Abstract
A 5-month-old female infant was admitted to hospital with a history of fever and rash during the recent coronavirus pandemic. She had significantly elevated inflammatory markers and the illness did not respond to first line broad spectrum antibiotics. The illness was later complicated by coronary artery aneurysms which were classified as giant despite treatment with intravenous immunoglobulin, steroids and immunomodulators. The infant had COVID-19 antibodies despite an initial negative COVID-19 PCR test. This case highlights the association of atypical Kawasaki like illness and paediatric multisystem inflammatory syndrome-temporarily associated with COVID-19 infection.
Keywords: cardiovascular medicine, paediatrics, COVID-19
Background
‘In March 2020, WHO declared coronavirus a global pandemic’.1 The infection transmitted by COVID-19 was first discovered in Wuhan China in December 2019 and then quickly spread worldwide.1 In children COVID-19 disease has been reported to be less frequent and usually less aggressive. However, in early April 2020, there were growing concerns regarding an increased number of children presenting with a paediatric multisystem inflammatory syndrome-temporarily associated with COVID-19 (PIMS-TS).2
The cases have in common overlapping clinical features of toxic shock syndrome and Kawasaki disease (KD) with blood parameters consistent with severe inflammation. This has been observed in children with confirmed PCR positive COVID-19 infection as well as children who were found to be PCR negative. Serological evidence of possible preceding COVID-19 infection has also been observed.
There is growing concern that a COVID-19-related inflammatory syndrome, with accompanying coronary artery aneurysm (CAA), is emerging in children in the UK or that there may be another unidentified infectious pathogen associated with these cases. However, there is also a concern that in certain cases children are developing complications despite prompt treatment—the case described here highlights this concern.3
Case presentation
A 5-month-old infant, the first of twins and previously healthy, developed a high-grade fever of 40 °C, followed by an erythematous rash on her trunk and extremities (figure 1A). On day 2 of fever, she presented to the local hospital—where she was also found to have two petechial spots (figure 1B) and was therefore admitted and treated for sepsis with intravenous Ceftriaxone. She remained persistently tachycardic and required a 40mL/kg crystalloid fluid bolus in the first 48 hours of her admission. Subsequently, on day 5 of illness, she was transferred to the regional paediatric intensive care unit for respiratory support with high flow oxygen. On admission, her acute serum phase reactants including C reactive protein (CRP), d-dimer and white cell count were significantly high (table 1); no bacterial growth was detected in either her blood, urine or cerebrospinal fluid (CSF) culture. An extended respiratory viral PCR was negative. The infant’s swab for COVID-19 was negative, however, an antibody test for COVID-19 was found positive a week after admission to hospital. She later developed peeling skin on her hands and feet (figure 1C) and cracked red lips (figure 1D).
Figure 1.
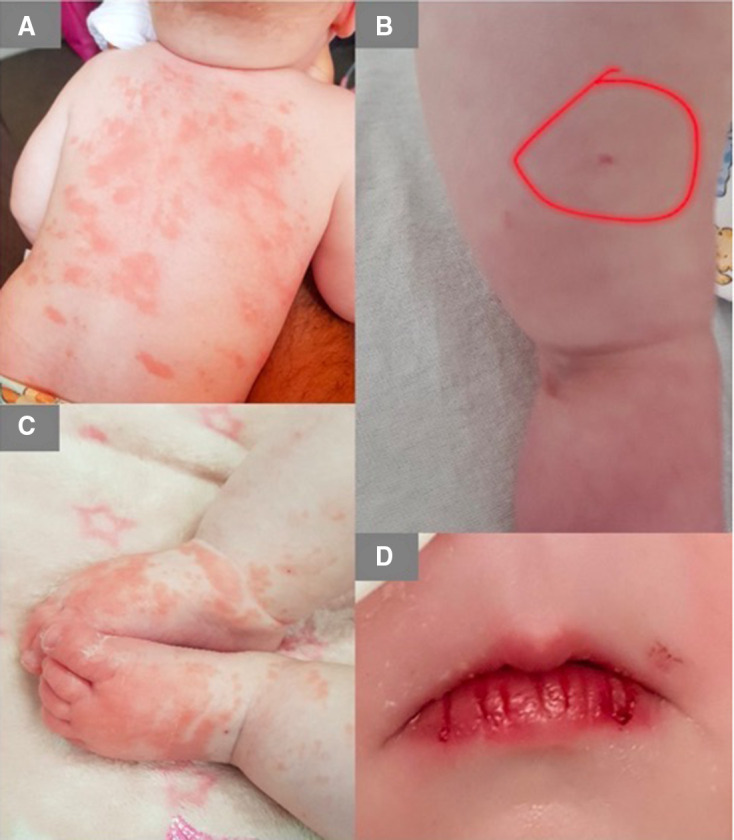
On day 2 of fever an erythematous rash was noted on the body (A) on admission to the local Hospital a petechial spot was noted on her leg (B) on day 6 she her skin began to peel on her feet (C) on day 10 her lips began to crack and bleed (D).
Table 1.
Laboratory results on admission and following each treatment
| Initial | 24 hours post-IVIG | 24 hours poststeroid | 24 hours postinfliximab | |
| WCC (x109/L) | 12.2 | 28.2 | 27.9 | 24.7 |
| Neutrophils | 28.1 | 15.7 | 20.1 | 11.0 |
| Lymphocytes | 2.9 | 9.0 | 4.6 | 8.7 |
| Platelets | 292 | 227 | 367 | 468 |
| CRP (mg/L) | 50 | 47 | 73 | 27 |
| Alanine transaminase (ALT) (U/L) | 89 | – | 21 | 14 |
| Ferritin (ug/L) | 937 | 550 | 315 | 205 |
| D-dimer (mcgm/L) | 6692 | 5601 | 5196 | 1514 |
| Troponin-I | – | – | 7 | <2 |
| Lactate dehydrogenase (LDH) (U/L) | 425 | 323 | 445 | |
| Albumin (g/L) | 22 | 19 | 20 | 26 |
| Fibrinogen (g/L) | 4.7 | – | 3.8 | 2.4 |
| Sodium (mmol/L) | 143 | 142 | 138 | 132 |
CRP, C reactive protein; IVIG, intravenous immunoglobulin; WCC, white cell count.
The child was suspected to have a diagnosis of PIMS-TS and therefore received intravenous immunoglobulin (IG) and methylprednisolone on day 5 of her illness. Her initial echocardiogram did not show any coronary changes, however, subsequent echocardiograms showed aneurysm of the coronary arteries—she, therefore, received further treatment with anakinra and infliximab. She was also commenced on aspirin and warfarin due to the associated risk of thrombus in CAA.
Investigations
Her inflammatory markers were raised on admission to the intensive care unit—CRP 50 mg/L, ferritin 937 ug/L, D-Dimer 6692—their response following treatment along with other parameters is outlined in table 1.
Her initial echocardiogram showed no dilatation of the coronary arteries, good ventricular function, and trivial mitral and aortic regurgitation. However, on day 12 of her illness, her repeat echocardiogram showed medium-sized CAA—her right coronary artery (RCA) measuring 3 mm (Z score 5) and left coronary artery (LCA) measuring 4 mm (Z score 7). A repeat scan on day 18 of her illness showed giant CAA; RCA 6 mm (Z score=14), left anterior descending 4 mm (Z score=9) and left circumflex 7 mm (Z score 19). She had normal left ventricular function throughout with fractional shortening (FS) 37%, ejection fraction (EF) 69%, mitral annular plane systolic excursion lateral 7 mm, septal 8 mm and tricuspid annular plane systolic excursion 15 mm. She had a physiological rim of pericardial fluid. On day 27 of her illness, her coronary arteries enlarged further with RCA 8 mm, LAD 8 mm and circumflex 10 mm (table 2 and figure 2).
Table 2.
Echocardiographic findings on admission and in the follow-up
| Initial | Day 12 | Day 18 | Day 27 | |
| ECHO -Right coronary artery (RCA) z score | <2 | 5 | 14 | 14 |
| ECHO - Left anterior descending (LAD) z score | <2 | 9 | 9 | 9 |
| ECHO - Left circumflex (LCX) z score | <2 | – | 19 | 19 |
| ECHO cardiac function | Normal LV function FS 41% EF 74% |
Normal LV function FS 37% EF 69% |
Normal LV function FS 36% EF 68% |
Normal LV function FS 33% EF 63% |
| Pericardial effusion | No | No | No | No |
FF, ejection fraction; FS, fractional shortening; LV, left ventricular.
Figure 2.
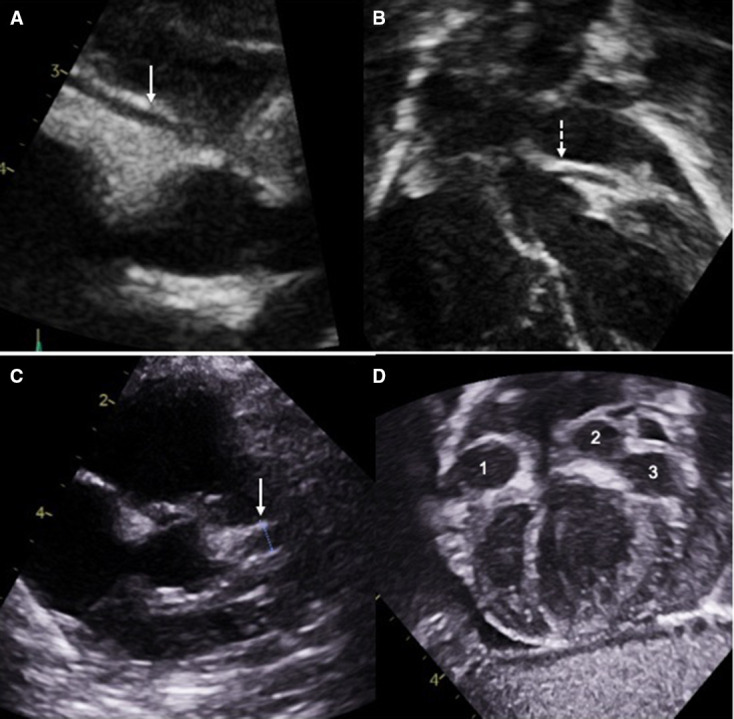
On admission the right (A) and left (B) coronary artery dimensions were normal. The LCA started to dilate (C) within 6 days after admission. Giant coronary artery aneurysms developed in the right (1) and left (2) coronary arteries and in the circumflex artery (3) in the next 3 weeks (D). LCA, left coronary artery.
Differential diagnosis
Sepsis.
Atypical KD.
COVID-19-related PIMS-TS.
-
Viral infections—Epstein-Barr virus, enterovirus, adenovirus, cytomegalovirus.
The above differential diagnosis was explored. In the absence of any positive cultures (blood, urine, sputum and CSF), and viral PCR and the presence of COVID-19 antibodies a diagnosis of COVID-19-related PIMS-TS was explored.
Treatment
She initially received treatment for sepsis with intravenous ceftriaxone. After PIMS-TS was suspected she received intravenous IG 2 gram/kg and low-dose aspirin 5 mg/kg/day on day 5. Methylprednisolone 10 mg/kg/day and anakinra 4 mg/kg/day on day 7 were commenced because of persistent temperature. She later received Infliximab on day 12 in view of her persistently high inflammatory markers and dilated coronary arteries. Timeline of medications as per table 3.
Table 3.
Treatments given and doses
| Dose per kg | Time given in relation to onset of fever | Comment | |
| IVIG | 2 g | Day 5 | Initial response of fever but symptoms returned within 24 hours |
| Methylprednisolone | 10 mg | Day 7 | Initially 2 mg/kg on day 7 increased to 10 mg/kg on day 10 |
| Infliximab | 5 mg | Day 12 | Commenced due to persistently high inflammatory markers |
| Anakinra | 4 mg | Day 7 | Commenced due to persistent temperature |
| Aspirin | 5 mg | Day 5 | |
| Warfarin | As per international normalised ratio (INR) | Day 30 | Target INR (2–3) |
IVIG, intravenous immunoglobulin.
Outcome and follow-up
The patient has been discharged from hospital and remains on aspirin, prednisolone and warfarin. She is being followed up weekly in the joint paediatric and paediatric cardiology clinic with repeat echocardiogram and monitoring of her international normalised ratio (INR). She remains well with normal blood test results, normal 12 lead ECG and good ventricular function on echocardiogram in spite of multiple giant CAA.
Discussion
This case report highlights that COVID-19 infection in children can lead to rapidly progressing CAA even in the absence of respiratory involvement or cardiac dysfunction. Contrary to published reports, haemodynamic instability, ventricular dysfunction, myocardial ischaemia or myopericarditis may not be evident in such cases.
There has been a significant rise in the number of children of all ages presenting with a multisystem inflammatory state requiring intensive care across the UK.4 5 As the inflammatory state has overlapping features with atypical KD many of the children have been medically managed according to KD protocols.
KD is an inflammatory disorder, mainly affecting young children and is associated with vasculitis of the coronary arteries with subsequent aneurysm formation in more than a third of untreated infants.6 7 Those who develop aneurysms are at lifelong risk of coronary thrombosis or the development of stenotic lesions, which may lead to myocardial ischaemia, infarction or death.8 Treatment of the acute illness with intravenous IG reduces the risk of CAA and is the standard recommended treatment. For patients who do not respond to IVIG the use of corticosteroids, infliximab or other immunosuppressive agents is recommended. Patients resistant to IVIG and immunomodulators are also at increased risk of developing CAA.9
The risk of thrombotic and stenotic complications is related to aneurysm size. Large or giant aneurysms (Z score ≧ 10) are the least likely to undergo resolution. These lesions are associated with up to 50% risk of thrombotic coronary occlusion leading to major adverse coronary event within 30 years after the acute illness.9
The risk of thrombosis within aneurysm is greatest in the first 2 years after the acute episode of KD but persists lifelong. Long-term management is based on the prevention of thrombosis, early detection of thrombosis or stenosis when they occur and general measures to lower cardiovascular risk.
The American heart association classifies giant CAA as a z score ≧10. This patient’s Z score of 19 for her left circumflex artery was significantly higher than the aforementioned cut-off. Whether COVID-19-related inflammatory syndrome is associated with an increased risk of very large or giant CAA would be a valuable area of future research and could help to guide the formulation of tailored management plans for such patients. Alternatively, it might be that KD is being initiated on exposure to COVID-19 and this might help us to understand the condition more clearly.10
Recent reports have, however, documented differences between PIMS-TS and KD—for example, a higher median age and higher inflammatory markers.2 Our case differs in this respect as they presented at the age of 5 months.
As more cases have been reported in the world, there will be clearer information available to elucidate the mechanism of PIMS-TS-related cardiovascular complications in children of various ages. As our patient has giant CAA, she will have lifelong cardiology follow-up, remain on aspirin and anticoagulation. She will require advice on avoidance of cardiac risk factors for example low fat diet and regular exercise. She will also require a person-specific protocol so that services have prior knowledge of the patient’s history and can act quickly in the event of a cardiac emergency.
Conclusion
In this case, the presence of temperature, rash, significantly elevated acute phase reactants in association with CAA and positive COVID-19 antibody test helped establish a diagnosis of PIMS-TS. This case highlights the importance of close monitoring of the heart with echocardiogram to detect any cardiac complication associated with PIMS-TS earlier. Earlier detection of such complications would allow the timely initiation of the most effective acute treatment, better parental counselling as well as devising an appropriate long-term management plan.
Patient’s perspective.
Day 1
Today was really hot weather, I spent the day with the children in the garden. The babies stayed in the tent so that they were shaded—but they did get hot and bothered so I brought them in the house. I noticed that our baby was a bit grizzly and warm but she didn’t have a temperature. Over the following evening she became even more unsettled and developed a temperature—her temperature responded to Calpol but she remained unsettled.
Day 2
In the early morning she remained unsettled—I undressed her and found a rash in the shape of her vest on her back. I initially thought that the rash looked like an allergic reaction but called 111 for advice. I discussed with the doctor over the phone and we discussed the difficulties with the current pandemic but they later advised that I bring her in for assessment. The out of hours GP sent us to A and E for assessment by the paediatric team. The senior paediatrican noted three petechial spots on her leg and advised to start treatment for sepsis. They also told me that she had tonsillitis. They took a urine sample, blood tests and a COVID-19 swab. The next few days were a blur so the below is as much as I can describe from updates I gave to family and friends.
Day 3
During the night our baby’s heart rate shot up sky high and her temperature was uncontrollable. She had an ECG which seemed clear, a chest X-ray that the team were happy with and a normal blood gas. She was struggling to take food orally so her medications were given IV and she had a nasogastric tube for her feeds. Her tongue developed little ulcers all over. She was transferred to high dependency and this is when the doctors said it looked like Kawasaki disease related to COVID-19. I explained that there was no way this could be due to coronavirus as we had been isolating. Later in the evening her blood gas had worsened and the anaesthetist came to talk to me incase she needed to be intubated. My heart was breaking.
Day 4
Her temperature seemed more reasonable and her colour was returning to normal, she seemed to look at me again when I spoke to her. She had an echo and ECG which both came back fine. Her blood gasses showed signs of improvement and she seemed to me to be returning to normal. Her COVID-19 results showed negative. As things seemed to be improving, I left her side to take a quick shower.
When I returned to the room the doctors were buzzing around and I was told she would need to be transferred to critical care in another hospital as some blood results had returned with alarming results, showing clotting issues and high infection markers. I was not allowed to travel with her in the ambulance due to the pandemic and travelling to the hospital away from her was the first time I broke down in fear for her future.
She was put into isolation and on high flow oxygen through nasal prongs into her nose and her heart rate was around the 200 bpm. She was really struggling to breathe. I had to wear gowns and protective equipment around her as did all the medical staff. She was on strong antibiotics for things such as meningitis and toxic shock. She was also given a mild sedative to help her to settle.
Day 5
She continued on high flow air to support her breathing. Various cannula’s she’d had were failing and it was becoming more difficult to get a vein each time. They set up a cannula in her head. This was the day I met the paediatric infectious disease consultant. She advised me that she believed the case to be the Kawasaki case that had been in the press lately as all the symptoms were similar and gave me information about three potential treatments. 1—Immunoglobin. 2—Anakinra. 3—Infliximab. I was very sceptical of this diagnoses at this point as I was adamant that she had not been in contact with COVID-19 and asked the doctor how she would have treated this illness had she come in 6 months earlier. The doctor explained that she had already thought about this and feels she would have followed exactly the same line of attack, but it may have taken her another week or so to get to the diagnosis. This reassured me and we started on the treatment that evening that had been recommended which was a dose of immunoglobin.
Day 6
She seemed to start to improve, her temperature levelled off, the high flow air was removed, and her heart rate calmed down. We were moved from the intensive care unit, to the high dependency unit. From the second we were out of isolation, our baby took a turn for the worse and didn’t stop crying. I was very scared at this point as nobody seemed to understand why she was so upset and just suggested maybe she was in pain from the multiple cannulas, blood tests etc. The next time I changed her nappy I noticed her feet were exceptionally swollen with a rash on them, this was also on her hands. I pointed this out to the doctors and they suggested potentially moving to the second stage of the Kawasaki treatment options.
Day 7
Her temperature was back up and no longer responding to paracetamol. Her heart rate was back up to 200bpm and all the positive improvements she had made in response to the immunoglobin seemed to have disappeared. She was back on ECG monitoring and her cannula in the head broke down. She was put on aspirin to thin her blood, steroids and the next course of Kawasaki treatment - Anakinra. Later that day they decided to put a line in her femoral artery through her groin. They advised me that she was anaemic and may require a blood transfusion at some point as well as potentially needing ventilation. Towards the evening she perked up, she took some feeds orally and it looked like ventilation and blood transfusions would not be required as improvements were being made. Her temperature and heart rate seemed to calm down.
Day 8
During the early hours of the morning, she vomited significantly, vomiting up her feeding tube. She was put back onto IV fluid. Her temperature and heart rate was back up. Later in the day, she had a lumbar puncture complete which initially showed a very slight raised white cell count but nothing too alarming that would indicate meningitis or sepsis and seemed in keeping with an infection. Doctors told me they felt that our baby was as bad as she was going to get and they felt she may start to make improvements going forward, though maybe not immediately.
Day 9
Her anaemia worsened, and a blood transfusion was complete.
Day 10.
Her lips cracked significantly and started bleeding but otherwise she seemed to improve.
Her heart rate and temperature calmed down. Doctors were now less concerned and decided it was safe to move her from High Dependency to the inpatient ward. I found this change very difficult to adapt to. I felt less supported on the ward and concerned about her feeding. It’s a massive change coming out of High Dependency. Our baby was really difficult to settle and spent the whole evening crying. The Oramorph and Calpol had limited effects and she seemed to struggle. Doctors did their rounds and told me that her blood results had worsened and her steroid dose would be increased to ten times what it was.
Day 11. (Coronary artery inflammation found)
The infection markers in her blood seemed to come down which was positive. I was told that her central line was starting to block but they would keep it in as long as they could without risking infection. She had been having echocardiograms regularly through her time in High Dependency but today was the first one that showed inflammation of her arteries. She was still difficult to settle, and the doctor told me that her lumbar had shown infection of her meninges which would be causing a very painful headache for her and could explain why she was in such discomfort.
Day 12. (Positive COVID-19 antibody result)
I was told today that her COVID-19 antibody test had come back positive and to be honest, I was in genuine shock. I still don’t understand how she can ever have been exposed to this disease. Her discomfort and pain levels were still high and this was when they decided to give her infliximab.
Day 13.
This was where she started making some real positive steps. She became happier overall, she was feeding orally, her temperature was becoming easier to manage and she genuinely seemed to be improving. I was feeling quite confident we were on the road to recovery, and from here on in, the days started to blend into one a bit as the doctors’ intervention was a little less and we seemed to be just there for monitoring rather than treatments so much.
Day 15.
She had another echocardiogram which showed her arteries worsening and an aneurysm. Doctors started Clexane to prevent clotting. She was still outwardly seeming much better and she was able to have her feeding tube removed.
Day 17.
Blood tests were on the path to improvement and her outward demeanour continued to improve. She was up playing and smiling regularly. The doctor advised me at this point however that while most blood tests had improved, he was concerned about clotting and potentially heart attacks, which put me back at a stage of worrying. It was difficult hearing this type of news when she seemed to be doing so well outwardly. I have been trained to administer Clexane via injection which is not nice but a necessary thing to do to be able to come home from hospital and keep her as healthy as possible.
Day 20
Regular monitoring via the echo has shown the coronary arteries measuring up to 8 mm. All blood results improving except her platelet levels. The cardiologist had a direct conversation with me warning me about the real possibility of a rupture which would result in her death as there would be nothing anyone could do about it. I nodded and took this in at the time. He left the room and I instantly broke down not being able to focus on anything other than the possibility of losing my baby. Having made such positive steps recently, I found it difficult to comprehend that she was in as much of a risky position now as she was when we were in critical care and she was obviously ill. I pulled myself together and phoned my husband to tell him and heard him break down as I said the words out loud. I think this was probably my most difficult time of our journey.
Day 26.
Having undertaken regular bloods and echo’s the doctors finally agreed that they believed her coronary arteries had stabilised at between 6 and 8 mm and we were free to go home for the day to return the following day as a couple for a meeting with the doctors. On returning home, she spent the whole evening grinning at her sisters, seeming very well and happy to be home.
Day 27.
We returned to the hospital for blood tests and an echo. Our doctors empathised with us having to give Clexane injections as she continued to bleed through her clothes after every one and made the decision to move her onto oral warfarin instead. We were given training to use a heel prick test to check her INR levels and a final echo, which reinforced stable arteries.
Day 38.
Having had a few echos and blood tests as an outpatient today we returned again. Warfarin has finally got the INR to within range of 2 and 3 so she is able to come off the Clexane injections which is a massive relief as we’ve been struggling with her bleeding through bedsheets, babygros and clothes since being home as well as the discomfort of administering the injections and the bruising to her legs. Today’s echo showed our baby’s coronary arteries measuring at 9 mm which is a slight growth but the cardiologist was not concerned by it so we’ve managed to put any fears we have to the back of our minds and hope that it’s just a blip. We have discussed the potential use of beta blockers to slow down her heart rate to relieve the pressure of blood through her arteries and hopefully give them a chance to shrink back down but it’s not been put in place yet. She is due another scan on Monday. It’s a constant journey, but we are hopeful that she is now on a path to recovery and conscious it may be a long road.
Learning points.
COVID-19 infection can cause a multisystem inflammatory syndrome in children (paediatric multisystem inflammatory syndrome, PIMS).
PIMS may trigger atypical Kawasaki-like illness resulting in haemodynamic compromise or coronary artery aneurysms.
COVID-19 infection presenting with Kawasaki-like illness requires close monitoring of the heart and coronary arteries with frequent echocardiogram.
COVID-19 triggered Kawasaki-like illness could be refractory to intravenous immunoglobulin and immunomodulators.
Footnotes
Contributors: KLR, OU, AJ and JE all contributed to the concept and writing of this case report. All authors critically appraised and edited the final version of the manuscript and have full responsibility of the data presented. All authors including the parents have seen and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
Ethics approval
In addition, the parents agreed to provide their own experience and account of the events throughout her illness. Their summary of events was not altered by the authors other than anonymising identifiable personal information.
References
- 1.World Health Organisation . Coronavirus, 2020. Available: https://www.who.int/health-topics/coronavirus#tab=tab_1 [Accessed 7 Feb2021].
- 2.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259–69. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harahsheh AS, Dahdah N, Newburger JW. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. JCJ Pediatr 2020;23:30556–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet 2020;395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergano S, Alders N, Armstrong C, et al. Severe refractory Kawasaki disease in 7 infants in the COVID 19 era. Lancet Rheumatol 2020;10:30231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brogan P, Burns JC, Cornish J, et al. Lifetime cardiovascular management of patients with previous Kawasaki disease. Heart 2020;106:1–10. 10.1136/heartjnl-2019-315925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tulloh RMR, Mayon-White R, Harnden A, et al. Kawasaki disease: a prospective population survey in the UK and ireland from 2013 to 2015. Arch Dis Child 2019;104:640–6. 10.1136/archdischild-2018-315087 [DOI] [PubMed] [Google Scholar]
- 8.Kato H, Sugimura T, Akagi T, et al. Long-Term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379–85. 10.1161/01.cir.94.6.1379 [DOI] [PubMed] [Google Scholar]
- 9.Uehara R, Belay ED, Maddox RA, et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J 2008;27:155–60. 10.1097/INF.0b013e31815922b5 [DOI] [PubMed] [Google Scholar]
- 10.Levin M. Childhood multisystem inflammatory – a new challenge in the pandemic. B Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


