Abstract
Background
Hyaluronic acid (HA) injection procedures has experienced an unprecedented increase.
Aims
To assess and determine, by using ultrasound examinations, the patterns corresponding to different dermal fillers.
Patients/Methods
Observational and retrospective bicenter study conducted on patients who underwent previous aesthetic treatments with dermal fillers. Ultrasound examinations were performed, at each study center, by one experienced observer.
Results
Sixty patients were included in the analysis. Among them, 48 patients showed a well‐defined ultrasound pattern, while 12 exhibited a mixed one. According to ultrasound images, 4 different patterns were identified: [1] Heterogeneous, characterized by alternating hyperechoic and anechoic areas, which are visualized in the tissue in a heterogeneous way. This pattern is associated with healthy skin/subcutaneous cellular tissue and with fully integrated HA fillers. [2] Fine grain snowfall, characterized by alternating hyperechoic imaging, with posterior echogenic shadows. It is typical of liquid injectable silicone. [3] Coarse grain snowfall, characterized by hyperechoic images distributed all over the tissue. This is typical of calcium hydroxyapatite and polymethyl methacrylate‐based fillers. [4] Globular, typical "cystic" imaging, with anechoic images indicative of liquid semi‐liquid content. This pattern is characteristic of polyalkylamides and polyacrylamides, and HA‐based fillers immediately after their injection. The presence of "mixed" patterns is mainly due to different aesthetic procedures performed at different times.
Conclusions
Ultrasound imaging may be a valuable tool for assessing the nature of former dermal filler procedures in daily practice. The identification of these patterns will allow specialists to choose the best therapeutic approach in patients who underwent previous aesthetictreatments.
Keywords: aesthetic medicine, dermal fillers, hyaluronic acid, minimally invasive procedures, ultrasounds
1. INTRODUCTION
Over the last several years, the demand for minimally invasive aesthetic procedures has experienced an unprecedented increase. 1 According to the data of the International Society of Aesthetic Plastic, hyaluronic acid (HA) injection procedures, increased 13.1% in 2018 compared to 2017. 1 This rise in the use of HA fillers in recent years is a testament to their safety and efficacy. 2
Despite HA fillers are the most widely used worldwide, other dermal fillers exist, such as collagen (either purified bovine or human); poly‐L‐lactic acid (PLLA); Calcium hydroxyapatite (CaHa); polymethyl methacrylate (PMMA) microspheres; polyacrylamide hydrogel; silicone; carboxymethyl cellulose; autologous fat, etc. 2 , 3 Although there are different methods for categorizing dermal fillers, they can be classified in two major types: temporary and permanent. 2 Each type of filler has specific requisites and treatment choice depends on the desired result and product longevity. Temporary includes HA‐based fillers, CaHA‐based fillers, poly‐L‐lactic acid (PLLA), and polycaprolactone (PCL). Among them, may be identified a subgroup of biostimulators, which use neocollagenesis as main modus operandi, such as CaHA, PLLA, and PCL.
Permanent injectable fillers include PMMA, polyacrylamide, polyalkylamides, and liquid injectable silicone (LIS), among others. 2
Different techniques for assessing filler performance are mostly focusing on skin surface topography but do not provide information to what is happening to the filler beneath the skin surface. Ultrasound examination is a non‐invasive, convenient, and rapid technique for the assessment of filler performance. Fillers are recognizable on ultrasound and generate different patterns of echogenicity and posterior acoustic artifacts. 4 , 5 , 6 , 7
Due to the increasing number of dermal filler procedures performed worldwide, 1 , 8 more and more aesthetic specialists are receiving previously treated patients, without these patients providing neither exact details of the procedure nor the agent used. For these situations, ultrasound examination may be an effective and reliable tool for clinical practice assessment of the type of filler, its location, and the possibility or need of extraction by different procedures. 9
This study aimed to evaluate and determine the ultrasound patterns corresponding to different dermal fillers.
2. METHODS
2.1. Design
Descriptive, retrospective, and bi‐center study.
This study adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by an independent ethics committee, which waives the need of informed consent for this study.
2.2. Patients
Consecutive patients who attended the study centers, between January 2017 and January 2020, for assessment of previous facial aesthetic filler procedures (either diagnosis or removal) were included in the study.
Those subjects who, in addition to filler treatment, underwent any other aesthetic procedure (either surgical or non‐surgical), such as botulinum toxin A injection, Laser, radiofrequency, or intense pulse light were excluded of the analysis.
Most of the patients were unaware of the nature of the implanted material and did not present any document, report, or invoice that could clarify, to a greater or lesser extent, the nature of the filler. Nevertheless, many subjects said that they were "verbally informed" about the fact that the dermal filler injected was "natural" or "resorbable".
2.3. Ultrasounds
Ultrasound examinations were performed by three experienced observers (FU; FDF; and IB). Three different ultrasound devices were used in the study: Samsung HT 30 ultrasound machine (Samsung Healthcare Global, Gangwon; South Korea) with a 12 MHz linear array transducer; General Electric Logiq S8 DX Clear and General Electric Logiq E (General Electric Healthcare, Chicago, IL; USA) with 18 MHz linear probe.
Ultrasound examinations were performed from 4 months to 15 years after treatment.
Ultrasound patterns of the different dermal fillers, as well as skin and subcutaneous cellular tissue, were evaluated.
3. RESULTS
Of the total 90 screened patients, 60 patients (46 women and 14 men) fulfilled the demands of the inclusion/exclusion criteria.
Regarding the nature of the dermal fillers, study sample had been injected with HA, CaHa, PMMA, PLLA, polyalkylamide, polyacrylamide, silicone, carboxymethylcellulose, and PCL‐based fillers. Thirty (50%) patients had received treatment with permanent fillers (PMMA‐, polyacrylamide‐, or polyalkylimide‐based fillers).
Forty‐eight (80%) patients exhibited a “well‐defined” ultrasound pattern, while 12 (20.0%) patients exhibited "mixed" patterns.
Some patients showed different ultrasound patterns according to the treated area, for example, one filler in lips (for example, silicone oil), and another totally different in cheek or lower third (Like a HA filler) (Figure 1).
FIGURE 1.
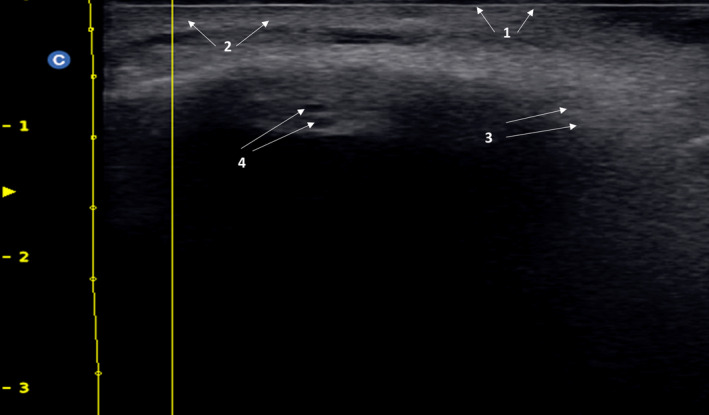
Combine or complex pattern. It is possible to see a combination of a “Fine‐grain snowfall” characteristic of silicone and a “globular pattern” typical of hyaluronic acid filler injected recently. 1: Epidermis; 2: Dermis; 3: Fine‐grain snowfall pattern; 4: Globular pattern
Four "well‐defined" ultrasound patterns were defined (Table 1).
TABLE 1.
Overview of the ultrasound patterns of the different dermal fillers
| Pattern | Ultrasound findings |
|---|---|
| Heterogeneous |
Presence of alternating anechoic/hyperechoic images, which that are visualized in the tissue in a heterogeneous way. This pattern is characteristic of healthy skin and subcutaneous cellular tissue. Additionally, it is observed after tissue biointegration of resorbable fillers, like hyaluronic acid‐based fillers. This pattern allows to see those fillers that, due to their chemical characteristics, are not collected and remain diffused into the tissue, causing fibrosis and hyperechoic images to varying degrees. |
| Fine‐grain snowfall | Presence of hyperechoic images with posterior echogenic shadow. This pattern is characteristic of oily silicone and biopolymers. There is an important infiltration of the tissue, which causes posterior reverberations. |
| Coarse grain snowfall | Presence of multiple hyperechoic images distributed into the tissue in a homogeneous way, which gives its typical coarse grain snowy appearance. Echogenic shadow is usually lower than in the "fine‐grain snowfall pattern", since the infiltration of the tissue by the material is usually less. This pattern is characteristic of calcium hydroxylapatite and polymethyl methacrylate. |
| Globular | Presence of cysts, with content of an anechoic nature to a greater or lesser extent, surrounded by a capsule of variable thickness. There is posterior reinforce suggestive of liquid or semi‐liquid content in the cyst, which causes a typical hyperechoic imaging. This pattern is characteristic of polyalkylamides and polyacrylamides (non‐resorbable materials that behave like a Endoprosthesis). In addition, this pattern is also observed in hyaluronic acid‐based filler immediately after their injection. |
Heterogeneous pattern: This is the characteristic pattern of healthy skin and subcutaneous cellular tissue (Figure 2). This pattern is characterized by alternating hyperechoic and anechoic areas, which are visualized in the tissue in a heterogeneous way. Additionally, this pattern is observed typically after integration of tissue resorbable materials, such as HA fillers (Figure 3B). This pattern is associated with those fillers that, due to their chemical characteristics, integrate into the tissues.
Fine‐grain snowfall pattern: This pattern is characterized by alternating hyperechoic imaging, with posterior echogenic shadows. This type of pattern is typical of silicone‐ or biopolymers‐based fillers. This pattern is frequently associated with lip injections and its high echogenic density prevents or, at least makes difficult, to see the teeth. The high tissue infiltration of the filler causes the characteristic fine grain snowy imaging (Figure 4).
Coarse grain snowfall pattern: It is characterized by hyperechoic images distributed all over the tissue, which give an imaging of snowfall, but with a coarse grain. Grains are more defined and have a higher brightness than those of the fine‐grain snowfall pattern. This is specific for particulate fillers, such as CaHa and PCL‐based fillers (Figure 5).
Globular pattern: Typical "cystic" imaging, with anechoic images indicative of liquid content. It is possible to see a posterior echogenic reinforcement, due mainly to the liquid semi‐liquid cyst content, which causes a typical hyperechoic imaging. These images are indicative of polyalkylamides and polyacrylamides, as well as non‐resorbable materials that behave like an Endoprosthesis (Figure 6). Additionally, HA‐based fillers may exhibit this pattern immediately after injection (Figure 3A).
FIGURE 2.
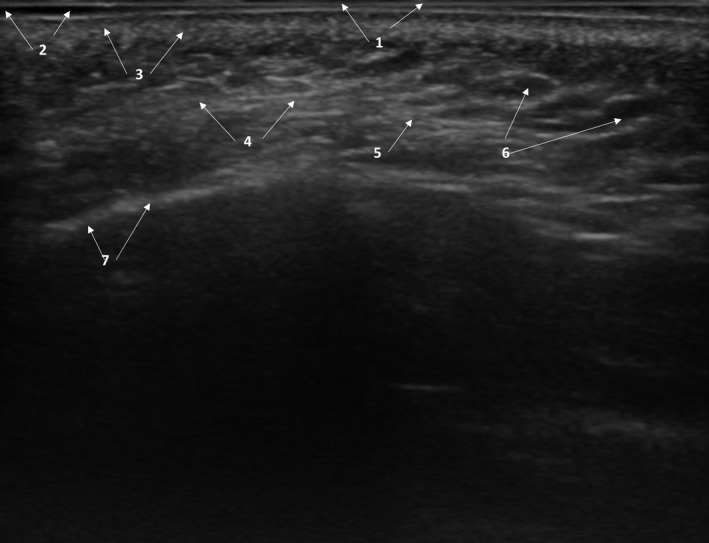
Heterogeneous pattern of healthy skin and subcutaneous cellular tissue. 1: Epidermis; 2: Subepidermal low‐echogenic band; 3: dermis; 4: Subcutaneous cellular tissue; 5: hyperechoic images;6: anechoic images; 7: Periosteum
FIGURE 3.
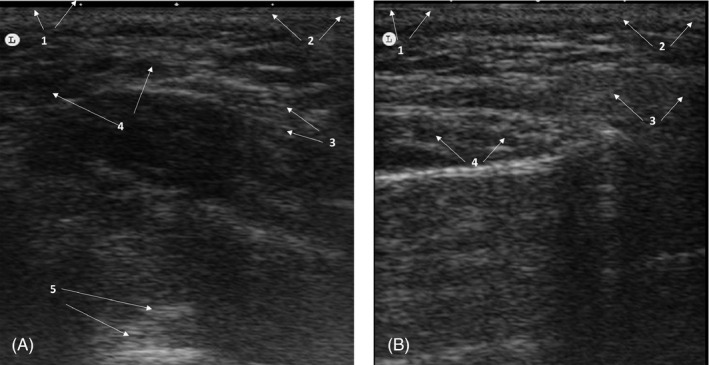
Heterogeneous pattern of a patient injected with hyaluronic acid filler. (A) Immediately after treatment. Poorly defined globular ultrasound pattern, with anechoic images indicative of liquid content. 1: Epidermis; 2: dermis; 3: Subcutaneous cellular tissue; 4: anechoic images; 5: Posterior echogenic reinforcement. (B) 1 month after treatment. Typical heterogeneous pattern, without residual anechoic areas, which was indicative of a total integration of Hyaluronic acid filler. 1. Epidermis; 2: Dermis; 3: Subcutaneous cellular tissue; 4: Heterogeneous pattern
FIGURE 4.
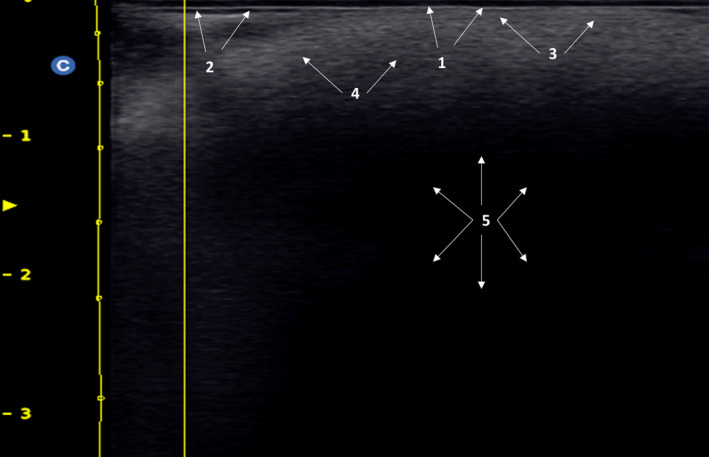
Fine‐grain snowfall patter in a patient who underwent a liquid injectable silicone. 1: Epidermis; 2: Subepidermal low‐echogenic band; 3: Dermis; 4: fine grain snowy pattern; 5: Posterior echogenic shadow
FIGURE 5.
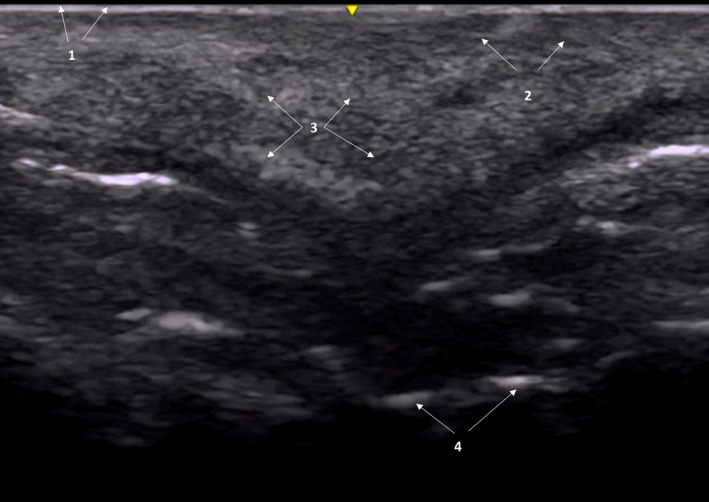
Coarse‐grain snowfall pattern in a patient who was injected with Calcium hydroxylapatite‐based fillers. 1: Epidermis; 2: Dermis; 3: Coarse‐grain snowfall pattern; 4: Periosteum
FIGURE 6.
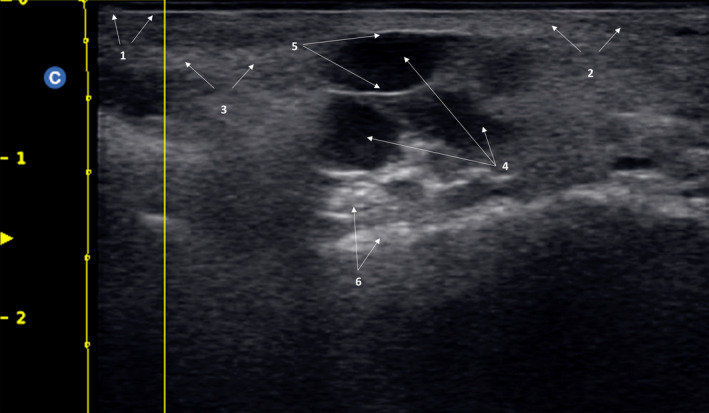
Globular pattern in a subject who underwent treatment with polyalkylamides and polyacrylamides. 1: Epidermis; 2: dermis; 3: Subcutaneous cellular tissue; 4: Cysts; 5: Cyst wall; 6: Posterior echogenic reinforcement
In the 12 patients who showed mixed patterns, ultrasound pattern varied according to the treated area; due mainly to successive treatments with a combination of fillers of different nature over time (Figure 1).
Ultrasound imaging also showed hyperechoic images, with different degrees of hyperechogenicity, which clearly suggested the presence of different amount of fibrosis at the reticular dermis and subcutaneous cellular tissue levels. These hyperechoic images were more perceptible in those material‐oriented mainly to induce fibrosis, such as CaHa, PLLA, PCL, and carboxymethylcellulose; than in those focusing on hydrating and volumizing treated areas, like HA‐based fillers (Figure 7).
FIGURE 7.
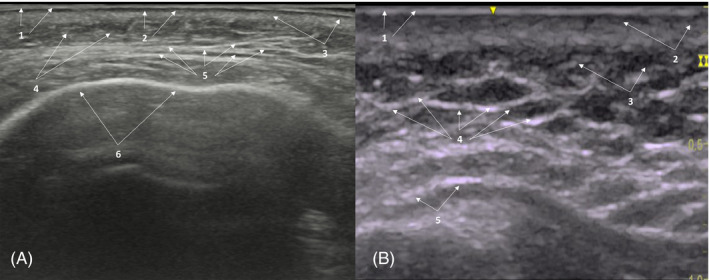
Combine heterogeneous pattern (hyperechoic/anechoic) in subcutaneous cellular tissue. (A) 56‐years‐old patient with hyperechoic/anechoic balance, with hyperechoic predominance in subcutaneous cellular tissue. 1. Epidermis; 2: Subepidermal low‐echogenic band; 3: dermis; 4: Subcutaneous cellular tissue; 5: hyperechoic/anechoic balance predominantly hyperechoic; 6: Periosteum. (B) Heterogeneous pattern in a patient treated with poly‐L‐lactic acid with hyperechoic/anechoic balance, with hyperechoic‐fibrotic predominance subcutaneous cell tissue. 1. Epidermis; 2: dermis; 3: Subcutaneous cellular tissue; 4: hyperechoic/anechoic balance predominantly hyperechoic‐fibrotic; 5: Periosteum
The typical heterogeneous pattern, characteristic of the health skin, points to either anechoic or hyperechoic depending on the amount of fibrosis induced by the product.
4. DISCUSSION
Ultrasound examination is a non‐invasive imaging method capable to obtain real‐time visualization of patients' anatomy. Skin ultrasound examinations are used both for assessing healthy skin and for evaluating pathological lesions. 10 , 11 Moreover, the development of more sensitive, accurate, and reliable ultrasound devices has allowed dynamic skin examinations. 10 , 11
The increasing number of minimally invasive aesthetic procedures, particularly dermal filler injections, makes necessary to have sensitive and reliable tools that allow the evaluation, in real‐time, of their results in daily clinical practice. 1 , 8 Furthermore, ultrasound devices for skin examinations more and more frequently constitute an integral element of the equipment used in aesthetic medicine. 10 , 11
Regarding aesthetics, ultrasound examinations are mainly focusing on two aspects: Monitoring the changes induced by aesthetic treatments, in this case, dermal fillers 10 , 11 ; and to evaluate and prevent the potential complications occurring with these treatments. 12 , 13 , 14 , 15
Because ultrasounds allow the study of skin and the underlying tissues, its use provides the capability to make a reliable and accurate assessment of facial anatomy. 14 , 15
Besides diagnosis and prevention of potential adverse events, 13 , 14 ultrasounds have been successfully used for managing such complications. Since fillers are easily seen in ultrasounds, it is possible to perform an ultrasound‐guided approach for addressing overcorrections, dislocations, or vascular adverse events (either intravascular injection or vascular compression). 12 , 14 , 15
According to the results of this study, it is possible to identify 4 "well‐defined" ultrasound patterns associated with the use of dermal fillers. These ultrasound patterns allow clinicians to identify the nature and behavior of the injected filler.
The fact that aesthetic filler injections are recognizable on ultrasound and generate different patterns of echogenicity and posterior acoustic artifacts is not new. 4 , 5 , 6 , 7
However, identification of such fillers was related more with appearance of adverse events 3 , 12 , 13 , 14 , 15 than with the possibility of performing a patient‐tailored new therapy.
Because patients who had previous filler treatments may not always remember the type of filler, and the place or plane of injection; an accurate diagnostic strategy that allows us to identify the nature of such filler/s is essential for achieving good and predictable aesthetic outcomes.
It was recently published a paper that proposed a standard nomenclature for the different ultrasound patterns generated by the different dermal fillers. 7 Description of dermal fillers was based on different ultrasound parameters, which included echogenicity, texture, border, shape, diameter, quantity, internal characteristics, and artifacts. 7
Despite the contribution made by Schelke et al was extremely important, they were much focused on describing the different ultrasound identity characteristics of the fillers (to describe “well‐defined” ultrasound patterns), while we were more focused on attempting to identify different ultrasound patterns and to stablish a relationship between them and the filler injected. Thus, for example, Shelke et al described the ultrasound pattern of the HA filler as: "Well‐defined oval‐ or round‐shaped anechoic homogeneous deposits without any signs of internal echoes". 7 In our study, we have seen this pattern in HA‐based fillers immediately after their injection. However, when HA‐based fillers are integrated their ultrasound pattern is similar to that observed in the healthy skin/subcutaneous tissue.
Moreover, due to the increasing number of patients undergoing minimally invasive procedures with dermal fillers, it is more common to receive patients who have undergone aesthetic treatments with different types of fillers and at different times. This fact makes it increasingly difficult to find “well‐defined” ultrasound patterns.
In our study, 12 (20.0%) patients exhibited "mixed" patterns, which means that they either were treated with different types of filler; were treated with the same type of filler, but at different times; or were treated with different types of fillers at different times.
Cosmetic fillers are biodegradable or nonbiodegradable (synthetic) biologically inert nanoparticles (1–100 nm in size) injected mainly to fill wrinkle or cutaneous defects, and for restoring/creating lost volumes. 16 , 17 , 18 Therefore, the commonly used term “dermal fillers” is confusing or, at least imprecise, because the injected material is detected mostly in the subcutaneous tissue.
Hyaluronic acid‐based dermal fillers are the most widely used worldwide, with more than 3.7 million procedures during 2018, followed, at a great distance, by CaHa‐based fillers (129 038 procedures), and PLLA‐based fillers (72 756). 1
Different non‐reasorbable fillers have been or are currently used, among them silicone (in pure or oily formulation), and PMMA may be considered as the main ones.
Although pure silicone is anechoic on ultrasound, oily silicone shows hyperechoic images, with posterior reverberations. 7 , 19 In our study, silicone oil ultrasound pattern was defined as "fine‐grain snowfall", which was characterized by alternating hyperechoic imaging, with posterior echogenic shadows.
The pattern of the PMMA‐based fillers was previously defined as hyperechoic dots with a mini‐tail reverberation artifact. 7 , 19 However, in our study, PMMA‐based fillers were associated with a "course‐grain snowy" pattern, which is characterized by hyperechoic images distributed all over the tissue, associated with posterior echogenic shadow.
Calcium hydroxyapatite‐based fillers appear sonographically as hyperechoic bands projecting posterior acoustic shadowing. 7 , 19 In our study, CaHa‐based fillers were associated with a “coarse‐grain snowfall” pattern, with alternating hyperechoic imaging, with posterior echogenic shadows.
Regarding HA‐based fillers, ultrasound pattern depends on time elapsed from injection. A recent study published by our group evaluated, by means ultrasound examinations, tissue biointegration of an HA‐based dermal filler (VYC‐25L, Juvéderm Volux®; Allergan plc, Dublin, Ireland). 20 The results of this found that, immediately after treatment, ultrasound images were characterized by a globular and poorly define pattern, with anechoic images indicative of liquid content. Nevertheless, 1‐month after treatment, ultrasound images showed a typical complete heterogeneous pattern, which indicated a total integration of the VYC‐25L into the tissue. 20
The HA‐based fillers ultrasound pattern found immediately after injection is similar to that described by Schelke et al. 7 However, the ultrasound pattern exhibited by HA‐based fillers once integrated into the tissue was totally different, becoming similar to the healthy skin/subcutaneous tissue pattern.
As far as we know, this is one of the first papers describing the ultrasound pattern of different dermal fillers used in minimally invasive aesthetic procedures. Despite we were able to identify four well‐defined ultrasound patterns, some patients exhibited "mixed" patterns, which indicated mainly the existence of different treatments, with different fillers, performed at different times.
This study is not free of limitations. The first one is its design. Retrospective observational studies have selection bias and confounding factors that need to be taken into consideration when interpreting the results. An additional limitation is the limited sample size. Another limitation is the lack of reproducibility studies among the three investigators. Finally, it should be mentioned that ultrasound examinations were no performed with the same device, but three devices were used (one by investigator). Nevertheless, ultrasound examinations precisely reflect structural changes in the skin. 21
5. CONCLUSIONS
Ultrasound imaging may be considered as a valuable tool for evaluating the nature of former dermal filler procedures in daily clinical practice. The presence of "combine or complex" patterns is mainly due to different aesthetic procedures performed at different times.
The identification of these patterns will allow aesthetic specialists to choose the best therapeutic approach in patients who underwent previous aesthetic treatments. Drawing an ultrasound map of previous dermal filler procedures (nature and place of filler) will be essential for selecting the best HA‐based filler for avoiding or minimizing the incidence of complications. An HA‐based filler product that shows good biointegration is crucial for achieving good aesthetic outcomes.
ACKNOWLEDGEMENTS
Medical writing and Editorial assistant services have been provided by Ciencia y Deporte S.L. Support for this assistance was funded by Allergan S.A. at the request of the investigator.
CONFLICT OF INTEREST
Dr Urdiales‐Gálvez has received a Grant from Allergan Aesthetics, an AbbVie company, for covering the medical writing services.
All the coauthors declare that they have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
All authors met the ICMJE authorship criteria. All authors contributed to the drafting and critical revision of the manuscript, commented on previous versions of the manuscript, and read and approved the final manuscript prior to submission.
ETHICAL APPROVAL
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards”.
STATEMENT OF HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
All patients were fully informed about the details of the study protocol. The need for informed consent was waived for this study.
Commercial interests: None.
Funding information
Medical writing services have been provided by Allergan Aesthetics, an AbbVie company. Allergan did not participate in either data collection, analysis, or redaction of the manuscript. Neither honoraria nor payments were made for authorship.
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. The international study on aesthetic/cosmetic procedures performed in 2018. https://www.isaps.org/medical‐professionals/isaps‐global‐statistics/. Accessed November 20, 2020.
- 2. Liu MH, Beynet DP, Gharavi NM. Overview of deep dermal fillers. Facial Plast Surg. 2019;35(3):224‐229. [DOI] [PubMed] [Google Scholar]
- 3. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young SR, Bolton PA, Downie J. Use of high‐frequency ultrasound in the assessment of injectable dermal fillers. Skin Res Technol. 2008;14(3):320‐323. [DOI] [PubMed] [Google Scholar]
- 5. Wortsman X, Wortsman J, Orlandi C, Cardenas G, Sazunic I, Jemec GB. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012;26(3):292‐301. [DOI] [PubMed] [Google Scholar]
- 6. Schelke LW, DenElzen HJ, Erkamp PP, Neumann HA. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatol Surg. 2010;36(suppl 3):1843‐1851. [DOI] [PubMed] [Google Scholar]
- 7. Schelke LW, Cassuto D, Velthuis P, Wortsman X. Nomenclature proposal for the sonographic description and reporting of soft tissue fillers. J Cosmet Dermatol. 2020;19(2):282‐288. [DOI] [PubMed] [Google Scholar]
- 8. [The socio‐economic impact of aesthetic medicine in Spain]. Available in: https://www.actasanitaria.com/un‐36‐de‐los‐espanoles‐recurre‐a‐la‐medicina‐estetica‐a‐partir‐de‐los‐26‐anos/. Accessed November 20, 2020.
- 9. Villegas Fernández C, Burón Álvarez I, Fernández‐Tresguerres Centeno A, Alfageme Roldán F, de Cabo FF. Cutaneous ultrasound and dermal fillers. Actas Dermosifiliogr. 2015;106(Suppl 1):87‐95. [DOI] [PubMed] [Google Scholar]
- 10. Mlosek RK, Malinowska S. Ultrasound image of the skin, apparatus and imaging basics. J Ultrason. 2013;13(53):212‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safran T, Gorsky K, Viezel‐Mathieu A, Kanevsky J, Gilardino MS. The role of ultrasound technology in plastic surgery. J Plast Reconstr Aesthet Surg. 2018;71(3):416‐424. [DOI] [PubMed] [Google Scholar]
- 12. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41(3):667‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17(6):1019‐1024. [DOI] [PubMed] [Google Scholar]
- 15. Schelke LW, Velthuis P, Kadouch J, Swift A. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol. 2019;S0190‐9622(19):32392‐8. [DOI] [PubMed] [Google Scholar]
- 16. Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011;38(3):379‐390. [DOI] [PubMed] [Google Scholar]
- 17. Greco TM, Antunes MB, Yellin SA. Injectable fillers for volume replacement in the aging face. Facial Plast Surg. 2012;28(1):8‐20. [DOI] [PubMed] [Google Scholar]
- 18. Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 19. Wortsman X, Wortsman J. Sonographic outcomes of cosmetic procedures. AJR Am J Roentgenol. 2011;197(5):W910‐W918. [DOI] [PubMed] [Google Scholar]
- 20. Urdiales‐Gálvez F, Barres‐Caballer J, Carrasco‐Sánchez S. Ultrasound assessment of tissue integration of the crosslinked hyaluronic acid filler VYC‐25L in facial lower‐third aesthetic treatment: a prospective multicenter study. J Cosmet Dermatol. 2020. 10.1111/jocd.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatta AK, Keyal U, Liu Y. Application of high frequency ultrasound in dermatology. Discov Med. 2018;26(145):237‐242. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


