Abstract
Aim
The aim of this study is to gain more insight into child and environmental factors that influence gross motor development (GMD) of healthy infants from birth until reaching the milestone of independent walking, based on longitudinal research.
Background
A systematic search was conducted using Scopus, PsycINFO, MEDLINE and CINAHL to identify studies from inception to February 2020. Studies that investigated the association between child or environmental factors and infant GMD using longitudinal measurements of infant GMD were eligible. Two independent reviewers extracted key information and assessed risk of bias of the selected studies, using the Quality in Prognostic Studies tool (QUIPS). Strength of evidence (strong, moderate, limited, conflicting and no evidence) for the factors identified was described according to a previously established classification.
Results
In 36 studies, six children and 11 environmental factors were identified. Five studies were categorized as having low risk of bias. Strong evidence was found for the association between birthweight and GMD in healthy full‐term and preterm infants. Moderate evidence was found for associations between gestational age and GMD, and sleeping position and GMD. There was conflicting evidence for associations between twinning and GMD, and breastfeeding and GMD. No evidence was found for an association between maternal postpartum depression and GMD. Evidence for the association of other factors with GMD was classified as ‘limited’ because each of these factors was examined in only one longitudinal study.
Conclusion
Infant GMD appears associated with two child factors (birthweight and gestational age) and one environmental factor (sleeping position). For the other factors identified in this review, insufficient evidence for an association with GMD was found. For those factors that were examined in only one longitudinal study, and are therefore classified as having limited evidence, more research would be needed to reach a conclusion.
Keywords: child and environmental factors, cohort studies, gross motor development, infant, longitudinal design, systematic review
Key messages
Low birthweight and short gestational age have a persisting negative association with infant gross motor development from birth to independent walking.
There is inconsistent evidence for an association of breastfeeding, supine sleeping and (occluded) baby‐walker use with infant gross motor development.
More robust measures for environmental factors are needed.
1. INTRODUCTION
Infants show great variability in the attainment of the milestones of gross motor development (GMD). For example, independent walking is achieved between the ages of 8 and 17 months (WHO Multicentre Growth Reference Study Group & de Onis, 2006). According to dynamics systems theory, infant motor development emerges from the interaction between factors within the child and in the environment (Adolph & Hoch, 2019). Therefore, many different factors are responsible for this variability in infant motor development (Thelen & Corbetta, 2002). Several studies have investigated the association between child factors and an infant's GMD. Some factors have been subjects of study in reviews, including gestational age (GA) and birthweight (BW). In three reviews on these factors, strong evidence was found for an association of motor impairment and infants born very preterm (VPT) or with a very low BW from birth till 16 years of age (de Kieviet, Piek et al., 2009; Golding et al., 2014; Pascal et al., 2018). The review by Pin et al. (2007), about the factors sleeping position and the use of equipment, showed evidence for a transient delay in motor development of both term—and low risk PT infants who were not exposed to prone position. The use of equipment does not seem to delay or advance motor development in healthy term born infants (Pin et al., 2007). Reviews on other child and/or environmental factors are lacking. Furthermore, in the above‐mentioned reviews, it was noted that many studies were of low methodological quality and most included studies had a cross‐sectional design. Because variability and time are key elements in GMD, studies with a repeated‐measures design are preferred to those that evaluate the association of a factor cross‐sectionally (Adolph et al., 2008). By examining the association between a factor and GMD over time using the same sample, findings based on sample differences are avoided. Hence, studies with longitudinal designs give a more reliable representation of factors associated with GMD than those with cross‐sectional designs (Twisk, 2013).
A better understanding of factors associated with GMD of infants is an important basis for clinical reasoning and for designing new interventions for infants lagging in their GMD (Lobo & Galloway, 2012). Given the small number of reviews on factors associated with GMD, their dates of publication and the limited scope of factors included, it is important to provide an update. Besides, longitudinal studies relating to child factors and environmental factors associated with infant GMD have not yet been considered systematically. Therefore, the aim of the present review is to provide an overview of child and environmental factors associated with GMD of infants from birth to independent walking, based on longitudinal studies.
2. METHODS
2.1. Data sources and searches
A systematic search was conducted to identify studies that met the inclusion criteria. MEDLINE, CINAHL, PsycINFO and SCOPUS were searched from inception to February 2020. The search contained three main terms: ‘motor development’, ‘infants’ and ‘cohort studies’. The search strategies, tailored to the different databases, are included in Appendix A. When a systematic review was found, all included studies were screened for eligibility for this review.
2.2. Study selection
Only studies published in peer‐reviewed journals in English, with full text available, were included. Two reviewers (I.S. and M.B.) selected the studies independently, first by title and abstract and then, if necessary, by reading the methods section of the study. If the reviewers could not reach consensus, a third independent reviewer (J.N. or M.V.) was consulted. All remaining studies were subsequently read in full text to determine eligibility according to inclusion and exclusion criteria.
For inclusion, a longitudinal design was required, meaning two or more repeated measurements of GMD. When the study outcome was the attainment of a motor milestone, only prospective parental reports were included. Participants had to be healthy PT or full‐term (FT) infants. PT infants with the following conditions were excluded: cystic periventricular leukomalacia; Grade III or IV haemorrhage according to Papile classification; post‐haemorrhagic ventricular dilation; bronchopulmonary dysplasia (defined as oxygen supplementation > 36 weeks postmenstrual age). Studies on pathology or medical intervention were excluded. If no description of important characteristics, such as GA, birth weight and the presence of pathology was available, the study was excluded. Only birth cohort studies with samples that included >1,500 infants, a maximum of 5% of infants with health conditions that may affect motor development were accepted. At least one measurement of a child factor or an environmental factor, hypothesized to have an association with GMD, had to be reported. The following factors were excluded: prenatal factors (e.g., intrauterine growth retardation) or specific maternal factors (e.g., drugs and intracytoplasmic injection) and interventions (e.g., zinc and baby massage).
2.3. Study quality/RoB
Critical appraisal of studies is essential to identify and assess biases that may have affected the study outcomes (Huguet et al., 2013). Therefore, two researchers (I.S. and M.B.) assessed all included studies (n = 36) independently with the Quality in Prognostic Studies tool (QUIPS). This tool is designed to assess the risk of bias (RoB) in studies with prognostic factors (Hayden et al., 2013). The QUIPS includes 31 questions on validity and bias in six areas: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. The items are scored as ‘yes’ (fulfilled), ‘partial’ (partially fulfilled), ‘no’ (not fulfilled) or ‘?’ (unclear whether criterion is fulfilled). Subsequently, based on individual items' scores within each domain, all six domains were labelled ‘low’, ‘moderate’ or ‘high’ RoB, according to the recommendations and prompts of Hayden et al. (2013). Disagreement on individual scores was resolved by discussion and consensus. If necessary, a third reviewer (J.N. or M.V.) was consulted. Finally, a total RoB score was composed for each study as a basis for the best evidence synthesis. A study had to score a low RoB in all six domains for the overall RoB to be judged low. If this requirement was not met, the study was rated as having a high overall RoB. This procedure was determined a priori by the reviewers and based on the procedure described by Hayden et al. (2013). All information and discussion about RoB assessment was reported in Review Manager (Cochrane Collaboration, 2012). A summary statement of the study quality is displayed in Section 3 (Table 1).
TABLE 1.
Strength of evidence (Hayden et al., 2019)
| Strength of evidence | Description |
|---|---|
| Strong | Defined as greater than 75% of studies showing the same direction of effect in multiple low RoB studies |
| Moderate | Findings in multiple high RoB studies and/or one study with low RoB |
| Limited | One study available |
| Conflicting | Inconsistent findings across studies |
| No evidence | No association between prognostic factor and outcome of interest |
Abbreviation: RoB, risk of bias.
2.4. Data extraction and data synthesis
The results were presented according to PRISMA guidelines (Moher et al., 2009). Factors with statistical significance (p < 0.05) were reported for each study. Analysing the data, it became evident that various types of analysis had been performed, for example, repeated‐measures analysis, cross‐sectional analysis and analysis of the mean age of reaching milestones as outcome measure (motor milestone studies). Because these outcomes were so heterogeneous, a meta‐analysis could not be conducted. Therefore, a qualitative synthesis was performed, and the strength of evidence assessed following the descriptions for prognostic studies according to Hayden et al. (2019), described in Table 1. Data extraction focused on population characteristics, ages and measurements for motor outcomes and factors. From the results, correlations, regression coefficients, odds ratios and other outcomes were extracted (Appendix B).
3. RESULTS
The search yielded 5,594 potentially relevant studies. After removing duplicates, 3,548 studies remained. These were screened independently by two reviewers on title and abstract, and 3,250 studies were excluded. Four studies were added from other sources. From the remaining 302 full text studies, 36 were eligible for this review. Reasons for exclusion are specified in the PRISMA flow chart (Moher et al., 2009) (Figure 1).
FIGURE 1.
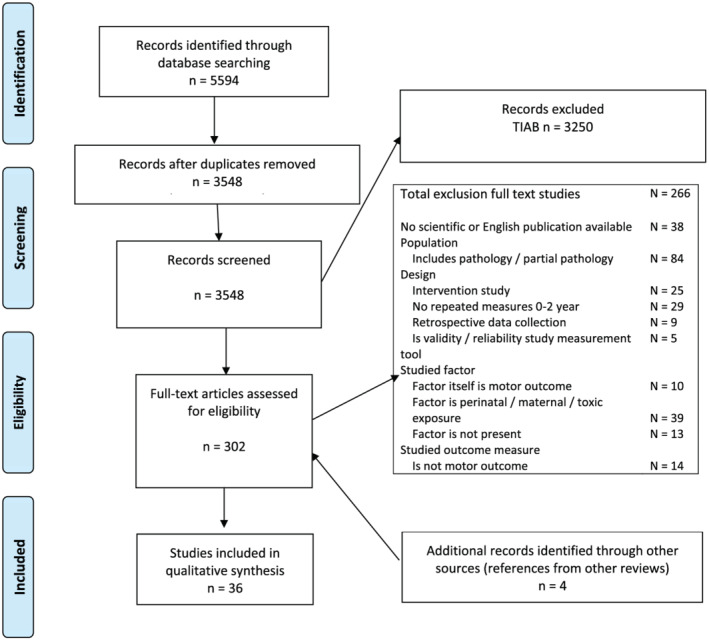
PRISMA flowchart [Colour figure can be viewed at wileyonlinelibrary.com]
3.1. Study characteristics
Included studies had their origin in 13 countries. Of the 36 studies, 25 were conducted in North America and Europe, the others being mainly carried out in Asia (Taiwan and Japan) and South America (Brazil). In total, the studies represent 71.546 infants with a median sample size of 261.5 (range 27–20.112). In 22 of the included studies, only FT infants (GA ≥ 37 weeks) participated. Mixed populations (both FT and PT infants) were examined in 13 studies, and one study included only PT infants (GA < 34 weeks). Six child factors were examined in 16 studies, and the association of 12 environmental factors was evaluated in 20 studies. The included studies table (Appendix B) provides information on the main population characteristics, study design, analyses performed and outcomes. The studies were grouped by type of factor (child, environmental or multiple factors), see Table 2. Studies were described by the main factor, which was the main objective of the research question. Studies examining multiple factors were grouped. Confounders that were considered and were significant in the final model are summarized in the included studies table. A summary of the significant associations of factors with GMD is displayed in Table 3.
TABLE 2.
Included studies and factors examined
| Single factor studies | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child factors | Environmental factors | ||||||||||||||||
| Author (year) | GA | BW | Twin | Anthropometry | Back‐ground | Motivation to move | Sleep position | BF | Mat. depression | Maternal mental health | Adolescent mother | Parental mental health | Parental neonatal perceptions | Baby walker | Day‐care attendance | Season of birth | Cultu‐ral context |
| Yaari et al. (2018) | Xc | ||||||||||||||||
| Espel et al. (2014) | Xc | ||||||||||||||||
| Field et al. (1978) | X | ||||||||||||||||
| Datar and Jacknowitz (2009) | Xc | ||||||||||||||||
| Grantham‐McGregor et al. (1998) | Xc | ||||||||||||||||
| Lung et al. (2009a) | Xc | ||||||||||||||||
| Nan et al. (2013) | Xc | ||||||||||||||||
| Brouwer et al. (2006) | Xc | ||||||||||||||||
| Goetghebuer et al. (2003) | Xc | ||||||||||||||||
| Wilson and Harpring(1972) | Xc | ||||||||||||||||
| Scharf et al. (2016) | Xc | ||||||||||||||||
| Slining et al. (2010) | Xc | ||||||||||||||||
| Bartlett (1998) | X | ||||||||||||||||
| Capute et al. (1985) | Xc | ||||||||||||||||
| Atun‐Einy et al. (2013) | X | ||||||||||||||||
| Majnemer and Barr (2006) | Xc | ||||||||||||||||
| Davis et al., 1998 | Xc | ||||||||||||||||
| Lung and Shu (2011) | Xc | ||||||||||||||||
| Ratliff‐Schaub et al. (2001) | Xc | ||||||||||||||||
| Jardí et al. (2018) | Xc | ||||||||||||||||
| Michels et al. (2017) | Xc | ||||||||||||||||
| Morris et al. (1999) | Xc | ||||||||||||||||
| Oddy et al. (2011) | Xc | ||||||||||||||||
| Smith‐Nielsen et al. (2016) | Xc | ||||||||||||||||
| Sutter‐Dallay et al. (2011) | Xc | ||||||||||||||||
| Lung and Shu (2011) | Xc | ||||||||||||||||
| de Borba and Valentini (2015) | X | ||||||||||||||||
| Lung et al. (2009b) | Xc | ||||||||||||||||
| Hernández‐Martínez et al. (2011) | Xc | ||||||||||||||||
| Siegel and Burton (1999) | Xc | ||||||||||||||||
| Souza et al. (2010) | X | ||||||||||||||||
| Tsuchiya et al. (2012) | Xc | ||||||||||||||||
| Vierhaus et al. (2011) | X | ||||||||||||||||
TABLE 3.
Associations between factors and infant GMD
| Child factors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of motor assessment outcome | Age of motor milestone outcome | ||||||||||
| RoB | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | |
| GA | |||||||||||
| Preterm | |||||||||||
| Yaari et al., 2018 | H | s** | |||||||||
| Term | |||||||||||
| Espel et al., 2014 | H | s* | s** | s** | |||||||
| Post‐term | |||||||||||
| Field et al., 1978 | H | ns | ns | ||||||||
| Birthweight | |||||||||||
| Low BW | |||||||||||
| Datar & Jacknowitz, 2009 | L | s *** | s** | ||||||||
|
Grantham‐McGregor et al., 1998 |
L | s *** | s *** | ||||||||
| Normal BW | |||||||||||
| Lung et al., 2009a | H | s *** | s *** | ||||||||
| Twin | |||||||||||
|
Nan et al., 2013 |
H | s *** | s *** | s *** | ns | ||||||
|
Brouwer et al., 2006 |
H | ns | ns | ||||||||
|
Goetghebuer et al., 2003 |
H | s** | s*/ns a | s*/ns a | |||||||
| Wilson & Harpring, 1972 | H | ns | s *** | ns | s *** | ||||||
| Anthropometry | |||||||||||
| Weight, length and head circumference | |||||||||||
| Scharf et al., 2016 | H | s *** | s *** | ||||||||
| Overweight | |||||||||||
|
Slining et al., 2010 |
L | s* | |||||||||
| Proportionately larger head | |||||||||||
| Bartlett, 1998 | H | ns/s *** | ns | ns | ns | ||||||
| Afro‐American background | |||||||||||
|
Capute et al., 1985 |
H | s** | s** | s** | s** | ||||||
| Motivation to move | |||||||||||
|
Atun‐Einy et al., 2013 |
H | s* | s* / ** / ** / ** | ||||||||
| Environmental factors | |||||||||||
| RoB | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | |
| Sleep position | |||||||||||
| Prone sleeping | |||||||||||
|
Majnemer and Barr, 2006 |
H | ns b /s b | ns | ||||||||
|
Davis et al., 1998 |
H | s**/**/ *** /ns | s**/**/ns | ||||||||
| Supine sleeping | |||||||||||
|
Lung and Shu, 2011 |
H | s *** | ns | ns | |||||||
|
Ratliff‐Schaub et al., 2001 |
H | ns | ns | ||||||||
| Breastfeeding | |||||||||||
| Jardí et al., 2018 | L | s* | s* | ||||||||
|
Michels et al., 2017 |
H | ns | ns | ns | |||||||
|
Morris et al., 1999 |
L | ns/sLBW/HBW * | ns | ||||||||
|
Oddy et al., 2011 |
H | ns | ns | ns | |||||||
| Maternal depression | |||||||||||
| Smith‐Nielsen et al., 2019 | H | ns | ns | ||||||||
|
Sutter‐Dallay et al., 2011 |
H | ns | ns | ns | ns | ||||||
| Maternal mental health | |||||||||||
| Lung, Shu, Chiang et al., 2011 | H | ns | ns | ns | |||||||
| Adolescent mother | |||||||||||
|
de Borba and Valentini, 2015 |
H | ns | |||||||||
| Parental mental health | |||||||||||
| Lung et al., 2009b | H | ns | sm * | ||||||||
| Parental neonatal perception | |||||||||||
| Hernández‐Martínez et al., 2011 | H | sm * | sp ** | ||||||||
| Babywalker | |||||||||||
| Siegel and Burton, 1999 | H | s *** | s *** / ** | s** | |||||||
| Daycare attendance | |||||||||||
|
Souza et al., 2010 |
H | s | s | ||||||||
| Season of birth winter | |||||||||||
| Tsuchiya et al., 2012 | H | s *** | s *** | ns | |||||||
| Cameroonian versus German culture | |||||||||||
| Vierhaus et al., 2011 | H | s ns | |||||||||
| Multiple factors | |||||||||||
| RoB | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | 0–3 m | 4–6 m | 7–12 m | 13–24 m | 25–60 m | |
| Bjarnadóttir et al., 2019 | H | ns | ns | ns | ns | ||||||
| Pereira et al., 2016 | H | s | |||||||||
| Flensborg‐Madsen & Mortensen, 2017 | H | s *** | s *** | s *** | |||||||
Note. L = low RoB (Risk of Bias), H = high RoB; ns = no significant association, s = significant association; s/ns = (no) associations longitudinally analysed; m = maternal; p = paternal.
Abbreviations: HBW, high birthweight; LBW, low birthweight.
Multiple motor milestones measured in same age‐range.
Multiple motor outcomes measured in same age‐range.
p<0.05.
p<0.01.
p<0.001.
3.2. RoB assessment
Major issues with study quality were related to study attrition and study participation. High RoB on the domain ‘statistical analysis and reporting’ was mainly found in research carried out before the year 2000 (n = 4). Five studies scored an overall low RoB, comprising 14% of included studies (Table 4).
TABLE 4.
Risk of bias assessment (QUIPS)
| Study participation | Study attrition | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and reporting | Overall RoB | |
|---|---|---|---|---|---|---|---|
| Child factors | |||||||
| GA | |||||||
| Preterm | |||||||
| Yaari et al., 2018 | ? | + | + | ? | + | + | H |
| Term | |||||||
|
Espel et al., 2014 |
+ | ? | ? | + | + | + | H |
| Post‐term | |||||||
|
Field et al., 1978 |
? | N/A | ‐ | + | ‐ | ‐ | H |
|
Birthweight Low birthweight | |||||||
| Datar & Jacknowitz, 2009 | + | + | + | + | + | + | L |
|
Grantham‐McGregor et al., 1998 |
+ | + | + | + | + | + | L |
| Normal birthweight | |||||||
| Lung et al., 2009a | + | + | + | ? | + | + | H |
| Twin | |||||||
|
Nan et al., 2013 |
+ | ‐ | + | ? | + | + | H |
|
Brouwer et al., 2006 |
? | N/A | + | ? | ? | ‐ | H |
|
Goetghebuer et al., 2003 |
? | ‐ | + | ? | + | + | H |
| Wilson & Harpring, 1972 | ‐ | ‐ | ‐ | + | ‐ | ‐ | H |
| Weight, length and head circumference | |||||||
| Scharf et al., 2016 | ? | ? | + | + | + | ? | H |
| Overweight | |||||||
|
Slining et al., 2010 |
+ | + | + | + | + | + | L |
| Proportionately larger head | |||||||
| Bartlett, 1998 | + | ‐ | + | + | ‐ | ‐ | H |
| Afro‐American background | |||||||
|
Capute et al., 1985 |
? | ‐ | ? | ‐ | ‐ | ‐ | H |
| Motivation to move | |||||||
|
Atun‐Einy et al., 2013 |
‐ | N/A | ‐ | + | ‐ | ? | H |
| Environmental factors | |||||||
| Sleep position | |||||||
| Prone sleeping | |||||||
|
Majnemer and Barr, 2006 |
‐ | ? | ? | + | + | + | H |
|
Davis et al., 1998 |
+ | ? | + | ? | ? | + | H |
| Supine sleeping | |||||||
|
Lung and Shu, 2011 |
? | ? | + | ? | + | + | H |
|
Ratliff‐Schaub et al., 2001 |
? | ‐ | ? | + | + | ‐ | H |
| Breastfeeding | |||||||
| Jardí et al., 2018 | + | N/A | + | + | + | + | L |
|
Michels et al., 2017 |
+ | N/A | + | ? | + | + | H |
|
Morris et al., 1999 |
+ | + | + | + | + | + | L |
|
Oddy et al., 2011 |
? | ? | ? | ? | + | + | H |
| Maternal depression | |||||||
| Smith‐Nielsen et al., 2019 | ? | + | + | + | ? | + | H |
|
Sutter‐Dallay et al., 2011 |
+ | ? | ? | + | + | + | H |
| Maternal mental health | |||||||
|
Lung and Shu, 2011 |
+ | ? | + | ? | + | + | H |
| Adolescent mother | |||||||
|
de Borba and Valentini, 2015 |
‐ | N/A | + | ‐ | ? | ‐ | H |
| Parental mental health | |||||||
| Lung et al., 2009b | + | + | + | ? | + | + | H |
| Parental neonatal perception | |||||||
| Hernández‐Martínez et al., 2011 | + | N/A | + | + | + | ? | H |
| Babywalker | |||||||
| Siegel and Burton, 1999 | ? | + | ? | + | ? | ? | H |
| Daycare attendance | |||||||
|
Souza et al., 2010 |
‐ | N/A | ? | + | ‐ | ‐ | H |
| Season of birth winter | |||||||
| Tsuchiya et al., 2012 | ? | + | + | ? | + | + | H |
| Cameroonian versus German culture | |||||||
| Vierhaus et al., 2011 | ? | ? | + | ? | + | + | H |
| Multiple factors | |||||||
| Bjarnadóttir et al., 2019 | + | + | + | ? | + | + | H |
| Flensborg‐Madsen & Mortensen, 2017 | + | ‐ | + | ? | + | ? | H |
| Pereira et al., 2016 | ‐ | ‐ | ? | + | + | + | H |
Note. L = low risk of bias (RoB), H = high RoB; ‐ = not reported, + = reported, ? = not sure; N/A = not applicable.
4. CHILD FACTORS
4.1. Gestational age
Four studies with high RoB examined the association of GA and GMD in various populations (Espel et al., 2014; Field et al., 1978; Flensborg‐Madsen & Mortensen, 2017; Yaari et al., 2018), finding moderate evidence that a shorter GA for infants is negatively associated with GMD in the age range 0–18 months. The study by Yaari et al. (2018) showed that moderately preterm (MPT) infants (GA 32–34 weeks) have persistently lower levels of GMD in the age range 1–18 months, compared with FT infants. However, because GA and BW were highly correlated, it is not clear whether these differences are primarily due to GA or BW (Yaari et al., 2018). Flensborg‐Madsen and Mortensen (2017) concluded that most of the variance (14.5%) in the achievement of motor milestones by infants, both FT and PT, is explained by GA and BW. In a sample of FT infants (37–41.6 weeks GA), longer pregnancy duration was also significantly associated with better motor scores at 3, 6 and 12 months, after adjusting for confounders (Espel et al., 2014). There is no evidence for an association between GA in infants born post‐term (>42 weeks) and GMD from 4 to 12 months (Field et al., 1978).
4.2. Birthweight
Four studies examined the association between BW and infant GMD (Datar & Jacknowitz, 2009; Flensborg‐Madsen & Mortensen, 2017; Grantham‐McGregor et al., 1998; Lung et al., 2009a). Two studies with a low RoB and one study with a high RoB examined infants with very low birthweight (VLBW) (<1,500 g) and found strong evidence that low birthweight (LBW) (<2,500 g) in both PT and FT infants is associated with a more delayed GMD in the age range 4–24 months (Datar & Jacknowitz, 2009; Grantham‐McGregor et al., 1998; Scharf et al., 2016). There is limited evidence that infants with normal BW (>2,500 g) have more advanced GMD than infants with LBW (Lung et al., 2009a). In a mixed population of infants (GA 27–46.5 weeks), Flensborg‐Madsen and Mortensen (2017) showed that BW in addition to GA explained most of the variance in motor milestone attainment (Flensborg‐Madsen & Mortensen, 2017). All studies that included PT infants accounted their outcomes to GA.
4.3. Anthropometry
Three studies investigated the association of anthropometric measures with infant GMD. The study with the factor ‘overweight’ (Slining et al., 2010) had a low RoB; the other two had high RoB (Bartlett, 1998; Scharf et al., 2016). Due to the heterogeneity of the populations and the difference in measures, the outcomes of these three studies could not be compared.
Regarding the factor overweight, there is moderately consistent evidence that overweight FT infants, measured from birth to 18 months, are more prone to delayed GMD in the age range 3–18 months, compared with infants of normal weight (Slining et al., 2010).
Limited evidence was found for the factors ‘proportionately larger head’, ‘body mass index (BMI)’ and ‘body length’. Infants with normal BW and a proportionately larger head showed lower motor scores at 6 weeks, but not at later ages (Bartlett, 1998). For the factors ‘body length’ and ‘BMI’, no association was found with infant motor outcome between 6 weeks and 15 months.
For VLBW infants, there is limited evidence that BMI and length are associated with more delayed GMD at 9 and 24 months (Scharf et al., 2016).
4.4. Twin
Four studies with high RoB investigated the association between twinning and GMD, allowing for BW and GA (Brouwer et al., 2006; Goetghebuer et al., 2003; Lung et al., 2009a; 2011; Nan et al., 2013). Overall, the evidence was inconsistent: either significantly negative associations or no associations between GMD and twinning were reported. The study by Brouwer et al. (2006) found no differences in the achievement of motor milestones between Dutch singletons and twins in the age range 0–24 months. Three other studies reported both significant and non‐significant associations at different ages. Nan et al. (2013) reported that twins from 0 to 12 months scored lower on GMD, compared with singletons. These outcomes are broadly in line with the study by Goetghebuer et al. (2003). After adjusting for the confounder BW, the age of milestone achievement was significantly later for twins in only three out of eight milestones in the first year. Lastly, Wilson and Harpring (1972) observed that twins had significantly lower motor scores compared with singletons at 6 and 18 months, but not at 3, 9 and 12 months.
4.5. Other child factors
For the child factors, ‘Afro‐American background’ and ‘motivation to move’, significant associations with infant GMD were reported but, as each factor was examined by only one longitudinal study, each with high RoB, these findings were interpreted as providing limited evidence. Infants with an Afro‐American background achieved most motor milestones at an earlier age compared with infants with other cultural backgrounds (Capute et al., 1985). Infants that were perceived to have a stronger motivation to move in the age range 7 to 12 months showed earlier achievement of five milestones (Atun‐Einy et al., 2013).
5. ENVIRONMENTAL FACTORS
5.1. Sleep position
In four studies, all high RoB, sleep position was examined in association with infant GMD. There is moderate evidence that prone sleeping is associated with a better GMD from 4 to 10 months (Davis et al., 1998; Majnemer & Barr, 2006). No association was found from 11 to 17 months. In a study of Majnemer and Barr (2006), prone‐sleeping infants showed more advanced GMD at 6 months, but not at 4 and 15 months. Davis et al. (1998) found that prone‐sleeping infants were faster in the attainment of several motor milestones in the age range of 4–10 months. No association was found between prone sleeping and the motor milestone ‘walking alone’.
Conflicting evidence was found for the association between supine sleeping and a delayed GMD at 4 and 6 months (Lung & Shu, 2011; Ratliff‐Schaub et al., 2001). No evidence was found for any association of supine sleeping and GMD in the age ranges 0–3 and 12–36 months. In a cohort study of Lung and Shu (2011), supine‐sleeping infants showed a delay in GMD at 6 months. At 18 and 36 months, this association was no longer present (Lung & Shu, 2011). Ratliff‐Schaub et al. (2001) studied a population of VPT infants. GMD at the corrected ages of 4 and 13 months was not associated with sleeping in supine (Ratliff‐Schaub et al., 2001).
5.2. Breastfeeding
Five studies, two with low RoB (Jardí et al., 2018; Michels et al., 2017) and three with high RoB (Bjarnadóttir et al., 2019; Morris et al., 1999; Oddy et al., 2011), investigated the association between breastfeeding (BF) and infant GMD. Two studies had mixed populations (PT/FT infants and LBW/HBW FT infants), one was a cohort study, and two studies examined FT infants. BF duration as a factor was defined differently in all studies, and overall, conflicting evidence was found regarding the role of BF. Jardí et al. (2018) reported a significantly advanced GMD at 6 months in FT infants that received both exclusive BF and mixed feeding till 4 months, as compared with infants who received only formula feeding (Jardí et al., 2018). These associations were only significant in an adjusted model when the factors BMI (at 6 months) and GA were added. At 12 months, a significant association of exclusive BF with advanced GMD was present when the factor iron status was added to the model.
In four studies, no evidence was found of an association between BF and GMD in the first 3 years of life in diverse populations (Michels et al., 2017; Morris et al., 1999; Oddy et al., 2011). Morris et al. (1999), a low RoB study, compared groups of FT infants with HBW and LBW and evaluated the frequency of BF in the first 4 weeks and between 5 and 26 weeks after birth. They found that BF intensity did not correlate with motor outcome at 6 and 12 months for both groups separately. Linear regression showed that in both LBW and HBW infants, BF intensity in the first 4 weeks of life was significantly associated with motor scores at 6 months, but this was no longer apparent at 12 months (Morris et al., 1999). Michels et al. (2017) did not find an association of exclusive BF and infant GMD, nor for PT infants (Michels et al., 2017). The study by Oddy et al. (2011) revealed that GMD scores in infants with BF < 4 months did not differ from those in infants with BF > 4 months. Only boys who were breastfed for less than 4 months had an increased risk of one atypical score on the Ages and Stages Questionnaire (ASQ) at any time‐point. In the group of FT infants with a normal BW, Bjarnadóttir et al. (2019) found no association between duration of BF (exclusive or total duration) and motor milestone achievement.
5.3. Maternal depression
In two studies, both with high RoB, maternal depression was examined in association with infant GMD. Overall, there was no evidence that postpartum depression is associated with GMD between the ages of 3 and 24 months (Smith‐Nielsen et al., 2016; Sutter‐Dallay et al., 2011). In the study of Smith‐Nielsen et al. (2016), 28 FT infants of mothers with a diagnosis of maternal depression were compared with a control group (n = 53). This revealed no association with motor scores at the ages of 4 and 13 months. Sutter‐Dallay et al. (2011) found no association between the depression score of the mother (at 6 weeks after giving birth and at follow‐up) and GMD from 3 to 24 months (Sutter‐Dallay et al., 2011).
5.4. Other environmental factors
The following environmental factors were examined by only one longitudinal study each and findings are therefore categorized as high RoB, interpreted as limited evidence.
For the environmental factors ‘use of an occluding baby walker’, ‘home environment’ and ‘daycare attendance’, significant associations with infant GMD were reported. The use of an occluding baby walker, a walker in which the infant is not able to see its own feet, is significantly associated with a delayed GMD between 6 and 15 months, in comparison with a see‐feet baby walker and no baby walker use (Siegel & Burton, 1999). Home environment, including a higher family income, more stimulation by putting the infant in independent positions, is significantly associated with higher motor performance in infants between 2 and 12 months (Pereira et al., 2016). About the factor ‘daycare attendance’ it was found that infants who were attending daycare full‐time, 13% (n = 4) were suspect for motor delays at 12 and 17 months (Souza et al., 2010).
For the factors that all were examined by one high RoB study: season of birth (Tsuchiya et al., 2012), parental mental health (Lung et al., 2009b), parental neonatal perceptions (Hernández‐Martínez et al., 2011), and cultural context (Vierhaus et al., 2011), the association with GMD changed over time. Infants born in spring have higher motor scores at 6 and 10 months of age than infants born in winter; at 14 months, no association with GMD was found (Tsuchiya et al., 2012). Better parental mental health was associated with better GMD at 18 months (Lunget al., 2009b). Concerning the factor ‘parental neonatal perceptions’, more negative maternal perceptions have a negative association with infant GMD at 4 months. At 12 months, positive paternal perceptions were associated with an advanced GMD (Hernández‐Martínez et al., 2011). Cameroonian infants have significantly higher motor scores than German infants at 3 and 6 months, implying an association between cultural context and GMD. At 9 months, this association was no longer present (Vierhaus et al., 2011).
No evidence was found for the factors adolescent mother. Motor scores of infants aged zero to 18 months did not differ significantly between infants who had an adolescent or adult mother (de Borba & Valentini, 2015). Also for the factor maternal mental health no evidence was found for an association with infant GMD at 6, 18 and 36 months (Lung, Shu, Chiang, et al., 2011).
6. DISCUSSION
This review aimed to provide an overview of factors associated with GMD of healthy FT and PT infants as examined in longitudinal studies. In total, 36 studies were identified of which 15 examined a child factor, 17 examined an environmental factor and 4 investigated multiple factors. Six child factors and 11 environmental factors were examined in the selected studies. Strong evidence was found for the association of the child factor ‘LBW’ with infant GMD. Moderate evidence was found for the child factors ‘overweight’ and ‘shorter GA’, and for the environmental factor ‘prone sleeping’. There was conflicting evidence for the factors ‘twinning’, ‘supine sleeping’ and ‘BF’. Regarding the other factors identified in this review, insufficient evidence for an association with GMD was found, and they were classified as having no or limited evidence. Only the factors that are examined in multiple studies and therefore enabling a qualitative synthesis will be discussed in more depth.
6.1. Child factors
This review included four longitudinal studies (Espel et al., 2014; Field et al., 1978; Flensborg‐Madsen & Mortensen, 2017; Scharf et al., 2016), all showing moderate evidence that a shorter GA is associated with a delay in GMD. The samples that were studied ranged from 26 to 42 weeks GA. This association is in line with the results from the meta‐analysis by de Kieviet et al. (2009) who reported a significant negative association between the GA of VPT children and GMD. The study of Espel et al. (2014) indicated that the duration of gestation is not only associated with GMD in PT infants but also, maybe less pronounced, in early FT, FT and late FT infants. Fundamentals about the association of GA with GMD presented in most of the included studies (Espel et al., 2014; Yaari et al., 2018) are that growth of the brain and neurological maturation of the brain during the prenatal period are linked to neurodevelopmental outcome.
This review provides strong evidence that both VLBW (<1,500 g) and LBW (1,500–2,500 g) are significantly associated with lower motor outcomes of PT and FT infants from 0 to 24 months. These findings concur with those of a systematic review on motor outcomes in VLBW and VPT children (de Kieviet et al., 2009), including a meta‐analysis on 9,653 VLBW children from 0 to 16 years. de Kievit et al. concluded that an increase in BW was related to better motor outcomes. The negative association of LBW and GMD was also reported in a cross‐sectional study of Hediger et al. (2002), who found delays in GMD in both FT and PT infants with LBW (Hediger et al., 2002). These outcomes show that the impact of BW on GMD transcends that of premature birth. Golding et al. (2014) concluded that LBW is a marker of intrauterine growth retardation rather than of PT delivery and therefore has a direct and strong impact on GMD. From the included studies, only the study of Datar and Jacknowitz provides an explanation of the relation between BW and GMD. Besides intrauterine malnutrition, low birth weight is also caused by genetic and environmental effects. These also may exert the negative effect on motor outcomes in the first year of life (Datar & Jacknowitz, 2009).
Regarding the factor twinning, it is known that twins are more prone to developmental delay from prematurity and LBW. The question arises of whether twinning is an independent risk factor. In this review, conflicting evidence was found in four studies (Brouwer et al., 2006; Goetghebuer et al., 2003; Nan et al., 2013; Wilson & Harpring, 1972). Differences in the sample and in the method of measuring GMD might play a role in this. Goetghebuer et al. (2003) found that Gambian twins were significantly delayed in reaching three of the eight milestones studied, after adjustment for the confounders BW and GA. However, the authors suggest that cultural factors may explain the observed delays in the twins' GMD. In the Dutch sample of Brouwer et al. (2006), no significant differences were observed in GMD between twins and singletons with normal BW and GA. Unlike the study of Goetghebuer et al. (2003), who used the mean age of reaching a milestone, Brouwer et al. (2006) used the percentage of twins who achieved a milestone at a fixed age, which is less accurate and might explain differences in outcomes. A study performed in the United Kingdom measured GMD of infants (GA 26–39 weeks), using the ASQ, and based the outcomes on the American norm scores of healthy FT singletons (Nan et al., 2013). This study found that UK twins scored below the normal range on GMD until 9 months of age. However, a singleton control group was not used. Recent research on the cross‐cultural validity of norm values of motor measurements shows that North American infants are ahead of European infants (De Kegel et al., 2013; Steenis et al., 2015a; 2015b; Suir et al., 2019). In this light, it might be debated whether the described results are indicators of delayed GMD in twins or merely a reflection of normal GMD in UK PT and FT infants. Overall, the evidence from these longitudinal studies does not show that twinning is an independent risk factor for GMD of infants.
6.2. Environmental factors
The included studies on the factor BF, all suggest that GMD may be positively affected by BF because (1) BF is a critical source of energy enabling motor development and (2) BF protects infants against gastrointestinal infections which optimizes health and therefore (motor) development. In this review, no evidence of an association with GMD was found (Bjarnadóttir et al., 2019; Michels et al., 2017; Morris et al., 1999; Oddy et al., 2011). This is in line with recent cross‐sectional studies (Khan et al., 2019; Leventakou et al., 2015). A review by Golding et al. (2014), which included six cross‐sectional studies, also found no clear evidence for any association of BF and GMD. However, Jardí et al. (2018) did find a positive association of BF (exclusive and mixed feeding) and GMD in a group of term born infants. As the outcomes were only significant in the adjusted model including GA and BMI at 6 months and iron status at 12 months, this might indicate that any existing relationship between BF and GMD is mainly indirect and based on infant anthropometry and important nutrients like iron. Considering the limitations that are mentioned in the included studies, it becomes evident that rigorous research in this field is a challenge. One reason for this is the many confounding factors, such as maternal cognition and socio‐economic effects. Besides, the effects of BF appear to be different in developing and developed countries and in term born and PT born infants with a low BW. Finally, several studies report that the lack of an association between GMD and BF might also be due to the formula feeding that improved so much over the last decades that it levels the quality of breastmilk (Bjarnadóttir et al., 2019; Oddy et al., 2011). Michels et al. (2017) concludes that the positive effects of BF go beyond motor development.
The moderate evidence found in this review for a positive association of prone sleeping and GMD from 4 to 10 months for both FT and PT infants was already signalled in the review of Pin et al. (2007), which included nine studies on the effects of sleeping position on GMD. Three of these studies were longitudinal and were included in this review (Davis et al., 1998; Majnemer & Barr, 2006; Ratliff‐Schaub et al., 2001). The study of Lung and Shu (2011) concluded that supine sleepers only showed a delayed GMD at 6 months, not at 18 and 36 months (Lung & Shu, 2011). It seems logical that the association between sleeping position and GMD is most present before 6 months when infants are dependent on their caregivers to change positions. There are also indications that more than 20 years after the ‘Back to sleep’ campaign was set up, the adverse effects of supine sleeping on GMD might have diminished due to adequate education about ‘tummy time’ (Carmeli et al., 2009; Hewitt et al., 2017).
There was no evidence found in the two included studies for an association of postpartum maternal depression (PPMD) and GMD in infants (Smith‐Nielsen et al., 2016; Sutter‐Dallay et al., 2011). A systematic review of nine studies by Aoyagi and Tsuchiya (2019), including the study of Smith‐Nielsen also found no association between GMD and PPMD (Aoyagi & Tsuchiya, 2019). The study of Smith‐Nielsen et al. (2016) and Sutter‐Dallay et al. (2011) do not explain the mechanism that links PPMD to a delayed motor development.
Regarding the other environmental factors that were examined in single studies with a high RoB, only the effect of baby walker use on GMD has been previously reviewed (Burrows & Griffiths, 2002; Pin et al., 2007). The cohort study of Siegel and Burton (1999), included in this review, was included in both reviews. Pin et al. (2007) reported conflicting evidence. Burrows and Griffiths (2002) conducted a pooled analysis of four studies and found a delay of 11 to 26 days in the onset of walking for infants using a baby walker, which is in line with the outcome of the study of Siegel and Burton (1999). Both reviews evaluated the overall study quality as poor.
6.3. Strengths and limitations
In 18 of 36 studies, mean BW and mean GA were not reported. The absence of these major characteristics made comparisons difficult. Furthermore, the characteristics of the samples varied between studies examining the same factor. This heterogeneity in population characteristics improves the generalizability of the outcomes found in this review. In addition, the QUIPS has proved to be a useful tool to assess the quality of observational studies. This approach is supported by Huguet et al. (2013) who, in addiction, advocate the use of modified GRADE standards to judge the quality of prognosis studies.
6.4. Future directions
In this review, inadequate study participation, high attrition and the lack of some robust measures for environmental factors seem to be the main causes of low study quality. Therefore, more high‐quality studies need to be performed and replicated in the field to increase the levels of evidence.
In future research, using clearly described population groups, a fixed set of confounders and measures regarding infant GMD would enable researchers to draw more firm conclusions. Results from this review suggest that BW and GA should be considered as confounders for their profound impact on GMD.
To increase the number of longitudinal studies including large cohorts of infants, feasibility should be improved by lowering the burden for both infants and parents in time and costs. Innovative and digital aids, like smartphone apps and activity trackers, are possible means for gathering large amounts of data to provide insight into the complex pathways of infant development (Boonzaaijer et al., 2017; Kwong et al., 2019; Spittle et al., 2016). Also, more robust measures for environmental factors, like the home situation, caregiving practices and parent–infant interaction, are needed. Outcomes of these ‘modifiable factors’ can be the building blocks in developing new effective interventions to improve infant GMD (Lobo & Galloway, 2012).
To date, evidence reveals that lower BW and shorter GA have a persisting negative association with GMD of infants over time. For many other factors, the association with GMD remains unclear. Overall, it can be concluded that our knowledge on what drives motor development in infants is still limited. To disentangle the complex interplay of genetic and environmental factors and their association with GMD, more research is needed.
AUTHOR CONTRIBUTIONS
All named authors have made an active contribution to the conception and design and/or analysis and interpretation of the data and/or the drafting of the paper, and all authors have critically reviewed its content and have approved the final version submitted for publication.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
he study was funded by The Netherlands Organization for Scientific Research (NWO). Both first authors conducted this research with a teacher grant (numbers 023.006.070 (MB)/023.008.043 (IS)). Proofreading by Les Hearn (Scientific Proofreading & Editing: les_hearn@yahoo.co.uk).
APPENDIX A.
SEARCH STRINGS DATABASES
Searchstring MedLine (PubMed)
Run: Feb 2016 to Feb 2020
((motor development*[tiab] OR “Motor Skills”[Mesh] OR motor skill*[tiab] OR motor milestone*[tiab] OR motor assessment*[tiab] OR motor behavior*[tiab] OR motor abilit*[tiab] OR motor growth[tiab] OR motor maturation[tiab] OR neuro maturation[tiab]) AND (“Child”[Mesh] OR “Infant”[Mesh] OR child*[tiab] OR newborn*[tiab] OR preschool*[tiab] OR infant*[tiab] OR neonate*[tiab]) AND (factor*[tiab] OR affordance*[tiab] OR constraint*[tiab] OR obstacle*[tiab] OR impediment*[tiab] OR enabler*[tiab] OR motivat*[tiab] OR inhibit*[tiab] OR stimulat*[tiab] OR correlat*[tiab] OR determin*[tiab] OR facilitat*[tiab] OR barrie*[tiab]) AND (“Cohort Studies”[Mesh] OR cohort stud*[tiab] OR concurrent stud*[tiab] OR cohort analys*[tiab] OR incidence stud*[tiab] OR cohort survey*[tiab] OR follow up stud*[tiab] OR followup stud*[tiab] OR follow up analys*[tiab] OR followup analys*[tiab] OR follow up survey*[tiab] OR followup survey*[tiab] OR longitudinal stud*[tiab] OR longitudinal analys*[tiab] OR longitudinal survey*[tiab] OR prospective stud*[tiab] OR prospective analys*[tiab] OR prospective survey*[tiab] OR retrospective stud*[tiab] OR retrospective analys*[tiab] OR retrospective survey*[tiab] OR repeated measure*[tiab])) AND (“2001/01/01”[PDat]: “3000/12/31”[PDat])
Searchstring CINAHL (EBSCO)
Run: Feb 2016 to Feb 2020
(“motor development*” OR MH “Motor Skills+” OR “motor skill*” OR “motor milestone*” OR “motor assessment*” OR “motor behavior*” OR “motor abilit*” OR “motor growth” OR “motor maturation” OR “neuro maturation”) AND (MH “Child+” OR MH “Infant+” OR child* OR newborn* OR preschool* OR infant* OR neonate*) AND (factor* OR affordance* OR constraint* OR obstacle* OR impediment* OR enabler* OR motivat* OR inhibit* OR stimulat* OR correlat* OR determin* OR facilitat* OR barrie*) AND (MH “Prospective Studies+” OR “cohort stud*” OR “concurrent stud*” OR “cohort analys*” OR “incidence stud*” OR “cohort survey*” OR “follow up stud*” OR “followup stud*” OR “follow up analys*” OR “followup analys*” OR “follow up survey*” OR “followup survey*” OR “longitudinal stud*” OR “longitudinal analys*” OR “longitudinal survey*” OR “prospective stud*” OR “prospective analys*” OR “prospective survey*” OR “retrospective stud*” OR “retrospective analys*” OR “retrospective survey*” OR MH “Repeated Measures” OR “repeated measure*”)
Limiters: Publication year 2001‐
Searchstring PsycInfo (EBSCO)
Run: Feb 2016 to Feb 2020
(DE “Motor Development” OR DE “Perceptual Motor Development” OR DE “Psychomotor Development” OR “motor development*” OR DE “Motor Skills” OR “motor skill*” OR.
“motor milestone*” OR “motor assessment*” OR “motor behavior*” OR “motor abilit*” OR “motor growth” OR “motor maturation” OR “neuro maturation”) AND (child* OR newborn* OR preschool* OR infant* OR neonate*) AND (factor* OR affordance* OR constraint* OR obstacle* OR impediment* OR enabler* OR motivat* OR inhibit* OR stimulat* OR correlat* OR determin* OR facilitat* OR barrie*) AND (ZC “prospective study” OR ZC “retrospective study” OR ZC “followup study” OR ZC “longitudinal study” OR “cohort stud*” OR “concurrent stud*” OR “cohort analys*” OR “incidence stud*” OR “cohort survey*” OR “follow up stud*” OR “followup stud*” OR “follow up analys*” OR “followup analys*” OR “follow up survey*” OR “followup survey*” OR “longitudinal stud*” OR “longitudinal analys*” OR “longitudinal survey*” OR “prospective stud*” OR “prospective analys*” OR “prospective survey*” OR “retrospective stud*” OR “retrospective analys*” OR “retrospective survey*” OR MH “Repeated Measures” OR “repeated measure*”)
Limiters: Publication year 2001‐
Searchstring SCOPUS (Elsevier)
Run: Feb 2016 to Feb 2020
TITLE‐ABS‐KEY (“motor development” OR “motor skill” OR “motor milestone” OR “motor assessment” OR “motor behavior” OR “motor ability” OR “motor growth” OR “motor maturation” OR “neuro maturation”) AND TITLE‐ABS‐KEY(“infant” OR “child” OR “newborn” OR “neonate” OR “preschool”) AND TITLE‐ABS‐KEY(“factor” OR “affordance” OR “constraint” OR “obstacle” OR “impediment” OR “enabler” OR “motivate” OR “inhibit” OR “stimulate” OR “correlate” OR “determiner” OR “facilitate” OR “barrier”) AND TITLE‐ABS‐KEY(“cohort study” OR “concurrent study” OR “cohort analysis” OR “incidence study” OR “cohort survey” OR “follow up study” OR “followup study” OR “follow up analysis” OR “followup analysis” OR “follow up survey” OR “followup survey” OR “longitudinal study” OR “longitudinal analysis” OR “longitudinal survey” OR “prospective study” OR “prospective analysis” OR “prospective survey” OR “retrospective study” OR “retrospective analysis” OR “retrospective survey” OR “repeated measure”) AND (PUBYEAR > 2000)
APPENDIX B.
Table B1. Table with characteristics and results of the included studies
| Author (year, country) | Number of participants and participant characteristics | Motor measures and ages at measurement | Factor measures and ages at measurement | Confounders (*in the final model) | Statistical analysis and results | |
|---|---|---|---|---|---|---|
| Child factors | ||||||
| Yaari (2018, Israel) |
n = 149 Groups: FT n = 39 M GA = 39.8 weeks (SD = 1.0) (range = 37.7–41.2) M BW = 3,373 g (SD = 346) MPT n = 57 M GA = 33.2 weeks (SD = 0.6) (range = 32.1–34) M BW = 1865 g (SD = 320) VPT n = 34 (NI) EPT n = 19 (NI) |
Motor: MSEL Age:1, 4, 8, 12 and 18 months |
Factor: GA Measurement: medical status FT (GA 37–41 weeks, BW > 2,500 g) MPT (GA 33–34 weeks, BW < 2,500 g) Age: at birth |
Sex* |
Regression with pairwise comparisons show that average level of gross motor outcome across time (from 1 to 18 months) is lower for MPT than for FT infants (b* = −2.19, SD = 1.09, p = 0.045). Pairwise comparisons between MPT and FT on differences between gross motor outcomes between 18 months and 1 month, shows that MPT are more delayed in GMD than FT infants (b* = − 6.60, SD = 2.23, p = 0.0036). |
|
| Espel et al. (2014, USA) |
n = 232 Groups: Early term: 27% Full term: 56% Late term: 6.6% M GA = 39.46 weeks (SD 1.06) (range = 37 0/7–41 6/7) M BW = 3,418 g (SD = 420) |
Motor: BSID‐II Age: 3, 6 12 months |
Factor: GA Measurement: ultrasound <20 weeks of gestation Early FT (37–38 weeks) FT (39–40 weeks) Late FT (41–42 weeks) Age: at birth |
BW GA Sex Birth order Ethnicity* |
ANCOVA reveals group differences in psychomotor development at each assessment age. At 3 months, PDI is lower for early FT infants than for late FT (F(2, 179) 54.01, p < 0.05). Early FT infants exhibit lower psychomotor development scores than FT and late FT infants at 6 months (F(2, 168) 56.69, p < 0.01) and 12 months (F(2,155) 55.32, p < 0.01). FT infants had lower psychomotor development scores than late FT infants at 12 months. | |
| Field et al. (1978, USA) |
n = 151 Groups: Post term n = 46 M GA = 42 weeks M BW = 3,600 g FT n = 59 M GA = 40 weeks M BW: 3,300 g Post‐term RDS n = 46 (NI) |
Motor: DDST, BSID Age: 4, 8 and 12 months |
Factor: GA Measurement: N/A Age: at birth |
No confounders considered | MANOVA showed that, at 4 months, post FT infants had inferior ratings on the DDST in comparison with the normal FT infants (p < 0.001). At 8 and 12 months, there were no significant differences between post FT and FT infants on the PDI scores. | |
| Datar & Jacknowitz (2009, USA) |
n = 7,425 Groups: Singletons n = 6,750 Twin pairs n = 625 Twins and other higher births whose siblings not included n = 50 M GA = 38.3 weeks M BW not reported |
Motor: BSID‐II SF Age: 9 and 24 months |
Factor: BW VLBW < 1,500 g MLBW 1,500–2,499 g NBW ≥ 2,500 g Measurement: weight Age: at birth |
BW GA* Sex Birth order* Height* Ethnicity* education* Income Marital status* Pregnancy/delivery risk factors* |
At 9 months, multiple linear regression revealed large and significant effects of VLBW (b* = −8,764; p < 0.001) and MLBW (b* = −2,901; p < 0.001) on GMD. At 2 years, the cross‐sectional estimates of VLBW (b* = −4.123; p < 0.001) and MLBW (b* = −1.383; p < 0.001) were considerably smaller, these changes being statistically significant at α = 0.01. This suggests some catch‐up is taking place between LBW and NBW children by age of 2 years. | |
| Grantham‐McGregor et al. (1998, Brazil) |
n = 262 Groups: ABW n = 131 LBW n = 131 GA > 37 weeks M GA and M BW not reported |
Motor: BSID Age: 6, 12 months |
Factor: BW ABW 3,000–3,499 g LBW 1,500–2,499 g Measurement: weight Age: at birth |
SES | At 6 months, multiple linear regression showed that LBW‐FT infants have significantly lower scores than ABW infants on the PDI (−7.3 points, p < 0.001). This difference increased by 12 months of age (PDI −9.9 points, p < 0.001). | |
| Lung et al. (2009a, Taiwan) |
n = 20,112 Groups: FT n = GA ≥ 37 weeks BW ≥ 2,500 g PT n = GA < 37 weeks BW < 2,500 g M GA and M BW not reported |
Motor: TBCS Age: 6, 18 months |
Factor: twin, BW Measurement: N/A Age: at birth |
BW* GA* Sex* Twin* Maternal education* Parental income* |
Using structural equation modelling at 6 months, infants of parents with a higher income and infants born FT or with normal BW showed advanced GMD (b* = 0.03, p < 0.001; b* = −0.11, p < 0.001; b* = −0.10, p < 0.001). At 18 months, infants of mothers with a higher education, and of parents with higher income, who were male, twin, born FT of normal BW, had better GMD (b* = 0.03, p < 0.001; b* = 0.06, p < 0.001; b* = 0.02, p = 0.019; b* = −0.02, p = 0.026; b* = −0.02, p = 0.036; b* = −0.05, p < 0.001). (model with p value = 0.227 and AGFI = 0.999). |
|
| Nan (2013, UK) |
n = 152 Twins M GA = 37 weeks (range = 26–39) M BW = 2,300 g (range = 940–3,500) |
Motor: ASQ Age: 3, 6, 9, 12, 18 and 24 months |
Factor: twin, BW Measurement: birth chart Age: at birth |
GA* BW Sex* |
Cross‐sectional multilevel linear regression analysis adjusted for sex and GA showed that twins scored lower on GMD than singletons (p < 0.001) during the first year of life. After the age of 12 months, twins catch up on GMD. BW was not a significant predictor of GMD at any age of measurement. | |
| Brouwer et al. (2006, The Netherlands) |
n = 3,490 Groups: Monozygotic twins n = 786 Dizygotic twins n = 1,645 Singletons n = 1,059 GA > 36.5 weeks BW > 2,500 g M GA and M BW not reported |
Motor: MM 4 milestones: turn, sit, crawl and walk Age: 0–24 months |
Factor: twin Measurement: questionnaires, blood typing Age: ≥ 3 years |
GA* BW (highly correlated with GA) |
ANOVA shows that no remarkable differences are seen between healthy singletons and healthy twins in the achievement of gross motor milestones within the normal range. Dizygotic twins were faster than monozygotic twins in reaching the moment for sit (p < 0.001), crawl (p = 0.013), stand (p < 0.001) and walk (p < 0.001). | |
| Goetghebuer et al. (2003, UK) |
n = 408 Groups: Twin pairs n = 168 M GA twins = 38.9 weeks (range = 38.7–39.2) M BW twins = 2,790 g (range = 2,700–2,800) Singletons n = 72 BW > 2,500 g M GA singletons = 38.3 weeks (range = 38.1–38.6) M BW = 3,240 g (range = 3,100–3,300) |
Motor: MM 8 milestones adapted from DDST Age: 1, 2, 3, 4, 5, 9, 12, 18 months |
Factor: twin Measurement: twin delivery Age: at birth |
BW* Number of siblings* Non‐independence within twin pairs* Length* |
Age of milestone achievement was higher in twins for each milestone and significant for: Maintaining head (p = 0.003), sitting without support (p = 0.03), walking (holding on) (p = 0.03). Age of milestone achievement was highly concordant within twins. The concordance was significantly higher (p < 0.05) in monozygotic than in dizygotic twins for crawling, sitting, standing holding on, and taking two steps. At 12 months, after adjustment for BW, length and sex, twin status and number of siblings were significantly associated with ‘parental report infant shows slower development than siblings’ (p = 0.05) and ‘maintaining head’ (p = 0.05). |
|
| Wilson and Harpring(1972, USA) |
n = 261 M GA and M BW not reported |
Motor: BSID Age: 3, 6, 9, 12 and 18 months |
Factor: Twin Measurement: blood typing Age: at birth |
BW* |
Correlations show that twins have significantly lower scores on the motor scale at 6 and 18 months. Low GA in twins has a major effect on developmental status in the first half year of life (correlations at 3, 6, 9 and 12 months r = 0.30, r = 0.40, r = 0.20, r = 0.20, by 18 and 24 months p < 0.001). |
|
| Scharf et al. (2016, USA) |
n = 950 GA: ≥37 weeks = 3% 32–37 weeks = 18% 28 < 32 weeks = 46% 22 < 28 weeks = 34% VLBW: <1,500 g Groups: Anthropometric scores < −2 SD Anthropometric scores > 2 SD |
Motor: BSID SF Age: 9 and 24 months |
Factor: weight, length and head circumference Measurement: weight Age: at birth, 9, 24 months |
BW* Sex GA |
Linear regression analysis adjusted for BW, sex and SES show that length and weight z‐scores at 9 months were correlated with (1) children's Bayley motor scores at 2 years and (2) the change in Bayley motor scores from 9 to 24 months. Children who scored more than 2 SDs below the mean in weight at 9 months showed a significant odds ratio (OR 2.64, p < 0.01) for Bayley motor scores of 2 SDs below the mean at 2 years. | |
| Slining et al. (2010, USA) |
n = 217 GA > 35 weeks M GA = 39.48 weeks (SD = 1.47) M BW = 3.23 g (SD = 0.48) |
Motor: BSID‐II Age: 3, 6, 9, 12 and 18 months |
Factor: weight Measurement: weight and subcutaneous fat Age: at birth, 3, 6, 9, 12 and 18 months |
Age* Age squared* Sex* Weight status |
Multivariate models showed that motor delay is 1.80 times more likely in overweight infants compared with nonoverweight infants (i.e., weight‐for‐length z‐score > 90th percentile) (95% CI [1.09, 2.97]) and 2.32 times as likely in infants with high subcutaneous fat compared with infants with lower subcutaneous fat (95% CI [1.26, 4.29]). High subcutaneous fat was also associated with delay in motor development (OR 2.27, 95% CI [1.08, 4.76]). | |
| Bartlett (1998, Canada) |
n = 132 BW > 2,500 g M GA and M BW not reported |
Motor: AIMS and PDMS Age: 6 weeks, 3, 5, 7, 10 and 15 months |
Factor: head proportion, BMI and body length Measurement: standard anthropometric measurements Age: 6 weeks, 3, 5, 7, 10 and 15 months |
No confounders considered | Pearson correlations between head proportion and AIMS total, and subscale scores, revealed that infants with proportionately larger heads had significant lower scores on the AIMS total (r = −0.38, p = 0.001), this outcome being fully explained by the prone motor scores at 6 weeks of age. There was no correlation between BMI and body length and motor outcome scores. | |
| Capute et al. (1985, USA) |
n = 381 M GA and M BW not reported |
Motor: MM 12 motor milestones Age: N/A |
Factor: ethnicity Measurement: N/A Age: time of recruitment |
Sex SES |
Analysis of variances show that infants with an Afro‐American background achieve motor milestones, on average, at an earlier age, except ‘roll prone to supine’. Between 4 and 5 months of age, the milestones ‘roll supine to prone’ was reached 0.5 month earlier by infants with Afro‐American background. This advantage increases to 1.1 months for the milestone ‘walk’ (10.9 months vs. 12 months). Association of ethnicity with motor gradient without adjustment is F(16.88 p < 0.01)). After adjusting for SES and sex, the association of ethnicity still exceeds p < 0.01‐level. | |
| Atun‐Einy et al. (2013, Israel) |
n = 27 M GA and M BW not reported |
Motor: AIMS (video) Age: 7–12 months/every 3 weeks |
Factor: MTM Measurement: MTM scale Age: 7–12 months/every 3 weeks. Seven measurements |
No confounders considered |
A repeated‐measures ANOVA on the MTM score over the course of the seven observations reveals a main effect for the group: F(1, 18) = 0.25, p = 0.11. A significant interaction effect (F(6, 108) = 2.96, p < 0.01) showed an increase in motivation scores by the lower scoring group across time and a decrease in motivation scores by the higher scoring group. No significant effect of time was found. Infants with higher AIMS scores had higher motivation to move scores than infants who scored lower on the AIMS. The t test shows that strongly motivated infants had earlier onset for all motor milestones (sitting, pulling‐to‐stand, hands‐and‐knees, crawling and cruising) than weakly motivated infants (t(13) = 2.39, 2.98, 2.25, 2.50 p < 0.05). Infants' MTM score was positively correlated with the AIMS percentile at the same and subsequent sessions (Pearson correlations ranging from r = 0.36 to 0.69; with only r = 0.36 ns (p = 0.06). |
|
| Environmental factors | ||||||
| Majnemer and Barr (2006, Canada) |
n = 155 GA > 38 weeks M GA and M BW not reported |
Motor: AIMS, PDMS, Battelle Developmental Inventory Age equivalent (mon) Age: 4 or 6 and 15 months |
Factor: sleep position Measurement: parental diary 3 consecutive days/24 h every 5 min Age: 4 or 6 months |
Sex* Parental education* Parental age* Parity* Weight at assessment* Age at testing* |
Linear regression showed there were no significant differences between sleep position on AIMS total score and PDMS score at 4 months. At 6 months of age, infants sleeping prone had significantly better motor scores on the AIMS total raw scores (p = 0.02) and PDMS (p = 0.03). At 15 months, no significant differences in PDMS score and Battelle developmental inventory age equivalent scores. Linear regression models at 4 months shows that the AIMS prone raw score (r 2 = 0.27, p = 0.0001) and the total raw score were predicted by sleep position (prone versus supine) (r 2 = 0.17, p = 0.0001), when adjusting for confounders. When adjusting for confounders on linear regression models, sleep position consistently predicted AIMS motor scores and Peabody gross motor quotient, accounting for 22% to 31% of the variance. Univariate analyses indicated that the Battelle gross motor subscale score was significantly associated (p = 0.05) with sleep position, which was further demonstrated on simple linear regression analysis (r 2 = 0.8, p = 0.048). At 15 months of age, prone sleepers attained motor milestones significantly earlier: walking upstairs (p = 0.04) and walking (p = 0.05). |
|
| Davis et al. (1998, USA) |
n = 351 M BW = 3,490 g (SD = 41) M GA not reported |
Motor: MM 9 motor milestones Age: 0–18 months |
Factor: sleep position Measurement: position recorded by parents: prone and supine Age measurement: 2–6 months |
BW* Sex* Maternal education* Ethnicity* Number of siblings* |
Linear regression analysis shows that prone sleepers acquire motor milestones significantly earlier for: rolling prone to supine (p < 0.002), sitting unsupported (p = 0.003), creeping (p = 0.0002), crawling (p = 0.003) and pulling to stand (p = 0.001). Walking alone was not associated with prone sleeping (p = 0.4). Increased prone playtime was associated with tripod sitting, sitting alone, crawling and pulling to stand (p < 0.05). After controlling for maternal education, ethnicity, sex, BW and number of siblings, only pulling to stand remained significant (p < 0.01). |
|
| Lung and Shu (2011, Taiwan) |
n = 1,630 Birth cohort with 7.1% infants with chronic illness included M GA and M BW not reported |
Motor: TBSC Age: 6, 18 and 36 months |
Factor: sleep position Measurement: interview at home Age: 6 months |
Maternal education* Paternal education* Acute hospital admissions* Chronic illness* |
At 6 months, structural equation model shows that infants sleeping supine had slower GMD (b* = −0.11, p < 0.001). Supine sleeping position did not affect infant development at 18 and 36 months. Other factors were associated with infant GMD at 6 months: acute hospital admission (b* = −0.07), chronic illness (b* = −0.05) and paternal education (b* = 0.06). At 18 and 36 months, maternal education (b* = 0.11 and b* = 0.07) and chronic illness (b* = −0.13 and b* = −0.05) were also associated. |
|
| Ratliff‐Schaub et al. (2001, USA) |
n = 205 GA < 34 weeks (range = 29.33–29.65) BW < 1,750 g (range = 174–1,257) M GA and M BW not reported |
Motor: BSID second edition Age: 4 and 13 months corrected age |
Factor: Sleep position Measurement: Question on infants' usual sleeping position Age: 4 and 13 months corrected age |
Maternal education* Ethnicity*, Days hospitalized*, Methyxanthine use*, Marital status* head circumference* Other maternal and infant characteristics were potential confounders, but were excluded from analysis due to p > 0.2 |
Multiple linear regression analyses show that the PDI scores of PT infants at 4 and 13 months corrected age did not differ significantly between prone sleepers and supine or side sleepers in both adjusted and unadjusted analyses (4 months: p = 0.7371; 13 months p = 0.1454). Individual items of the BSID show that supine sleepers were less likely than prone sleepers to receive credit for: maintaining head at 45° and 90° (p = 0.021) and lowering the head with control (p = 0.001). |
|
| Jardí et al. (2018, Spain) |
n = 154 GA ≥ 37 weeks BW ≥ 2,500 g |
Motor: BSID second Edition Age: 6 and 12 months |
Factor: BF (exclusive BF, mixed feeding and total time BF) Measurement: 24‐h food diary and questionnaires Age: at birth, 1, 4, 6 and 12 months |
BW* GA* Sex* Maternal education Maternal age* Maternal SES* Head circumference at birth, 6 and at 12 months* Height at birth, 6 and at 12 months* Iron status at 6 and 12 months* Infant haemoglobin at 6 and 12 months* BMI at 6 and 12 months* |
Multiple linear regression showed in the adjusted model, that exclusive BF during the first 4 months increased the PDI by 7.712 points (p = 0.019), while mixed feeding increased it by 6.393 points (p = 0.039) at 6 months. Higher GA and higher BMI increased the PDI scores (p = 0.005 and p = 0.024 respectively) At 12 months, the adjusted model showed that exclusive BF during the first 4 months increased the PDI by 7.223 points (p = 0.033), while mixed feeding did not significantly increase the PDI (b* = 4.620; p = 0.160). Higher iron status at 6 months increased the PDI scores (p = 0.015). |
|
| Michels et al. (2017, USA) |
n = 4,270 Groups: PT = 17%) FT = 83% M GA and M BW not reported |
Motor: MM Age: 4, 8, 12, 18 and 24 months |
Factor: BF (exclusive BF, mixed feeding) Measurement: Parent report Age: 4 months |
Maternal factors Ethnicity* Education* Age* BMI* PPD* Paternal factors Education* Age* Infant characteristics Sex* Plurality* Rapid weight gain until 4 months postpartum* ASQ pass/failure at 4 months* Postpartum* Conception via fertility treatment* |
Accelerated failure time models reveal that feeding differences at 4 months do not greatly affect the timing of gross motor milestone achievement. After adjustment for confounders, infants who were fed solids in addition to breastfeeding achieved standing faster than infants exclusively breastfed at 4 months (AF: 0.93; 95% CI [0.87, 0.99]). After controlling for multiple testing, these differences were no longer significant. No differences were found for PT and FT infants. | |
| Morris et al. (1999, Brazil) |
n = 262 Groups: LBW (1,500–2,499 g) n = 131 GA ≥ 37 weeks M LBW = 2,338 g (SD = 152) HBW (3,000–3,499 g) n = 131 GA ≥ 37 weeks M HBW = 3,210 g (SD = 142) |
Motor: BSID Age: 6, 12 months |
Factor: BF intensity in first 4 weeks or 5–26 weeks Measurement: frequency of BF Age: at birth, 6 and 12 months |
BW* SES* Diarrhoea morbidity* |
Weak and non‐significant correlations were observed between BF intensity in weeks 1–4 and 6 months. There was no association between BF intensity over weeks 5–26 and PDI scores at 6 and 12 months. Multiple linear regression models, adjusted for confounders, showed that BF frequency over the first 4 weeks of life was significantly associated with motor development at 6 months in both LBW and HBW infants (b* = 0.23; 95% CI [0.00–0.45]; p = 0.047). |
|
| Oddy et al. (2011, Australia) |
n = 2,868 All infants eligible M GA = 38.8 weeks (SD = 2.13) M BW not reported |
Motor: IMQ Age: 24, 26 and 36 months |
Factor: BF duration Measurement: parental questionnaire Age: 0–12 months |
GA* Sex* Maternal education* Maternal age* Maternal smoking in pregnancy* Biological father living with family* Total family income* Total amount of stressful life events during pregnancy* Apgar score infant at 5 min* |
Overall, t tests show no significant differences in GMD of infants who were breastfed <4 months and >4 months. In subsequent analysis separated by sex, boys receiving BF < 4 months did have an increased risk for one atypical score on GMD at one time point between 0 and 3 years (OR 2.03; 95% CI [1.17, 3.50]; p = 0.011). | |
| Smith‐Nielsen et al. (2016, Denmark) |
n = 83 Groups: PPD‐group n = 53: M GA 40.2 weeks (SD = 1.3) M BW 3,466 g (SD = 450) Control group n = 83 M GA = 40.6 weeks (SD = 1.2) M BW = 3,583 g (SD = 526) |
Motor: BSID‐III Age: 4, 13 months |
Factor: maternal PPD Measurement: EPDS Age of measurement: 4, 13 months |
Sex* Maternal co‐morbid personality disorder* |
Multivariate analyses of variance (MANOVA) showed no significant effects of PPD on motor scales at 4 and 13 months. Also, after adjustment for confounders, the effect remained non‐significant (at 4 months p = 0.187; at 13 months p = 0.562). |
|
| Sutter‐Dallay et al. (2011, France) |
n = 515 BW < 2,500 g = <1% M GA and M BW not reported |
Motor: BSID‐II Age:3, 6, 12, 18 and 24 months |
Factor: maternal depression Measurement: EPDS Age: 6 weeks, 3, 6, 12, 18 and 24 months |
GA* Maternal education level* Maternal age* Mean income* Parity* EPDS score* |
Multivariate regression models revealed no concurrent association between EPDS scores and infant motor scores over the follow up (b* = 0.60; 95% CI [−0.40, 1.60]; p = 0.24). This association remained non‐significant after adjustment for EPDS score at the time of infant assessment. | |
| Lung, Shu, Chiang et al. (2011, Taiwan) |
n = 1,693 All infants eligible M GA and M BW not reported |
Motor: BSID Age: 6, 18 and 36 months |
Factor: maternal mental health Measurement: Interview, SF‐36 Age: 6 months |
Maternal education* Parental income* Family support* |
Structural equation analysis showed that maternal mental health at 6 months was not significantly associated with GMD of infants at 6, 18 and 36 months. The study revealed the association of GMD with several other factors like family support, prenatal income and maternal education. | |
| de Borba and Valentini (2015, Brazil) |
n = 40 Groups: Infants with adolescent mothers M GA = 37.3 (SD = 2.7) M BW = 2,914 (SD = 734) Infants with adult mothers M GA = 38.7 (SD 2.4) M BW = 3,194 (SD 539) |
Motor: AIMS Age: three assessments with an interval of 2 months between 0 and 18 months |
Factor: maternal age Adolescent: 15–19 years Adult: 25–39 years Measurement: questionnaire Age: maternal age at infant birth |
No confounders considered |
Generalized estimated equations showed that AIMS percentile (F(938.2) = 0.003, p = 0.874) and total AIMS score (F(38.2) = 0.085; p = 0.755) did not differ between infants of adolescent mothers and adult mothers. Infants of adolescent mothers had lower scores in the third evaluation in supine position (p = 0.046). |
|
| Lung et al. (2009b, Taiwan) |
n = 17,595 M GA and M BW not reported |
Motor: TBCS Age: 6,18 months |
Factor: Parental mental health Measurement: SF‐36 Age: 6 months |
Parental education* Parental age* |
Multiple linear regression showed that parental mental health (paternal and maternal) was not significantly associated with children's 6‐month development (paternal b* = −0.01, t = 1.04, p = 0.298; maternal b* = 0.01, t = 0.74, p = 0.458). At 18 months, only maternal mental health was predictive of infants' GMD (maternal b* = 0.017, p = 0.01). When the covariates of parental education and age of childbirth were added, the effect of maternal mental health decreased (b* = 0.02, t = 2.12, p = 0.034). |
|
| Hernández‐Martínez et al. (2011, Spain) |
n = 72 M GA = 39.8 weeks (SD = 1.32) M BW = 3,277.7 g (SD = 456.23) |
Motor: BSID Age: birth, 12 months |
Factor: parental neonatal perceptions Measurement: NPI Age: 3 days, 3 months |
GA* BW SES Father and mother neonatal perception scores* NBAS (endurance item)* |
Using stepwise multiple regression models, more negative maternal neonatal perceptions (b* = −0.325, p = 0.024) and a higher GA (b* = 0.340, p = 0.018) predicted psychomotor development at 4 months and accounted for 21.8% of the variance. At 12 months, paternal neonatal perceptions (b* = 0.383, p = 0.010), together with the NBAS endurance item (b* = 0.339, p = 0.021) were significant in accounting for 17.2% variance of the psychomotor development. | |
| Siegel and Burton, (1999, USA) |
n = 109 M GA and M BW not reported |
Motor: BSID, MM Age: 6 and 9 months (n = 34) 9 and 12 months (n = 35) 12 and 15 months (n = 40) |
Factor: use of a baby walker Measurement: exposure baby walker from parent interview Age: 6 and 9, 9 and 12, and 12 and 15 months |
Parental education | A three‐by‐three between‐subjects MANCOVA showed a significant effect of walker experience on infants' motor milestones in general (multivariate F(6,154) = 4.81 p = <0.0005). The univariate test showed that the use of a baby walker significantly affects the developmental onset of sitting, crawling and walking (F(2,79) = 11.07, 4.97 and 4.25, p = 0.0005, p = 0.01 and p = 0.02), with a later onset of the motor milestones. A significant main effect of the use of a baby walker was observed for motor and mental scores considered together (multivariate F(4,196) = 6.16 p < 0.0005). The univariate tests showed significant effects for motor development (F(2,99) = 6.06, p < 0.03). Parental education was added as a covariate in the analyses. | |
| Souza et al. (2010, Brazil) |
n = 30 Groups: FT = 86.2% PT = 13.8% M GA, M BW not reported |
Motor: BSID‐III Age: 12, 17 months |
Factor: daycare attendance Measurement: full time daycare attendance Age: 0–17 months |
No confounders considered | Descriptive statistics showed that 13% (n = 4) of the infants attending daycare full‐time had suspected delays in GMD at 12 and 17 months, according to the reference means of the BSID. Of these four infants, one infant was PT with LBW. | |
| Tsuchiya et al. (2012, Japan) |
n = 742 GA = 39.0–39.2 weeks BW = 2,948–2,985 g Infants with pathology affecting motor function n = 5 M GA and M BW not reported |
Motor: MSEL Age: 6, 10, 14 months |
Factor: seasonal variation Measurement: month of birth Age: at birth |
GA BW Sex SES Parental ages Parity |
In a linear regression model (month of birth is transformed to a trigonometric form), the season of birth was significantly associated with GMD at 6 months (F(2,736) = 21.71, p < 0.001 and 10 months (F(2,736) = 12.36, p < 0.001. At 14 months, the season of birth was not significantly associated with gross motor score (F(2,736) = 1.21, p = 0.30). Infants born in Mar to Apr show a peak in GMD and those born in autumn (Sep to Oct) show the lowest GMD scores. The cyclic fluctuation of motor development according to month of birth disappears at 14 months of age. | |
| Vierhaus et al. (2011, Cameroon/Germany) |
n = 345 Groups: Cameroonian infants n = 73 German infants n = 272 M GA and M BW not reported |
Motor: BSID III Age: 3, 6 and 9 months |
Factor: cultural context Measurement: N/A Age: time of inclusion |
No confounders considered | Univariate analysis of variance of the BSID outcomes, depending on cultural background (Cameroonian Nso versus Germans, between subjects factor) and cultural background by age (3, 6 and 9 months, within subjects factor), showed large differences between the two cultural backgrounds (F = 65.58; df 1/251; p < 0.001; η 2 = 0.207) in favour of the Cameroonian infants at 3 months. These differences decrease over time and are almost non‐existent at 9 months (F = 23.63; df 2/502; p = <0.001; η 2 = 0.086). The largest deviance is related to GMD at 6 months due to items as sitting and standing being reached by Cameroonian infants much earlier than German ones. The sequence of BSID items differ between the groups. | |
| Multiple factors | ||||||
| Bjarnadóttir et al. (2019, Denmark) |
n = 650 GA > 37 weeks BW > 2,500 g |
Motor: MM 13 predefined milestones Age: N/A |
Factor: BF, predictors pregnancy and birth, home environment Measurement: interviews/questionnaires Age: ongoing parental interviews from 1 week to 24 months |
GA* BW Sex* SES Maternal age* Maternal education Paternity leave* |
Principal components analysis was used to analyse motor milestone outcomes, grouping the milestones into ‘late’ and ‘early with late in opposite directions’. Multivariate analysis showed that sex, GA and maternal age (M = 0.32, p = 0.05. b* = −0.23, p = < 0.001 and b* = 0.05, p = 0.02, respectively) were significant predictors for the achievement of later milestones (crawling, walking and standing). Boys achieved these late milestones at an earlier age. For the early milestones, GA (b* = −0.11, p = 0.01) and paternity leave (M = −0.28, p = 0.01) were significant predictors. Linear and logistic regression analysis revealed that motor milestone achievement from 1 to 24 months was not significantly related to BF duration (exclusive or total). |
|
| Flensborg‐Madsen & Mortensen (2017, Denmark) |
n = 5,601 M GA = 39.1 weeks (range = 27–46.5) M BW = 3,250 g (range = 850–5,450) |
Motor: MM Age: N/A |
Factor: GA, BW and other predictors Measurement: questionnaire, measurements Age: at birth and 12 months |
Sex | Multiple linear regression analysis showed that most of explained variance (14.5%) in motor milestone attainment is due to GA (b* = −0.15; p < 0.001) and BW (b* = −0.16; p ≤ 0.001), after adjustment for confounders. Other predictors (p values ≤ 0.10 were considered significant) in the final model were: BF, paternal age, higher birth order, weight increase (all negative associations) and larger head (positive association). | |
| Pereira et al. (2016, Brazil) |
n = 49 Groups: Preterm n = 12 (24.5%) Term n = 37 (75.5%) M GA = 38.20 weeks (range = 32–42 weeks M BW = 3,156 g (range = 2,200–3,995 g |
Motor: AIMS Age: three assessments from 2 to 12 months |
Factor: home environment, maternal practices, cognition Measurement: DAIS, A‐HEMD‐IS and KIDI Age: between 2 and 12 months |
GA BW Sex* Cognition* DAIS score* Family income* Mechanical ventilation* |
Generalized estimating equations used for longitudinal analysis showed that the scores on motor development increased over time and strongly significant correlations were found between the motor outcomes at the three time points. Multivariate analysis revealed at assessment 1 that: family income (p = 0.011), score on cognition (p > 0.001), days of mechanical ventilation (p = 0.099) and being put in more stimulating and independent positions (p = 0.037) explained motor performance significantly (Adj R 2 = 0.876). At assessment 2, the multivariate model included cognition (p > 0.001) and family income (p = 0.003, Adj R 2 = 0.860); at the third assessment, only cognition (p > 0.001) remained in the model (Adj R 2 = 0.751). Variability in motor development is better explained by environment and parental knowledge and practice. | |
Abbreviations: ABW, adequate birthweight; AHEMD‐IS, Affordances of the home environment—Infant‐Scale; AIMS, Alberta Infant Motor Scale; ASQ, Ages and Stages Questionnaire; BSID, Bayley Scales of Infant Development; BW, birthweight; DAIS, Daily Activities of Infants Scale; DDST, Denver Developmental Screening Test; EPDS, Edinburgh Postnatal Depression Scale; EPT, extremely preterm; FT, full term; HBW, high birthweight; GA, gestational age; IMQ, Infant Motor Quotient (now ASQ); KIDI, Knowledge Infant Development Inventory; LBW, low birthweight; M, mean; M‐ABC, Movement‐ABC; MM, motor milestones; MLBW, medium low birthweight; MPT, moderately preterm; MSEL, Mullen Scale of Early Learning; MTM scale, motivation to move scale; N/A, not applicable; NBAS, Neonatal Behavioural Assessment Scale; NBW, normal birthweight; NI, not included in the results of this review due to pathology; NPI, Neonatal Perception Inventory; PDI, Psychomotor Developmental Index; PDMS, Peabody Developmental Motor Scales; PPD, postpartum depression; PT, preterm; RDS, respiratory distress syndrome; SES, socio‐economic status; SF‐36, 36‐item Short Form Health Survey; TBCS, Taiwanese Birth Cohort Study developmental instrument; VLBW, very low birthweight; VPT, very preterm.
Table B2. Table with confounders of the included studies
| Child confounders | Environmental confouders | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Age | Anthropometry | Birth order | BW | Ethnicity | GA | Plurality | Sex | Other child factors | Marital status | Maternal age | Maternal education | Number of siblings | Parity | Parental income/SES | Paternal age | Paternal education | Other environmental factors |
| Child factors | ||||||||||||||||||
| GA | ||||||||||||||||||
| Preterm | ||||||||||||||||||
| Yaari et al. (2018) | X | |||||||||||||||||
| Term | ||||||||||||||||||
| Espel et al. (2014) | – | – | X | – | – | |||||||||||||
| Post‐term | ||||||||||||||||||
| Field et al. (1978) | ||||||||||||||||||
| Birthweight | ||||||||||||||||||
| Low BW | ||||||||||||||||||
|
Datar and Jacknowitz (2009) |
X1 | X | – | X | X | X2 | X | X | – | X | ||||||||
| Grantham‐McGregor et al. (1998) | ‐s | |||||||||||||||||
| Normal BW | ||||||||||||||||||
| Lung et al. (2009a) | X | X | Xtwin | X | X | X | ||||||||||||
| Twin | ||||||||||||||||||
| Nan et al. (2013) | – | X | X | |||||||||||||||
| Brouwer et al. (2006) | – | X | ||||||||||||||||
| Goetghebuer et al. (2003) | X3 | X | X4 | X | ||||||||||||||
| Wilson and Harpring (1972) | X | |||||||||||||||||
| Anthropometry | ||||||||||||||||||
| Weight, length and head circumference | ||||||||||||||||||
| Scharf et al. (2016) | X | – | – | |||||||||||||||
| Overweight | ||||||||||||||||||
| Slining et al. (2010) | X | – | X | X5 | ||||||||||||||
| Proportional larger head | ||||||||||||||||||
| Bartlett (1998) | ||||||||||||||||||
| Afro‐American background | ||||||||||||||||||
| Capute et al. (1985) | ‐ | ‐s | ||||||||||||||||
| Motivation to move | ||||||||||||||||||
| Atun‐Einy et al. (2013) | ||||||||||||||||||
| Environmental factors | ||||||||||||||||||
| Sleep position | ||||||||||||||||||
| Prone sleeping | ||||||||||||||||||
|
Majnemer and Barr (2006) |
Xtest | X6 | X | X | X | X | X | X | ||||||||||
| Davis et al. (1998) | X | X | X | X | X | |||||||||||||
| Supine sleeping | ||||||||||||||||||
| Lung and Shu (2011) | X7,8 | X | X | X7,8 | ||||||||||||||
|
Ratliff‐Schaub et al. (2001) |
X9 | X | X10 | X | X | X11 | ||||||||||||
| Breastfeeding | ||||||||||||||||||
| Jardí et al. (2018) | X12,13, 14, 15, 16 | X | X | X | X | – | Xs | |||||||||||
| Michels et al. (2017) | X | X | X20,21, 22 | X | X | X | X | X17,18,19 | ||||||||||
| Morris et al. (1999) | X | X23 | Xs | |||||||||||||||
| Oddy et al. (2011) | X | X | X24 | X | X | X | X25,26,27 | |||||||||||
| Maternal depression | ||||||||||||||||||
| Smith‐Nielsen et al. (2016) | X | X28 | ||||||||||||||||
| Sutter‐Dallay et al. (2011) | X | X | X | X | X | X29 | ||||||||||||
| Maternal mental health | ||||||||||||||||||
| Lung, Shu, Chiang et al. (2011) | X | Xpaternal | X30 | |||||||||||||||
| Adolescent mother | ||||||||||||||||||
|
de Borba and Valentini (2015) |
||||||||||||||||||
| Parental mental health | ||||||||||||||||||
| Lung et al. (2009b) | X | X | X | X | ||||||||||||||
| Parental neonatal perception | ||||||||||||||||||
| Hernández‐Martínez et al. (2011) | – | X | – | X31,32 | ||||||||||||||
| Babywalker | ||||||||||||||||||
|
Siegel and Burton (1999) |
X | X | ||||||||||||||||
| Daycare attendance | ||||||||||||||||||
| Souza et al. (2010) | ||||||||||||||||||
| Season of birth | ||||||||||||||||||
| Tsuchiya et al. (2012) | – | – | – | – | – | ‐s | – | |||||||||||
| Cameroonian versus German culture | ||||||||||||||||||
| Vierhaus et al. (2011) | ||||||||||||||||||
| Multiple factors | ||||||||||||||||||
| Bjarnadóttir et al. (2019) | – | X | X | X | X33 | |||||||||||||
| Flensborg‐Madsen & Mortensen, 2017 | – | |||||||||||||||||
| Pereira et al. (2016) | X34,35 | – | – | X | X36 | |||||||||||||
Note. X = factor in final model; – = factor considered, but not in final model; ‐s = factor SES considered, but not in final model; 1 = height; 2 = pregnancy/delivery risk factors; 3 = length; 4 = non‐independence within twin pairs; 5 = age squared; 6 = weight at all assessments; 7 = acute hospital admission; 8 = chronic illness; 9 = head circumference; 10 = days hospitalized; 11 = methyxanthine use; 12 = infant iron status; 13 = infant haemoglobin level; 14 = head circumference at birth, 6 and 12 months; 15 = height at birth, 6 and at 12 months; 16 = BMI at 6 and 12 months; 17 = maternal BMI; 18 = maternal postpartum depression; 19 = maternal ethnicity; 20 = rapid weight gain until 4 months postpartum; 21 = ASQ pass/failure at 4 months; 22 = conception via fertility treatment; 23 = diarrhoea morbidity; 24 = APGAR score infant at 5 min; 25 = total amount of stressful life events during pregnancy; 26 = biological father living with family; 27 = maternal smoking; 28 = maternal co‐morbid personality disorder; 29 = EPDS score mother; 30 = family support; 31 = parental neonatal perceptions; 32 = NBAS (Neonatal Behavioural Assessment Scale) endurance item; 33 = paternity leave; 34 = cognition; 35 = Mechanical ventilation; 36 = DAIS score (Daily Activities of Infants Scale).
Boonzaaijer M, Suir I, Mollema J, Nuysink J, Volman M, Jongmans M. Factors associated with gross motor development from birth to independent walking: A systematic review of longitudinal research. Child Care Health Dev. 2021;47:525–561. 10.1111/cch.12830
Imke Suir and Marike Boonzaaijer should be considered joint first authors.
Funding information Netherlands Organization for Scientific Research (NWO), Grant/Award Number: 023.006.070 (MB)/023.008.043 (IS)
Contributor Information
Marike Boonzaaijer, Email: m.boonzaaijer@hu.nl.
Imke Suir, Email: imke.suir@hu.nl.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Adolph, K. E. , & Hoch, J. E. (2019). Motor development: Embodied, embedded, enculturated, and enabling. Annual Review of Psychology, 70, 141–164. 10.1146/annurev-psych-010418-102836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph, K. E. , Robinson, S. R. , Young, J. W. , & Gill‐Alvarez, F. (2008). What is the shape of developmental change? Psychological Review, 115(3), 527. 10.1037/0033-295X.115.3.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi, S. , & Tsuchiya, K. J. (2019). Does maternal postpartum depression affect children's developmental outcomes? Journal of Obstetrics and Gynaecology Research, 45(9), 1809–1820. 10.1111/jog.14064 [DOI] [PubMed] [Google Scholar]
- Atun‐Einy, O. , Berger, S. E. , & Scher, A. (2013). Assessing motivation to move and its relationship to motor development in infancy. Infant Behavior and Development, 36(3), 457–469. 10.1016/j.infbeh.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Bartlett, D. J. (1998). Relationship between selected anthropometric characteristics and gross motor development among infants developing typically. Pediatric Physical Therapy, 10(3), 114–119. Retrieved from https://journals.lww.com/pedpt/pages/articleviewer.aspx?year=1998&issue=01030&article=00005&type=abstract [Google Scholar]
- Bjarnadóttir, E. , Stokholm, J. , Chawes, B. , Thorsen, J. , Mora‐Jensen, A. C. , Deleuran, M. , & Bisgaard, H. (2019). Determinants of neurodevelopment in early childhood—Results from the Copenhagen prospective studies on asthma in childhood (COPSAC2010) mother‐child cohort. Acta Paediatrica, 108(9), 1632–1641. 10.1111/apa.14753 [DOI] [PubMed] [Google Scholar]
- Boonzaaijer, M. , van Dam, E. , van Haastert, I. C. , & Nuysink, J. (2017). Concurrent validity between live and home video observations using the Alberta infant motor scale. Pediatric Physical Therapy, 29(2), 146–151. 10.1097/PEP.0000000000000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, S. I. , van Beijsterveldt, T. C. , Bartels, M. , Hudziak, J. J. , & Boomsma, D. I. (2006). Influences on achieving motor milestones: A twin–singleton study. Twin Research and Human Genetics, 9(3), 424–430. 10.1375/twin.9.3.424 [DOI] [PubMed] [Google Scholar]
- Burrows, P. , & Griffiths, P. (2002). Do baby walkers delay onset of walking in young children? British Journal of Community Nursing, 7(11), 581–586. 10.12968/bjcn.2002.7.11.10889 [DOI] [PubMed] [Google Scholar]
- Capute, A. J. , Shapiro, B. K. , Palmer, F. B. , Ross, A. , & Wachtel, R. C. (1985). Normal gross motor development: The influences of race, sex and socio‐economic status. Developmental Medicine and Child Neurology, 27(5), 635–643. 10.1111/j.1469-8749.1985.tb14136.x [DOI] [PubMed] [Google Scholar]
- Carmeli, E. , Marmur, R. , Cohen, A. , & Tirosh, E. (2009). Preferred sleep position and gross motor achievement in early infancy. European Journal of Pediatrics, 168(6), 711–715. 10.1007/s00431-008-0829-4 [DOI] [PubMed] [Google Scholar]
- Cochrane Collaboration . (2012). Review manager (RevMan) [Computer program]. Version 5.2 Copenhagen: The Nordic Cochrane Centre. [Google Scholar]
- Datar, A. , & Jacknowitz, A. (2009). Birth weight effects on children's mental, motor, and physical development: Evidence from twins data. Maternal and Child Health Journal, 13(6), 780. 10.1007/s10995-009-0461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, B. E. , Moon, R. Y. , Sachs, H. C. , & Ottolini, M. C. (1998). Effects of sleep position on infant motor development. Pediatrics, 102(5), 1135–1140. 10.1542/peds.102.5.1135 [DOI] [PubMed] [Google Scholar]
- de Borba, L. S. , & Valentini, N. C. (2015). Motor and cognitive development of infants of adolescent and adult mothers: Longitudinal study. Brazilian Journal of Kinanthropometry and Human Performance, 17(4), 438–449. 10.5007/1980-0037.2015v17n4p438 [DOI] [Google Scholar]
- De Kegel, A. , Peersman, W. , Onderbeke, K. , Baetens, T. , Dhooge, I. , & Van Waelvelde, H. (2013). New reference values must be established for the Alberta infant motor scales for accurate identification of infants at risk for motor developmental delay in Flanders. Child: Care, Health and Development, 39(2), 260–267. 10.1111/j.1365-2214.2012.01384.x [DOI] [PubMed] [Google Scholar]
- de Kieviet, J. F. , Piek, J. P. , Aarnoudse‐Moens, C. S. , & Oosterlaan, J. (2009). Motor development in very preterm and very low‐birth‐weight children from birth to adolescence: A meta‐analysis. JAMA, 302(20), 2235–2242. 10.1001/jama.2009.1708 [DOI] [PubMed] [Google Scholar]
- Espel, E. V. , Glynn, L. M. , Sandman, C. A. , & Davis, E. P. (2014). Longer gestation among children born full term influences cognitive and motor development. PLoS ONE, 9(11), e113758. 10.1371/journal.pone.0113758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, T. , Hallock, N. , Ting, G. , Dempsey, J. , Dabiri, C. , & Shuman, H. H. (1978). A first‐year follow‐up of high‐risk infants: Formulating a cumulative risk index. Child Development, 49(1), 119–131. Retrieved from https://www.jstor.org/stable/pdf/1128600 [PubMed] [Google Scholar]
- Flensborg‐Madsen, T. , & Mortensen, E. L. (2017). Predictors of motor developmental milestones during the first year of life. European Journal of Pediatrics, 176(1), 109–119. 10.1007/s00431-016-2817-4 [DOI] [PubMed] [Google Scholar]
- Goetghebuer, T. , Ota, M. O. , Kebbeh, B. , John, M. , Jackson‐Sillah, D. , Vekemans, J. , Marchant, A. , Newport, M. , & Weiss, H. A. (2003). Delay in motor development of twins in Africa: A prospective cohort study. Twin Research and Human Genetics, 6(4), 279–284. Retrieved from https://search.informit.com.au/documentSummary;dn=976292568792151;res=IELHEA ISSN: 1832‐4274. [cited 08 Apr 20]. 10.1375/136905203322296629 [DOI] [PubMed] [Google Scholar]
- Golding, J. , Emmett, P. , Iles‐Caven, Y. , Steer, C. , & Lingam, R. (2014). A review of environmental contributions to childhood motor skills. Journal of Child Neurology, 29(11), 1531–1547. 10.1177/883073813507483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham‐McGregor, S. M. , Lira, P. I. , Ashworth, A. , Morris, S. S. , & Assunçao, A. M. (1998). The development of low birth weight term infants and the effects of the environment in Northeast Brazil. The Journal of Pediatrics, 132(4), 661–666. 10.1016/S0022-3476(98)70357‐9 [DOI] [PubMed] [Google Scholar]
- Hayden, J. A. , van der Windt, D. A. , Cartwright, J. L. , Côté, P. , & Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Annals of Internal Medicine, 158(4), 280–286. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- Hayden, J. A. , Wilson, M. N. , Riley, R. D. , Iles, R. , Pincus, T. , & Ogilvie, R. (2019). Individual recovery expectations and prognosis of outcomes in non‐specific low back pain: Prognostic factor review. Cochrane Database of Systematic Reviews, 11, 1–126. 10.1002/14651858.CD011284.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger, M. L. , Overpeck, M. D. , Ruan, W. J. , & Troendle, J. F. (2002). Birthweight and gestational age effects on motor and social development. Paediatric and Perinatal Epidemiology, 16(1), 33–46. 10.1046/j.1365-3016.2002.00393.x [DOI] [PubMed] [Google Scholar]
- Hernández‐Martínez, C. , Canals Sans, J. , & Fernández‐Ballart, J. (2011). Parents' perceptions of their neonates and their relation to infant development. Child: Care, Health and Development, 37(4), 484–492. 10.1111/j.1365-2214.2011.01210.x [DOI] [PubMed] [Google Scholar]
- Hewitt, L. , Stanley, R. M. , & Okely, A. D. (2017). Correlates of tummy time in infants aged 0–12 months old: A systematic review. Infant Behavior and Development, 49, 310–321. 10.1016/j.infbeh.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Huguet, A. , Hayden, J. A. , Stinson, J. , McGrath, P. J. , Chambers, C. T. , Tougas, M. E. , & Wozney, L. (2013). Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Systematic Reviews, 2(1), 71. 10.1186/2046-4053-2-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardí, C. , Hernández‐Martínez, C. , Canals, J. , Arija, V. , Bedmar, C. , Voltas, N. , & Aranda, N. (2018). Influence of breastfeeding and iron status on mental and psychomotor development during the first year of life. Infant Behaviour Development, 50, 300–310. 10.1016/j.infbeh.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Khan, A. A. , Mohiuddin, O. , Wahid, I. , Khan, B. S. , & Khan, S. H. (2019). Predicting the relationship between breastfeeding and gross motor milestones development: The practice and prevalence of breastfeeding in metropolitan areas of Sindh, Pakistan. Cureus, 11(2), e4039. 10.7759/cureus.4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, A. K. , Eeles, A. L. , Olsen, J. E. , Cheong, J. L. , Doyle, L. W. , & Spittle, A. J. (2019). The Baby Moves smartphone app for general movements assessment: Engagement amongst extremely preterm and term‐born infants in a state‐wide geographical study. Journal of Paediatrics and Child Health, 55(5), 548–554. 10.1111/jpc.14240 [DOI] [PubMed] [Google Scholar]
- Leventakou, V. , Roumeliotaki, T. , Koutra, K. , Vassilaki, M. , Mantzouranis, E. , Bitsios, P. , & Chatzi, L. (2015). Breastfeeding duration and cognitive, language and motor development at 18 months of age: Rhea mother‐child cohort in Crete, Greece. Journal of Epidemiology and Community Health, 69(3), 232–239. 10.1136/jech-2013-202500 [DOI] [PubMed] [Google Scholar]
- Lobo, M. A. , & Galloway, J. C. (2012). Enhanced handling and positioning in early infancy advances development throughout the first year. Child Development, 83(4), 1290–1302. 10.1111/j.1467-8624.2012.01772.x [DOI] [PubMed] [Google Scholar]
- Lung, F. , & Shu, B. (2011). Sleeping position and health status of children at six‐, eighteen‐and thirty‐six‐month development. Research in Developmental Disabilities, 32(2), 713–718. 10.1016/j.ridd.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Lung, F. , Shu, B. , Chiang, T. , & Lin, S. (2009a). Twin–singleton influence on infant development: A national birth cohort study. Child: Care, Health and Development, 35(3), 409–418. 10.1111/j.1365-2214.2009.00963.x [DOI] [PubMed] [Google Scholar]
- Lung, F. , Shu, B. , Chiang, T. , & Lin, S. (2009b). Parental mental health, education, age at childbirth and child development from six to 18 months. Acta Paediatrica, 98(5), 834–841. 10.1111/j.1651-2227.2008.01166.x [DOI] [PubMed] [Google Scholar]
- Lung, F. W. , Shu, B. C. , Chiang, T. L. , & Lin, S. J. (2011). Maternal mental health and childrearing context in the development of children at 6, 18 and 36 months: a Taiwan birth cohort pilot study. Child Care Health Development, 37(2), 211–223. 10.1111/j.1365-2214.2010.01163 [DOI] [PubMed] [Google Scholar]
- Majnemer, A. , & Barr, R. G. (2006). Association between sleep position and early motor development. The Journal of Pediatrics, 149(5), 623–629. 10.1016/j.jpeds.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Michels, K. A. , Ghassabian, A. , Mumford, S. L. , Sundaram, R. , Bell, E. M. , Bello, S. C. , & Yeung, E. H. (2017). Breastfeeding and motor development in term and preterm infants in a longitudinal US cohort. The American Journal of Clinical Nutrition, 106(6), 1456–1462. 10.3945/ajcn.116.144279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- * Morris, S. S. , Grantham‐McGregor, S. M. , Lira, P. I. , Assunçãao, A. , & Ashworth, A. (1999). Effect of breastfeeding and morbidity on the development of low birthweight term babies in Brazil. Acta Paediatrica, 88(10), 1101–1106. 10.1080/08035259950168162 [DOI] [PubMed] [Google Scholar]
- Nan, C. , Piek, J. , Warner, C. , Mellers, D. , Krone, R. E. , Barrett, T. , & Zeegers, M. P. (2013). Trajectories and predictors of developmental skills in healthy twins up to 24 months of age. Infant Behavior and Development, 36(4), 670–678. 10.1016/j.infbeh.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Oddy, W. H. , Robinson, M. , Kendall, G. E. , Li, J. , Zubrick, S. R. , & Stanley, F. J. (2011). Breastfeeding and early child development: A prospective cohort study. Acta Paediatrica, 100(7), 992–999. 10.1111/j.1651-2227.2011.02199.x [DOI] [PubMed] [Google Scholar]
- Pascal, A. , Govaert, P. , Oostra, A. , Naulaers, G. , Ortibus, E. , & Van den Broeck, C. (2018). Neurodevelopmental outcome in very preterm and very‐low‐birthweight infants born over the past decade: A meta‐analytic review. Developmental Medicine and Child Neurology, 60(4), 342–355. 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- Pereira, K. R. , Valentini, N. C. , & Saccani, R. (2016). Brazilian infant motor and cognitive development: Longitudinal influence of risk factors. Pediatrics International, 58(12), 1297–1306. 10.1111/ped.13021 [DOI] [PubMed] [Google Scholar]
- Pin, T. , Eldridge, B. , & Galea, M. P. (2007). A review of the effects of sleep position, play position, and equipment use on motor development in infants. Developmental Medicine and Child Neurology, 49(11), 858–867. 10.1111/j.1469-8749.2007.00858.x [DOI] [PubMed] [Google Scholar]
- Ratliff‐Schaub, K. , Hunt, C. E. , Crowell, D. , Golub, H. , Smok‐Pearsall, S. , Palmer, P. , & O'bell, R. (2001). Relationship between infant sleep position and motor development in preterm infants. Journal of Developmental and Behavioral Pediatrics, 22(5), 293–299. 10.1097/00004703-200110000-00003 [DOI] [PubMed] [Google Scholar]
- Scharf, R. J. , Stroustrup, A. , Conaway, M. R. , & DeBoer, M. D. (2016). Growth and development in children born very low birthweight. Archives of Disease in Childhood. Fetal and Neonatal Edition, 101(5), 433. 10.1136/archdischild-2015-309427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, A. C. , & Burton, R. V. (1999). Effects of baby walkers on motor and mental development in human infants. Journal of Developmental and Behavioral Pediatrics: JDBP, 20(5), 355–361. 10.1097/00004703-199910000-00010 [DOI] [PubMed] [Google Scholar]
- Slining, M. , Adair, L. S. , Goldman, B. D. , Borja, J. B. , & Bentley, M. (2010). Infant overweight is associated with delayed motor development. The Journal of Pediatrics, 157(1), 20–25.e1. 10.1016/j.jpeds.2009.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Nielsen, J. , Tharner, A. , Krogh, M. T. , & Vaever, M. S. (2016). Effects of maternal postpartum depression in a well‐resourced sample: Early concurrent and long‐term effects on infant cognitive, language, and motor development. Scandinavian Journal of Psychology, 57(6), 571–583. 10.1111/sjop.12321 [DOI] [PubMed] [Google Scholar]
- Souza, C. T. , Santos, D. C. , Tolocka, R. E. , Baltieri, L. , Gibim, N. C. , & Habechian, F. A. (2010). Assessment of global motor performance and gross and fine motor skills of infants attending day care centers. Brazilian Journal of Physical Therapy, 14(4), 309–315. Retrieved from https://coffito.gov.br/nsite/wp‐content/uploads/comunicao/RevistasCientificas/revistaBrasileiraFisioterapia/VOL_4_julhoAgosto_2010_eng.pdf#page=41 [PubMed] [Google Scholar]
- Spittle, A. J. , Olsen, J. , Kwong, A. , Doyle, L. W. , Marschik, P. B. , Einspieler, C. , & Cheong, J. (2016). The baby moves prospective cohort study protocol: Using a smartphone application with the general movements assessment to predict neurodevelopmental outcomes at age 2 years for extremely preterm or extremely low birthweight infants. BMJ Open, 6(10), e013446. 10.1136/bmjopen-2016-013446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenis, L. J. , Verhoeven, M. , Hessen, D. J. , & van Baar, A. L. (2015b). Performance of Dutch children on the Bayley III: A comparison study of US and Dutch norms. PLoS ONE, 10(8), e0132871. 10.1371/journal.pone.0132871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenis, L. J. P. , Verhoeven, M. , Hessen, D. J. , & van Baar, A. L. (2015a). Parental and professional assessment of early child development: The ASQ‐3 and the Bayley‐III‐NL. Early Human Development, 91(3), 217–225. 10.1016/j.earlhumdev.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Suir, I. , Boonzaaijer, M. , Nijmolen, P. , Westers, P. , & Nuysink, J. (2019). Cross‐cultural validity: Canadian norm values of the Alberta infant motor scale evaluated for Dutch infants. Pediatric Physical Therapy, 31(4), 354–358. 10.1097/PEP.0000000000000637 [DOI] [PubMed] [Google Scholar]
- Sutter‐Dallay, A. L. , Murray, L. , Dequae‐Merchadou, L. , Glatigny‐Dallay, E. , Bourgeois, M. L. , & Verdoux, H. (2011). A prospective longitudinal study of the impact of early postnatal vs. chronic maternal depressive symptoms on child development. European Psychiatry, 26(8), 484–489. 10.1016/j.eurpsy.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Thelen, E. , & Corbetta, D. (2002). Microdevelopment and dynamic systems: Applications to infant motor development. In Granott N., & Parziale J. (Eds.), Microdevelopment: Transition processes in development and learning. Chap 2 (pp. 59–79). Cambridge: Cambridge University Press. [Google Scholar]
- Tsuchiya, K.J. , Tsutsumi, H. , Matsumoto, K. , Takei, N. , Narumiya, M. , Honda, M. , Thanseem, I. , Anitha, A. , Suzuki, K. , Matsuzaki, H. , Iwata, Y. , Nakamura, K. , Mori, N. , & For H. B. C. Study Team (2012). Seasonal variations of neuromotor development by 14 months of age: Hamamatsu Birth Cohort for mothers and children (HBC Study). PLoS One, 7(12), e52057. 10.1371/annotation/ce119959-9626-4a3e-a30f-5b9f1bb40a2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk, J. W. (2013). Applied longitudinal data analysis for epidemiology: A practical guide (2nd ed.). Cambridge: Cambridge University Press. [Google Scholar]
- Vierhaus, M. , Lohaus, A. , Kolling, T. , Teubert, M. , Keller, H. , Fassbender, I. , Freitag, C. , Goertz, C. , Graf, F. , Lamm, B. , Spangler, S.M. , Knopf, M. , & Schwarzer, G. (2011). The development of 3‐ to 9‐month‐old infants in two cultural contexts: Bayley longitudinal results for Cameroonian and German infants. European Journal of Developmental Psychology, 8(3), 349–366. 10.1080/17405629.2010.505392 [DOI] [Google Scholar]
- WHO Multicentre Growth Reference Study Group , & de Onis, M. (2006). WHO motor development study: Windows of achievement for six gross motor development milestones. Acta Paediatrica, 95, 86–95. 10.1111/j.1651-2227.2006.tb02379.x [DOI] [PubMed] [Google Scholar]
- Wilson, R. S. , & Harpring, E. B. (1972). Mental and motor development in infant twins. Developmental Psychology, 7(3), 277. https://psycnet.apa.org/doi/10.1037/h0033357 [Google Scholar]
- Yaari, M. , Mankuta, D. , Harel‐Gadassi, A. , Friedlander, E. , Bar‐Oz, B. , Eventov‐Friedman, S. , & Yirmiya, N. (2018). Early developmental trajectories of preterm infants. Research in Developmental Disabilities, 81, 12–23. 10.1016/j.ridd.2017.10.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


