Abstract
Leprosy is a chronic granulomatous infectious disease caused by the pathogen, Mycobacterium leprae, and the more recently discovered, M. lepromatosis. Described in 1873, M. leprae was among the first microorganisms to be proposed as a cause of a human infectious disease. As an obligate intracellular bacterium, it has still not thus far been reproducibly cultivated in axenic medium or cell cultures. Shepard's mouse footpad assay, therefore, was truly a breakthrough in leprosy research. The generation of immunosuppressed and genetically engineered mice, along with advances in molecular and cellular techniques, has since offered more tools for the study of the M. leprae–induced granuloma. While far from perfect, these new mouse models have provided insights into the immunoregulatory mechanisms responsible for the spectrum of this complex disease.
Keywords: clofazimine, interferon‐gamma, interleukin‐17, M. leprae, M. lepromatosis, NOS2
1. ANIMAL MODELS FOR LEPROSY
For nearly 150 years, there have been numerous attempts by scientists to propagate Mycobacteriu M. lepromatosis and find a suitable animal model representative of the spectrum of leprosy. Johnstone 1 reviewed the early endeavors to cultivate M. leprae in various animals, which included a vast array of mammals, birds, and cold‐blooded animals (Table 1). Most of these attempts yielded disappointing results, with either no response by the host or only a mild inflammation at the site of inoculation. This may have been due simply to the natural resistance of these hosts to M. leprae infection. However, in many of the early efforts the M. leprae inoculum that was used was likely of poor quality as it was crude material of unknown viability and concentration derived from patient biopsies. Furthermore, M. leprae is a rather fastidious organism and little was known at the time regarding its long doubling time of 12‐14 days, its requirement for cool temperatures, or an appropriate infection route.
TABLE 1.
Early attempts to develop animal models for leprosy
| Animal | First known attempt |
|---|---|
| Mammals | |
| Rabbits | Niesser (1881) a |
| Dogs | Niesser (1881) |
| Guinea pigs | Kobner (1882) |
| Rats | Kobner (1882) |
| Cats | Damsch (1883) |
| Pigs | Arning (1885) |
| Monkeys | Tedeschi (1893) |
| Java | Kobner (1882) |
| Macaque | Nicolle (1906) |
| Rhesus | Reenstierna (1926) |
| Wedge‐capped capuchin | McKinley (1932) |
| Patas | Roffo (1927) |
| Japanese | Saito (1949) |
| Mice | Sugai (1909) |
| Chimpanzees | Marchoux and Bourret (1907) |
| Hamsters | Adler (1937) |
| Gerbils | Saito (1949) |
| Korean Chipmunks | Lew et al (1973) |
| Slender Lorises | Narayanan (1976) |
| Armadillos | Kirchheimer and Storrs (1971) |
| Hedgehogs | Klingmuller 1979) |
| Chinchillas | Binford (1987) |
| Fruit bats | Binford (1987) |
| Lemmings | Binford (1987) |
| Meadow voles | Binford (1987) |
| Opossums | Binford (1987) |
| Birds | |
| Pigeons | Kobner (1882) |
| “Fowls” | Ota (1939) |
| Chickens | Nonaka (1940) |
| Paddy birds | Saito (1949) |
| Canaries | Saito (1949) |
| Parrots | Saito (1949) |
| Love birds | Saito (1949) |
| Chick embryos | Nakagawa and Nakamura (1954) |
| Fish | |
| Eels | Kobner (1882) |
| Loaches | Kobner (1882) |
| Goldfish | Couret (1911) |
| Saltwater fish | Couret (1911) |
| Rainbow Perch | Chaussinand and Besse (1951) |
| Amphibians | |
| Frogs | Kobner (1882) |
| Tadpoles | Couret (1911) |
| Toads | Saito (1949) |
| Reptiles | |
| Turtles | Couret (1911) |
| Snakes | Couret (1911) |
| Lizards | Fite et al (1964) |
| Alligators | Fite et al (1964) |
Complete citations can be found in ref. 1.
Based on the knowledge of the preference of M. leprae for the cooler areas of the body, Charles Shepard 2 inoculated M. leprae into the footpads of mice (Mus musculus) and demonstrated limited growth of the organisms over several months. Importantly, he was able to reproducibly passage and propagate the bacilli into subsequent mice. This mouse footpad assay has permitted the culture of multiple isolates of M. leprae and M. lepromatosis 3 from human lesions, as well as the evaluation of new leprosy drugs and regimens, the documentation of drug resistant strains, and fundamental immunological studies including testing the efficacy of vaccines. 4 While this review will focus primarily on the mouse model, a brief overview of other useful animal models is provided here.
The nine‐banded armadillo (Dasypus novemcinctus) was demonstrated in 1971 5 to develop leprosy after inoculation with M. leprae. Their low body temperature of 32‐35°C underlies the fact that infection disseminates to all of the tissues, resulting in massive numbers of organisms in the spleen, liver, and lymph nodes. Histopathological elements across the spectrum of leprosy have been described, 6 although most armadillos are susceptible to M. leprae infection and only 15%‐20% of the animals are resistant. For years, the armadillo has been used to cultivate large numbers of M. leprae for the purification of leprosy research reagents, 7 which can be obtained through the Biodefense and Emerging Infections Research Resources Repository (https://www.beiresources.org/). More recently, the armadillo is advancing as a model for leprosy‐specific neuropathy. 8 , 9 As an experimental animal, the armadillo remains an exotic species requiring capture in the wild. However, gravid females will successfully give birth in captivity, and their genetically identical quadruplicate offspring are excellent models for the study of host genetics. 10 , 11 Since the sequencing of the armadillo genome in 2011, 12 some immunological reagents, including recombinant interleukin‐2 (IL‐2) 13 and recombinant interferon‐gamma (IFN‐γ), 14 have been generated. Numerous RT‐PCR reagents 15 necessary to probe the model for clues to leprosy pathogenesis have been developed as well. Armadillos naturally infected with M. leprae are found in the Gulf Coast area of the United States and in other parts of the Americas, 16 , 17 and their role as reservoir hosts for transmission of leprosy to humans has been documented. 18 , 19 , 20
Among non‐human primates, sooty mangabey monkeys (Cercocebus atys) have been shown to harbor natural infection with M. leprae. 21 Natural infection in chimpanzees (Pan troglodytes) has also been documented. 22 , 23 , 24 In these animals, however, it was not certain whether they had been infected while feral, or if infection occurred upon exposure to M. leprae–infected humans while being temporarily housed for shipment after their capture. Serological 25 and molecular 26 studies have now provided evidence that M. leprae can be transmitted from humans to non‐human primates, and between non‐human primates. Recently, leprosy in wild chimpanzees has been observed (https://www.biorxiv.org/content/10.1101/2020.11.10.374371v1). Experimentally, mangabey monkeys inoculated with M. leprae developed a disseminated disease which resembles human leprosy, both clinically and histologically. 27 Experimental leprosy has also been established in African green monkeys (Cercopithecus aethiops), 28 , 29 the white‐handed gibbon (Hylobatus lar), 30 rhesus monkeys (Macaca mulatta), 28 and a chimpanzee. 31 Immunological reagents that react in primates 32 are becoming increasingly available, but acquisition and long‐term maintenance of non‐human primates require specialized facilities and can be prohibitively expensive.
Recently, adult zebrafish (Danio rerio) were used to study M. leprae–induced granulomas. Upon infection, histopathological examination revealed well‐formed, non‐caseating granulomas, which developed rapidly and controlled M. leprae growth. 33 Early nerve damage was examined in zebrafish larvae, which have the added advantages of being optically transparent and lacking in adaptive immunity at this stage. 34 In this model, PGL‐1–induced nitric oxide synthase, produced by M. leprae–infected macrophages, generated reactive nitrogen species which caused demyelination of adjacent nerves. While the study of peripheral nerves is difficult in this non‐mammalian model, zebrafish are relatively inexpensive, have a low body temperature, and many genetic mutants are available. 35
Red squirrels (Sciurus vulgaris) in Great Britain having lesions resembling those of leprosy have been reported. 36 , 37 , 38 , 39 Red squirrels in Brownsea Island, England, were found to harbor a natural infection with M. leprae, whereas M. lepromatosis infection was found in red squirrels in England, Scotland, and Ireland. Leprosy infection has not been detected in squirrels in other parts of Europe. 40 , 41 Therefore, red squirrels appear to be a reservoir of infection in the British Isles, although current inter‐ and intra‐species transmission patterns are unknown. Red squirrels are not currently being used as leprosy research models, primarily because of their protected species status. 42
2. LEPROSY SPECTRUM
Leprosy is predominantly a disease of skin, mucous membranes of the upper respiratory tract, and peripheral nerves, the cooler parts of the body. 43 There is a considerable range in the manifestations of clinical leprosy as the course of the disease is largely a result of the cell‐mediated immune response, or lack thereof, of the host toward the antigens of M. leprae. The World Health Organization (WHO) developed a disease classification scheme which, based on the number of lesions and the presence of bacilli in skin smears, describes patients as either paucibacillary or multibacillary to aid in the recommendation of the course of therapy. A more detailed five‐stage clinical‐immuno‐pathological spectrum was developed by Ridley and Jopling. 44 The Ridley‐Jopling classification of leprosy considers the appearance of skin lesions, the presence of acid‐fast bacilli in the skin and nerves, motor and sensory nerve changes, and histopathological findings. This scheme describes a comprehensive spectrum consisting of indeterminate, tuberculoid (TT), borderline tuberculoid (BT), mid‐borderline (BB), borderline lepromatous (BL), and lepromatous (LL) phases.
The earliest recognizable stage of leprosy is indeterminate leprosy. It is important to understand that indeterminate does not mean that one is unsure of the diagnosis of leprosy; rather, it means that it is not yet known where in the spectrum the disease should be classified. Indeterminate leprosy often goes unrecognized as it presents with a single or a few hypopigmented lesions, perhaps with some sensory loss, minimal histological changes, and rare acid‐fast bacilli. Spontaneous healing may occur. Some individuals, however, progress into one of the established polar forms of leprosy (TT or LL) or to an unstable borderline stage.
TT leprosy is a localized disease with one, or at most, a few well‐circumscribed skin lesions with extreme anesthesia. Peripheral nerves in the vicinity of the skin lesion(s) may be enlarged. Histologically, there are very few demonstrable acid‐fast bacilli. Well‐organized granulomas consisting of epithelioid cells, multinucleated giant cells, and a distinct organization of CD4+ and CD8+ T cells are observed. 45 Individuals with TT leprosy manifest a strong cell‐mediated immunity to M. leprae antigens but produce relatively low levels of antibody. Much of the clinical picture in TT leprosy is due to the cell‐mediated immune responses of the host to the relatively few bacilli in sites adjacent to nerves.
LL leprosy is the widespread, anergic form of the disease. Lepromatous granulomas, or lepromas, in advanced LL leprosy may contain 1010 M. leprae per gram of tissue. Histologically, they are characterized by an enormous infiltration of macrophages filled with large numbers of acid‐fast bacilli. A foamy appearance of the cells is due to copious amounts of lipids. CD8+ cells predominate among the few T cells that are found in the LL lesions. Nerve destruction is characterized by the massive numbers of bacilli seen in surrounding macrophages, perineural cells, and Schwann cells. There is a strong antibody response. Although LL leprosy patients are not afflicted with a general immunosuppression, there is a striking and clearly identified specific T cell anergy for the antigens of M. leprae.
Borderline leprosy includes those presentations of disease between LL leprosy and TT leprosy. The majority of persons with leprosy fall into this area of the spectrum. The signs and symptoms of borderline leprosy are due to a combination of bacterial multiplication and the host's cell‐mediated immune response to M. leprae. BT leprosy exhibits more skin lesions than TT leprosy. Satellite lesions develop near the edges of the larger lesions, and individual lesions are larger but still with well‐defined edges. Peripheral nerve damage is generally more prevalent and more severe in BT leprosy than in TT leprosy. Histopathologically, BT leprosy skin lesions are similar to those of TT leprosy except that the granulomas are less organized and do not extend up to involve the basal layer of the epidermis. The numbers of M. leprae in BT lesions vary from undetectable to a few bacilli. In contrast, BL leprosy is closer to the LL end of the disease spectrum. Skin lesions of BL leprosy contain predominantly macrophages but with far more lymphocytes than in LL leprosy. The numbers of acid‐fast bacilli are greater than in BT leprosy but less than in LL leprosy. BB leprosy is rare because it is the most unstable. A patient with BB leprosy can develop clinical, bacteriological, and histopathologic features of more tuberculoid disease, and this shift is termed “upgrading.” A shift from the BB leprosy region to more lepromatous disease is called “downgrading.”
3. MOUSE MODELS
3.1. Conventional and athymic nude mice
Immunocompetent mice, such as C57Bl/6 or BALB/c, are quite resistant to infection with M. leprae. Using Shepard's infection model, a low‐dose suspension of a few thousand bacilli is inoculated into the hind footpads where it grows locally and plateaus at approximately 105‐106 organisms by 4‐6 months postinfection. It is postulated that infection during the first few months is governed primarily by innate immunity. The growth peak, or plateau phase, is indicative of the onset of adaptive cell‐mediated immunity that results in killing of the bacilli. 46 It is important to note that if >106 M. leprae are inoculated into the footpad, there is no subsequent growth as this dose immunizes the mouse. In our studies, we inoculate 6 × 103 freshly harvested, viable M. leprae into each hind footpad and monitor bacterial growth (Figure 1A) and granuloma development, both histopathologically and by immunohistochemical staining, over a 12‐ to 18‐month infection period. Immunocompetent mice develop a modest granulomatous response, consisting of a mild lymphocytic infiltrate, few epithelioid cells, and small focal collections of macrophages, located immediately underneath the epidermis with minimal invasion of deeper tissue (Figure 1B). These properties of limited growth and mild pathology led researchers to regard immunocompetent mice as resembling TT leprosy, but it is actually more of a self‐healing type of presentation.
FIGURE 1.
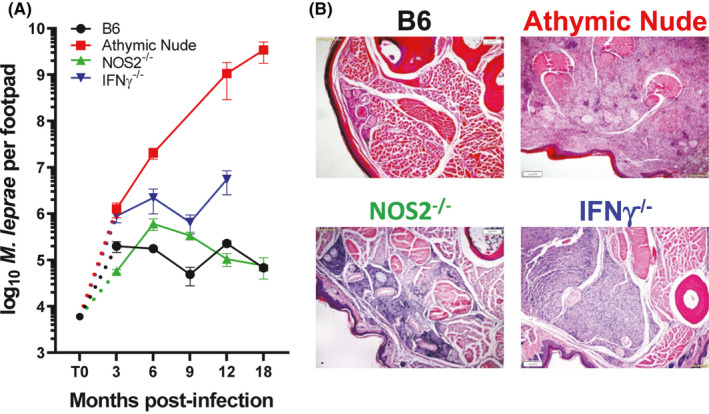
Growth of M. leprae and granulomatous response in infected footpads. A, B6, athymic nude, NOS2−/−, and IFN‐γ−/− mice were infected in the hind footpads with 6 × 103 M. leprae, and growth was monitored for 18 mo. B, Histological examination (hematoxylin and eosin stains) of M. leprae–infected footpads at 9 mo postinfection. B6 develop a small granuloma comprised of lymphocytes, epithelioid cells, and macrophages. Athymic nude mouse footpads become engorged with M. leprae filled macrophages. In NOS2−/− mouse footpads, the large granulomatous response infiltrated the perineurium and muscle bundles, whereas in IFN‐γ−/− footpads, the large unorganized infiltration was composed of epithelioid macrophages with randomly interspersed lymphocytes
In contrast, athymic nude mice cannot mount a cell‐mediated immune response and M. leprae grow ostensibly without constraint (Figure 1A). Bacterial numbers can reach 1 × 1010 or more acid‐fast bacilli per footpad. The granuloma in athymic nude mice resemble LL. In the absence of mature T cells, infected footpad tissue becomes a massive leproma (Figure 1B), where the macrophages are packed with bacilli. Local fibroblasts, striated muscle cells, and nerves are heavily infected as well. The athymic nude mouse model has been used for years to culture M. leprae, and along with studies in thymectomized‐irradiated mice and rats, nude rats, and severe combined immunodeficiency (SCID) mice, 47 , 48 , 49 this strain defined the role of the T cell in cell‐mediated immunity to leprosy. Furthermore, it enabled detailed evaluation of drug regimens, and allowed for detection of persisting drug sensitive M. leprae from treated patients. We currently use these mice to routinely propagate M. leprae and M. lepromatosis. 3
In the course of our studies using M. leprae infection of mice, we also developed a companion strategy to the Shepard growth method that would allow us to better assess the immune responsiveness to M. leprae at the site of infection and permit a more in‐depth exploration of the cellular composition of the footpad lesion. This protocol utilized a higher dose of M. leprae and was loosely based on the lepromin test. The lepromin test 43 is a skin test previously used to classify clinical disease in humans. In general, a large dose of killed, whole bacilli were injected intradermally, and the ensuing granulomatous response was examined grossly for induration and histologically for cellular composition. A variety of M. leprae preparations, obtained from human or armadillo tissues and subjected to different purification protocols, were used over the years for the lepromin test. The lepromin test is not a diagnostic test for leprosy and bears no similarity to the tuberculin skin test for tuberculosis. The lepromin test is read at ~4 weeks, not 48 h, and it is not a delayed‐type hypersensitivity response. Rather, a positive lepromin test is prognostic and determines whether or not one can mount a successful granulomatous response to M. leprae. Persons who have never been exposed to M. leprae can mount a positive lepromin test response. A negative response means one cannot mount a granulomatous response to the organism. If the individual is a patient, a positive lepromin test classifies the patient in the TT‐BT range of the spectrum. A negative test suggests BL‐LL.
In our footpad induration protocol, mice are injected in the footpad with a high dose of M. leprae, and induration is measured weekly as an external determination of cellular infiltration. As shown in Figure 2A, immunocompetent B6 mice develop significant footpad induration over the first few weeks which peaks at ~4 weeks, similarly to a positive lepromin test. In contrast, there is virtually no early induration in athymic nude mice, and the footpad only slowly increases in size as the macrophages infiltrate the tissue to accommodate the growing M. leprae. Using this strategy, we developed procedures to isolate the cells from the granulomas and culture them in vitro. Immunocompetent mouse footpad granulomas contained mostly macrophages and T cells, while cells isolated from M. leprae–infected athymic nude mouse footpads consisted primarily of engorged macrophages (Figure 2B). Furthermore, these footpad granuloma cells could be analyzed for cytokine and chemokine expression and production by RT‐PCR and ELISA, respectively. These cells can also be characterized via extracellular and intracellular flow cytometry.
FIGURE 2.
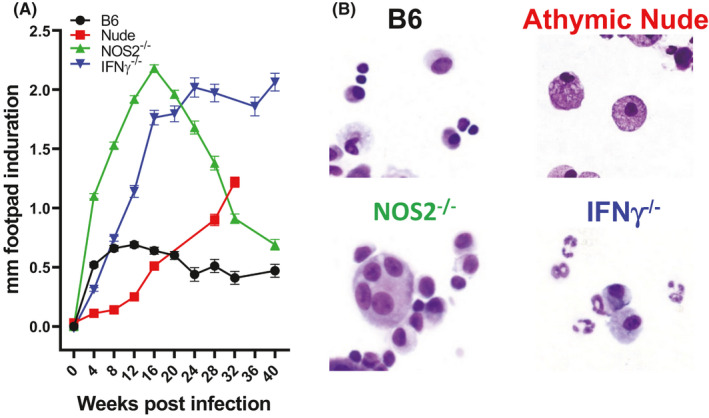
Footpad induration and isolated granuloma cells. A, B6, athymic nude, NOS2−/−, and IFN‐γ−/− mice were infected in the hind footpads with 30 × 106 M. leprae, and induration was measured with a Vernier caliper. B, Cells were isolated from the footpad granulomas at 16 wk postinfection, and cytospin preparations were stained with a Diff‐Quik staining kit. B6 footpad granulomas contained macrophages and T cells. Athymic nude footpads are comprised of heavily infected macrophages (photograph originally published in ref. 103). T cells, macrophages, and multinucleated giant cells were recovered from NOS2−/− footpads. IFN‐γ−/− footpads contained large numbers of neutrophils
3.2. Knockout mice
The extremes provided by the immunocompetent B6 and BALB/c strains and the immunosuppressed athymic nude strain have been productively employed in various chemotherapeutic and immunotherapeutic leprosy studies. However, we wanted to explore the possibility of mouse models that may represent borderline disease. Immunologically, the borderline area of the leprosy spectrum is the most interesting, as BT, BB, and BL leprosy can be clinically unstable and prone to inadequately understood immunological complications. 43 In order to modify the immune response in immunocompetent mice, we tried several chemical, for example, aminoguanidine, L‐N6‐(1‐iminoethyl)‐lysine, and immunological, for example, antibodies, cytokines, and manipulations with some success, but the protracted nature of M. leprae infection made these studies difficult to carry out long term. The widespread availability of genetically engineered mice, therefore, aided our studies immensely.
There are now literally hundreds of commercially available genetically engineered mouse strains, including targeted gene knockouts, conditional knockouts, tissue‐specific knockouts, knock‐in mutations, and humanized mice. Using such mice afforded us more options for analyzing the effects of infection compared with the extremes of the immunocompetent and athymic nude mouse strains. Of course, the mechanisms that regulate inflammation and immunity are complex and changeable. They can be influenced not only by genetic factors but also by a number of intrinsic qualities, such as age, hormones, or microbiome, as well as external burdens such as poor nutrition, co‐infections with immunomodulating parasites, viruses, or worms, or exposure to other mycobacteria. Any of these conditions can affect both short‐ and long‐term immune status. 50 Yet, single‐gene knockout mice with deficiencies at specific points of the immune cascade provided a good starting point for dissecting responses to infection with M. leprae. Furthermore, these strains could suggest targets for prevention, early diagnosis, and improved treatments. Therefore, we chose to first examine mice with knockouts in genes important for cell‐mediated immunity.
3.2.1. IFN‐γ knockout (IFN‐γ−/−) mice
One of the most interesting strains has been the IFN‐γ−/− mice. 51 , 52 IFN‐γ production by CD4+ T cells plays a key role in immunity to mycobacterial infections. Studies with M. tuberculosis were done with mice having disruptions in the IFN‐γ gene (IFN‐γ−/−) or the IFN‐γ receptor 53 , 54 , 55 gene. Infection of IFN‐γ−/− mice with M. tuberculosis resulted in decreased survival time, unrestricted growth of the bacilli, and necrotic granulomas. Adoptive transfer experiments from IFN‐γ−/− mice to TB‐infected nude mice showed that protection afforded by CD8+ T cells required IFN‐γ−/− production. 56
When IFN‐γ−/− mice were infected with M. leprae, the bacilli multiplied beyond the peak reached in wild type mice to reach a plateau approximately one log higher (Figure 1A); however, the bacilli did not grow unchecked as with the athymic nude mice. By 9 months postinfection, the footpads of the M. leprae–infected IFN‐γ−/− mice were visibly enlarged. Histopathologically, the footpads of IFN‐γ−/− mice exhibited large aggregates of mononuclear cells replacing 40%‐75% of the soft tissue (Figure 1B). The infiltrates were not organized into well‐formed granulomas but were composed primarily of epithelioid macrophages with small aggregates of lymphocytes randomly interspersed.
We also examined the host response of the IFN‐γ−/− mouse to M. leprae infection using the high dose footpad induration protocol. Early in our studies, we intended to harvest the tissue only at four weeks postinoculation, as is done in the human lepromin test. However, as studies with our various knockout mice progressed, we discovered that the granulomatous response to the bacilli evolved quite strikingly over the course of several months and was distinct among the various strains. Therefore, we extended these studies to evaluate the evolution of the granulomatous response at early (4 weeks), mid (16‐20 weeks)‐, and late (>32 weeks) time points. While the induration plateau was steadily maintained in control mice, induration of the IFN‐γ−/− footpads increased steadily over a 16‐week period (Figure 2A). At this time, it reached a plateau and maintained this level of induration for 40 weeks. IFN‐γ−/− footpad cells harvested at 4 months postinfection were analyzed by RT‐PCR, ELISA, and flow cytometry. Interestingly, while few Ly6G+ neutrophils were found in the B6 footpads, there was a substantial increase in both the number and percentage of neutrophils in the IFN‐γ−/− footpads (Figure 2B). While similar levels of IL‐10 were expressed, significantly higher levels of IL‐17A and CXCL‐5 were expressed in IFN‐γ−/− granuloma footpads (Figure 3A). Flow cytometric analyses showed a higher percentage of both CD4+ IL‐17+ and CD8+ IL‐17+ cells in the IFN‐γ−/− mice (Figure 3B) compared with B6 mice. Furthermore, high amounts of IL‐17 were generated by IFN‐γ−/− footpad cells upon stimulation with M. leprae membrane antigen (MLMA) (Figure 3C). These data indicate a predominantly Th17 response to M. leprae in these mice. The properties of augmented but not uncontrolled growth of M. leprae, enhanced granulomatous infiltration with only scattered lymphocytes present, and reduced Th1‐type cytokine production are attributes present in BL leprosy. Moreover, the infiltration of neutrophils suggests the possibility that this strain may be manipulated for leprosy reactions (see below).
FIGURE 3.
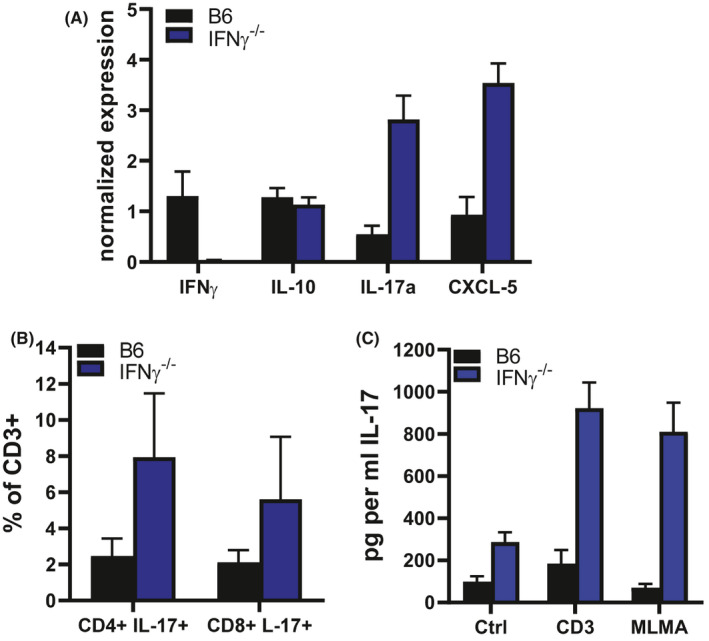
IL‐17 generation in M. leprae–infected INF‐γ−/− footpads. B6 and IFN‐γ−/− mice were infected in the hind footpads with 30 × 106 M. leprae. A, IFN‐γ, IL‐10, IL‐17, and CXCL‐5 expression determined by RT‐PCR on footpad tissue. Granuloma cells were isolated and IL‐17 production was determined by B. flow cytometry and C. ELISA on supernatants from isolated cells cultured in vitro with MLMA
3.2.2. NOS2−/− mice
Another mouse strain which showed some features of borderline leprosy was the inducible nitric oxide synthase knockout (NOS2−/−). Cytokine (primarily IFN‐γ)‐activated macrophages are the primary effector cells in the control of intracellular pathogens, including M. leprae. 57 Two powerful products produced by activated macrophages are toxic radicals of nitrogen and oxygen.
Reactive nitrogen intermediates (RNI) generated from L‐arginine 58 by a high‐output, cytokine‐inducible isoform of nitric oxide synthase (NOS2) are principal components of the antimicrobial effector mechanisms of activated macrophages. Competitive inhibitors of L‐arginine, such as NG‐monomethyl‐L‐arginine (L‐NMA), aminoguanidine (AG), and L‐N6‐(1‐iminoethyl)‐lysine (L‐NIL) have been employed to demonstrate the role of RNI in macrophage‐mediated resistance to infection with M. leprae and M. tuberculosis in vitro, 59 , 60 and their in vivo administration has confirmed the importance of this pathway in resistance to M. tuberculosis. 61 , 62 , 63 The generation of knockout mouse strains with targeted disruptions in the NOS2 gene has enabled long‐term M. leprae infection studies without the possible side effects of chemical inhibitors. As shown in our own studies 64 and those of Nathan's group, 63 growth of M. tuberculosis and the granulomatous response in the tissues was markedly enhanced in NOS2−/− mice, while time‐to‐death following infection was decreased. In contrast, NOS2‐deficient mice could control M avium growth in vivo. 65
We had previously shown the importance of RNI in IFN‐γ–induced macrophage‐mediated resistance to M. leprae in vitro using both L‐NMA–treated control macrophages 59 and macrophages from NOS2−/− mice. 66 Therefore, M. leprae growth in vivo was assessed in an effort to determine whether, as with M. tuberculosis infection, macrophage incapacity in vitro would correlate with exacerbated growth in vivo. NOS2−/− mice were infected with M. leprae using the low‐dose growth protocol and bacterial multiplication was monitored over several months. 67 There was a slightly enhanced growth of M. leprae at 6‐9 months postinfection, but the number of M. leprae in the footpads then declined yielding a marked reduction in the number of bacilli at 15‐17 months postinfection (Figure 1A). Interestingly, NOS2−/− mice exhibited a 10‐fold larger, organized granuloma, composed of numerous epithelioid cells, a few macrophages, and dense collections of lymphocytes. In areas, the granuloma infiltrated the perineurium and muscle bundles (Figure 1B). Using the footpad induration protocol, there was a rapid enlargement of the footpads in NOS2−/− mice (Figure 2A) which peaked at 16 weeks but then began to decline. The majority of the lymphocytes which infiltrated the footpads of both the B6 and NOS2−/− strains were CD3+ T cells; few B220+ B cells were present. However, both the number and percentage of CD3+ cells were augmented in NOS2−/− footpads. Furthermore, in the NOS2−/− footpads, a higher percentage of these cells displayed activation markers. Cytokine gene expression in the developing granuloma of the footpad tissues of NOS2−/− mice was examined by quantitative RT‐PCR. While present in control mice, cytokines crucial to an effective Th1 response, such as IFN‐γ, IL‐2, and tumor necrosis factor (TNF) and chemokines important in granuloma formation, including CCL‐3 and CCL4, were expressed at significantly higher levels in NOS2−/− footpads. Interestingly, higher levels of IL‐10 were also generated in NOS2−/− footpads. The CD4+ CD44hi cells from NOS2−/− footpads also produced higher levels of IFN‐γ when stimulated in vitro with M. leprae membrane antigen. NOS2−/− mice, therefore, develop more rapid, extensive chronic inflammation with the presence of multinucleated giant cells (Figure 2B). These attributes, along with the controlled growth of M. leprae, led us to categorize this strain as BT leprosy.
3.2.3. phox91 −/− mice
In contrast, we saw little effect on M. leprae infection in the absence of reactive oxygen intermediates (ROI). Early studies by Sharp 68 showed that M. leprae was susceptible to killing by hydrogen peroxide in a cell‐free system. More recently, Cole's group 69 , 70 determined that the genes for KatG, a catalase‐peroxidase enzyme, and AhpD, part of a peroxidase and peroxynitrite reductase system, are pseudogenes in M. leprae, verifying the reason that neither a catalase nor a peroxidase is generated. Yet, M. leprae is able to remain viable and proliferate inside the macrophage, the primary host cell. How M. leprae survives inside the macrophage, especially with regard to defense against ROI, has been attributed to a number of factors. First, M. leprae is only a weak stimulus of the macrophage oxidative burst. This may be due its entry via the complement (C) 3 receptor 71 or a scavenging of ROI by cell wall glycolipids such as PGL‐1 72 and LAM. 73 M. leprae also expresses both sodC and sodA 74 and produces a superoxide dismutase. Thus, the toxic effects of superoxide should be effectively suppressed by M. leprae and the production of the subsequent ROI should be greatly reduced. Taken together, these in vitro studies suggest that M. leprae should be competent in dealing with the ROI host defense mechanism.
We examined the role of ROI for the effective host response to experimental leprosy using mice which have a disruption in the 91 kD subunit of the NAPDH oxidase cytochrome b (phox91 −/−). 75 Multiplication of M. leprae in phox91 −/− footpads was elevated early in infection compared with B6 mice, but growth was subsequently controlled as counts similar to those observed in B6 mice were obtained later in infection. Histopathologically, a mild granulomatous response developed in both strains with no tissue destruction. Footpad induration was similar in B6 and phox91 −/− with the lymphocyte infiltration into the granuloma consisting primarily of activated effector cells. Th1 cytokines, chemokines, and inducible nitric oxide synthase were expressed at in phox91 −/− mice footpads at levels comparable to the immunocompetent strains. When infected with M. leprae in vitro, normal macrophages from B6 and phox91 −/− mice supported bacterial viability, whereas IFN‐γ–activated macrophage killed M. leprae in a RNI‐dependent manner, confirming that production of RNI is the more important effector mechanism utilized by activated murine macrophages to inhibit M. leprae.
3.2.4. p40−/− mice
The cytokines, IL‐12, produced primarily by macrophages and monocytes, and IL‐23, produced by macrophages and dendritic cells, are important in the regulation of innate and adaptive immunity. IL‐12 induces the production of IFN‐γ by T and NK cells early in infections and promotes the development of a Th1‐type cell–mediated immune response, whereas IL‐23 stimulates the production of IL‐17 by Th17 cells and is important for the development of chronic inflammation and granuloma formation. 76 IL‐12 and IL‐23 are heterodimeric cytokines which share a common p40 subunit, and p40 has been shown to be important in host defense against mycobacterial disease. 77 , 78
To study the roles of IL‐12/23 in experimental leprosy, M. leprae infection was evaluated in p40−/− mice. 79 p40−/− mice infected using the growth protocol exhibited significantly enhanced growth of M. leprae, reaching 105 by 3 months postinfection and continuing to multiply to reach 106 by 12 months postinfection. However, p40−/− mice developed only a mild inflammation. Likewise, footpad induration was markedly decreased in p40−/− compared with that of B6 mice. Similarly to B6 footpads, leukocyte accumulation into p40−/− footpads consisted primarily of CD4+ T cells and CD11b+ macrophages; however, the percent CD4+ T cell infiltration was reduced and the percent CD8+ cell infiltration was augmented in p40−/− mice. Expression of cytokines crucial to an effective Th1 response (IFN‐γ and TNF) and chemokines important in granuloma formation (CCL2, CCL3, CCL4) were significantly lower in p40−/− footpads compared with B6 footpads. In summary, compared with B6 mice, p40−/− mice exhibited a decreased ability to control M. leprae growth and evidenced reduced footpad induration with altered CD4+ and CD8+ T cell composition, presumably due to the lack of protective IL‐12 and proinflammatory IL‐23, respectively.
3.2.5. IL‐10−/− mice
IL‐10, generated primarily by T cells and macrophages, exerts immunosuppressive and anti‐inflammatory actions. IL‐10−/− mice experimentally infected with various pathogens tend to succumb rapidly from a severe pathology associated with an intense production of inflammatory mediators. Studies in several leprosy endemic populations have revealed IL‐10 polymorphisms associated with both susceptibility and resistance to leprosy. 80 IL‐10 has been detected in lesions of multibacillary patients 81 and likely contributes to the leprosy‐specific unresponsiveness seen in LL leprosy. 82
Upon infection, M. leprae growth in IL‐10−/− mice showed similar growth kinetics as control mice. 67 However, this deficiency in IL‐10 did not further restrict growth or augment inflammation in the already highly resistant B6 mice. Histopathologically, IL‐10−/− mice developed a slight increase in the infiltration of lymphocytes, macrophages, and especially epithelioid cells to the site of infection. Mild, yet significant, increases in both footpad induration and in the numbers of cells isolated from the footpad were observed in IL‐10−/− mice.
3.2.6. TNF−/− and TNFR1−/− mice
TNF plays a pivotal role in inflammatory phenomena that culminate in either pathogenesis or resistance in mycobacterial disease. The classic studies by Kindler et al 83 showed that TNF is produced by many cell types and demonstrated a role in regulating granuloma structural organization and maintenance through the recruitment and migration of leukocytes into lesions. In addition, TNF is important for triggering activation of IFN‐γ–primed macrophages for antimicrobial activity, inducing apoptosis or necrosis, and inducing cellular differentiation. In leprosy, TNF expression has been detected in leprosy lesions. 84 In general, the amount of TNF has correlated with cell‐mediated immunity, with higher levels expressed in TT leprosy as compared to LL leprosy. In addition, studies have associated TNF gene alleles as relevant to increased clinical susceptibility to M. leprae infection, 80 although the actual role of TNF in cellular dynamics within that microenvironment of long‐term infection remains experimentally undefined.
To study the role of TNF in experimental leprosy, M. leprae footpad infection was evaluated in TNF−/− and TNFR1−/− mice. 85 M. leprae growth was augmented 10‐fold throughout the 9‐12 months infection period compared with control mice. Histopathologically, TNF−/− and TNFR1−/− mice developed an extensive but diffuse lymphocytic infiltration. Footpad induration was initially delayed in both knockout strains but did develop by 28 days postinfection. Flow cytometric analyses demonstrated an increased activated CD4+ T cells and I‐Ab+ macrophage accumulation in TNF−/− and TNFR1−/− footpads. Expression of inflammatory cytokines and chemokines was elevated in TNF−/− and TNFR1−/− footpads compared with control mice. These data indicate that TNF plays a role in the development of an organized and protective granuloma in experimental leprosy.
3.2.7. Lymphotoxin‐alpha (LTα−/−) mice
An absence of the related cytokine, LTα, had quite a different effect. 85 LTα is implicated in the development of secondary lymphoid organs and in cell‐mediated immunity against intracellular pathogens. Previous studies had shown that although LTα‐deficient mice can amass similar accumulations of T cells at the site of M. tuberculosis lung infection, T cells fail to properly co‐localize with granuloma macrophages, resulting in abnormal granulomas with elevated bacterial load. 86 LTα deficiency does not impair TNF production, and conversely, LTα production is independent of TNF. A polymorphism, LTAα+ 80, which has been found within the promoter region of the gene encoding LTα, is an important risk factor for developing leprosy per se. 87 LTα has been detected in TT leprosy lesions and in type 1 reactions, which supports its purported role as a proinflammatory cytokine.
Mycobacteriu M. lepromatosis footpad infection was evaluated in LTα‐deficient chimeric (cLTα−/−) mice. 85 Targeted disruption of LTα results in the loss of secondary lymphoid tissue; therefore, in order to have mice with peripheral lymph nodes populated with LTα −/− lymphocytes, chimeric knockout mice were generated by reconstitution of irradiated RAG mice with LTα −/− bone marrow (cLTα −/−). Irradiated RAG mice reconstituted with B6 bone marrow (cB6) served as controls. cLTα−/− mice were deficient in both the soluble and membrane‐bound forms of LTα. Multiplication of M. leprae in the footpad of cB6 mice reached on the order of 105 bacilli by three months postinfection and remained at this level up to nine months postinfection. There was no difference between cB6 and cLTα−/− mice in the number of acid‐fast bacilli recovered from footpads at three and six months postinfection. However, acid‐fast bacilli counts were slightly but significantly higher in the cLTα−/− mice at nine months postinfection. These data show that LTα is not essential for controlling the growth of M. leprae during early infection, but may influence survival of M. leprae late infection. Histopathologically, few lymphocytes accumulated in the footpads of cLTα−/− mice and footpad induration could not be sustained. Flow cytometric analyses demonstrated that few lymphocytes could be recovered from cLTα−/− footpads, but the popliteal lymph nodes contained more CD3+ cells compared with cB6 mice suggesting an aberration in cell trafficking. In addition, there was a five‐ to 50‐fold lower level of expression of inflammatory cytokines and chemokines in M. leprae–infected cLTα−/− footpads. These data indicate that LTα regulates the induction and maintenance of the granulomatous response during chronic M. leprae infection, and this may underlie the association of genetic variants in the LTα gene and human leprosy.
4. LEPROSY REACTIONS
Perhaps the most difficult and challenging aspect in the treatment of leprosy is the care and management of reactional episodes. Up to 40% of leprosy patients will undergo these painful and debilitating immunological events. Both type 1 and type 2 reactions can cause severe inflammation, require prolonged and recurrent specialized inpatient care, and can exacerbate and lead to irreversible nerve damage, paralysis, and deformity. 43 Leprosy reactions typically occur in the borderline and LL leprosy areas of the disease spectrum. They can occur during leprosy drug treatment and even after completion of the treatment regimen when patients are technically cured of leprosy. Little is known about what induces reactions. Dead bacteria can remain in skin and delicate nerve tissues for years following treatment completion, representing a hoard of persistent antigens. It has also been observed that certain “stresses,” for example, puberty, menstruation, pregnancy, postpartum, vaccination, illness, and psychological strain that may alter complex immunological balances can precipitate a reactional episode. 88 The mechanisms that underlie and modulate the onset of leprosy reactions are not well understood but are likely manifestations of immunoregulatory changes, both systemically and in the microenvironment of the leprosy granulomas. Predicting and preventing reactional episodes would be of great benefit 89 as existing treatments often have side effects which limit application and effectiveness, especially when they must be administered long term.
Type 1 (reversal) reactions appear as the result of an increase in cell‐mediated immunity and affect patients classified in the BT to BL areas of the spectrum. They are characterized by their localized and gradual onset. Histologically, type 1 reactions consist of an influx of mononuclear cells, edema, and erythema in pre‐existing lesions. If a Type 1 reaction upgrades, lymphocyte numbers may increase. In intense type 1 reactions, necrosis accompanied by severe nerve damage may occur.
Type 2 (erythema nodosum leprosum [ENL]) reactions are characterized by the abrupt development of groups of tender, erythematous skin nodules and fever. Because type 2 reactions can involve any tissue containing M. leprae antigens, ENL lesions are not confined to the skin or pre‐existing lesions, but can involve the eye, joints, nasal mucosa, and other tissues. Histologically, type 2 reactions often present an influx of neutrophils on a BL or LL background. A vasculitis involving arterioles or venules may be demonstrable. Immunologically, type 2 reactions have long been considered manifestations of an Arthus type of hypersensitivity reaction.
Global transcriptional profiles of peripheral blood mononuclear cells from leprosy patients in reaction revealed immunity‐related pathways. The “complement and coagulation” pathway was common in both type 1 and type 2 reactions, as was IFN‐γ as an upstream regulator. 90 An increase in CXCL‐10 expression was uniquely associated with type 1 reactions. 91 , 92 Decreased C4 and increased IgM, IgG1, and C3d‐associated immune complexes in patients newly diagnosed with leprosy were associated with subsequent development of type 2 reaction. 93 Decreased C4 at diagnosis was also found in those who developed type 1 reaction. Another study examining gene expression profiles in leprosy lesions found “cell movement” pathways associated with neutrophil recruitment as a major factor in Type 2 reactions. 94 Recently, studies using longitudinal analyses of leprosy patients before, during, and after type 1 reaction revealed an upregulation of IFN‐γ downstream genes 95 and identified a five‐gene transcriptomic signature for onset type 1 reactions. 89
4.1. Immunotherapy in leprosy patients
A number of studies attempted to modify the anergic responses of LL patients to M. leprae and augment cell‐mediated immunity by means of local administration of purified recombinant human cytokines. Multiple intradermal injections of IFN‐γ into the lesions of LL and BL patients 96 evoked a notable migration of T cells, predominantly CD4+, and monocytes into the dermis. The bacillary load was reported to have also decreased. A persistent granulomatous response showing characteristic epithelioid and multinucleated giant cells was observed. In addition, repeated intramuscular injections of IFN‐γ induced a generalized, intradermal mononuclear cell infiltration of CD3+, CD4+, CD8+, and CD1a+ T cells and Leu‐M5+ mononuclear phagocytes. Unfortunately, IFN‐γ administration induced an ENL reaction in 60% of the LL patients. 97 Upon intradermal injection of IL‐2 into LL lesions, 98 both CD4+ and CD8+ cells migrated into the lesion, and epidermal keratinocytes expressed activation markers. There was also evidence of the destruction of M. leprae–parasitized granuloma macrophages and a reduction in the bacterial index in the lesions. More recently, as the use of biologics for conditions such as arthritis has increased, incidences of leprosy reactions have occurred. 99 For example, Scollard et al 100 reported two patients who developed clinical borderline leprosy while being treated with Infliximab, an anti‐TNF antibody therapy they had been prescribed for arthritis. The development of leprosy under these circumstances highlighted the protective aspects of TNF which had likely kept a subclinical infection with M. leprae under control. More intriguing, however, was that when the infliximab therapy was discontinued, both patients developed type 1 reactions within one month. Thus, fluctuations in TNF can profoundly affect the development, progression, and stability of borderline leprosy. These immunotherapy studies in humans undeniably demonstrated that the position on the leprosy spectrum could be purposely upgraded to augment cell‐mediated immunity, but at the risk of harmful consequences such as the induction of reactions.
5. MOUSE MANIPULATIONS
We have attempted several manipulations, in both in vitro models and in vivo models, to disrupt the status quo of the mouse response to M. leprae infection.
5.1. Reconstitution with IFN‐γ–activated macrophages in an in vitro model of LL leprosy
Krahenbuhl's group 101 , 102 showed that macrophages heavily infected with M. leprae, either derived from the athymic nude mouse footpad granuloma or infected in vitro, were refractory to activation with IFN‐γ. From these data, he proposed that any killing of the bacilli in LL leprosy lesions would have to be accomplished by newly arriving, previously activated macrophages infiltrating the granuloma. To further test this hypothesis, we developed an in vitro model of the LL granuloma and used it to study the fate of M. leprae in a LL leprosy lesion with and without immunotherapeutic intervention. 103 Target cells, consisting of granuloma macrophages harvested from the footpad of M. leprae–infected athymic nude mice, were co‐cultured with normal or IFN‐γ–activated effector macrophages. The bacilli were recovered and assessed for viability. M. leprae recovered from target macrophages, either cultured alone or in the presence of normal effector macrophages, exhibited high viability. In contrast, bacilli recovered from target macrophages co‐cultured with IFN‐γ–activated macrophages were killed. An effector:target ratio of at least 5:1, a co‐culture incubation period of 3‐5 days, and production of RNI, but not ROI, were required. Neither IFN‐γ nor TNF was required during the co‐cultivation period for killing of the bacilli, but cell‐to‐cell contact was necessary for both augmentation of bacterial metabolism by normal macrophages and inhibition of M. leprae by activated effector macrophages. Conventional and confocal fluorescent microscopy revealed that the bacilli from target macrophages were acquired by the effector macrophages. These data suggest that, in the absence of cell‐mediated immunity, the influx of fresh macrophages into a LL leprosy lesion sustains growth and viability. However, if the new effector macrophages are activated beforehand, they have an opportunity to kill the M. leprae they acquire from the infected macrophages. These studies were performed in the absence of cytotoxic T cells. Exactly how the new macrophages acquire M. leprae from the infected granuloma macrophages is currently being studied.
5.2. Overcoming anergy in an in vivo model of LL leprosy
Reasoning that the overwhelming growth of M. leprae in the footpads of athymic nude mice could be an intriguing model on which to exert an abrupt induction of cell‐mediated immunity to M. leprae antigens in a granuloma that resembled LL, we performed adoptive transfer of M. leprae–sensitized T cells. However, we were disappointed to observe remarkably little effect in the recipient athymic nude mice with regard to inflammation or killing of M. leprae. Previous studies had shown prominent secretion of prostaglandin (PG) E2 by ex vivo cultured granuloma cells from M. leprae–infected athymic nude footpads as well as from human LL leprosy biopsies. 101 Based on this experience and on reports that essential fatty acid deficiency enhances cell‐mediated immunity by reducing production of prostaglandins with immunosuppressive actions, these adoptive transfer experiments were redesigned to suppress PGE2 production in M. leprae–infected recipient athymic nude mice prior to adoptive transfer of activated T cells. M. leprae–infected mice were fed either a control or linoleic acid‐free diet for 3 months prior to adoptive transfer of M. leprae–primed, T‐enriched lymphocytes. 104 In brief, activated T cells adoptively transferred into recipient mice fed control diets induced little reduction in M. leprae viability. In contrast, M. leprae from recipient mice fed the essential fatty acid–deficient diet exhibited a markedly reduced viability upon adoptive transfer of activated T cells. These data showed that induction of PGE2 production by M. leprae or its constituents could negatively modulate the function of the T cells in the microenvironment of the lepromatous granuloma and result in a localized immune deficiency in the vicinity of the bacilli.
5.3. Modification of neutrophil infiltration in M. leprae–infected IFN‐γ−/− footpads
Upon infection of IFN‐γ−/− mice with M. leprae, there was a persistent and long‐term infiltration of Ly6G+ neutrophils into the footpads, a response seen in neither B6 nor athymic nude mouse footpads, nor any other knockout mouse strain that we had examined. This response was especially interesting since the presence of neutrophils is a hallmark of type 2 reactions. In our initial attempts to modify this cellular response, we treated IFN‐γ−/− mice with intra‐footpad inoculations of recombinant IFN‐γ. We observed a reduction in the size of the footpads, emphasizing the dynamic nature of this granulomatous response. However, we know that macrophages from IFN‐γ−/− mice are functionally normal and can be activated by IFN‐γ to kill M. leprae, 51 so this decrease in the induration of IFN‐γ−/− footpads may simply have been due to the killing of the bacilli in these mice. Therefore, we tried an alternate approach. The incidence of type 2 reactions has been reported to be lower in leprosy patients treated with clofazimine. 105 Therefore, we treated mice via gastric gavage with 10 daily doses of a very low, non‐bactericidal dose of 1 mg/kg Clofazimine or the standard bactericidal dose of 10 mg/mL Rifampin. Footpad induration was monitored over six weeks. As shown in Figure 4A, significant reductions in footpad induration were observed with both drug treatments. Flow cytometric analyses of granuloma footpad cells (Figure 4B) showed that the high infiltration of neutrophils seen in sham‐treated IFN‐γ−/− mice were significantly reduced upon both clofazimine and rifampin treatment. Interestingly, this reduction in neutrophils in clofazimine‐treated mice occurred without bacterial killing (Figure 4C). These data suggest that clofazimine may subdue type 2 reactions by directly regulating neutrophil trafficking. The mechanisms of this process are currently being studied.
FIGURE 4.
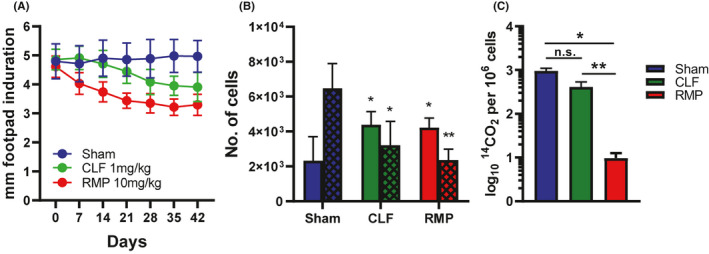
Modulation of M. leprae–infected INF‐γ−/− footpad induration and cellular composition by Clofazimine and rifampin. IFN‐γ−/− mice were infected in the hind footpads with 30 × 106 M. leprae. At 24 wk postinfection, mice were treated daily for 10 d with vehicle (sham), 1 mg/mL clofazimine (CLF), or 10 mg/kg rifampin (RMP). A, Footpad induration was measured with a vernier caliper. B, At 42 d, footpads cells were isolated and the number of macrophages (open bars) and neutrophils (hatched bars) were determined by flow cytometry. C, M. leprae viability was assessed by radiorespirometry
5.4. Generation of double knockout mouse strains via cross‐breeding
As noted earlier, the NOS2−/− mouse strain responded to M. leprae infection in a manner that resembled BT leprosy. They developed a large granulomatous response, generated high levels of TH1 cytokines and chemokines, and restricted bacterial growth; however, elevated levels of IL‐10 were also detected at the site of infection. While IL‐10 is an immunosuppressive cytokine that has been associated with multibacillary leprosy, variations in IL‐10 expression have been observed upon the occurrence or treatment of reactional episodes. 92 , 106 , 107 , 108 It has been proposed 109 that the presence of IL‐10 in reaction lesions is an attempt to activate regulatory pathways which can suppress proinflammatory pathways and temper tissue damage from excessive inflammation. Therefore, we questioned whether disruption of IL‐10 in the NOS2‐/‐ strain would exacerbate the model further toward a more inflammatory or even ‘‘reactional’’ state. To study this, we cross‐bred the NOS2−/− and IL‐10−/− strains to create a double knockout strain, 10NOS2−/−.
Like the control and parent strains, 10NOS2−/− mice controlled growth of M. leprae. 67 Similar to the NOS2−/− strain, inflammatory infiltrates replaced muscle bundles in the footpad granuloma, replacing nearly 50% of the muscle mass. Induration and the number of cells isolated from the footpad were also significantly higher in 10NOS2−/− mice than in either B6 or IL10−/−, although similar to NOS2−/−. In addition, 10NOS2−/− footpads yielded increased numbers of CD4+ and CD8+ lymphocytes. Upon examination of T cells isolated from the granulomas of M. leprae–infected mice and re‐stimulated in vitro with various M. leprae native or recombinant antigen preparations, 10NOS2−/− CD4+ and CD8+ T cells produced elevated levels of IFN‐γ compared with the other three strains. Furthermore, CD4+ T cells were present inside fragmented nerves. It is important to point out that the numbers of M. leprae present in the footpads of these different strains were the same. In fact, all four strains killed the bacilli. The difference in pathology and nerve damage was due to the host response. While the magnitude of T cell infiltration into the granuloma was primarily regulated by NOS2, a concurrent lack of IL‐10 resulted in an intensified M. leprae antigen responsiveness and T cell invasion of nerves.
6. CONTRIBUTIONS OF THE MOUSE MODEL
6.1. Molecular enumeration and viability of M. leprae
Mycobacteriu M. lepromatosis is uncultivable in axenic media and must be enumerated by direct microscopic counting of the bacilli. This procedure is complex, labor‐intensive, and suffers from limited sensitivity and specificity. Using primers to amplify a shared region of the multicopy repeat sequence (RLEP) specific to M. leprae, we have developed real‐time PCR assays for quantifying M. leprae DNA in biological samples. 110 Because of the intermediate level of growth of M. leprae in the TNFR1−/− footpad, this knockout strain, along with immunocompetent mice, athymic nude mice, and armadillo tissue, was used to assess the validity, accuracy, and reproducibility of this molecular enumeration assay. The RLEP assay was sensitive and showed excellent correlation with microscopic counting. We have also developed a molecular enumeration assay for M. lepromatosis. 3 Molecular enumeration is a rapid and objective means to estimate the numbers of M. leprae in tissues, and application of the technique can facilitate work with this agent in many laboratories.
We have also spent a considerable amount of time over the years to develop a number of surrogate viability assays for M. leprae. One of our most widely used assays is a radiorespirometry assay, which measures bacterial metabolism of palmitic acid. With a sensitivity of approximately one million organisms, this assay can determine viability from bacilli harvested from in vitro cultures and was one of the first assays used to show that activated macrophages can kill M. leprae. 57 Another technique is a live‐dead fluorescent staining procedure based on Syto9 and propidium iodide. 111 Perhaps the most exciting development is a molecular viability assay based on the expression of hsp18 and esxA transcripts from a defined number of M. leprae. 112 An advantage of this assay is that it can be performed on fixed tissues, lending it well to mouse footpad tissues or human biopsies. Furthermore, since it utilizes a standard curve, it determines absolute viability at time of harvest and does not rely on a paired “pretreatment” sample. The outcome of the molecular viability assay correlates with both radiorespirometry and the mouse footpad assay. Lastly, the molecular viability assay can detect M. leprae killing by either immunological, that is, activated macrophages, or chemotherapeutic means. In drug studies, it can differentiate between bactericidal and bacteriostatic drug activities, enabling even short‐term efficacy studies without the need for sub‐passage of M. leprae in mice. Development and optimization of the molecular enumeration and viability assays depended on the mouse model, and with these advances, we have developed a drug evaluation pipeline 113 which evaluates a new drug candidate against non‐dividing M. leprae in a cell‐free axenic analysis, non‐dividing M. leprae in intracellular macrophage cultures, and multiplying M. leprae in the mouse footpad assay.
6.2. Antigen responsiveness and diagnostic tests
A top priority in leprosy research is the implementation of a field‐friendly diagnostic test for detection of early leprosy, 114 and the development and assessment of such a test is an area of intense research. 115 , 116 , 117 To aid in this endeavor, animal models have been used to screen immune responsiveness to various M. leprae antigens with a goal of providing an interface between the production of these test antigens and their evaluation in human field trials. Lahiri et al 118 tested the sensitivity for antigen‐induced IFN‐γ production in a carefully defined mouse model of early leprosy infection. Since there may be no lesion present in subclinical leprosy, they examined splenic T cells for a systemic response to footpad infection and found that several M. leprae recombinant proteins induced significant levels of IFN‐γ secretion. Hagge et al 67 investigated the CD4+ and CD8+ T cell responses at the site of infection in an inflammatory footpad model of infection. They found upregulated IFN‐γ responses in the granuloma by CD4+, and especially CD8+, T cells to ML0380 (GroES), ML2038 (bacterioferritin), and ML1877 (EF‐Tu) in lesions associated with nerve damage. Antigen responsiveness to a variety of recombinant M. leprae proteins has also been evaluated in peripheral blood mononuclear cells from M. leprae–infected armadillos. 15 M. leprae antigens used in this study were specifically chosen based on their recognition by T cells derived from M. leprae exposed humans. 119 , 120 They found that armadillos expressed a strong IFN‐γ response to these same antigens. Armadillos have also been recently used to test new skin test reagents. 121 Collectively, these studies support the utility of these defined animal models of M. leprae infection for examining antigens currently under consideration for improved T cell–based diagnostic assays for leprosy in humans.
6.3. Vaccines
There is ample evidence that cell‐mediated immunity, effected by both CD4+ and CD8+ T cells, is instrumental in host defense against mycobacterial diseases. It is commonly stated that 95% of people are resistant to developing leprosy upon exposure to M. leprae. This rather anecdotal estimate is largely based on subjective observed incidences of disease in close contacts of leprosy patients in highly endemic areas. A review of epidemiological studies in the pre‐antibiotic era supports this conclusion 122 ; however, this phenomenon remains difficult to investigate and quantify as leprosy is often unrecognized and undiagnosed for years. Indeterminate leprosy has been known to resolve or progress into the leprosy spectrum. Furthermore, TT leprosy is considered the highly resistant form of the disease, but it is still leprosy. Viable organisms are found in the lesions and nerve damage often accompanies this resistance. So, what actually constitutes protection in leprosy?
Initial attempts to define protective immunity to M. leprae with the goal of producing a vaccine were studied in the mouse footpad by Shepard 123 who determined that heat‐killed M. leprae (HKML) stimulated the protection against subsequent footpad challenge with live M. leprae. Walker et al 124 demonstrated that HKML induced a Th1‐type cytokine response. In a study utilizing CD4+ T cell knockout (CD4−/−) mice and CD8+ T cell knockout (CD8−/−) mice (manuscript in preparation), vaccination with HKML primed spleen cells from both the CD4−/− and CD8−/− strains as well as control mice for substantial IFN‐γ production upon in vitro stimulation with M. leprae antigen. Moreover, vaccination with HKML induced a substantial inhibition of M. leprae growth in control and CD8−/− footpads. In contrast, however, growth of M. leprae in HKML‐vaccinated CD4−/− footpads was not inhibited but similar to non‐vaccinated mice.
Live, but not dead, BCG can confer protective immunity against M. leprae challenge and induce immunotherapeutic protection when administered after footpad infection. However, field trials of BCG as a vaccine for leprosy have yielded widely variable results (reviewed in ref. 125, 126 Other whole cell vaccine candidates for leprosy include M habana, M vaccae, M indicus pranii, and ICRC bacilli. 127
New defined vaccines are also being developed and tested in animal models. The LepVax vaccine is composed of a hybrid recombinant protein made of four M. leprae antigens, ML2531, ML2380, ML2055, and ML2028 (LEP‐F1) which are prepared as a stable emulsion with a synthetic, TLR4 agonist (GLA‐SE) as adjuvant. 128 LepVax reduced growth of M. leprae in mouse footpads; furthermore, LepVax, administered as postexposure immunoprophylaxis to armadillos infected with high doses of M. leprae, reduced sensory nerve damage and both delayed and alleviated motor nerve damage. A phase 1 trial with LepVax was recently completed and reported to be well‐tolerated. 129 Another attractive option in vaccine development is a vaccine that is cross‐protective for both leprosy and tuberculosis. Vaccination with M. tuberculosis Ag85B‐ESAT6 or M. leprae Ag85B‐ESAT6, both formulated in GLA‐SE, reduced growth of both organisms in mouse footpads. 130 In fact, M. leprae infection was controlled better by the M. tuberculosis recombinant vaccine than by the M. leprae recombinant vaccine.
6.4. Chemotherapeutics
Currently, treatment for leprosy in most parts of the world entails a WHO‐recommended multidrug therapy comprised of three drugs: dapsone, a weakly bactericidal sulfone drug which interferes with dihydrofolic acid synthesis by blocking dihydroteroate synthetase; rifampicin, a bactericidal drug which binds to the β‐subunit of the bacterial RNA polymerase and interferes with RNA synthesis; and clofazimine, a riminophenazine dye with immunosuppressive properties. While quite effective, the leprosy treatment regimens are quite long, at least six months for paucibacillary leprosy and 12 months for multibacillary leprosy. This lengthy course of therapy can exacerbate adverse drug side effects and make compliance difficult. This in turn can lead to relapse and drug resistance. Hence, better drugs with shorter regimens would be beneficial for control measures.
Testing new drugs for anti–M. leprae activity is not an easy undertaking. One must have a ready supply of viable leprosy bacilli against which to assay the test compounds. Through an interagency agreement with National Institutes of Allergy and Infectious Diseases, the NHDP maintains four strains of M. leprae and two strains of M. lepromatosis. As M. leprae only multiplies in living hosts, for example, humans, armadillos, and mice, these strains are carefully maintained through continuous passage in athymic nude mice. Aliquots of leprosy bacilli with a viability of >90% are routinely harvested and supplied to the leprosy research community for a wide variety of studies (https://www.hrsa.gov/hansens‐disease/research/index.html). In addition, because of the slow growth and long generation time drug studies in the mouse footpad can take months to complete.
Early drug studies were performed using immunocompetent mice. It is important to note that drug therapy for any infection does not likely kill every single pathogenic organism, but instead decreases the number of bacteria to a less detrimental level so that a developing immune response can complete the job. Therefore, we and others use the athymic nude mouse for drug efficacy studies because it increases the sensitivity of the assay by allowing evaluation solely of the drug's antimicrobial properties without contribution from an acquired cell‐mediated immune response. We also employ a variation of Shepard's kinetic mouse footpad model. With this method, footpads of athymic nude mice are infected with M. leprae and treatment begins one to two months postinfection, when the bacteria are in exponential growth. Footpads are harvested at an early and later time point post‐treatment completion, and M. leprae growth and viability are measured using molecular techniques. One measures the delay of growth in the treated group compared with control mice, and thereby bactericidal versus bacteriostatic outcomes can be differentiated. 131
However, could other immunological aspects benefit drug efficacy studies? One such contribution could lie in the development of chemoprophylaxis regimens for contacts of those diagnosed with leprosy. Ideally, postexposure prophylaxis should be safe, effective, easy to administer, and not costly. 114 It should also be a brief and simple regimen so as not to promote any untoward anxiety or stigma that may ensue from a confusion between prophylaxis and treatment for leprosy. With this in mind, a variety of proposed treatment regimens could be carried out in mice with differing levels of immunity, for example, immunocompetent, athymic nude, 132 or even knockout strains, to determine the least intrusive, yet effective, chemoprophylaxis regimen for different levels of immunity. Then, as immunodiagnostic assays for leprosy become more refined and predictive of susceptibility to M. leprae infection, 133 a more personalized chemoprophylaxis regimen could be recommended based on a multibacillary or paucibacillary disease prediction.
7. CONCLUSIONS AND OUTLOOK
An essential feature in the successful containment of chronic intracellular infections is the ability to develop and maintain an effective granulomatous response. While examination of patient biopsies has implicated several cytokines as responsible for disease susceptibility and pathology, it is difficult to characterize the longitudinal development of the chronic granuloma microenvironment generated in response to M. leprae infection when such infection may have existed sub‐clinically for years prior to diagnosis. The advantage of animal models is that we know the dose, time, and site of infection and can, for the most part, plan accordingly.
Over the years, we have evaluated, in vivo and in vitro, the host cell–mediated immune response to mouse footpad infection with M. leprae in more than 10 different strains of mice, many of which are presented here. These studies focused on the M. leprae–induced granuloma, its histopathology, cellular composition, the immunological products the cells generate, and their ability to kill or, conversely, provide a niche, for M. leprae. Interestingly, comparing our findings in the knockout mouse strains with intact immunocompetent control mice at one end of the leprosy spectrum and athymic nude mice on the other, no knockout of a single cytokine, T cell type, or antimicrobial mechanism transformed them into an immunosuppressed model like the athymic nude mouse. Nevertheless, based on their unique characteristic response, some mouse strains could be placed in the leprosy spectrum (Table 2). These findings underscore the presence of alternative mechanisms of host resistance in these animals, the very mechanisms which may be functioning in borderline leprosy.
TABLE 2.
Attributes for classification of mouse strains in the leprosy spectrum
| Characteristic | B6 | 10NOS2−/− | NOS2−/− | IFN‐γ−/− | Athymic nude |
|---|---|---|---|---|---|
| ML growth | + | + | + | ++ | +++ |
| Granuloma | Minimal | Destructive | Destructive | Unorganized | Leproma |
| FP induration | + | +++ | +++ | ++ | − |
| Lymphoid cells | CD4 > CD8 | ↑CD4↑CD8 | ↑CD4 | ↑CD4 | − |
| Myeloid cells | Epithelioid | Epithelioid | Epithelioid | ↓MΦ/↑PMN | Foamy |
| Cytokine response | TH1 | ↑TH1 | ↑TH1 | TH2/TH17 | TH2 |
| Classification | Self‐healing | TT | BT | BL | LL |
An ideal animal model of leprosy would recapitulate the clinical and histopathological spectrum of disease, the peripheral neuritis, and the reactional episodes. The current animal models each contribute to some of these aspects. There is as of yet no really good animal model, mouse or otherwise, for leprosy reactions, although ENL has been described in a sooty mangabey monkey. 27 The closest we have come so far to a reaction in a mouse is with our double knockout 10NOS2−/− strain. However, with the ever‐increasing numbers of genetically altered animals and the sophisticated methods used to generate such strains, the potential for reaction models and better representatives of the leprosy spectrum is definitely possible.
Disclaimer
The views expressed in this publication are solely the opinions of the author and do not necessarily reflect the official policies of the U.S. Department of Health and Human Services, the Health Resources and Services Administration, or the Agency for Healthcare Research and Quality, nor does mention of the department or agency names imply endorsement by the U.S. Government.
ACKNOWLEDGEMENTS
This work was supported by the NIH National Institute of Allergy and Infectious Diseases (AI50027 and AAI15006), and the Leprosy Research Initiative (LRI) and the Turing Foundation under LRI Grant number 703.15.43.
Adams LB. Susceptibility and resistance in leprosy: Studies in the mouse model. Immunol Rev. 2021;301:157–174. 10.1111/imr.12960
This article is part of a series of reviews covering Immunity to Mycobacteria appearing in Volume 301 of Immunological Reviews.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Johnstone PA. The search for animal models of leprosy. Int J Lepr Other Mycobact Dis. 1987;55(3):535‐547. [PubMed] [Google Scholar]
- 2. Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into foot‐pads of mice. J Exp Med. 1960;112:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma R, Singh P, McCoy RC, et al. Isolation of Mycobacterium lepromatosis and development of molecular diagnostic assays to distinguish Mycobacterium leprae and M. lepromatosis . Clin Infect Dis. 2020;71(8):e262‐e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levy L, Ji B. The mouse foot‐pad technique for cultivation of Mycobacterium leprae . Lepr Rev. 2006;77(1):5‐24. [PubMed] [Google Scholar]
- 5. Kirchheimer WF, Storrs EE. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int J Lepr Other Mycobact Dis. 1971;39(3):693‐702. [PubMed] [Google Scholar]
- 6. Job CK, Truman RW. Comparative study of Mitsuda reaction to nude mouse and armadillo lepromin preparations using nine‐banded armadillos. Int J Lepr Other Mycobact Dis. 2000;68(1):18‐22. [PubMed] [Google Scholar]
- 7. Hazbon MH, Rigouts L, Schito M, et al. Mycobacterial biomaterials and resources for researchers. Pathog Dis. 2018;76(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma R, Lahiri R, Scollard DM, et al. The armadillo: a model for the neuropathy of leprosy and potentially other neurodegenerative diseases. Dis Models Mech. 2013;6(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Truman R, Ebenezer G, Pena M, et al. The armadillo as a model for peripheral neuropathy in leprosy. ILAR J. 2014;54(3):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prodohl PA, Loughry WJ, McDonough CM, Nelson WS, Avise JC. Molecular documentation of polyembryony and the micro‐spatial dispersion of clonal sibships in the nine‐banded armadillo, Dasypus novemcinctus . Proc Biol Sci. 1996;263(1377):1643‐1649. [DOI] [PubMed] [Google Scholar]
- 11. Fichtner AS, Karunakaran MM, Starick L, Truman RW, Herrmann T. The Armadillo (Dasypus novemcinctus): a witness but not a functional example for the emergence of the butyrophilin 3/Vgamma9Vdelta2 system in placental mammals. Front Immunol. 2018;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindblad‐Toh K, Garber M, Zuk O, et al. A high‐resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478(7370):476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams JE, Pena MT, Gillis TP, Williams DL, Adams LB, Truman RW. Expression of nine‐banded armadillo (Dasypus novemcinctus) interleukin‐2 in E coli . Cytokine. 2005;32(5):219‐225. [DOI] [PubMed] [Google Scholar]
- 14. Pena MT, Adams JE, Adams LB, et al. Expression and characterization of recombinant interferon gamma (IFN‐gamma) from the nine‐banded armadillo (Dasypus novemcinctus) and its effect on Mycobacterium leprae‐infected macrophages. Cytokine. 2008;43(2):124‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pena M, Geluk A, Van Der Ploeg‐Van Schip JJ, Franken KL, Sharma R, Truman R. Cytokine responses to Mycobacterium leprae unique proteins differentiate between Mycobacterium leprae infected and naive armadillos. Lepr Rev. 2011;82(4):422‐431. [PubMed] [Google Scholar]
- 16. Schaub R, Avanzi C, Singh P, et al. Leprosy transmission in Amazonian countries: current status and future trends. Curr Trop Med Reports. 2020;7:79‐91. [Google Scholar]
- 17. Ploemacher T, Faber WR, Menke H, Rutten V, Pieters T. Reservoirs and transmission routes of leprosy; a systematic review. PLoS Negl Trop Dis. 2020;14(4):e0008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364(17):1626‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma R, Singh P, Loughry WJ, et al. Zoonotic leprosy in the southeastern United States. Emerg Infect Dis. 2015;21(12):2127‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. da Silva MB, Portela JM, Li W, et al. Evidence of zoonotic leprosy in Para, Brazilian Amazon, and risks associated with human contact or consumption of armadillos. PLoS Negl Trop Dis. 2018;12(6):e0006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyers WM, Walsh GP, Brown HL, et al. Leprosy in a mangabey monkey–naturally acquired infection. Int J Lepr Other Mycobact Dis. 1985;53(1):1‐14. [PubMed] [Google Scholar]
- 22. Donham KJ, Leininger JR. Spontaneous leprosy‐like disease in a chimpanzee. J Infect Dis. 1977;136(1):132‐136. [DOI] [PubMed] [Google Scholar]
- 23. Hubbard GB, Lee DR, Eichberg JW, Gormus BJ, Xu K, Meyers WM. Spontaneous leprosy in a chimpanzee (Pan troglodytes). Vet Pathol. 1991;28(6):546‐548. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki K, Udono T, Fujisawa M, Tanigawa K, Idani G, Ishii N. Infection during infancy and long incubation period of leprosy suggested in a case of a chimpanzee used for medical research. J Clin Microbiol. 2010;48(9):3432‐3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gormus BJ, Xu KY, Alford PL, et al. A serologic study of naturally acquired leprosy in chimpanzees. Int J Lepr Other Mycobact Dis. 1991;59(3):450‐457. [PubMed] [Google Scholar]
- 26. Honap TP, Pfister LA, Housman G, et al. Mycobacterium leprae genomes from naturally infected nonhuman primates. PLoS Negl Trop Dis. 2018;12(1):e0006190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baskin GB, Gormus BJ, Xu K, et al. Experimental borderline lepromatous leprosy with intraneural erythema nodosum leprosum in a mangabey monkey (Cercocebus atys). Int J Lepr Other Mycobact Dis. 1991;59(4):618‐623. [PubMed] [Google Scholar]
- 28. Wolf RH, Gormus BJ, Martin LN, et al. Experimental leprosy in three species of monkeys. Science. 1985;227(4686):529‐531. [DOI] [PubMed] [Google Scholar]
- 29. Baskin GB, Gormus BJ, Martin LN, et al. Experimental leprosy in African green monkeys (Cercopithecus aethiops): a model for polyneuritic leprosy. Am J Trop Med Hyg. 1987;37(2):385‐391. [DOI] [PubMed] [Google Scholar]
- 30. Waters MF, Bakri IB, Isa HJ, Rees RJ, McDougall AC. Experimental lepromatous leprosy in the white‐handed gibbon (Hylobatus lar): successful inoculation with leprosy bacilli of human origin. Brit J Exp Pathol. 1978;59(6):551‐557. [PMC free article] [PubMed] [Google Scholar]
- 31. Gunders AE. Progressive experimental infection with Mycobacterium leprae in a chimpanzee; a preliminary report. J Trop Med Hyg. 1958;61(9):228‐230. [PubMed] [Google Scholar]
- 32. Wong EA, Evans S, Kraus CR, et al. IL‐10 impairs local immune response in lung granulomas and lymph nodes during early Mycobacterium tuberculosis Infection. J Immunol. 2020;204(3):644‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madigan CA, Cameron J, Ramakrishnan L. A zebrafish model of Mycobacterium leprae granulomatous infection. J Infect Dis. 2017;216(6):776‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madigan CA, Cambier CJ, Kelly‐Scumpia KM, et al. A macrophage response to Mycobacterium leprae phenolic glycolipid initiates nerve damage in leprosy. Cell. 2017;170(5):973‐985 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teame T, Zhang Z, Ran C, et al. The use of zebrafish (Danio rerio) as biomedical models. Anim Front. 2019;9(3):68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meredith A, Del Pozo J, Smith S, Milne E, Stevenson K, McLuckie J. Leprosy in red squirrels in Scotland. Vet Rec. 2014;175(11):285‐286. [DOI] [PubMed] [Google Scholar]
- 37. Avanzi C, Del‐Pozo J, Benjak A, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science. 2016;354(6313):744‐747. [DOI] [PubMed] [Google Scholar]
- 38. Schilling AK, Del‐Pozo J, Lurz PWW, et al. Leprosy in red squirrels in the UK. Vet Rec. 2019;184(13):416. [DOI] [PubMed] [Google Scholar]
- 39. Schilling A, van Hooij A, Corstjens PLAM, et al. Detection of humoral immunity to mycobacteria causing leprosy in Eurasian red squirrels (Sciurus vulgaris) using a quantitative rapid test. Eur J Wildlife Res. 2019;65:5. [Google Scholar]
- 40. Schilling AK, Avanzi C, Ulrich RG, et al. British red squirrels remain the only known wild rodent host for leprosy Bacilli. Front Vet Sci. 2019;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tio‐Coma M, Sprong H, Kik M, et al. Lack of evidence for the presence of leprosy bacilli in red squirrels from North‐West Europe. Transbound Emerg Dis. 2020;67:1032‐1034. [DOI] [PubMed] [Google Scholar]
- 42. Hardouin EA, Baltazar‐Soares M, Schilling AK, et al. Conservation of genetic uniqueness in remaining populations of red squirrels (Sciurus vulgaris L.) in the South of England. Ecol Evol. 2019;9(11):6547‐6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five‐group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255‐273. [PubMed] [Google Scholar]
- 45. Modlin RL, Melancon‐Kaplan J, Young SM, et al. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci USA. 1988;85(4):1213‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welch TM, Gelber RH, Murray LP, Ng H, O'Neill SM, Levy L. Viability of Mycobacterium leprae after multiplication in mice. Infect Immun. 1980;30(2):325‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chehl S, Ruby J, Job CK, Hastings RC. The growth of Mycobacterium leprae in nude mice. Lepr Rev. 1983;54(4):283‐304. [DOI] [PubMed] [Google Scholar]
- 48. Colston MJ, Hilson GR. Growth of Mycobacterium leprae and M marinum in congenitally athymic (nude) mice. Nature. 1976;262(5567):399‐401. [DOI] [PubMed] [Google Scholar]
- 49. Converse PJ, Haines VL, Wondimu A, Craig LE, Meyers WM. Infection of SCID mice with Mycobacterium leprae and control with antigen‐activated "immune" human peripheral blood mononuclear cells. Infect Immun. 1995;63(3):1047‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hagge DA, Parajuli P, Kunwar CB, et al. Opening a can of worms: leprosy reactions and complicit soil‐transmitted helminths. EBioMed. 2017;23:119‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adams LB, Scollard DM, Ray NA, et al. The study of Mycobacterium leprae infection in interferon‐gamma gene–disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J Infect Dis. 2002;185(Suppl 1):S1‐8. [DOI] [PubMed] [Google Scholar]
- 52. Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN‐gamma and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10(5):221‐226. [DOI] [PubMed] [Google Scholar]
- 53. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene‐disrupted mice. J Exp Med. 1993;178(6):2243‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249‐2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kamijo R, Le J, Shapiro D, et al. Mice that lack the interferon‐gamma receptor have profoundly altered responses to infection with Bacillus Calmette‐Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178(4):1435‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66(2):830‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramasesh N, Adams LB, Franzblau SG, Krahenbuhl JL. Effects of activated macrophages on Mycobacterium leprae . Infect Immun. 1991;59(9):2864‐2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hibbs JB Jr, Vavrin Z, Taintor RR. L‐arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987;138(2):550‐565. [PubMed] [Google Scholar]
- 59. Adams LB, Franzblau SG, Vavrin Z, Hibbs JB Jr, Krahenbuhl JL. L‐arginine‐dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae . J Immunol. 1991;147(5):1642‐1646. [PubMed] [Google Scholar]
- 60. Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175(4):1111‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis . Infect Immun. 1995;63(2):736‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160(4):1796‐1803. [PubMed] [Google Scholar]
- 63. MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94(10):5243‐5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tubercle Lung Dis. 1997;78(5–6):237‐246. [DOI] [PubMed] [Google Scholar]
- 65. Gomes MS, Florido M, Pais TF, Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J Immunol. 1999;162(11):6734‐6739. [PubMed] [Google Scholar]
- 66. Adams LB, Job CK, Krahenbuhl JL. Role of inducible nitric oxide synthase in resistance to Mycobacterium leprae in mice. Infect Immun. 2000;68(9):5462‐5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagge DA, Scollard DM, Ray NA, et al. IL‐10 and NOS2 modulate antigen‐specific reactivity and nerve infiltration by T cells in experimental leprosy. PLoS Negl Trop Dis. 2014;8(9):e3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sharp AK, Colston MJ, Banerjee DK. Susceptibility of Mycobacterium leprae to the bactericidal activity of mouse peritoneal macrophages and to hydrogen peroxide. J Med Microbiol. 1985;19(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 69. Eiglmeier K, Fsihi H, Heym B, Cole ST. On the catalase‐peroxidase gene, katG, of Mycobacterium leprae and the implications for treatment of leprosy with isoniazid. FEMS Microbiol Lett. 1997;149(2):273‐278. [DOI] [PubMed] [Google Scholar]
- 70. Pym AS, Domenech P, Honore N, Song J, Deretic V, Cole ST. Regulation of catalase‐peroxidase (KatG) expression, isoniazid sensitivity and virulence by furA of Mycobacterium tuberculosis . Mol Microbiol. 2001;40(4):879‐889. [DOI] [PubMed] [Google Scholar]
- 71. Schlesinger LS, Horwitz MA. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J Clin Invest. 1990;85(4):1304‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vachula M, Holzer TJ, Andersen BR. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae . J Immunol. 1989;142(5):1696‐1701. [PubMed] [Google Scholar]
- 73. Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59(5):1755‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Williams DL, Torrero M, Wheeler PR, et al. Biological implications of Mycobacterium leprae gene expression during infection. J Mol Microbiol Biotechnol. 2004;8(1):58‐72. [DOI] [PubMed] [Google Scholar]
- 75. Hagge DA, Marks VT, Ray NA, et al. Emergence of an effective adaptive cell mediated immune response to Mycobacterium leprae is not impaired in reactive oxygen intermediate‐deficient mice. FEMS Immunol Med Microbiol. 2007;51(1):92‐101. [DOI] [PubMed] [Google Scholar]
- 76. Matucci A, Maggi E, Vultaggio A. Cellular and humoral immune responses during tuberculosis infection: useful knowledge in the era of biological agents. J Rheumatol Suppl. 2014;91:17‐23. [DOI] [PubMed] [Google Scholar]
- 77. Khader SA, Pearl JE, Sakamoto K, et al. IL‐23 compensates for the absence of IL‐12p70 and is essential for the IL‐17 response during tuberculosis but is dispensable for protection and antigen‐specific IFN‐gamma responses if IL‐12p70 is available. J Immunol. 2005;175(2):788‐795. [DOI] [PubMed] [Google Scholar]
- 78. Khader SA, Gopal R. IL‐17 in protective immunity to intracellular pathogens. Virulence. 2010;1(5):423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adams LB, Pena MT, Sharma R, Hagge DA, Schurr E, Truman RW. Insights from animal models on the immunogenetics of leprosy: a review. Mem Inst Oswaldo Cruz. 2012;107(Suppl 1):197‐208. [DOI] [PubMed] [Google Scholar]
- 80. Fava VM, Schurr E. The complexity of the host genetic contribution to the human response to Mycobacterium leprae, Chapter 8.1. In: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Greenville: American Leprosy Missions; 2016. https://www.internationaltextbookofleprosy.org [Google Scholar]
- 81. Palermo ML, Pagliari C, Trindade MA, et al. Increased expression of regulatory T cells and down‐regulatory molecules in lepromatous leprosy. Am J Trop Med Hyg. 2012;86(5):878‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montoya D, Cruz D, Teles RM, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6(4):343‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56(5):731‐740. [DOI] [PubMed] [Google Scholar]
- 84. Khanolkar‐Young S, Rayment N, Brickell PM, et al. Tumour necrosis factor‐alpha (TNF‐alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99(2):196‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hagge DA, Saunders BM, Ebenezer GJ, et al. Lymphotoxin‐alpha and TNF have essential but independent roles in the evolution of the granulomatous response in experimental leprosy. Amer J Pathol. 2009;174(4):1379‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin‐alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193(2):239‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alcais A, Alter A, Antoni G, et al. Stepwise replication identifies a low‐producing lymphotoxin‐alpha allele as a major risk factor for early‐onset leprosy. Nat Genet. 2007;39(4):517‐522. [DOI] [PubMed] [Google Scholar]
- 88. Geluk A. Correlates of immune exacerbations in leprosy. Semin Immunol. 2018;39:111‐118. [DOI] [PubMed] [Google Scholar]
- 89. Tio‐Coma M, van Hooij A, Bobosha K, et al. Whole blood RNA signatures in leprosy patients identify reversal reactions before clinical onset: a prospective, multicenter study. Sci Rep. 2019;9(1):17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dupnik KM, Bair TB, Maia AO, et al. Transcriptional changes that characterize the immune reactions of leprosy. J Infect Dis. 2015;211(10):1658‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scollard DM, Chaduvula MV, Martinez A, et al. Increased CXC ligand 10 levels and gene expression in type 1 leprosy reactions. Clin Vacc Immunol. 2011;18(6):947‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Geluk A, van Meijgaarden KE, Wilson L, et al. Longitudinal immune responses and gene expression profiles in type 1 leprosy reactions. J Clin Immunol. 2014;34(2):245‐255. [DOI] [PubMed] [Google Scholar]
- 93. Amorim FM, Nobre ML, Nascimento LS, et al. Differential immunoglobulin and complement levels in leprosy prior to development of reversal reaction and erythema nodosum leprosum. PLoS Negl Trop Dis. 2019;13(1):e0007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee DJ, Li H, Ochoa MT, et al. Integrated pathways for neutrophil recruitment and inflammation in leprosy. J Infect Dis. 2010;201(4):558‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teles RMB, Lu J, Tio‐Coma M, et al. Identification of a systemic interferon‐gamma inducible antimicrobial gene signature in leprosy patients undergoing reversal reaction. PLoS Negl Trop Dis. 2019;13(10):e0007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nathan C, Squires K, Griffo W, et al. Widespread intradermal accumulation of mononuclear leukocytes in lepromatous leprosy patients treated systemically with recombinant interferon gamma. J Exp Med. 1990;172(5):1509‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992;175(6):1729‐1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kaplan G, Sampaio EP, Walsh GP, et al. Influence of Mycobacterium leprae and its soluble products on the cutaneous responsiveness of leprosy patients to antigen and recombinant interleukin 2. Proc Natl Acad Sci USA. 1989;86(16):6269‐6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cogen AL, Lebas E, De Barros B, et al. Biologics in leprosy: a systematic review and case report. Am J Trop Med Hyg. 2020;102(5):1131‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Scollard DM, Joyce MP, Gillis TP. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of 2 cases. Clin Infect Dis. 2006;43(2):e19‐22. [DOI] [PubMed] [Google Scholar]
- 101. Sibley LD, Krahenbuhl JL. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae . Infect Immun. 1988;56(8):1912‐1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sibley LD, Krahenbuhl JL. Defective activation of granuloma macrophages from Mycobacterium leprae‐infected nude mice. J Leukoc Biol. 1988;43(1):60‐66. [DOI] [PubMed] [Google Scholar]
- 103. Hagge DA, Ray NA, Krahenbuhl JL, Adams LB. An in vitro model for the lepromatous leprosy granuloma: fate of Mycobacterium leprae from target macrophages after interaction with normal and activated effector macrophages. J Immunol. 2004;172(12):7771‐7779. [DOI] [PubMed] [Google Scholar]
- 104. Adams LB, Gillis TP, Hwang DH, Krahenbuhl JL. Effects of essential fatty acid deficiency on prostaglandin E2 production and cell‐mediated immunity in a mouse model of leprosy. Infect Immun. 1997;65(4):1152‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Balagon M, Saunderson PR, Gelber RH. Does clofazimine prevent erythema nodosum leprosum (ENL) in leprosy? A retrospective study, comparing the experience of multibacillary patients receiving either 12 or 24 months WHO‐MDT. Lepr Rev. 2011;82(3):213‐221. [PubMed] [Google Scholar]
- 106. Sreenivasan P, Misra RS, Wilfred D, Nath I. Lepromatous leprosy patients show T helper 1‐like cytokine profile with differential expression of interleukin‐10 during type 1 and 2 reactions. Immunology. 1998;95(4):529‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Manandhar R, Shrestha N, Butlin CR, Roche PW. High levels of inflammatory cytokines are associated with poor clinical response to steroid treatment and recurrent episodes of type 1 reactions in leprosy. Clin Exp Immunol. 2002;128(2):333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Khadge S, Banu S, Bobosha K, et al. Longitudinal immune profiles in type 1 leprosy reactions in Bangladesh, Brazil, Ethiopia and Nepal. BMC Infect Dis. 2015;15:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Iyer A, Hatta M, Usman R, et al. Serum levels of interferon‐gamma, tumour necrosis factor‐alpha, soluble interleukin‐6R and soluble cell activation markers for monitoring response to treatment of leprosy reactions. Clin Exp Immunol. 2007;150(2):210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL, Gillis TP. Enumeration of Mycobacterium leprae using real‐time PCR. PLoS Negl Trop Dis. 2008;2(11):e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lahiri R, Randhawa B, Krahenbuhl J. Application of a viability‐staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J Med Microbiol. 2005;54(Pt 3):235‐242. [DOI] [PubMed] [Google Scholar]
- 112. Davis GL, Ray NA, Lahiri R, et al. Molecular assays for determining Mycobacterium leprae viability in tissues of experimentally infected mice. PLoS Negl Trop Dis. 2013;7(8):e2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lenz SM, Collins JH, Lahiri R, & Adams LB. Rodent models in leprosy research, Chapter 10.3. In: Scollard DM & Gillis TP, eds. International Textbook of Leprosy. Greenville: American Leprosy Missions; 2020. https://www.internationaltextbookofleprosy.org [Google Scholar]
- 114. Steinmann P, Dusenbury C, Addiss D, Mirza F, Smith WCS. A comprehensive research agenda for zero leprosy. Infect Dis Pov. 2020;9(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Hooij A, Geluk A. Immunodiagnostics for leprosy, Chapter 7.1. In: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Greenville: American Leprosy Missions; 2016. https://www.internationaltextbookofleprosy.org [Google Scholar]
- 116. Gama RS, Leite LA, Colombo LT, Fraga LAO. Prospects for new leprosy diagnostic tools, a narrative review considering ELISA and PCR assays. Rev Soc Bras Med Trop. 2020;53:e20200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van Hooij A, van den Eeden S, Richardus R, et al. Application of new host biomarker profiles in quantitative point‐of‐care tests facilitates leprosy diagnosis in the field. EBioMed. 2019;47:301‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lahiri R, Randhawa B, Franken KL, et al. Development of a mouse food pad model for detection of sub clinical leprosy. Lepr Rev. 2011;82(4):432‐444. [PubMed] [Google Scholar]
- 119. Bobosha K, Van Der Ploeg‐Van Schip JJ, Zewdie M, et al. Immunogenicity of Mycobacterium leprae unique antigens in leprosy endemic populations in Asia and Africa. Lepr Rev. 2011;82(4):445‐458. [PubMed] [Google Scholar]
- 120. Geluk A, Bobosha K, van der Ploeg‐van Schip JJ, et al. New biomarkers with relevance to leprosy diagnosis applicable in areas hyperendemic for leprosy. J Immunol. 2012;188(10):4782‐4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Duthie MS, Pena MT, Khandhar AP, et al. Development of LepReact, a defined skin test for paucibacillary leprosy and low‐level M. leprae infection. Appl Microbiol Biotechnol. 2020;104(9):3971‐3979. [DOI] [PubMed] [Google Scholar]
- 122. Joyce MP. Historic aspects of human susceptibility to leprosy and the risk of conjugal transmission. Mem Inst Oswaldo Cruz. 2012;107(Suppl 1):17‐21. [DOI] [PubMed] [Google Scholar]
- 123. Shepard CC, Walker LL, van Landingham R. Heat stability of Mycobacterium leprae immunogenicity. Infect Immun. 1978;22(1):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Walker LL, Colston MJ. Role of Th‐1 lymphocytes in the development of protective immunity against M leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine mRNA. J Immunol. 1992;148:1885‐1889. [PubMed] [Google Scholar]
- 125. Merle CSC, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Exp Rev Vacc. 2010;9(2):209‐222. [DOI] [PubMed] [Google Scholar]
- 126. Schoenmakers A, Mieras L, Budiawan T, van Brakel WH. The state of affairs in post‐exposure leprosy prevention: a descriptive meta‐analysis on immuno‐ and chemo‐prophylaxis. Res Rep Trop Med. 2020;11:97‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Reed SG, Duthie MS. Vaccines for prevention of leprosy, Chapter 6.3. In: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Greenville: American Leprosy Missions; 2016. https://www.internationaltextbookofleprosy.org [Google Scholar]
- 128. Duthie MS, Pena MT, Ebenezer GJ, et al. LepVax, a defined subunit vaccine that provides effective pre‐exposure and post‐exposure prophylaxis of M leprae infection. NPJ Vacc. 2018;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Duthie MS, Frevol A, Day T, et al. A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP‐F1 + GLA‐SE) in healthy adults. Vaccine. 2020;38(7):1700‐1707. [DOI] [PubMed] [Google Scholar]
- 130. Coppola M, van den Eeden SJF, Robbins N, et al. Vaccines for leprosy and tuberculosis: opportunities for shared research, development, and application. Front Immunol. 2018;9:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lahiri R, Adams LB. Cultivation and viability determination of Mycobacterium leprae, Chapter 5.3. In: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Greenville: American Leprosy Missions; 2016. https://www.internationaltextbookofleprosy.org [Google Scholar]
- 132. Lenz SM, Collins JH, Ray NA, Hagge DA, Lahiri R, Adams LB. Post‐exposure prophylaxis (PEP) efficacy of rifampin, rifapentine, moxifloxacin, minocycline, and clarithromycin in a susceptible‐subclinical model of leprosy. PLoS Negl Trop Dis. 2020;14(9):e0008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. van Hooij A, Tio‐Coma M, Verhard EM, et al. Household contacts of leprosy patients in endemic areas display a specific innate immunity profile. Front Immunol. 2020;11:1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


