholar]
- 15. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017;19:627–634. [DOI] [PubMed] [Google Scholar]
- 16. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 17. Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient‐reported outcome instruments in heart failure. JACC Heart Fail 2016;4:165–175. [DOI] [PubMed] [Google Scholar]
- 18. Giles TD, Katz R, Sullivan JM, Wolfson P, Haugland M, Kirlin P, Powers E, Rich S, Hackshaw B, Chiaramida A, Rouleau JL, Fisher MB, Pigeon J, Rush JE. Short‐ and long‐acting angiotensin‐converting enzyme inhibitors: a randomized trial of lisinopril versus captopril in the treatment of congestive heart failure. The Multicenter Lisinopril‐Captopril Congestive Heart Failure Study Group. J Am Coll Cardiol 1989;13:1240–1247. [DOI] [PubMed] [Google Scholar]
- 19. Rogers WJ, Johnstone DE, Yusuf S, Weiner DH, Gallagher P, Bittner VA, Ahn S, Schron E, Shumaker SA, Sheffield LT. Quality of life among 5,025 patients with left ventricular dysfunction randomized between placebo and enalapril: the Studies of Left Ventricular Dysfunction. The SOLVD Investigators. J Am Coll Cardiol 1994;23:393–400. [DOI] [PubMed] [Google Scholar]
- 20. Majani G, Giardini A, Opasich C, Glazer R, Hester A, Tognoni G, Cohn JN, Tavazzi L. Effect of valsartan on quality of life when added to usual therapy for heart failure: results from the Valsartan Heart Failure Trial. J Card Fail 2005;11:253–259. [DOI] [PubMed] [Google Scholar]
- 21. O'Meara E, Lewis E, Granger C, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Ostergren J, Carlsson J, Olofsson B, McMurray J, Yusuf S, Swedberg K, Pfeffer MA. Patient perception of the effect of treatment with candesartan in heart failure. Results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Programme. Eur J Heart Fail 2005;7:650–656. [DOI] [PubMed] [Google Scholar]
- 22. Gallanagh S, Castagno D, Wilson B, Erdmann E, Zannad F, Remme WJ, Lopez‐Sendon JL, Lechat P, Follath F, Höglund C, Mareev V, Sadowski Z, Seabra‐Gomes RJ, Dargie HJ, McMurray JJ. Evaluation of the functional status questionnaire in heart failure: a sub‐study of the Second Cardiac Insufficiency Bisoprolol Survival Study (CIBIS‐II). Cardiovasc Drugs Ther 2011;25:77–85. [DOI] [PubMed] [Google Scholar]
- 23. Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus KL, Jánosi A, Thorgeirsson G, Dunselman PH, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT‐HF). MERIT‐HF Study Group. JAMA 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 24. Santema BT, Ouwerkerk W, Tromp J, Sama IE, Ravera A, Regitz‐Zagrosek V, Hillege H, Samani NJ, Zannad F, Dickstein K, Lang CC, Cleland JG, Ter Maaten JM, Metra M, Anker SD, van der Harst P, Ng LL, van der Meer P, van Veldhuisen DJ, Meyer S, Lam CS, Voors AA; ASIAN‐HF Investigators ; Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019;394:1254–1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Recommended doses of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers (ARB) and beta‐blockers in European Society of Cardiology guidelines for patients with left ventricular ejection fraction <40%.
Table S2. Baseline characteristics stratified by sex.
Table S3. Variation in quality of life summary scores between baseline and 9 months in the total population (a), and in men (b) and women (c) separately, who received 0, 1–49%, 50–99%, or 100% or more of guideline‐recommended beta‐blocker dose.
Table S4. Variation in quality of life summary scores between baseline and 9 months in the total population (a), and in men (b) and women (c) separately, who received 0, 1–49%, 50–99%, or 100% or more of guideline‐recommended angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker dose.
Table S5. Variation of parameters of functional capacity between baseline and 9 months in the total study population and in men and women separately, median (interquartile range).
Table S6. Unadjusted and adjusted association of 6‐min walk test distance at baseline with outcome.
Table S7. Comparison between time‐dependent receiver operating characteristic curves' area under the curve for different quality of life measures‐based models (unadjusted) for predicting mortality (a), heart failure hospitalization (b), and the composite mortality and/or heart failure hospitalization.
Table S8. Reclassification among people who experienced or did not experience each studied outcome at 1 year, comparing different quality of life measures (A,C,E) or the use of quality of life measures on top of the BIOSTAT risk model for each explored outcome (B,D,F). (A,B) All‐cause death; (C,D) heart failure hospitalization; (E,F) All‐cause death and/or heart failure hospitalization.
Figure S1. Study flow diagram.
Figure S2. Sex differences in the answers to the items of the Kansas City Cardiomyopathy Questionnaire at baseline.
Figure S3. Sex differences in the answers to the items of the Kansas City Cardiomyopathy Questionnaire at 9 months.
Figure S4. Sex differences in the answers to the items of the EuroQol 5 dimensions questionnaire at 9 months.
Figure S5. Shift analysis showing sex differences in fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers at baseline.
Figure S6. Shift analysis showing sex differences in achieved fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers at 3 months.
Figure S7. Shift analysis showing sex differences between the quantiles of Kansas City Cardiomyopathy Questionnaire change (Delta KCCQ‐OS) from baseline to 9 months, at varying levels of achieved fraction target dose of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and beta‐blockers.
Figure S8. Effect plots for all‐cause mortality by (a) Kansas City Cardiomyopathy Questionnaire overall score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding median quality of life measure as reference.
Figure S9. Effect plots for heart failure hospitalization by (a) Kansas City Cardiomyopathy Questionnaire Overall Score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding median quality of life measure as reference.
Figure S10. Effect plots for all‐cause mortality and/or heart failure hospitalization by (a) Kansas City Cardiomyopathy Questionnaire overall score, EuroQoL 5 dimensions (b) visual analogue scale, and (c) utility score at baseline, using adjusted Cox model with the corresponding quality of life measure as reference.


