Abstract
Background
The arrival of immunotherapies and targeted therapies challenged the authorities to make them available as soon as possible. France has effective tools, such as clinical trials (CTs) and a national early access program (temporary authorizations for use [ATUs] and temporary recommendations for use [RTUs]), allowing the use of innovative drugs, whether or not they have been authorized or used off‐label, for cases that have reached a therapeutic impasse.
Methods
The methodology involved real‐time data collection from ATUs, RTUs (between September 1, 2009 and September 1, 2019), and CT authorizations (from December 1, 2017 to September 1, 2019) that were filed and reviewed by the French National Agency for Medicines for metastatic melanoma (MM).
Results
In total, 45 CTs were authorized for MM (51% early phase trials and 44% phase 2 and 3 trials), mainly for the metastatic line (86%) and with an industrial sponsor (73%). Immunotherapies and targeted therapies (63% and 24%, respectively) mostly were used in combination. Three RTUs were authorized for the adjuvant treatment of MM, whereas 13 drugs were available through nominal ATUs (nATUs), of which 5 were awarded a cohort ATU (cATU). This enabled the treatment of 6538 patients (28% through nATUs and 72% through cATUs). All of these drugs were granted marketing authorization and were included in the reimbursement list.
Conclusions
Thanks to CTs and the national early access program, patients in France have been able to benefit from innovative MM treatments.
Lay Summary
Several tools allow the use of innovative drugs in France, even if they are not yet authorized or used off‐label.
From December 1, 2017 to September 1, 2019, 45 clinical trials have been authorized for metastatic melanoma, mostly using immunotherapy (63%) and targeted therapy (24%) at an early phase (51%).
Since 2010, the national early access program has treated 6538 patients, including 28% under nominative temporary authorizations for use and 72% under cohort temporary authorizations for use.
Fourteen drugs are available through nominative temporary authorizations for use, and 5 are available through cohort temporary authorizations for use, and all of these drugs were granted marketing authorization.
Keywords: clinical trials, compassionate use trials, data collection, delivery of health care, drugs investigational, empathy, France, humans, malignant melanoma
Short abstract
France has effective tools, such as clinical trials and a national early access program (temporary authorizations for use and temporary recommendations for use), allowing the use of innovative drugs that either are not authorized or are used off‐label in cases at a therapeutic impasse. Thanks to these tools, patients with malignant melanoma have early access to innovative treatments.
Introduction
The incidence of melanoma has been rising rapidly since 1980, doubling every 20 years and making it the tumor with the highest growth rate in terms of incidence in France. 1 Surgery is the standard treatment for local melanoma. It is also the only curative treatment. From 1980 until the arrival of new therapies, dacarbazine and fotemustine were the standard chemotherapy regimens for the treatment of metastatic melanoma (MM) in France. However, because melanoma tumors have low chemosensitivity, the response rate to these alkylating agents is low (approximately 10% according to studies), and the complete response rate is only 2%. 2 , 3 , 4
An understanding of the pathogenesis of melanoma through knowledge of molecular medicine and immunology has enabled the development of much more effective new therapeutic approaches. The modulation of cellular immune defense against tumors is exploited using specific anticytotoxic T‐lymphocyte‐associated protein 4 (anti‐CTLA4) and anti‐programmed cell death protein 1 (anti‐PD1)/anti‐programmed death‐ligand 1 (anti‐PDL1) immunotherapies (ITs). Furthermore, the development of targeted therapies (TTs) has allowed tumor growth inhibition through specific transduction pathways, mainly those of mitogen‐activated protein kinases, which are highly implicated in the oncogenesis of approximately one‐half of melanomas. Therefore, the arrival in 2011 of the anti‐CTLA4 antibody ipilimumab and the selective BRAF inhibitor vemurafenib revolutionized the management of patients with MM. Today, innovative therapies are used mainly in combination.
There are several possible ways of gaining early access to innovative therapies. Clinical trials (CTs) are governed by European Directive 2001/20/EC. 5 In France, setting up a CT requires prior authorization from the French National Agency for Medicines and Health Products Safety (ANSM) and approval by an institutional review board (referred to as a CPP). The ANSM conducts a scientific review of the CT in terms of pharmaceutical and biologic quality and from nonclinical and clinical perspectives. On the basis of the oncohematologic application type, a criticality scale can be used to adjust the review according to the risk level. The review process usually takes 60 days but has been reduced to <30 days using new methodology.
The French national early access program (NEAP) provides a pathway for patients to gain access to innovative drugs that are not authorized and are outside the framework of CTs. Temporary authorizations for use (ATUs) are regulated by article L.5121‐12 of the French Public Health Code 6 and are granted exceptionally by the ANSM under the following conditions: treatment, prevention, or diagnosis of serious or rare diseases; cases at a therapeutic impasse with a lack of suitable alternatives available on the market in France; strong presumed efficacy and safety based on current scientific knowledge; and impossibility to defer implementation of the treatment. 7
The use of ATUs should not replace CTs because they do not have an investigative purpose. They can only be granted to patients who cannot be included in a CT. Compared with clinical trials, ATUs and temporary recommendations for use (RTUs) are not competitive mechanisms of early access. The focus of an ATU is a patient with unmet medical needs. There are different types of ATUs.
Nominative ATUs (nATU) are awarded to a designated patient at the request and under the responsibility of the prescribing physician. The review is carried out on a case‐by‐case basis by the ANSM, based: 1) on the clinical information 2 provided by the prescriber, and 2) conversely, on the product information provided by the stakeholder.
Cohort ATUs (cATUs) are requested by a stakeholder and intended for a group or subgroup of patients and for a specific indication. When filing the application, the stakeholder undertakes to apply for marketing authorization (MA) within a specified period. A critical analysis of the data submitted to the ANSM is carried out to define the population whose therapeutic need is unmet. After this review, a multidisciplinary working group made up of external experts from the agency may be consulted on the application. When a cATU is granted, like for an MA, this authorization includes a summary of product characteristics, a patient information leaflet, and labeling in French.
RTUs are another kind of pathway for patient access to products; however, in this case, the products are authorized within an MA and are used off‐label (prescriptions that do not fall within the scope of the MA). This scheme secures the off‐label use of drugs in response to an unmet therapeutic need once the benefit:risk ratio is presumed to be favorable for the indication concerned. RTUs are generally accompanied by a specific follow‐up of the patients treated within this framework, notwithstanding the MA. An RTU is valid for a renewable period of 3 years. The fundamental difference between an ATU and an RTU is that a medicine under an RTU already has an MA for another indication. Another difference is that, in the case of an ATU, the manufacturer intends to apply for an MA.
In France, prices of ATUs are unregulated, and hospital pharmacies, which are exclusively in charge of supplying ATUs, directly negotiate the price freely set by the pharmaceutical company. Some ATUs may be available free of charge. The purchase of medicines under ATUs does not affect the hospital's budget because the cost is entirely reimbursed by a dedicated national fund called the pharmaceutical innovation financing fund.
Once the MA has been obtained, a national review by the French health technology assessment body (the French National Authority for Health) examines the clinical added value of the new drug, assessing the actual clinical benefit (referred to as the SMR) and the improvement in actual clinical benefit (referred to as the ASMR). The SMR is used to study whether the drug is of sufficient benefit to be covered by public funding, and the ASMR assesses the added value offered by the drug over the available treatments. These 2 criteria are used for the pricing and reimbursement scheme. The price is fixed further to a negotiation between the pharmaceutical companies and the Healthcare Products Pricing Committee. In its pricing decision, this committee considers several factors, including the ASMR and the price in reference countries.
After the MA has been granted, availability of the product through the ATU system comes to an end, but the product is still available for patients and is reimbursed pending the health technology assessment and national agreements on reimbursement and pricing. For patients whose treatment was initiated under the ATU regime, the drug will be covered unless the MA process yields an unfavorable assessment. Treatment in the indications of the cATU can be initiated if they are mentioned in the wording of the MA. However, the initiation of specialties that have only been covered by an nATU is not reimbursed. This period, referred to as post‐ATU, is framed by Article L.162‐16‐5‐2 of the Social Security Code and should not exceed 180 days. 8
Materials and Methods
The methodology for the current study involved real‐time data collection of authorizations for CTs, nominative and cohort ATUs, and RTUs reviewed by the ANSM for the treatment of MM. The results were collected over 10 years for ATUs (between September 1, 2009 and September 1, 2019) and over 21 months for CT authorizations (from December 1, 2017 to September 1, 2019) from the agency's internal databases and from the European CT registry (radiopharmaceutical, gene therapy, and cell therapy CTs were excluded). Clinical data on the drugs granted a cATU are based on the periodic summary reports produced by pharmaceutical firms.
Results
Clinical Trials
Over the 21‐month study period, the ANSM reviewed 468 CTs for solid oncology, including 49 (10.5%) for MM. Of the 49 trials reviewed, 45 were authorized, and the other 4 were withdrawn by the sponsor. Of these, 36% trials had a complex design with several indications (basket CTs). Most of the trials were early phase CTs (51%) and phase 2 and 3 CTs (44%). Phase 4 CTs accounted for 5%.
CTs relating to melanoma were mainly sponsored by pharmaceutical companies (73%), and 27% originated from academic sponsors. Most were multicenter trials (93%).
The drugs used in the experimental arms of the CTs submitted and examined mainly comprised ITs (63%), followed by trials involving TTs (24%). These therapies were used mainly in combination in the experimental arms (Fig. 1).
Figure 1.
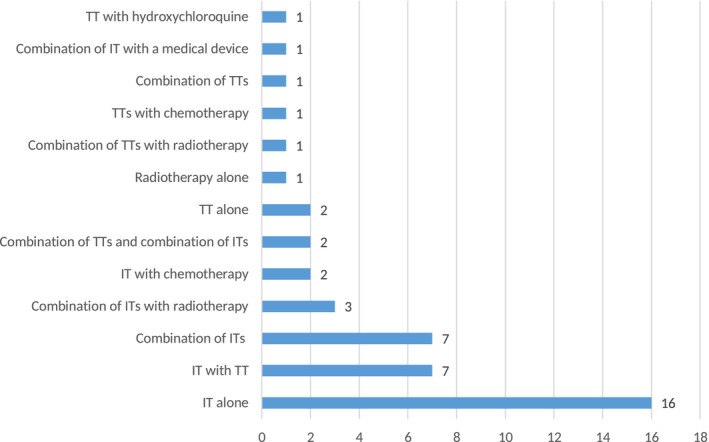
The most common classes of drugs found in malignant melanoma clinical trials are shown according to the number of trials between December 1, 2017 and September 1, 2019. IT indicates immunotherapy; TT, targeted therapy.
Sixty‐nine percent of the CTs did not include comparator arms. Among the CTs with a comparator arm (31%), 86% were compared with the standard monotherapy treatment, and 14% were compared with a combination.
Most of the trials reviewed (86%) were indicated for metastatic stages of melanoma, whereas 14% of the treatments were used at the adjuvant or neoadjuvant stage (Fig. 2). These 45 CTs would represent 2279 French patients, or 15.4% of the total number of patients worldwide.
Figure 2.
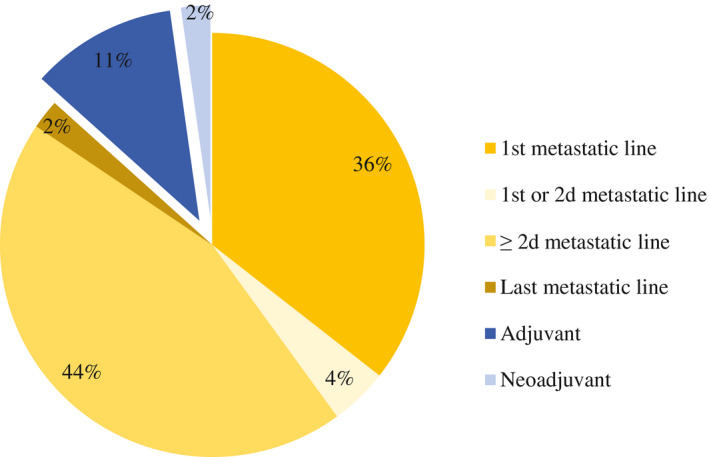
This is a breakdown of clinical trials for the treatment of malignant melanoma according to treatment lines between December 1, 2017 and September 1, 2019.
Because the prevalence of stage III and IV melanoma was 6342 cases recorded in France over 21 months (an estimate based on the prevalence between 2012 and 2018), 35.9% of patients benefited from therapeutic innovation through a CT.
Only 9% of the CTs allowed the inclusion of patients with brain metastases. Eighty‐three percent of CTs were aimed at patients aged >18 years, and only 17% allowed the inclusion of children aged >12 years.
Temporary Authorizations for Use
Over the 10‐year study period, an nATU application was filed for 13 drugs. The ANSM received 2597 nATU applications, including 2268 initial applications and 329 renewal applications. Of the initial applications, 1840 were approved, 220 were rejected, and 208 were canceled or inadmissible.
Among the 13 drugs that were granted an nATU, 10 were also the subject of a cATU application, but only 5 were notified by the agency, and the other 5 applications were withdrawn by the pharmaceutical firm or were not approved (Fig. 3).
Figure 3.

This is a breakdown by drug of the number of patients who were included in a cohort temporary authorization for use (cATU) or who were granted a nominative temporary authorization for use (nATU) for the treatment of malignant melanoma between September 1, 2009 and September 1, 2019. LAG 525 indicates lymphocyte‐activation gene 525; MA, market authorization.
Among the drugs that were covered by ATUs, the most highly represented classes were anti‐PD1 ITs (23%), and TTs acting on the BRAF (23%) and mitogen‐activated protein kinase kinase (23%) intracellular kinases. These are used mainly in combination (vemurafenib plus cobimetinib, encorafenib plus binimetinib, and dabrafenib plus trametinib). The other drugs acted on the LAG3 (15%) and PIK3CA (8%) receptors or were anti‐CTLA4 ITs (8%).
Eighty percent of indications were for the treatment of advanced melanoma (unresectable or metastatic) in adult patients who already had received treatment in a metastatic context. Conversely, for these products, 78% of the indications that were validated when the MA was granted were for use from the first metastatic line, and the remaining 22% of indications were for uses in an adjuvant context after complete resection (Table 1).
TABLE 1.
Upgrade of Approved Indications From Cohort Temporary Authorizations for Use (Delivered Only in Case of Unmet Medical Need) to Marketing Authorization (Dedicated to the Population at Large) Between September 1, 2009 and September 1, 2019
| Drug | Wording of cATU Indication | Wording of MA Indication |
|---|---|---|
| Ipilimumab | Treatment of advanced (unresectable or metastatic) melanoma, in adults who have already received treatment in a metastatic context | YERVOY as monotherapy is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults and adolescents aged ≥12 y |
| Vemurafenib | Treatment of metastatic melanoma in BRAF V600E mutation‐positive patients after failure of at least 1 line of treatment at the metastatic stage | Vemurafenib is indicated as monotherapy for the treatment of adult patients with BRAF V600 mutation‐positive, unresectable or metastatic melanoma |
| Pembrolizumab | Treatment of unresectable (stage III) or metastatic (stage IV) melanoma in adults and in children aged >12 y who have already received treatment with ipilimumab; patients must have an ECOG performance index of 0 or 1 and adequate organ function, defined by hematologic and biochemical criteria | KEYTRUDA as monotherapy is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults |
| Nivolumab | First indication: Treatment of adult patients (aged ≥18 y) with unresectable (stage III) or metastatic (stage IV) melanoma: | OPDIVO as monotherapy or in combination with ipilimumab is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults |
|
||
| Extension of the indication: Treatment of adult patients (aged ≥18 y) with unresectable (stage III) or metastatic (stage IV) melanoma: | ||
|
||
| ||
| Cobimetinib | First‐line treatment in combination with vemurafenib of adult patients with unresectable (stage III) or metastatic (stage IV) melanoma with a BRAF V600 mutation and with an ECOG performance index of 0 to 1 | Cotellic is indicated for use in combination with vemurafenib for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation |
Abbreviations: cATU, cohort temporary authorization for use; ECOG, Eastern Cooperative Oncology Group.
In 10 years, 6538 French patients benefited from therapeutic innovations for the treatment of melanoma in the context of an ATU. Of these patients, 1840 were covered by an nATU (28%), and 4698 were covered by a cATU (72%).
cATUs for vemurafenib and cobimetinib were made available free of charge. A cATU for nivolumab was initially free of charge and then became chargeable in 2015. The cATUs for pembrolizumab and ipilimumab were chargeable.
Four drugs have not been granted MA to date and thus are still available through an nATU (alpelisib, spartalizumab, LAG525, and relatlimab), and 38% of the drugs available under the nATU scheme have led to cATUs. Between 2009 and 2019, the average duration between the granting of the first nATU and the availability of the drug in the context of a cATU was 6.78 months (95% CI, −0.79 to 7.57 months).
The average time between the granting of the first nATU and the availability of the drug within the scope of its MA was 9.85 months (95% CI, 3.98‐5.87 months), and the average time between the awarding of the MA and the closure of the ATU scheme (nominative and cohort) was 2.27 months (95% CI, 0.64‐1.64 months) (Fig. 4). The average time between the end of the ATU and publication of the drug price in the Official Journal of the French Republic was 15.35 months (95% CI, 5.39‐9.96 months), and the average time was 16.88 months (95% CI, 5.17‐11.71 months) between the awarding of the MA and publication of the drug price in the journal.
Figure 4.
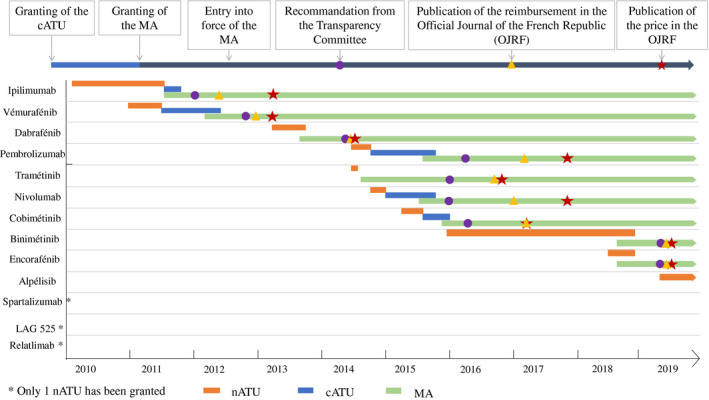
This is a timeline of granting nominative temporary authorization for use (nATU) and providing cohort temporary authorization for use (cATU), followed by awarding of marketing authorization (MA), and pricing and reimbursement schemes for the drugs used for the treatment of malignant melanoma between September 1, 2009 and September 1, 2019. LAG 525 indicates lymphocyte‐activation gene 525.
Of the 9 products that were granted an ATU for the treatment of MM, 4 were awarded an ASMR rating of III after the clinical benefit review (vemurafenib, trametinib, nivolumab, and cobimetinib), meaning that the drugs had moderate clinical added value, 2 had an ASMR rating of IV (minor clinical added value; ipilimumab and pembrolizumab), and 3 had an ASMR rating of V (lack of clinical added value; dabrafenib, binimetinib, and encorafenib). All of the drugs were approved for inclusion in the list of reimbursable medicines because 7 had a significant SMR rating (ipilimumab, vemurafenib, dabrafenib, pembrolizumab, trametinib, nivolumab, and cobimetinib), and 2 had a moderate SMR rating (binimetinib and encorafenib).
Efficacy data for patients covered by cATUs are available for cobimetinib, vemurafenib, nivolumab, and pembrolizumab (Fig. 5). Some data are missing because efficacy data collection is not the aim of an ATU. The average age of these patients (ratio of men to women, 1.4) was 61 years. Of these, 11% were at unresectable stage III, and 88% had stage IV disease; 66% had an Eastern Cooperative Oncology Group performance status of 0, and 34% had a performance status of 1. Among the patients who received cobimetinib and vemurafenib, 56% had more than 3 different metastatic sites. On average, 245 adverse events were observed for these drugs, and there were 73 severe adverse events. The system organ classes most encountered (>10%) were the same as those that were relevant in pivotal CTs.
Figure 5.

Efficacy data are illustrated for patients who were covered by cobimetinib, vemurafenib, and pembrolizumab cohort temporary authorizations for use according to Immune‐related Response Criteria and Response Criteria. ORR indicates overall response rate; W, week.
Temporary Recommendations for Use
In July 2018, 3 RTUs were granted (nivolumab, dabrafenib plus trametinib, and pembrolizumab) for the adjuvant treatment of resectable melanoma. At the time those applications were examined, the indication extensions for each product were in progress at the European level. Nivolumab, combined dabrafenib and trametinib, and pembrolizumab were approved by the Committee for Medicinal Products for Human Use in June 2018, July 2018, and October 2018, respectively; and the authorizations by the European Commission were issued in July 2018, August 2018, and December 2018, respectively. In the absence of funding for these RTUs, no patient had officially been treated within this framework; however, since then, these products fortunately have been authorized and reimbursed in France for MM.
Discussion
A better understanding of the melanoma development process and the mutational profile of various genes has led to the development of new treatments. Thus the management of MM has been revolutionized through the arrival of TTs and ITs since 2010. The positive results of pivotal CTs were published at an early stage. Different forms of leverage had to be identified to ensure that delays in European review procedures did not delay the availability of innovations for patients.
French recommendations include placing patients in CTs whenever possible. At the ANSM, a risk analysis‐based, multidisciplinary training model has been developed specifically for the evaluation of CTs in oncology, with the aim of accelerating patient inclusion. In recent years, protocols have allowed the inclusion of an increasingly large population with a view toward approaching patients who are treated at the post‐MA stage. Since March 2018, European regulations have notably encouraged the inclusion of children aged >12 years to harmonize them with those of the US Food and Drug Administration (FDA). 9 , 10 Likewise, patients with brain metastases are being included more often in CT target populations, although they are still mostly excluded, thereby optimizing CT results and not reflecting the general melanoma population (with a high frequency of stage IV brain metastasis). The new European CT regulation in force in 2020 (regulation 536/2014 11 ) will facilitate access to innovative treatments for European patients while protecting their safety and increasing transparency between member states for the conduct of CTs.
Early access is associated with a strong economic impact. Initially, in France, the financial effect of ATUs prompted policymakers to implement regulation. Since 2007, pharmaceutical firms have had to pay the difference between the MA price and the ATU price to the national health insurance. Next, because ATUs precede MAs, these prices serve as a reference for other markets. Although French ATUs seem marginal on an international level, they have an effect on international prices and, indirectly, on French prices because of the reference pricing mechanism.
The NEAP provides a more efficient starting point for the ordinary market access procedure. Patients have been able to benefit from all the innovations revolutionizing the management of melanoma before the MA in question has been granted.
Conditions for the provision of a product through an ATU are usually more restrictive than that those for the MA granted for the same product. Indeed, the ATU principle consists of making a product available to patients who are at a therapeutic impasse, whereas MA targets a much larger population. Therefore, the wording of the cATU Summary of Product Characteristics in MM is never identical, but the indications of the ATU are always covered by those of the MA.
In Europe, the use of compassionate use programs (CUP) is not homogeneous and has led to inequalities in the availability of innovative therapies. Less than 25% of European patients have been treated within the framework of these programs. CUPs are more readily accessible in western European countries than in eastern European countries. It is estimated that 35% of French patients with MM benefit from treatment through a CUP. By comparison, in Germany, only 10% of patients with MM benefit from a CUP, and 15% benefit from a CUP in the United Kingdom, but this rate stands at 80% in Belgium. 12 One of the major limitations of these programs is that they often are active only until registration, whereas reimbursement decisions arrive later. In France, the post‐ATU status serves to overcome this limit. In our data collection regarding the prevalence of MM in France, it was estimated that approximately 20% of patients with MM benefited from an ATU.
However, these measures to improve access to medicines are only useful if they are accompanied by coverage for these innovative medicines through public funding. Even when this is the case, the financial sustainability of the system is threatened by the expansion of early access schemes, which are not always supervised. The example of RTUs granted for the treatment of melanoma is an illustration of this because, in the absence of a funding order, they lack efficacy. The regulatory change regarding cATUs in early 2019 now allows for the granting and management of new indications for drugs that have already been granted MA in other situations.
Likewise, the criteria for inclusion in and withdrawal from the list allowing the funding of expensive medicines in hospital settings are now under debate. Ipilimumab has been withdrawn from the supplementary list, whereas its indication in combination with nivolumab is still included in the list. This results in regional treatment inequality scenarios based on hospital resources.
Collecting efficacy and safety data for medicines under an ATU is not the objective of ATUs and is not an obligation for the industrial firms. However, a significant limitation of this system is the inability to interpret ATU data collected on melanoma. Therefore, the ANSM is working on an efficient and consistent, real‐life data collection method. The systematic use of the Therapeutic Use and Information Collection Protocol could be extended to the collection of real‐time efficacy data, in addition to safety data, up to and including management. These data would serve as the basis for a performance‐based reimbursement scheme.
Conclusion
With recent advances in ITs and TTs, the MM treatment landscape has changed radically, thanks to the CUP and the inclusion of French patients in clinical trials. Since 2009, 2279 French patients have been included in CTs. These patients have been able to benefit mainly from ITs and TTs used on the first metastatic line. Recently, trials have been carried out at an increasingly early stage of the disease for adjuvant or neoadjuvant use of these therapies.
Over the same period, 6538 patients have benefited from therapeutic innovation under the nATU and cATU schemes. Thirteen products were involved in this early access program. The validated indications were more restrictive than the MA subsequently granted because they were mainly used after the second metastatic line, whereas the MA was granted for the first line. After the French Health Technology Assessment review, 4 products were awarded an ASMR rating of III, proving their added clinical benefit.
In view of these data, France, like other western European countries, is proving to be an innovative country in which committed policies have enabled early access to revolutionary new therapies that have emerged for the treatment of melanoma over the past 10 years. One of France's major strengths is the public funding coverage for products available under ATUs.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Claire Christen: Conception and design, collection and assembly of data, data analysis and interpretation, writing–initial draft, approved the final version, and accountable for all aspects of the work. Laetitia Belgodère: Approved the final version and accountable for all aspects of the work. Bernard Guillot: Approved the final version and accountable for all aspects of the work. Céline Jumeau: Approved the final version and accountable for all aspects of the work. Annie Lorence: Approved the final version and accountable for all aspects of the work. Ghania Kerouani‐Lafaye: Approved the final version and accountable for all aspects of the work. Liora Brunel: Approved the final version and accountable for all aspects of the work. Florence Turcry: Approved the final version and accountable for all aspects of the work. Adrien Monard: Provision of study materials, writing–initial draft, approved the final version, and accountable for all aspects of the work. Francoise Grudé: Approved the final version and accountable for all aspects of the work. Gaëlle Guyader: Approved the final version and accountable for all aspects of the work. Lotfi Boudali: Approved the final version and accountable for all aspects of the work. Nicolas Albin: Conception and design, provision of study materials, writing–initial draft, approved the final version, and accountable for all aspects of the work.
Christen C, Belgodère L, Guillot B, Jumeau C, Lorence A, Kerouani‐Lafaye G, Brunel L, Turcry F, Monard A, Grudé F, Guyader G, Boudali L, Albin N. Access to innovation through the national early access program and clinical trials for patients with malignant melanoma. Cancer. 2021. 10.1002/cncr.33492
See editorial on pages 2181‐2183, this issue.
We are grateful to Céleste Lebbé (University Department of Dermatology, Public Hospital Network of Paris, Saint‐Louis Hospital, INSERM Unit 976, Paris, France) for providing access to real‐world information collected in MELBASE, the French National Biobank dedicated to advanced melanoma.
References
- 1. Societe Francaise de Dermatologie . Patients Atteints de Melanome de Stade III Inoperable ou de Stade IV—Recommandations et Referentiels. I'Institut National du Cancer (INCa); 2017. [Google Scholar]
- 2. Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma: European consensus‐based interdisciplinary guideline. Eur J Cancer. 2010;46:270‐283.doi: 10.1016/j.ejca.2009.10.032 [DOI] [PubMed] [Google Scholar]
- 3. Foletto MC, Haas SE. Cutaneous melanoma: new advances in treatment. An Bras Dermatol. 2014;89:301‐310.doi: 10.1590/abd1806-4841.20142540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ugurel S, Loquai C, Terheyden P, et al. Chemosensitivity‐directed therapy compared to dacarbazine in chemo‐naive advanced metastatic melanoma: a multicenter randomized phase‐3 DeCOG trial. Oncotarget. 2017;8:76029‐76043.doi: 10.18632/oncotarget.18635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The European Parliament and the Council of the European Union . Directive 2001/20/EC on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Communities; 2001. Accessed September 23, 2019. https://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF [PubMed] [Google Scholar]
- 6. Republique Francaise . Code de La Sante Publique. Article L5121‐12.121‐12. Accessed September 23, 2019. https://www.codes‐et‐lois.fr/code‐de‐la‐sante‐publique/article‐l5121‐12
- 7. Agence Nationale de Securite du Medicament et des Produits de Sante (ANSM) . Qu'est ce qu'une autorisation temporaire d'utilisation (ATU)? Accessed September 23, 2019. https://www.ansm.sante.fr/Activites/Autorisations‐temporaires‐d‐utilisation‐ATU/Qu‐est‐ce‐qu‐une‐autorisation‐temporaire‐d‐utilisation/(offset)/0
- 8. Premier Ministre . LOI no. 2013‐1203 du 23 Decembre 2013 de Financement de la Securite Sociale pour 2014. Journal Officiel de la Republique Francaise; 2013. [Google Scholar]
- 9. Gaspar N, Marshall LV, Binner D, et al. Joint adolescent‐adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi‐stakeholder platform—ACCELERATE. Ann Oncol. 2018;29:766‐771.doi: 10.1093/annonc/mdy002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Department of Health and Human Services , Food and Drug Administration (FDA) . Considerations for the Inclusion of Adolescent Patients in Adult Oncology Clinical Trials. Guidance for Industry. FDA; 2019. Accessed September 23, 2019. https://www.fda.gov/media/113499/download [Google Scholar]
- 11. Official Journal of the European Union . Regulation (EU) No. 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. Accessed September 23, 2019. https://eur‐lex.europa.eu/legal‐content/EN/TXT/HTML/?uri=CELEX:32014R0536&from=FR
- 12. Kandolf Sekulovic L, Peris K, Hauschild A. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first‐line innovative treatments. Eur J Cancer. 2018;75:313‐322.doi: 10.1016/j.ejca.2017.01.012 [DOI] [PubMed] [Google Scholar]


