Abstract
Speech-language pathology caseloads often include individuals with hearing loss and a coexisting neurogenic communication disorder. However, specific treatment techniques and modifications designed to accommodate this population are understudied. Using a single-case experimental design, the current study investigated the utility of modified Video Implemented Script Training for Aphasia (VISTA) for an individual with nonfluent/agrammatic variant primary progressive aphasia and severe-to-profound, bilateral hearing loss. We analyzed the impact of this intervention, which incorporates orthographic input and rehearsal, on script production accuracy, speech intelligibility, grammatical complexity, mean length of utterance, and speech rate. Treatment resulted in comparable positive outcomes relative to a previous study evaluating script training in nonfluent/agrammatic primary progressive aphasia patients with functional hearing. Follow-up data obtained at three months, six months, and one year post-treatment confirmed maintenance of treatment effects for trained scripts. To our knowledge, this is the first study to investigate a modified speech-language intervention tailored to the needs of an individual with PPA and hearing loss, with findings confirming that simple treatment modifications may serve to broaden the range of treatment options available to those with concomitant sensory and communication impairments.
Keywords: Primary progressive aphasia, hearing loss, script training, treatment, single-case experimental design
Introduction
Hearing loss is one of the most common chronic impairments in older adults, affecting between 63%-68% of individuals over the age of 70 in the United States (Goman & Lin, 2016; Lin, Thorpe, et al., 2011). This sensory deficit often leads to a negative, multifactorial impact on quality of life, even in the absence of medical comorbidities (Dalton et al., 2007; Lin, Niparko, et al., 2011). Degraded hearing may limit an individual’s ability to successfully complete instrumental activities of daily living, such as using the telephone (Gopinath et al., 2012), and greater degree of hearing impairment is associated with increased disruption to an individual’s overall quality of life (Ciorba et al., 2012; Dalton et al., 2007).
The prevalence of hearing loss among individuals with stroke-induced aphasia is similar to that in the general population (Rankin et al., 2014; Formby et al., 1987). With a concomitant diagnosis of a neurogenic communication disorder such as aphasia, hearing loss may serve as an additional obstacle to establishing successful communication. Nonetheless, speech-language treatment options for adults with aphasia and concomitant hearing loss are understudied (Rankin et al., 2014; Formby et al., 1987; Zhang et al., 2018). For individuals with aphasia and concomitant hearing loss, linguistic deficits are compounded by degraded hearing acuity and undetected or unaddressed hearing loss can lead to suboptimal outcomes in treatment. Given that speech-language intervention often involves auditorily presented stimuli, it is imperative that treating speech-language pathologists (SLPs) make therapeutic accommodations that address concomitant hearing impairment (Silkes & Winterstein, 2016). Evidence-based practice for individuals with stroke-induced and progressive aphasia requires systematic investigation into the utility of speech-language intervention techniques and modifications that allow individuals with significant hearing loss to participate optimally in treatment.
Primary progressive aphasia
Primary progressive aphasia (PPA) is a type of aphasia that manifests as a gradual deterioration of speech and language skills, with relative sparing of other cognitive and motoric functions in early stages (Mesulam, 1982; Gorno-Tempini et al., 2011). The disorder is caused by underlying neurodegenerative disease, with speech and language deficits occurring as the earliest and most prominent symptoms. However, over time, PPA progresses to a more global dementia and/or motor syndrome, with emerging cognitive deficits, behavioral/personality changes, or non-speech motoric impairments (Dickerson, 2012; Harciarek et al., 2014; Rogalski & Mesulam, 2009).
Current consensus criteria for PPA diagnosis identify three distinct clinical phenotypes: semantic, logopenic, and nonfluent/agrammatic variants. These variants typically align with specific patterns of underlying brain atrophy, supported by neuroimaging, and/or specific pathological findings (Gorno-Tempini et al., 2004; Gorno-Tempini et al., 2011). The nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA), which is the focus of the current study, is characterized by the core clinical features of agrammatism and/or apraxia of speech (Ash et al., 2010; Gorno-Tempini et al., 2011; Grossman, 2012; Montembeault et al., 2018). As such, speech is typically slow, halting, distorted, and telegraphic. Additionally, at least two of the following associated characteristics must be present: impaired comprehension for syntactically complex sentences, spared comprehension at the single-word level, and intact object knowledge. Neuroimaging studies associate this phenotype with prominent atrophy in left posterior fronto-insular regions and tauopathy is often the underlying pathological finding at autopsy (Gorno-Tempini et al., 2011; Grossman, 2012; Spinelli et al., 2017).
Speech-language intervention for primary progressive aphasia
A number of studies have indicated that behavioral intervention targeting improved communication is beneficial for individuals with PPA. For individuals with nfvPPA, interventions targeting the core clinical features have proven beneficial, even in the face of disease progression (Schneider et al., 1996; Machado et al., 2014; Hameister et al., 2017; Henry et al., 2013; Henry et al., 2018). Several studies have evaluated treatments for grammatical sentence production. A study by Schneider and colleagues (1996) examined the outcomes of a treatment utilizing verbal and gestural cues that targeted the accurate production of verb tenses within sentences. The authors reported improved performance on trained verb tenses, generalization to untrained verbs, and some maintenance three months post-intervention. Another study examined training of verb inflections within simple canonical sentences using a cloze technique in one individual with nfvPPA (Machado et al., 2014). The authors found that this intervention resulted in significantly improved performance on trained structures, generalization to untrained structures, and maintenance of the primary outcome measure one month post-treatment. Hameister and colleagues (2017) implemented a modified constraint-induced language treatment for two individuals with nfvPPA, which resulted in significantly improved grammatical production, with one participant demonstrating generalization to untrained items. Taken together, these studies constitute a modest but growing body of evidence for the successful treatment of agrammatism in nfvPPA.
Fewer studies have examined the effect of treatment targeting motor speech impairment in nfvPPA, which is the other core clinical feature of this phenotype. One study targeted multisyllabic word production via an oral reading protocol with an individual with progressive apraxia of speech in the context of nfvPPA (Henry et al., 2013). The authors found that treatment resulted in a reduction in speech production errors and improved ability to self-correct speech errors in untrained reading passages, with maintenance of treatment gains one year post-treatment.
Many individuals with nfvPPA exhibit both of the core clinical features, so an ideal treatment approach is one that addresses both agrammatism and motor speech impairment. Henry et al. (2018) evaluated a treatment targeting speech production and fluency called Video Implemented Script Training for Aphasia (VISTA) in 10 individuals with nfvPPA. VISTA is a form of script training that involves a combination of clinician-directed, structured intervention targeting the accurate production and conversational usage of scripts and daily “speech-entrainment” (Fridriksson et al., 2012) home practice, in which individuals engage in unison production of scripted content with an audiovisual model of a healthy adult speaker. VISTA resulted in significantly improved production of correct, intelligible scripted words and reduced grammatical errors for trained script topics, as well as improved speech intelligibility for trained and untrained script topics at post-treatment. Performance on trained scripts was maintained through one year post-treatment. Moreover, performance on standardized speech/language measures and untrained scripts remained relatively stable through the one year follow-up, indicating that the treatment may serve to stabilize speech and language skills in the context of neurodegeneration.
Importantly, to our knowledge, none of the participants in the aforementioned treatment studies presented with significant, uncorrected hearing impairment. Participants were reported to have hearing that was at a functional level or was corrected to functional status via hearing aids or other assistive technology. For some participants, hearing status was unreported. Thus, it is unknown whether current, evidence-based treatment protocols are beneficial for individuals with severe or profound hearing loss that is either uncorrected or not corrected to a functional level appropriate for treatment. It is particularly important to study interventions that accommodate for hearing loss in individuals with nfvPPA given the finding that central auditory pathway involvement may be part of the neurodegenerative process in this syndrome (Cope et al., 2017; Goll et al., 2010; Grube et al., 2016; Hardy et al., 2016; Hardy et al., 2019).
Script training in neurogenic communication disorders
Beyond its implementation in nfvPPA (Henry et al., 2018), script training has proven effective for individuals with stroke-induced aphasia (e.g., Cherney et al., 2008; Holland et al., 2002; Goldberg et al., 2012; Grasso et al., 2019; Lee et al., 2009; Bilda, 2011; Youmans et al., 2005) and apraxia of speech (Youmans et al., 2011). Studies have documented several linguistic and motoric benefits of this intervention, including improved speech intelligibility, mean length of utterance, grammatical complexity, and speech rate. Script training interventions typically target patient-centered, personally relevant scripts that are intensively practiced to promote automaticity in production of scripted content (Cherney et al., 2008). Script training aligns with the International Classification of Functioning, Disability, and Health (ICF; World Health Organization, 2013) framework as it not only offers a restitutive approach to maximize speech and language status, but the scripted material is designed to be personally relevant. This enables the individual to participate in various functional communication contexts with different communication partners. Thus, this treatment adopts a person-centered approach that addresses both restoration of speech/language skills as well as activity/participation limitations imposed by aphasia.
“Speech entrainment,” a facilitation and practice technique used in some script training interventions, involves unison speech production with an audiovisual model. As a script training tool, it has proven efficacious for improving speech fluency in individuals with stroke-induced aphasia and PPA (Fridriksson et al., 2012, Fridriksson et al., 2015; Grasso et al., 2019; Henry et al., 2018). The relative importance of auditory and visual components of speech entrainment stimuli for promoting speech fluency has been investigated in aphasia caused by stroke, but not in nfvPPA. For individuals with stroke-induced aphasia, combined auditory and visual input was found to be most beneficial for promoting speech fluency (Fridriksson et al., 2012). This finding brings into question the utility of interventions such as VISTA for individuals with hearing loss that is not corrected to a functional level, as they are not able to adequately perceive auditory components of training stimuli. To our knowledge, there are no treatment studies that have investigated whether modifications to speech entrainment practice designed to accommodate for degraded auditory input may be of benefit to individuals with uncorrected or suboptimally-corrected hearing loss.
Existing script training interventions have utilized multimodal stimulation, incorporating written word stimuli as a complement to audiovisual components of script practice (Cherney et al., 2008). More specifically, the AphasiaScripts™ program features a video display of an animated therapist producing scripted material along with the text of the script. Thus, participants attend to auditory, visual, and textual content simultaneously to promote script learning and automaticity. In this way, orthographic input may serve as a potential supportive input modality to accommodate for impaired auditory perception. However, no studies have evaluated whether providing complementary orthographic input may compensate for hearing loss in an intervention that relies heavily on audiovisual training stimuli, such as VISTA.
Current study
In this study, we examined the utility of modified VISTA (VISTA with reading, hereafter VISTA-R) for an individual with nfvPPA and severe-to-profound hearing impairment. The original VISTA treatment was modified for this study by providing exposure to and practice with orthographic input as a complement to audiovisual input during script practice. Reading tasks were adapted from Oral Reading for Language in Aphasia (ORLA; Cherney, 1995; Cherney et al., 1986), a reading intervention that involves repeated oral reading of sentence-level text, initially with the clinician and then independently. ORLA has proven beneficial for reading comprehension as well as other aspects of spoken and written language (Cherney, 2010). VISTA-R included a reading component that was modeled after ORLA rather than other reading interventions (e.g., Brookshire & Nicholas, 1984; Kim & Russo, 2010; Moyer, 1979; Rogalski & Edmonds, 2008) because ORLA involves straightforward repeated rehearsal of written content, whereas other approaches engage additional cognitive or linguistic processes to target comprehension.
The goal of this study was to assess the impact of VISTA-R on treatment outcomes (script production accuracy, speech intelligibility, grammatical complexity, mean length of utterance, and speech rate) for an individual with nfvPPA and severe-to-profound hearing loss that was not fully corrected. Specifically, our research questions and hypotheses were: Question 1) Will an individual with residual, uncorrected hearing loss and nfvPPA benefit from VISTA with multimodality input to compensate for hearing loss? We predicted that the participant would demonstrate a positive treatment response, supported by a large effect size and significant improvement on the primary outcome measure, with maintenance up to one year post-treatment. Additionally, we predicted that the participant’s standardized test performance at post-treatment and follow-up time points would be relatively stable, as was observed in the previous VISTA study. Question 2) Will this participant’s treatment response on the primary outcome measure and other discourse measures be comparable to a cohort of individuals with functional hearing who participated in VISTA previously (Henry et al., 2018)? We predicted that pre- to post-treatment change on the primary and secondary outcome measures would be commensurate with outcomes from the original VISTA cohort.
Methods
Participant
The participant was a 72 year-old monolingual English speaking male. He was a retired accountant with thirteen years of formal education (Table 1). Enrollment in the treatment study was conducted through the Aphasia Research and Treatment Lab at the University of Texas at Austin. The study was approved by the Institutional Review Board at the University of Texas at Austin and written informed consent was obtained from the participant.
Table 1.
Demographics for the VISTA-R participant and the original VISTA cohort (Henry et al., 2018; n = 10).
| Demographics | VISTA-R Participant | VISTA Cohort |
|---|---|---|
| Age | 72 | Mean (SD): 67.7 (5.5) |
| Gender | Male | 4 M; 6 F |
| Education (years) | 13 | Mean (SD): 15.6 (2.1) |
| Handedness | Right | Right (all participants) |
The participant was diagnosed with nfvPPA one year prior to participating in this study, after undergoing comprehensive neurological and neuropsychological testing. At the time of the study, the participant presented with a five-year history of speech and language decline. Comprehensive pre-treatment assessment corroborated the nfvPPA diagnosis per current clinical criteria (Gorno-Tempini et al., 2011), revealing agrammatic language and halting speech, with moderate apraxia and mild dysarthria. Additionally, the participant presented with all three secondary features of nfvPPA (impaired comprehension of syntactically complex sentences, spared single word comprehension, and intact object knowledge).
Scores from standardized speech, language, and cognitive measures are presented in Table 2 along with the mean scores of the participants (n = 10) in the original VISTA cohort (Henry et al., 2018) for comparison. At pre-treatment, cognition was grossly intact, as demonstrated by the participant’s Mini Mental State Exam (MMSE; Folstein et al., 1975) score (29/30). Modified t-tests (Crawford & Howell, 1998) indicate that the participant’s global language, cognitive, and motor speech skills were not significantly different from the original cohort on the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006; t = −1.46; p = .178), the MMSE (t = .51; p = .623), or the Motor Speech Examination (MSE; Wertz et al., 1984; t = .22; p = .831 for apraxia of speech severity rating; t = −.50; p = .729 for dysarthria severity rating). However, a statistically significant difference was found on the Northwestern Anagram Test (NAT; Thompson et al., 2012; t = −2.53; p = .032), with the participant in the current study performing significantly lower than the participants in the original VISTA cohort. Additionally, oral reading was a relative strength for the participant. We observed high accuracy during oral reading tasks, including the Grandfather Passage (88.71% correct and intelligible words read; Darley et al., 1975) and an informal probe comprising sentences ranging in length from four to eight words and including complex words (i.e., multisyllabic words of up to four syllables; 88.89% correct and intelligible words read).
Table 2.
Speech, language, and cognitive assessment performance at pre- and post-treatment for the VISTA-R participant. Mean scores and standard deviations (SD) from the original VISTA cohort reported in Henry et al., 2018 are included for comparison.
| Test | Time Point |
VISTA-R Participant |
Mean (SD) from VISTA Cohort |
|---|---|---|---|
| Western Aphasia Battery-Revised Aphasia Quotient (WAB-R AQ; 100) | Pre | 74.5 | 84.3 (6.4) |
| Post | 76.8 | 85.7 (6.1) | |
| Mini Mental Status Examination (MMSE; out of 30) | Pre | 28 | 26.8 (2.3) |
| Post | 29 | 27.3 (1.8) | |
| Apraxia of Speech Rating* (0=none; 7=profound) | Pre | 4 | 3.7 (1.3) |
| Post | 4 | 4.3 (1.3) | |
| Dysarthria Rating* (0=none; 7=profound) | Pre | 2 | 2.9 (1.7) |
| Post | 2 | 3.1 (1.9) | |
| Northwestern Anagram Test (NAT, %) | Pre | 6.7♦ | 63.7 (21.5) |
| Post | 3.3♦ | 74.3 (20.0) |
from Wertz et al., 1984
significantly different from published VISTA cohort using modified t-test (Crawford & Howell, 1998)
The participant qualified for enrollment based on cognitive-linguistic inclusionary criteria (diagnosis of nfvPPA, an MMSE score of ≥ 15, and intact repetition of at least three syllables on the repetition subtest of the WAB-R) that were applied in the original study (Henry et al., 2018); however, hearing status was initially deemed exclusionary. The participant wore bilateral hearing aids and reported longstanding, bilateral, severe-to-profound hearing loss that emerged during adulthood. Audiometric evaluation results were requested for aided and unaided conditions; however, only unaided hearing thresholds were available (Table 3). During the initial tele-assessment screening for study participation, the participant performed at chance on a minimal pairs discrimination task, despite wearing hearing aids bilaterally. Of note, participants in the original study were required to perform (with or without hearing aids) at a minimum of 70% correct on this measure (mean for Real Words = 9.56/10, SD = 1.01; mean for Non-Words = 9.67/10; SD = 1) and failure on this task was considered exclusionary. In addition to poor performance on the minimal pairs task, the participant frequently required multiple spoken repetitions of task instructions from both the clinician and his spouse. To optimize hearing during sessions in hopes of qualifying for study participation, he obtained a Phonak ComPilot II device, which connected his computer’s audio signal directly to his hearing aids via Bluetooth. With this device, repetition of task instructions was required less frequently; however, hearing status was still not corrected to a fully functional level. In the original VISTA study, significantly impaired hearing or vision that was not corrected to a functional level was considered exclusionary, as the treatment procedures require adequate perception of audiovisual practice stimuli. However, because this potential participant was otherwise an excellent candidate for intervention, and because he was extremely motivated to undergo treatment, modifications to the VISTA protocol were implemented and he was enrolled in treatment.
Table 3.
The VISTA-R participant’s unaided hearing profile.
| Frequency (in Hz) | Right Ear Severity (in dB) | Left Ear Severity (in dB) |
|---|---|---|
| 250 | 55/Moderate-to-Severe | 55/Moderate-to-Severe |
| 500 | 55/Moderate-to-Severe | 60/Moderate-to-Severe |
| 1000 | 55/Moderate-to-Severe | 60/Moderate-to-Severe |
| 1500 | 70/Severe | 60/Moderate-to-Severe |
| 2000 | 85/Severe | 80/Severe |
| 3000 | 100/Profound | 100/Profound |
| 4000 | 105/Profound | 105/Profound |
| 6000 | 105/Profound | 105/Profound |
| 8000 | 105/Profound | 105/Profound |
The participant lived remotely from the research site; therefore, all phases of intervention were conducted via telerehabilitation. Notably, in the original VISTA cohort, half of the participants also received treatment via telerehabilitation, and all treatment components were identical, regardless of service delivery modality. Likewise, in the current study, no modifications were made to the telehealth-based intervention beyond the addition of reading procedures. Telerehabilitation is growing in popularity and has proven to be as effective as face-to-face speech/language intervention for individuals with stroke-induced aphasia and PPA (Agostini et al., 2014; Dechêne et al., 2011; Dial et al., 2019; Fridler et al., 2012; Furnas & Edmonds, 2014; Meyer et al., 2016; Woolf et al., 2016). In addition to the importance of accurate perception of audiovisual practice stimuli, the potentially degraded auditory and visual signals that result from conducting treatment sessions using teleconference software (Dial et al., 2019) were also considerations for treatment design with this participant, underscoring the need for modifications to the original VISTA protocol.
Experimental design
A multiple-baseline design across scripts was used for this single-case experiment. Six scripts were developed in total. Four scripts were randomly selected for training and two scripts remained untrained, serving as controls. The participant and his spouse were asked to generate script topics that were functionally relevant and important to target in treatment. Script content was then developed via a collaborative process with the participant and clinician. In accordance with procedures from the original VISTA study, each script was constructed to be challenging but attainable, given the participant’s language and motor speech profile. In particular, script sentences were designed to be a few words beyond the participant’s mean length of utterance (MLU; from the Spontaneous Speech subtest of the WAB-R) and to avoid a high proportion of multisyllabic words. Content was generated via an initial script development probe, wherein the participant was asked to speak at length about each selected topic. During this initial probe, the participant demonstrated moderate apraxia and mild dysarthria, consistent with results from the MSE. Additionally, his responses were characterized by telegraphic, one- to three-word utterances that were not consistently effective in communicating the intended thought. Thus, the clinician asked clarifying questions and confirmed details with the spouse to aid comprehension of the participant’s spoken production. The script development probe was used to generate specific information and to gain a sense of the participant’s preferred word choice and phrasing. Through this process, scripts were developed that were factually accurate, grammatically correct, and consistent with the participant’s unique “voice.” All scripts were reviewed with and approved by the participant and his spouse prior to treatment implementation.
All scripts, both trained and untrained, were balanced for linguistic parameters (via t-tests comparing each metric between scripts; all p-values greater than .05), including total number of sentences, number of words in sentences, average words per sentence, average syllables per word, number of complex words (i.e., measured as number of words with three or more syllables that are not proper nouns, a combination of easy or hyphenated words, or two-syllable verbs made into three-syllables by adding -es or -ed endings), mean length of utterance in morphemes, and Flesch Kincaid Reading Ease score (Flesch, 1948). The average number of words per script was 25.83 (additional details regarding linguistic characteristics can be found in the Appendix).
After script content was finalized, practice videos were created in which a healthy, gender-matched model slowly produced the words in each script using exaggerated articulatory gestures. The videos were filmed and edited to include a close-up of the healthy speaker’s mouth rather than the whole face. The speech rate used in the videos was based on the participant’s spontaneous speech rate during pre-treatment oral reading and picture description tasks.
After script creation but before any exposure to practice videos, two baseline probes were collected for each script topic. During baseline probes, the participant was asked to recall the scripted content for each topic following the prompt, “Tell me about (specific topic).” After baseline probing was complete and prior to starting home practice, guided speech entrainment practice with the first script video was attempted and clinician feedback was provided following each practice opportunity. The participant was instructed to keep his eyes open, look at the mouth model, and listen to the recording as he attempted unison speech production. He received clinician feedback regarding adherence to these guidelines. Subsequently, the clinician emailed the participant a homework link for Script 1. The homework interface featured a screen showing the text of the script, followed by a separate page with the practice video (delivered via QualtricsXM online survey platform). The original VISTA home practice regimen was modified in that the participant was instructed to read the script out loud four times before engaging in 30 minutes of unison speech production practice. The participant was asked to complete this home practice regimen daily. Practice frequency and duration were tracked via the survey platform.
Treatment sessions with the clinician took place twice weekly over six weeks, (12 sessions total), with sessions lasting approximately 45-60 minutes (see Box 1 for the complete treatment regimen). Only one script was practiced at a time, with each of the four trained scripts targeted over three sessions with the clinician. Each session began with the collection of a spontaneous probe for the script-in-training as well as other selected script topics. Each script that was not in training was probed once per week. As during baseline probing, these probes were collected using the prompt, “Tell me about (specific topic).” The participant also completed a unison speech production probe for the script-in-training, so that the clinician could observe how the participant was practicing with his video during home practice. This task also allowed the clinician to provide supportive or constructive feedback regarding the mechanics of unison speech production practice.
Box 1. VISTA-R treatment regimen, adapted from Henry et al., 2018.
| Probing: Participant completes trained and untrained script probes. If criterion is met (during sessions 1 and 2 for a given script) on primary outcome measure and participant is able to speak in unison during speech entrainment for trained script, then speaking rate of VISTA video is increased by 10% for home practice | |
| Treatment Steps: | |
| 1. Oral Reading of Script Text | Participant reads written script aloud, sentence by sentence, and points to each word simultaneously, with fading visual and verbal cues provided by clinician. This step ends with participant reading entire script aloud independently |
| 2. Recall/Recognize | Participant chooses each correct script sentence from four foil sentences |
| 3. Organize/Construct | Participant puts script sentences in order |
| 4. Read | Participant reads script aloud |
| 5. Respond to Questions in Scripted Order | Participant produces scripted sentences from memory in response to questions (in order of script) |
| 6. Produce Script from Memory | Participant recites entire script |
| 7. Respond to Questions with Scripted Sentences | Participant responds to questions with scripted sentences (not in order of script) |
| Structured Conversation: During the second treatment session for each script, participant engages in conversation with a naïve communication partner regarding the script topic | |
| Phone Call: On a non-treatment day, participant engages in structured conversation pertaining to script-in-training for 5-10 minutes with clinician | |
| Homework: Daily home practice with script-in-training, reading script out loud four times prior to engaging in 30 minutes of speech entrainment practice with the video | |
After probes were obtained, each training session started with choral and independent reading of script text and then transitioned to the original VISTA training tasks (Box 1). Reading tasks were included to promote script learning via complementary orthographic input and repeated exposure to written (in addition to auditory) stimuli. During the reading portion of the session, the participant was presented with the entire text of the script. Each sentence was produced aloud in turn. The clinician used a digital pointer to highlight each word on the computer screen while reading aloud and then instructed the participant to read the sentence aloud in unison with her while also pointing to each word (Figure 1). Next, the participant independently read each word of the current sentence aloud. After completing this sequence for each sentence, he independently read the entire script aloud.
Figure 1.

In-session visual display for reading practice. Both the clinician and the participant read the script aloud in unison. The clinician highlighted each word in the script and the participant simultaneously pointed to the word on his computer screen. Videoconference software also showed the faces of the clinician and participant on the right side of the screen (not depicted).
After completing the reading steps, the participant engaged in the VISTA training tasks (Henry et al., 2018), which are designed to target memorization, functional script usage, grammatical correctness, and articulatory accuracy (Box 1). First, individual scripted sentences were selected from foil sentences and arranged in the correct order, followed by oral reading of the entire script. Next, the clinician asked conversational questions to elicit single sentences from the script, and the participant provided the responses from memory in the order of his script. Subsequently the participant was instructed to recite his entire script from memory. Finally, in a second round of conversational questions, the participant responded to questions by producing scripted sentences from memory out of scripted order. Articulatory, grammatical, and general wording errors were targeted throughout the session via visual, verbal, and phonetic placement cues1.
Consistent with the original VISTA protocol, teletherapy sessions with the clinician and home practice procedures were complemented by generalization tasks. During the second teletherapy session for each script, a naïve communication partner engaged in a brief, informal conversation with the participant about the script topic in training. The communication partners were research assistants who were informed of the script topic but were not familiar with the script content. This conversational task provided an opportunity for the participant to practice using his script dynamically in a less structured conversational context. Additionally, home practice with training videos was complemented by generalization practice. Specifically, the participant engaged in a brief (5-10 minute) phone call once per week during which the clinician elicited spontaneous production of single sentences from the script-in-training using conversational questions (e.g., “How long have you been married?”). The phone call was included to provide the participant with an opportunity to practice using scripted material in a less structured context and to encourage generalized use of the script in everyday conversation.
As a means to ensure that audiovisual practice stimuli are maximally challenging to VISTA participants, we now incrementally adapt the speaking rate of training stimuli in response to each individual’s performance (Schaffer et al., 2018). Specifically, the speaking rate of each practice video is increased by 10% between sessions if the participant demonstrates at least 90% correct and intelligible scripted words during the spontaneous speech probe and successfully engages in unison speech production with the video at the beginning of the session. Adobe software (After Effects© and Audition©) was used to adjust the audio and video tracks while preserving pitch. There are three possible video rates for each trained script (i.e. original rate, first rate increase, second rate increase). Based on his performance during treatment probes, the current participant practiced with one rate increase for scripts 1 and 4, and two rate increases for scripts 2 and 3.
At the conclusion of the treatment phase, the participant was provided with videos for all trained scripts. In an effort to maximize treatment gains longitudinally, he was encouraged to continue engaging in home practice post-treatment, as were participants in the original study. Follow-up speech, language, and cognitive testing were completed three, six, and 12 months post-treatment in order to evaluate stability of treatment effects.
Outcome measures and statistical analysis
In order to address research question 1, regarding the participant’s response to treatment on the primary outcome measure (percent correct, intelligible scripted words), we calculated a standardized effect size and conducted a simulation analysis to determine the significance of the change in behavior from pre- to post-treatment and each subsequent time point. Effect sizes were calculated for trained and untrained scripts using d-statistics, wherein the mean pre-treatment performance was subtracted from the post-treatment performance and divided by the pre-treatment standard deviation (Beeson & Robey, 2006). Consistent with the original VISTA study (Henry et al., 2018), pre-treatment performance was calculated as average percent accuracy from the two baseline probes and post-treatment performance was calculated as average percent accuracy from two probes conducted once all script training was completed. Follow-up scores comprised a single probe from each trained and untrained script.
To conduct significance testing, we used a simulation technique that involved random sampling with replacement on the basis of the participant’s performance in each condition (trained versus untrained topics), and at each time point (Dial & Martin, 2017). The participant’s percent accuracy was obtained from each time point/condition, and random sampling with replacement of the actual data occurred on an item-by-item basis, using probabilities of correct and incorrect responses to create simulated datasets with parameters that were identical to the observed data. This process was conducted 10,000 times, thus creating 10,000 simulated distributions of performance accuracy scores at each time point (pre-treatment, post-treatment, and each follow-up) for trained scripts and separately for untrained scripts. A comparison of the distributions from two time points within a trained or untrained condition were then made, in order to calculate a p-value. Additionally, simulated data were used to calculate difference scores to determine 95% confidence intervals. Furthermore, to contextualize the direct benefits of treatment, we report outcomes on standardized assessments over time.
In order to address research question 2, to determine whether the participant’s treatment response was comparable to the previous cohort with functional hearing, we analyzed the participant’s change in performance on the primary outcome measure and several additional outcome measures from pre- to post-treatment compared with the original VISTA cohort’s change in performance. An unstandardized difference test (Crawford & Garthwaite, 2005) was utilized for statistical comparison of the magnitude of change in performance. This test examines whether the difference between two scores differs significantly in a single individual from the distribution of differences in controls. It has been found to control the Type 1 error rate, even when using a comparison cohort with a small n (in our case, n = 10). For these statistical tests, a significance level of p < 0.05 (two-tailed) was used. Two outcome measures reported in the original VISTA study (Henry et al., 2018) are reported in the current study: percent correct, intelligible scripted words and overall speech intelligibility (defined as the percent intelligible words produced during script probes, regardless of the targeted scripted content). Grammatical errors per 100 words was included as a third outcome measure in the original VISTA study. However, for individuals such as the current participant, who present with a very reduced mean length of utterance, this measure does not adequately capture grammatical impairment, since it is difficult to judge the number of grammatical “errors” in an utterance consisting of only a couple words.
To provide insight into other possible linguistic and motoric benefits of the intervention, additional outcome measures were analyzed using previously collected data from the original VISTA cohort and the current participant. Because grammatical errors per 100 words was not a meaningful outcome measure for this participant, the grammatical complexity index from the Computerized Language Analysis software (CLAN, MacWhinney, 2000) was used as an alternative assessment of grammatical ability. This index compares the number of complex grammatical relations2 to the total number of grammatical relations. Additional outcome measures that were calculated for the original cohort (and not previously reported), as well as the current participant, included speech rate in words per minute (WPM), mean length of utterance in morphemes, and mean length of utterance in words, each of which was derived using CLAN.
Interrater reliability and treatment fidelity
Interrater reliability was evaluated using ratings from the treating clinician and a trained undergraduate research assistant for the primary outcome measure (correct, intelligible scripted words). The treating clinician recorded the participant’s performance online during the treatment session, whereas the research assistant conducted ratings after being blinded to the treatment condition (trained versus untrained script). For 25% of the total number of treatment sessions, the research assistant watched and transcribed recorded videos of script probes, coding each word as intelligible or unintelligible and correct relative to the script target. Interrater reliability was calculated using point-by-point agreement (Kazdin, 1982). The number of agreements and disagreements between the two raters was calculated and the number of agreements was divided by the number of agreements and disagreements combined. The total value was multiplied by 100 to determine the reliability score. Interrater reliability was found to be high, at 98.18%.
Fidelity ratings were conducted by two trained undergraduate research assistants to determine the treating clinician’s adherence to the treatment protocol during sessions. The two raters independently viewed 33.33% of randomly selected treatment sessions and indicated whether or not the clinician correctly followed each step of the treatment hierarchy. Fidelity was high, at 100%.
Descriptive surveys
As a means of assessing change in functional communication status, the participant’s spouse completed the Communication Effectiveness Index (CETI) at pre- and post-treatment (Lomas et al., 1989). This 16-item questionnaire requires the communication partner to rate the participant’s current communication status relative to premorbid communication functioning using a visual analog scale. For this measure, the participant’s spouse placed a mark along a continuum ranging from “not at all able” (0) to “as able as before” (100).
To capture change in communication contexts and skills more closely linked to the specific intervention, a 21-item in-house survey (Henry et al., 2018) was administered to the participant and his spouse post-treatment. The survey gathered qualitative data regarding the participant and spouse’s perception of his communication skills and affective disposition as a result of participating in treatment. Specifically, communication-centered questions encompassed perceptions of speech fluency and grammatical abilities, articulatory precision, success with engaging in VISTA unison speech production tasks, and speech/language self-monitoring abilities with familiar and unfamiliar communication partners. Affect-based questions associated with the act of speaking pertained to level of stress, comfort, and confidence with familiar and unfamiliar communication partners. A seven-point Likert scale was used to rate responses to each item. Response choices on this continuum ranged from “a lot worse” (−3) to “a lot better” (+3).
Results
The participant attended each scheduled treatment session as well as all follow-up assessments. He completed daily home practice throughout the treatment period, with the exception of two days of missed practice due to personal factors. Digital records of practice frequency and duration revealed that, in total, the participant spent 24.45 hours engaged in his home practice over the course of the intervention. Moreover, from the end of treatment to the one year follow-up time point, the participant completed 11.29 hours of home practice.
The results, indicating immediate treatment effects, longitudinal stability of treatment effects, comparison to the original VISTA cohort, and patient and care partner perceptions of treatment response, are described below.
Individual response to treatment
Treatment effects from pre- to post-treatment
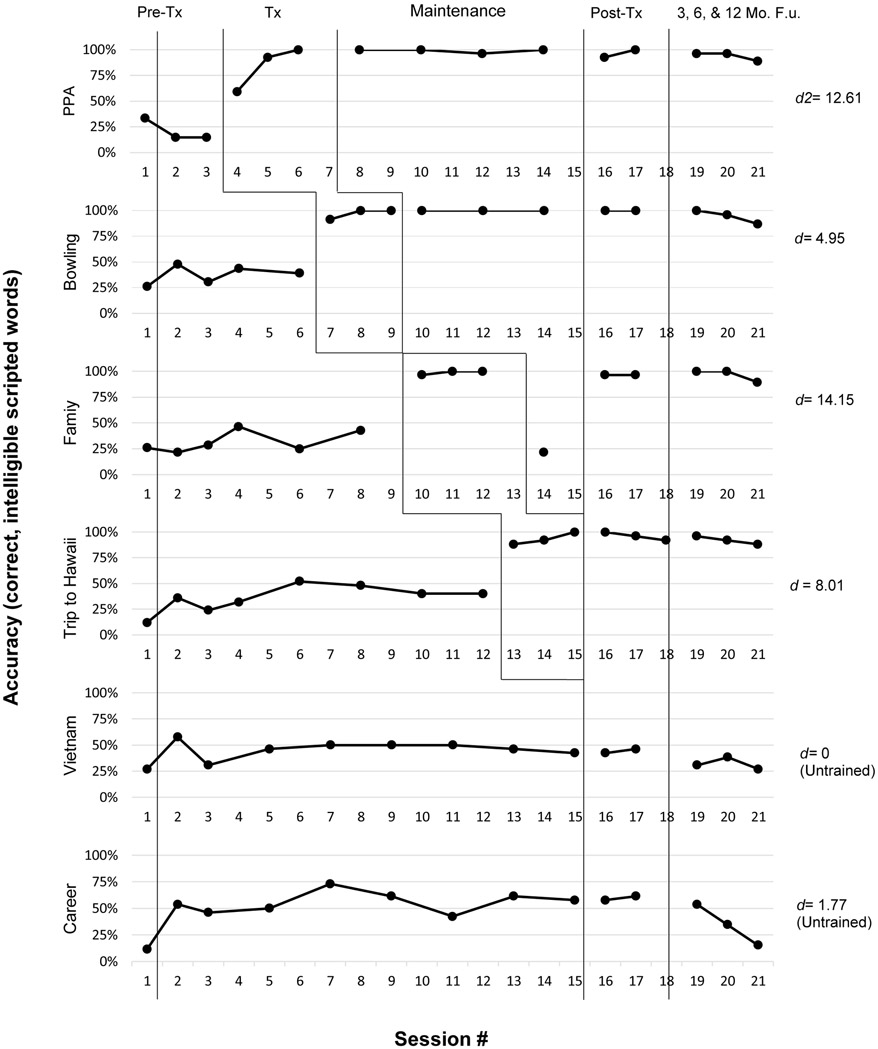
With respect to research question 1, we examined the participant’s performance on the primary outcome measure. He met the established mastery criterion of 90% correct, intelligible words for all trained scripts (Figure 2), with an average effect size (d-statistic; Beeson & Robey, 2006) of 9.93 for trained scripts (SD = 4.22). This robust effect size (Beeson & Robey, 2006; Robey et al., 1999) demonstrates that the participant benefitted from the intervention, mastering the ability to produce trained content intelligibly and with a high degree of accuracy. The participant’s mean d-statistic for untrained scripts was small at .88 (SD = 1.25). Simulation tests confirmed a significant difference from pre- to post-treatment on the primary outcome measure ( p <.0001, 95% CI [62.14-79.61]) for trained scripts only. Untrained script performance was not significantly different at post-treatment relative to pre-treatment (p = .301, 95% CI [−13.46-25.00]).
Figure 2.
Multiple baseline data for the VISTA-R participant’s performance across treatment phases for trained and untrained scripts. Vertical lines indicate treatment phase, which includes pre-treatment (first time point shows initial script development probe, which is not calculated in the d-statistic, as this probe served as the basis for script creation, followed by two baseline probes), treatment, maintenance, post-treatment, and follow-up phases. d2 was calculated using mean standard deviation from the baseline phase as the denominator (Beeson & Robey, 2006). Tx = Treatment; Mo.=Month; F.U.=Follow-Up.
To contextualize the participant’s performance on the primary outcome measure, we report standardized test scores from pre- to post-treatment (Table 2). Results indicate general stability on the WAB-R AQ, MMSE, and motor speech evaluation (apraxia and dysarthria). On the NAT, the participant demonstrated significant grammatical impairment at both time points, with an accuracy of 2/30 at pre-treatment and 1/30 at post-treatment. Notably, the participant’s MLU in morphemes on the WAB-R Picture Description Task improved from 4.22 at pre-treatment to 7.38 at post-treatment. Similarly, MLU in words on this task improved from 3.33 to 6.25 pre- to post-treatment, suggesting generalized improvement in connected speech for a non-scripted linguistic task.
Maintenance of treatment effects
Simulation tests comparing performance on the primary outcome measure relative to pre-treatment confirmed maintenance of gains at each follow-up time point (p <.0001, 95% CI [62.14-79.61] at the 3-month time point; p <.0001, 95% CI [59.22-77.67] at the 6-month time point; p <.0001, 95% CI [50.49-70.87] at the 12-month time point). Performance on untrained scripts was not significantly different from pre-treatment at any follow-up, indicating neither significant improvement nor decline relative to baseline status.
Despite the stability of script performance over time, results from standardized testing indicate a gradual decline in linguistic ability on the WAB-R AQ (from 74.5 at pre-treatment to 70.5 at the 12-month follow-up) and increased motor speech impairment on the MSE (on a severity scale of 0= no impairment to 7=severe, apraxia rating changed from a 4 at pre-treatment to a 6 at the 12-month follow-up; dysarthria rating changed from a 2 at pre-treatment to a 4 at the 12-month follow-up). The participant demonstrated a consistently impaired pattern of performance on the NAT across all time points (6.67% or 2/30 accuracy at pre-treatment and 10% or 3/30 accuracy at the 12-month follow-up). Although language deficits persisted or gradually progressed longitudinally, the participant’s cognition remained grossly intact, per the MMSE (28 at pre-treatment and 26 at the annual follow-up). Of note, this gradual decline on standardized testing measures was also observed in the original VISTA cohort, although changes at one-year post-treatment in the comparison cohort were not significantly different from pre-treatment.
Participant performance relative to comparison cohort
In order to address research question 2, we compared pre- to post-treatment performance for the VISTA-R participant to those of the original VISTA cohort (Table 4) using unstandardized difference tests (Crawford & Garthwaite, 2005). Results indicated no significant difference between the current participant and the original cohort for change in performance from pre- to post-treatment in percent correct, intelligible words for trained and untrained scripts (trained: t(9) = −.95, p = .366; untrained: t(9) = .16, p = .877). Additionally, no significant difference was found between the participant and the comparison cohort for pre- to post-treatment difference in the grammatical complexity index (trained sets: t(9) = 1.51, p = .166; untrained sets: t(9) = −.77, p = .464); MLU in morphemes (trained: t(9) = −.46, p = .659; untrained: t(9) = .27, p = .792); MLU in words (trained: t(9) = −.41, p = .695; untrained: t(9) = .38, p = .714); speech rate (trained: t(9) = −.44, p = .668; untrained: t(9) = −1.64, p = .136) or speech intelligibility (trained: t(9) = −.26, p = .800; untrained: t(9) = −.62, p = .549).
Table 4.
Secondary outcome measures for trained and untrained conditions at pre- and post-treatment for the VISTA-R participant and the original VISTA cohort (Henry et al., 2018).
| VISTA-R Participant | Original VISTA Cohort | ||||
|---|---|---|---|---|---|
| Outcome Measure |
Condition | Pre- Treatment |
Post- Treatment |
Pre- Treatment Mean (SD) |
Post- Treatment Mean (SD) |
| Grammatical Complexity | Trained | .03 | .04 | .06 (.03) | .09 (.02) |
| Untrained | .02 | .04 | .07 (.01) | .07 (.03) | |
| MLU (words) | Trained | 4.08 | 6.44 | 7.96 (3.13) | 9.44 (3.49) |
| Untrained | 5.71 | 6.21 | 7.73 (3.09) | 8.98 (4.01) | |
| MLU (morphemes) | Trained | 5.13 | 7.94 | 9.21 (3.84) | 10.89 (4.48) |
| Untrained | 6.34 | 7.38 | 8.94 (3.65) | 10.24 (4.86) | |
| Speech Rate | Trained | 33.42 | 56.49 | 58.49 (26.09) | 69.13 (27.35) |
| Untrained | 24.21 | 45.47 | 66.18 (36.16) | 60.99 (29.32) | |
| Speech Intelligibility (%) | Trained | 95 | 100.00 | 95.75 (5.09) | 99.25 (1.85) |
| Untrained | 97 | 100.00 | 97.38 (3.83) | 98.38 (2.35) | |
On standardized speech, language, and cognitive testing, the participant’s change in performance from pre- to post-treatment (Table 2) was not significantly different from the original cohort (WAB-R AQ: t(9) = −.31, p= .767; MMSE: t(9) = −.18, p= .859; apraxia of speech rating on the MSE: t(9) = 1.04, p= .327; dysarthria rating on the MSE: t(9) = .46, p= .654; NAT: t(9) = .85, p= .416).
Survey results
Pre- and post-treatment CETI results, completed by the participant’s spouse, are shown in Table 5. Responses were indicated using a visual analog scale, ranging from 0 (“not at all able”) to 100 (“as able as before” [onset of communication difficulties]). The spouses’ mean response at the pre-treatment time point was 45.06 and 47.19 at post-treatment, which indicates a change of 2.13. The magnitude of this change is not greater than the standard error of the measure for this assessment (5.87; Lomas et al., 1989); however, it is illustrative to examine the pattern of responses. Nine responses indicated a positive change in communication effectiveness following treatment, five responses indicated a negative change in communication effectiveness following treatment, and two responses indicated no change. Positive change scores ranged from 4 to 29 and were reflected in communication contexts such as “getting somebody’s attention,” “getting involved in group conversations that are about him,” and “communicating physical problems such as aches and pains.” Negative change scores ranged from −4 to −39. These negative scores indicate the spouse’s perception that the participant demonstrated less effective communication in these contexts following treatment. Negative change scores were noted in contexts such as “indicating that he understands what is being said to him” and “participating in conversations with strangers.” No change in communication effectiveness was noted in the contexts of “communicating his emotions” and “having a spontaneous conversation.”
Table 5.
Pre- and post-treatment survey results on the CETI (Lomas et al., 1989), as completed by the participant’s spouse. Scores range from 0 to 100, with 0 indicating “not at all able” and 100 indicating “as able as before” [onset of communication difficulties].
| Question | Pre- Treatment |
Post- Treatment |
Change Score |
|---|---|---|---|
| Getting somebody’s attention | 86 | 100 | 14 |
| Getting involved in group conversations that are about him or her | 21 | 46 | 25 |
| Giving yes and no answers appropriately | 21 | 25 | 4 |
| Communicating his or her emotions | 64 | 64 | 0 |
| Indicating that he or she understands what is being said to him or her | 72 | 64 | −8 |
| Having coffee time visits and conversations with friends and neighbors | 29 | 25 | −4 |
| Having a one-to-one conversation with you | 57 | 50 | −7 |
| Saying the name of someone whose face is in front of him or her | 68 | 72 | 4 |
| Communicating physical problems such as aches and pains | 43 | 72 | 29 |
| Having a spontaneous conversation | 29 | 29 | 0 |
| Responding to or communicating anything (including yes and no) without words | 61 | 68 | 7 |
| Starting a conversations with people who are not close family | 21 | 25 | 4 |
| Understanding writing | 78 | 68 | −10 |
| Being part of a conversation when it is fast and a number of people are involved | 7 | 11 | 4 |
| Participating in a conversation with strangers | 57 | 18 | −39 |
| Describing or discussing something in-depth | 7 | 18 | 11 |
Results from the in-house qualitative post-treatment survey, which was completed by both the participant and his spouse, are shown in Figure 3. Both individuals indicated their responses along a seven-point continuum. The participant’s average response was 1.86, which falls between “somewhat better” and “better.” In general, the participant indicated that, after completing the intervention, his communication and emotional disposition ranged from “somewhat better” to “a lot better.” Specifically, the participant indicated that his ability to speak in unison with the mouth model during speech entrainment was “a lot better.” He selected the “better” response for 16 items on the survey (e.g., articulation during practiced scripts; hesitations or pauses when speaking; overall speaking ability; confidence in communication with familiar and unfamiliar people). For the remaining four items, the participant selected the response of “somewhat better” (e.g., ability to speak smoothly and without errors when reciting practiced scripts; ability to speak in complete sentences during normal conversation).
Figure 3.
Post-treatment survey results for the participant and his spouse. Ratings on the X-axis are shown for each survey question, using the following scale: 3= “A lot better,” 2= “Better,” 1= “Somewhat better,” 0= “Unchanged,” −1= “Somewhat worse,” −2= “Worse,” and −3 = “A lot worse.”
The spouse’s average response rating was 1.43, which also falls between “somewhat better” and “better.” Responses from the spouse ranged from “unchanged” to “a lot better” along the seven-point continuum. In particular, the spouse noted that the participant’s confidence in communication with his primary communication partner and with familiar people was “a lot better.” She selected the “better” response for eight items (e.g., ability to speak smoothly and without errors when reciting practiced scripts; stress level during conversation; overall comfort level while speaking), the “somewhat better” response for eight items (e.g., ability to correct errors as they occur; ability to communicate thoughts; overall speaking ability), and the “unchanged” response for three items (e.g., overall number of hesitations or pauses while speaking; overall number of hesitations or pauses in normal conversation).
Discussion
Research evaluating the efficacy of speech-language interventions for individuals with nfvPPA is lacking, especially when compared to the large corpus of studies evaluating treatment for stroke-induced aphasia. To our knowledge, literature addressing interventions for individuals with aphasia and concomitant hearing impairment is nonexistent. Thus, the current study is a first step toward establishing whether evidence-based aphasia treatments that are modified to accommodate hearing loss can result in positive outcomes that are comparable to those with functional hearing status.
The results from this study confirm our participant’s positive response to VISTA-R. Specifically, he demonstrated improved performance and maintenance of gains on the primary outcome measure, indicating that the addition of orthographic input may have helped to compensate for the effects of reduced auditory acuity within treatment sessions and during speech entrainment home practice. Additionally, the participant showed comparable improvement on discourse measures capturing grammatical complexity, utterance length, and speech rate that have proven to be sensitive in traditional VISTA treatment (Berstis, 2020).
Furthermore, the participant in the current study demonstrated increased MLU in spontaneous language samples (WAB-R Picture Description) from pre- to post-treatment, suggesting that the intervention may promote generalized language gains. Follow-up data, collected through 12-months post-treatment, indicate that gains for accurate and intelligible production of trained scripts were maintained over time, despite limited (less than one hour per month) ongoing practice with training videos. To the authors’ knowledge, these promising findings are the first to be reported regarding the treatment of individuals with nfvPPA and concomitant severe-to-profound hearing loss.
From a qualitative perspective, the participant and his spouse indicated their satisfaction with the intervention, from both a communication and psychosocial perspective. They observed that the treatment resulted in positive gains across structured and functional speaking contexts, while also increasing the participant’s overall sense of self-efficacy. These survey results underscore improvements in functional communication and enhanced participation in meaningful activities, as situated within the ICF framework (World Health Organization, 2013). Moreover, these qualitative findings complement the quantitative results and support the personal utility of this intervention for the participant, especially considering the well-established link between a person’s sense of self-efficacy and their decisions to participate in life activities despite challenges (Bandura & Adams, 1977).
It is important to emphasize that the participant in the current study would have been excluded from our larger VISTA treatment study given his impaired hearing status. Many treatment studies outline exclusionary criteria which prohibit individuals from enrollment based on visual or hearing deficits that are not fully corrected. However, within the older adult demographic, these impairments are extremely common (Dalton et al., 2007; Lin, Niparko, et al., 2011), and it may not always be feasible to fully correct a significant sensory impairment. Moreover, a growing body of evidence suggests that neurodegeneration associated with nfvPPA may affect the auditory pathway (Cope et al., 2017; Goll et al., 2010; Grube et al., 2016; Hardy et al., 2016; Hardy et al., 2019). Thus, while only one participant was recruited for the current study, his profile may be similar to a significant proportion of other individuals with nfvPPA.
Given that the prevalence of hearing impairments among older adults is high, it is important that hearing status is confirmed in patients with aphasia and that evidence-based behavioral interventions are modified to account for this sensory impairment (Street, 1957). This study found that the augmentation of traditional VISTA with oral reading was effective in supporting the memorization and production of scripts for an individual with concomitant nfvPPA and severe-to-profound bilateral hearing loss that was not corrected to a functional level. It is likely that the reading procedures were beneficial in that they provided complementary orthographic input that supported the audiovisual input from VISTA. Thus, from a clinical perspective, SLPs may consider embedding orthographic input or stimulation within treatment approaches that require auditory input (such as speech entrainment) when working with patients with hearing loss and concomitant aphasia. As long as reading skills are relatively intact, this modification may serve to promote comprehension and memorization of content for maximal treatment response. Conversely, if reading skills are severely impaired, it is unlikely that an individual with hearing loss would benefit from multimodal input provided by orthography.
The addition of orthographic input to VISTA renders it feasible and beneficial for an individual with hearing loss, enabling them to participate in treatment despite the critical auditory component of the treatment (Fridriksson et al., 2012). Other research has utilized text in the context of script training, specifically AphasiaScripts™. Of note, the intervention and home practice used in the current study differ from the AphasiaScripts™ training program in several ways. First, the written text of the script and the audiovisual model were always presented separately to ensure the participant’s attention to each mode without distraction from the other. We considered this important in order to minimize cognitive-linguistic demands and to allow the participant to focus on both audio and video elements of the speaker model consistently and simultaneously. Secondly, the video model for this study was a healthy adult speaker producing exaggerated articulatory gestures, rather than an animated therapist. This afforded realistic and salient articulatory placement cues to facilitate speech production in our participant with concomitant motor speech deficits.
VISTA-R intervention is multifaceted, incorporating audio, visual, and reading components, daily home practice, rate manipulation of videos, as well as generalization tasks. Isolating which element or elements of treatment most significantly impacted treatment outcomes is not feasible without a priori experimental manipulation of each factor. However, each element of the intervention was included in an effort to maximize treatment outcomes and it is likely that the combination of these variables confers multifactorial benefit for an individual with nfvPPA and hearing loss. Audiovisual stimulation is augmented by orthographic input in order to provide redundant avenues for script learning and accommodate the participant’s hearing loss. Daily home practice with incremental rate manipulation and regular engagement in dynamic generalization activities ensure that practice will be intensive and maximally challenging throughout the training regimen. This notion shares similarities with principles of exercise science suggesting that implementing variability in exercise programs enables maximized health outcomes, fosters adaptability, promotes engagement, and mitigates plateau (Glaros & Janelle, 2001; Rajiv & Newell, 2013).
Several outcome measures were utilized in the current study, in order to holistically capture treatment response. The primary outcome measure corresponds with the metric that was used in the comparison VISTA cohort (Henry et al., 2018) and additional speech and language outcomes were incorporated to evaluate discourse-level change on scripted performance (Berstis, 2020). As a means of obtaining descriptive outcome data, the CETI and an in-house post-treatment survey were administered. As with any survey, there is a potential for bias. While the CETI was administered during both pre- and post-treatment time points, this survey provided a gross measure of functional communication outcomes and is not specific to the intervention used in this study. By contrast, the in-house post-treatment survey completed by both the participant and his spouse served to capture the ecological validity of this intervention with greater sensitivity. Other psychometrically validated scales may be used in future studies to provide further contextualization of the broader impact of treatment, as it relates to communication participation in daily life (e.g., ASHA Quality of Communication Life, Paul-Brown et al., 2004, or subscales of the Burden of Stroke Scale, Doyle et al., 2004).
An additional important consideration is the study’s utilization of telerehabilitation rather than in-person treatment. The positive outcomes are consistent with previous findings (e.g. Dial et al., 2019), which showed comparable outcomes for individuals with PPA who completed in-person sessions versus telerehabilitation. However, Dial et al. (2019) did not examine treatment outcomes for individuals with hearing loss that was not corrected to a functional level. Thus, the outcomes from this study support the implementation of this intervention, even in a telerehabilitation context, where the acuity of the auditory signal may be negatively impacted even for individuals with normal or corrected hearing. Future studies with adapted interventions for individuals with hearing loss will benefit from recruiting participants who receive in-person treatment as well as those receiving treatment remotely, to further contextualize treatment responses relative to different service delivery options.
The current study has several limitations. First, only two baseline probes were obtained during the pre-treatment phase. Our participant’s performance was generally stable during the baseline period. However, best practice in single-subject experimental research requires collection of at least three data points prior to advancement to the treatment phase (Kennedy, 2005. In future studies, additional sampling of performance at pre-treatment will ensure that a stable baseline has been obtained and pre-treatment variability accurately represented.
The inclusion of linguistically matched scripts that were randomly assigned to treatment condition (trained or untrained) and the documentation of overall language and cognitive performance over time via standardized testing serve to confirm the specificity of the treatment effect for the participant in this study. Nonetheless, given the heterogeneity of nfvPPA and this participant’s unique clinical profile, results cannot be assumed to generalize to the broader nfvPPA population. Future studies will benefit from inclusion of a larger number of participants with nfvPPA and hearing loss. While it is not feasible to recruit individuals who share identical speech, language, and hearing profiles, we believe it will be possible to enroll individuals who broadly share clinical features. By replicating this study with additional individuals, results may provide greater support for the utility and generalizability of this treatment.
Another limitation of this study is the lack of precise characterization of the participant’s aided hearing profile. Aided thresholds were not available due to difficulty with obtaining his audiological records and the fact that treatment was offered at a distance, precluding an in-person audiological assessment. Additionally, the use of a minimal pairs discrimination task as a screening measure for peripheral hearing status is problematic, as phoneme discrimination performance is susceptible to auditory cortical dysfunction (Miceli et al., 1980). This type of task cannot be assumed to holistically capture peripheral hearing function in isolation. Thus, it is possible that the interaction between the participant’s longstanding hearing loss and auditory dysfunction due to nfvPPA may have contributed to reduced performance on this measure. In light of these limitations, we can only report that, even with hearing aids and a hearing aid Bluetooth accessory, the participant’s hearing impairment rendered him functionally unable to participate in unmodified treatment tasks.
In conclusion, these results add to the growing evidence base supporting speech-language treatment for individuals with nfvPPA. In concordance with other findings, our results indicate that telerehabilitation is a viable service delivery option in this population, even in the presence of significant hearing loss. Findings confirm that, even for an individual with sensory impairment and the need for treatment delivered remotely, relatively simple treatment modifications may facilitate intervention that results in immediate and lasting gains in communication functioning.
Supplementary Material
Continuing Education Questions:
1. How did the findings in this study at the single-case level compare to the findings from the Henry et al. (2018) comparison cohort?
2. What are the clinical implications from this study for speech-language pathologists who are treating individuals with nfvPPA and significant hearing loss that is not fully corrected?
3. What are appropriate treatment goals that align with a modified VISTA with reading intervention?
Acknowledgements
We thank the participant and his spouse for their time and dedication to this research study. We also thank the members of the Aphasia Research and Treatment Lab at the University of Texas at Austin, particularly Sydney Hamilton, Willa Keegan-Rodewald, Kirsten Laursen, and Sanika Nayak for completing speech sample transcription, coding, reliability analyses, and fidelity analyses.
Disclosure of Interest: This research project is funded by the NIH/NIDCD R01DC016291, R03DC013403-02S1, and the Darrell K Royal Research Fund for Alzheimer’s Disease.
Footnotes
The participant was provided visual and phonetic placement cues, as needed, to ensure accurate script production. These cues offered multisensory support to facilitate speech production. For example, in cueing production of the /p/ phoneme, a visual cue would involve the clinician moving her mouth close to the camera as she produced the /p/ sound, and a phonetic placement cue would be “purse your lips tightly together and then release.” Gradually, the clinician scaled back visual and verbal cues, in alignment with the participant’s performance.
Complex grammatical relations include finite clausal subject of another clause, clausal complement of a verb, full clause that serves as the predicate of nominal of verbs, full clause that serves as the object of a preposition, full clause that serves as the direct object, finite clause that attaches to a verb, adjective, or adverb, head of a complex noun phrase with a personal pronoun attached as an adjunct of a noun, a finite clause that is a nominal modifier or complement, or a non-finite clause that is a nominal modifier or compliment.
References
- Agostini M, Garzon M, Benavides-Varela S, De-Pellegrin S, Bencini G, Rossi G, Rosadoni S, Mancuso M, Turolla A, Meneghello F, & Tonin P (2014). Telerehabilitation in poststroke anomia. BioMed Research International, 16, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A Moore P, Gee J, & Grossman M (2010). Speech errors in progressive non-fluent aphasia. Brain and Language, 113(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, & Adams NE (1977). Analysis of Self-Efficacy Theory of Behavioral Change. Cognitive Therapy and Research, 1(4), 287–310. [Google Scholar]
- Beeson PM, & Robey RR (2006). Evaluating single-subject treatment research: Lessons learned from the aphasia literature. Neuropsychology Review, 16(4), 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstis KV (2020). Investigating changes in connected speech in nonfluent/agrammatic primary progressive aphasia following script training (Publication No.) [Master’s thesis, The University of Texas at Austin]. Texas Digital Library. [Google Scholar]
- Bilda K (2011). Video-based conversational script training for aphasia: A therapy study. Aphasiology, 25(2), 191–201. [Google Scholar]
- Brookshire RH, & Nicholas LE (1984). Comprehension of directly and indirectly stated main ideas and details in discourse by brain-damaged and non-brain-damaged listeners. Brain and Language, 21(1), 21–36. [DOI] [PubMed] [Google Scholar]
- Cherney LR (1995). Efficacy of oral reading in the treatment of two patients with chronic Broca’s aphasia. Topics in Stroke Rehabilitation, 2(1), 57–67. [DOI] [PubMed] [Google Scholar]
- Cherney LR (2010). Oral Reading for Language in Aphasia: Impact of Aphasia Severity on Cross-Modal Outcomes in Chronic Nonfluent Aphasia. Seminars in Speech and Language, 31(1), 42–51. 10.1310/tsr1706-423 [DOI] [PubMed] [Google Scholar]
- Cherney LR, Halper AS, Holland AL, & Cole R (2008). Computerized script training for aphasia: Preliminary results. American Journal of Speech-Language Pathology, 17(1), 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney L,R, Merbitz C,T, & Grip JC (1986). Efficacy of oral reading in aphasia treatment outcome. Rehabilitation Literature, 47(5-6), 112–118. [PubMed] [Google Scholar]
- Ciorba A, Bianchini C, Pelucchi S, & Pastore A (2012). The impact of hearing loss on the quality of life of elderly adults. Clinical Interventions in Aging, 7, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TE, Sohoglu E, Sedley W, Patterson K, Jones PS, Wiggins J, Dawson C, Grube M, Carlyon RP Griffiths TD, Davis MH, & Rowe JB (2017). Evidence for causal top-down frontal contributions to predictive processes in speech perception. Nature Communications, 8(1). 10.1038/s41467-017-01958-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, & Garthwaite P (2005). Testing for suspected impairments and dissociations in single-case studies in neuropsychology: Evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations. Neuropsychology, 19(3), 313–331. [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Howell DC (1998). Comparing an individual’s test score against norms derived from small samples. Clinical Neuropsychologist, 12(4), 482–486. 10.1076/clin.12.4.482.7241 [DOI] [Google Scholar]
- Dalton DS, Cruickshanks K,J, Klein BEK, Klein R, Wiley TL, & Nondahl DM (2007). The impact of hearing loss on quality of life in older adults, Journal of the American Academy of Audiology, 18(3), 257–266. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, & Brown JR (1975). Motor speech disorders (3rd ed.). Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- Dechêne L, Tousignant M, Boissy P, Macoir J, Héroux S, Hamel M, Briere S, & Page C (2011). Simulated in-home teletreatment for anomia. International Journal of Telerehabilitation, 3(2), 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial HR, Hinshelwood HA, Grasso SM, Hubbard HI, Gorno-Tempini M-L, & Henry ML (2019). Investigating the utility of teletherapy in individuals with primary progressive aphasia. Clinical Interventions in Aging, 14, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial HR, & Martin R (2017). Evaluating the relationship between sublexical and lexical processing in speech perception: Evidence from aphasia. Neuropsychologia, 96, 192–212. [DOI] [PubMed] [Google Scholar]
- Dickerson BC (2012). Quantitating severity and progression in primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle PJ, McNeil MR, Mikolic JM, Prieto L, Hula WD, Lustig AP, Ross K, Wambaugh J, Gonzalez-Rothi LJ, & Elman RJ (2004). The Burden of Stroke Scale (BOSS) provides valid and reliable score estimates of functioning and well-being in stroke survivors with and without communication disorders. Journal of Clinical Epidemiology, 57(10), 997–1007. 10.1016/j.jclinepi.2003.11.016 [DOI] [PubMed] [Google Scholar]
- Flesch R (1948). A new readability yardstick. Journal of Applied Psychology, 32, 221–233. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state: A practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Formby C, Phillips DE, & Thomas RG (1987). Hearing loss among stroke patients. Ear and Hearing, 8(6), 326–332. [DOI] [PubMed] [Google Scholar]
- Fridler N, Rosen K, Menahemi-Falkov M, Herzberg O, Lev A, Kaplan D, Feldman Y, Grosberg D, Hildesheimer M, & Shani M (2012). Tele-rehabilitation therapy vs face-to-face therapy for aphasic patients. eTELEMED 2012: The Fourth International Conference on eHealth, Telemedicine, and Social Medicine, Valencia: IARIA, 18–23. [Google Scholar]
- Fridriksson J, Basilakos A, Hickok G, Bonilha L, & Rorden C (2015). Speech entrainment compensates for Broca’s area damage. Cortex, 69, 68–75. 10.1016/j.cortex.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Hubbard HI, Hudspeth SG, Holland AL, Bonilha L, Fromm D, & Rorden C (2012). Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain, 135(12), 3815–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnas DW, & Edmonds LA (2014). The effect of computerised Verb Network Strengthening Treatment on lexical retrieval in aphasia. Aphasiology, 28(4), 401–420. [Google Scholar]
- Goldberg S, Haley KL, & Jacks A (2012). Script training and generalization for people with aphasia. American Journal of Speech-Language Pathology, 21(3), 222–38. [DOI] [PubMed] [Google Scholar]
- Goll JC, Crutch SJ, Loo JHY, Rohrer JD, Frost C, Bamiou DE, & Warren JD (2010). Non-verbal sound processing in the primary progressive aphasias. Brain, 133(1), 272–285. 10.1093/brain/awp235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goman AM, & Lin FR (2016). Prevalence of hearing loss by severity in the United States. American Journal of Public Health, 106(10), 1820–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B, Schneider J, McMahon CM, Teber E, Leeder SR, & Mitchell P (2012). Severity of age-related hearing loss is associated with impaired activities of daily living. Age and Ageing, 41, 195–200. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, & Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, & Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaros NM, & Janelle CM (2001). Varying the mode of cardiovascular exercise to increase adherence. Journal of Sport Behavior, 24(1), 42–62. [Google Scholar]
- Grasso SM, Cruz DF, Benavidez R, Peña ED, & Henry ML (2019). Video- implemented script training in a bilingual Spanish–English speaker with aphasia. Journal of Speech, Language, and Hearing Research, 62(7), 2295–2316. 10.1044/2018_JSLHR-L-18-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M (2012). The nonfluent/agrammatic variant of primary progressive aphasia. The Lancet Neurology, 11(6), 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Bruffaerts R, Schaeverbeke J, Neyens V, De Weer AS, Seghers A, Bergmans B, Dries E, Griffiths T, & Vandenberghe R (2016). Core auditory processing deficits in primary progressive aphasia. Brain, 1339(6), 1817–1829. 10.1093/brain/aww067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameister I, Nickels L, Abel S, & Croot K (2017). “Do you have mowing the lawn?”- Improvements in word retrieval and grammar following constraint-induced language therapy in primary progressive aphasia. Aphasiology, 31(3), 308–331. [Google Scholar]
- Harciarek M, Sitek EJ, & Kertesz A (2014). The patterns of progression in primary progressive aphasia—Implications for assessment and management. Aphasiology, 28(8-9), 964–980. [Google Scholar]
- Hardy CJD, Frost C, Sivasathiaseelan H, Johnson JCS, Agustus JL, Bond RL, Benhamous E, Russell LL, Marshall CR, Rohrer JD, Bamious DE, & Warren JD (2019). Findings of Impaired Hearing in Patients with Nonfluent/Agrammatic Variant Primary Progressive Aphasia. JAMA Neurology, 76(5), 607–611. 10.1001/jamaneurol.2018.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJD, Marshall CR, Golden HL, Clark CN, Mummery CJ, Griffiths TD, Bamious DE, & Warren JD (2016). Hearing and dementia. Journal of Neurology, 263(11), 2339–2354. 10.1007/s00415-016-8208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Hubbard HI, Grasso SM, Mandelli ML, Wilson SM, Sathishkumar M, Fridriksson J, Daigle W, Boxer AL, Miller BL, & Gorno-Tempini ML (2018). Retraining speech production and fluency in nonfluent/agrammatic primary progressive aphasia. Brain, 141(6), 1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Meese MV, Truong S, Babiak MC, Miller BL, & Gorno-Tempini ML (2013). Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behavioral Neurology, 26(1-2), 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A, Milman L, Munoz M, & Bays G (2002). Scripts in the management of aphasia. Paper presented at The World Federation of Neurology, Aphasia & Cognitive Disorders Section Meeting, Villefranche, France. [Google Scholar]
- Kazdin AE (1982). Single-case experimental designs: Methods for clinical and applied settings. New York: Oxford University Press. [Google Scholar]
- Kennedy CH (2005). Single-Case Designs for Educational Research. Boston, Massachusetts: Pearson. [Google Scholar]
- Kertesz A (2006). Western Aphasia BatteryTM – Revised (WAB-RTM). https://www.pearsonclinical.com/language/products/100000194/western-aphasia-batteryrevised.html [Google Scholar]
- Kim M, & Russo S (2010). Multiple oral rereading (MOR) treatment: Who is it for?. Contemporary Issues in Communication Science & Disorders, 37, 58–68. [Google Scholar]
- Lee JB, Kaye RC, & Cherney LR (2009). Conversational script performance in adults with non-fluent aphasia: Treatment intensity and aphasia severity. Aphasiology, 23(7-8), 885–897. [Google Scholar]
- Lin FR, Niparko JK, & Ferrucci L (2011). Hearing loss prevalence in the United States. Archives of Internal Medicine, 171(20), 1851–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, & Ferrucci L (2011). Hearing loss prevalence and risk factors among older adults in the United States. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 66 A(5), 582–590. 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas J, Pickard L, Bester S, Elbard H, Finlayson A, & Zoghaib C (1989). The Communicative Effectiveness Index: Development and psychometric evaluation of a functional communication measure for adult aphasia. Journal of Speech and Hearing Disorders, 54(1), 113–124. 10.1044/jshd.5401.113 [DOI] [PubMed] [Google Scholar]
- Machado TH, Campanha AC, Caramelli P, & Cathery-Goulart MT (2014). Brief intervention for agrammatism in primary progressive nonfluent aphasia: A case report. Dementia and Neuropsychologia, 8(3), 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWhinney B (2000). The CHILDES Project: Tools for Analyzing Talk. 3rd Edition. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan DM, Hu T,M, & Friedman RB (2016). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology, 30(4), 483–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Gainotti G, Caltagirone C, & Masullo C (1980). Some aspects of phonological impairment in aphasia. Brain and Language, 11(1), 159–169. 10.1016/0093-934X(80)90117-0 [DOI] [PubMed] [Google Scholar]
- Montembeault M, Brambati SM, Gorno-Tempini ML, & Migliaccio R (2018). Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: A review. Frontiers in Neurology, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S (1979). Rehabilitation of alexia: A case study. Cortex, 14, 139–144. 10.1016/S0010-9452(79)80015-5 [DOI] [PubMed] [Google Scholar]
- Paul-Brown D, Holland A, Frattali C, & Thompson CK (2004). Quality of Communication Life Scales (QCL). American Speech-Language-Hearing Association. [Google Scholar]
- Rajiv R, & Newell K (2013). Changing up the routine: Intervention induced variability in motor learning. Exercise and Sport Sciences Review 41(1), 65–70 DOI: 10.1097/JES.0b013e318259beb5. [DOI] [PubMed] [Google Scholar]
- Rankin E, Newton C, Parker A, & Bruce C (2014). Hearing loss and auditory processing ability in people with aphasia. Aphasiology, 28(5), 576–595. [Google Scholar]
- Robey RR, Schultz MC, Crawford AB, & Sinner CA (1999). Single-subject clinical- outcome research: Designs, data, effect sizes, and analyses. Aphasiology, 13(6), 445–473. [Google Scholar]
- Rogalski Y, & Edmonds LA (2008). Attentive reading and constrained summarisation (ARCS) treatment in primary progressive aphasia: A case study. Aphasiology, 22, 763–775. [Google Scholar]
- Rogalski EJ, & Mesulam MM (2009). Clinical trajectories and biological features of primary progressive aphasia (PPA). Current Alzheimer Research, 6(4), 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer KM, Grasso SM, Hubbard HI, Berstis K, Gorno-Tempini ML, & Henry ML (2018). Speech-language treatment in nonfluent/agrammatic primary progressive aphasia: A modified video implemented script training approach. Poster presentation at the American Speech-Language Hearing Association Convention, Boston, Massachusetts. [Google Scholar]
- Schneider SL, Thompson CK, & Luring B (1996). Effects of verbal plus gestural matrix training on sentence production in a patient with primary progressive aphasia. Aphasiology, 10(3), 297–317. [Google Scholar]
- Silkes JP, & Winterstein K (2017). Speech-language pathologists use of hearing screening for clients with aphasia: Challenges, potential solutions, and future directions. American Journal of Speech-Language Pathology, 26(1), 11–28. [DOI] [PubMed] [Google Scholar]
- Spinelli E,G, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, Grinberg LT, Huang EJ, Trojanowski JQ, Meyer M, Henry ML, Comi G, Rabinovici G, Rosen HJ, Filippi M, Miller BL, Seeley WW, & Gorno-Tempini ML (2017). Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology, 81(3), 430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street BS (1957). Hearing loss in aphasia. Journal of Speech and Hearing Disorders, 22(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, & Weintraub S (2012). Verbal and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery, Aphasiology, 26(5), 632–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, & Rosenbek JC (1984). Apraxia of speech in adults: The disorder and its management. New York, NY: Grune and Stratton. [Google Scholar]
- Woolf C, Caute A, Haigh Z, Galliers J, Wilson S, Kessie A Hirani S, Hegarty B, & Marshall J (2016). A comparison of remote therapy, face to face therapy and an attention control intervention for people with aphasia: A quasi-randomised controlled feasibility study. Clinical Rehabilitation, 30(4), 359–373. [DOI] [PubMed] [Google Scholar]
- World Health Organization. How to use the ICF: A practical manual for using the International Classification of Functioning, Disability and Health (ICF). Exposure draft for comment. October 2013. Geneva: WHO [Google Scholar]
- Youmans G, Holland A, Munoz M, & Bourgeois M (2005). Script training and automaticity in two individuals with aphasia. Aphasiology, 19(3-5), 435–450. [Google Scholar]
- Youmans G, Youmans SR, & Hancock AB (2011). Script training treatment for adults with apraxia of speech. American Journal of Speech-Language Pathology, 20(1), 23–37. [DOI] [PubMed] [Google Scholar]
- Zhang M, Pratt SR, Doyle PJ, McNeil MR, Durrant JD, Roxberg J, & Ortmann A (2018). Audiological assessment of word recognition skills in persons with aphasia. American Journal of Audiology, 27(1), 1–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.