Abstract
A 10-year-old boy treated for alkali injury with multiple interventions presented with a perforated corneal ulcer with clinically suspected bacterial aetiology. Cornea scraping and tissue adhesive application were planned. During surgery, an eyelash was found embedded at the perforated site. Gram staining of corneal scraping revealed the presence of Gram-positive bacilli on the first day which later was identified as Turicella otitidis with culture followed by VITEK V.2.0 (Biomerieux) identification. The bacterium was found to be sensitive to amikacin, ciprofloxacin, cefazolin, gatifloxacin, moxifloxacin, ofloxacin and vancomycin antibiotics as per Clinical and Laboratory Standards Institute guidelines. Coryneform bacteria is a rare cause of keratitis, and this is the first reported case of microbial keratitis caused by one of the rare corynebacterium species T. otitidis to the best of our knowledge. Literature search does not reveal any specific ocular features typical to this organism. This case supports the growing evidence for pathogenicity of T. otitidis in ocular samples. This study demonstrates the utility of VITEK for the identification of rare pathogen and may facilitate the use of certain antibiotics in the treatment regimen of T. otitidis infections.
Keywords: ophthalmology, anterior chamber
Background
Turicella otitidis, an aerobic, Gram-positive bacillus; catalase positive and oxidase negative biochemically was first described by Funke et al.1 It was earlier considered as an unidentified coryneform, phenotypically similar to Corynebacterium afermentans primarily isolated from middle ear fluid of children with acute otitis media infections. It differs from most Corynebacterium sp in lacking mycolic acids and in producing the major menaquinones MK-10 and MK-11.2 T. otitidis is a commensal primarily associated with the external and middle auditory canal; and sometimes may become pathogenic causing otitis media, otitis externa, mastoiditis infections.3 4 The pathogenicity of T. otitidis in otitis remains controversial, and there are only a few cases published of bacteraemia.5–7 A case report published recently has revealed the association of T. otitidis with palmoplantar eczema in a 74-year-old woman.8 The first case report revealing the association of T. otitidis with endophthalmitis in a 71-year-old man has been established in 2020.9 To the best of our knowledge, this is the first report of T. otitidis causing microbial keratitis in a corneal graft. In this case report, we review the clinical presentation, microbial diagnosis and response to treatment.
Case presentation
A 10-year-old boy presented to us with redness, pain and loss of vision in the left eye following accidental lime injury. Visual acuity could not be assessed as the child was very symptomatic. On examination, cornea appeared ground glass pattern with total limbal stem cell deficiency (LSCD). He was started on an intensive topical steroid (prednisolone acetate 1% eyedrop every 2 hourly) and underwent amniotic membrane transplantation (AMG) twice within 1 month of injury. On subsequent visits, LSCD with leucomatous opacity and symblepharon formation was noticed for which he underwent symblepharon release and simple limbal epithelial transplantation (SLET) after 6 months of injury. Exactly after 1 year, because of persisting symblepharon, he underwent a mucus membrane grafting (MMG). Postoperatively, he was started on moxifloxacin 0.5% eyedrop four times per day, topical prednisolone 1% eyedrop six times per day in tapering doses, and chloramphenicol–dexamethasone–polymyxin eye ointment over lid margins two times per day.
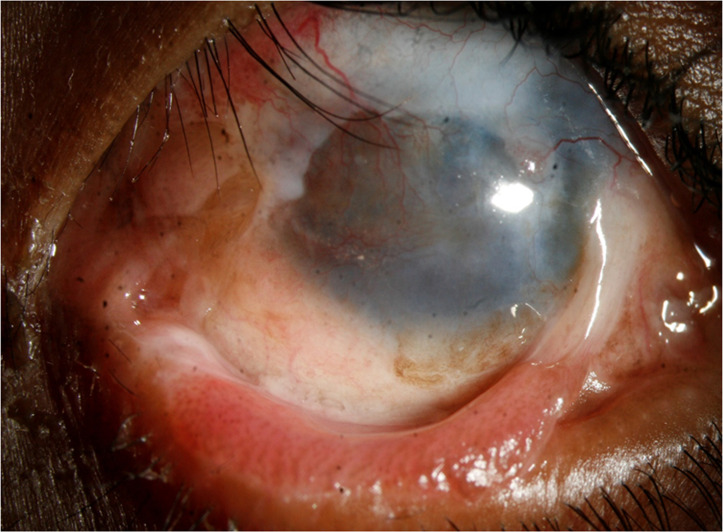
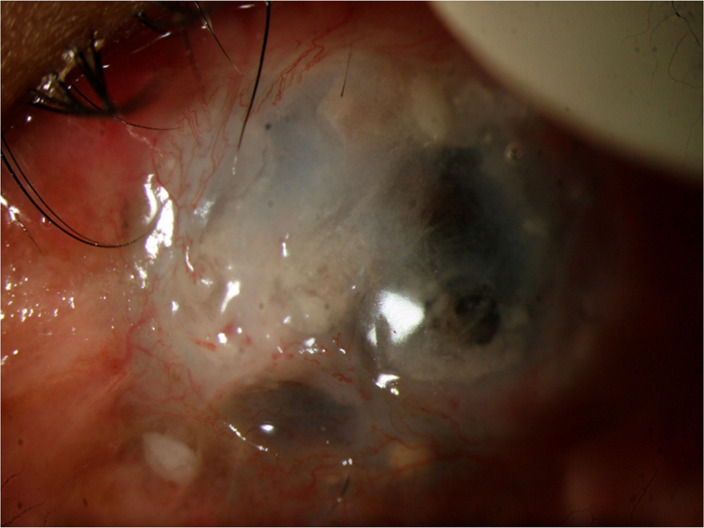
On subsequent follow-up visits, recurrence of symblepharon with LSCD was noted after 2.5 years (figure 1) for which he underwent Symblepharon release with repeat MMG in the same eye. The ocular surface was stable for the next 14 months, and the child underwent repeat SLET, lamellar keratoplasty, conjunctival autograft and tarsorrhaphy to address the corneal scarring. Postoperatively moxifloxacin eyedrop (0.5%) four times/day, prednisolone acetate 1% eyedrop eight times per day along with carboxymethylcellulose sodium 1% eyedrop were started. Topical antibiotics were discontinued after 2 weeks of keratoplasty. After the keratoplasty procedure, the excised corneal button was sent for histopathological study as per institute protocol. Unfortunately, after 1 month, he developed a large corneal melt for which a tectonic keratoplasty was planned immediately. Postoperatively, topical moxifloxacin 0.5% eyedrops every three hourly and carboxymethyl cellulose sodium 1% four times per day were started. About 5 months later, he presented to us again with a central corneal perforation for which multilayered AMG+permanent tarsorrhaphy was performed. He further presented after 6 months with graft perforation and misdirected eyelashes (figure 2) secondary to upper lid entropion for which he underwent corneal scraping with cyanoacrylate glue application and upper lid entropion correction. Gentle ultrasound B scan done preoperatively was echo-free and showed no evidence of posterior segment involvement. As per published literature, T. otitidis typically occurs in the human ear. But in our case, the child did not have an ear infection and the cornea was found to be affected primarily.
Figure 1.
Following symblepharon release and mucus membrane grafting—partial limbal stem cell deficiency, recurrent symblepharon and upper lid entropion can be seen.
Figure 2.
Diffuse areas of thinning with perforation and infiltration.
Investigations
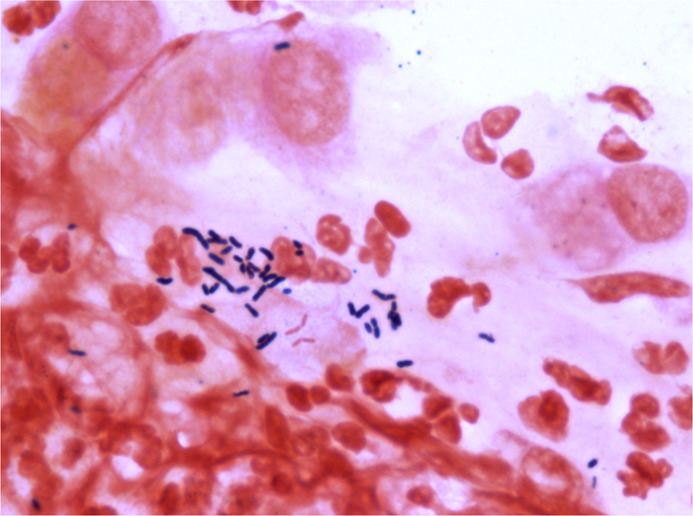
Corneal scraping was performed and sample collected on glass slides and blood agar, chocolate agar, brain heart infusion broth, thioglycolate broth, Robertson’s cooked meat, Sabouraud dextrose agar, non-nutrient agar (NNA) for finding the aetiology of infection. An eyelash was noticed at the area of perforation during scraping which was placed on blood agar medium along with routine cornea scraping for culture. Smear taken from corneal scraping turned out to be positive for Gram-positive Bacilli (figure 3). There was growth of white, dry, opaque bacterial colony on blood agar plates after 48 hours of incubation (figure 4) which was identified as T. otitidis (99% probability, excellent identification) with VITEK 2 microbial identification system (bioMérieux, Marcy L'Etoile, France).
Figure 3.
Gram positive bacilli after Gram staining of corneal scraping.
Figure 4.
White, dry, opaque bacterial colony growth on blood agar plates after 48 hours of incubation.
Treatment
The bacterium was found sensitive to amikacin, ciprofloxacin, cefazolin, gatifloxacin, moxifloxacin, ofloxacin and vancomycin with disc diffusion assay. He was prescribed topical gatifloxacin 0.5% eye-drops, cycloplegics and oral analgesics.
Outcome and follow-up
Following cyanoacrylate glue application, the eye was found to be tectonically stable, and infiltrate started resolving although without much visual potential.
Discussion
It has been previously established that T. otitidis is a commensal of the external and middle ear of children. The organism was first isolated in culture from the middle ear fluid of a child with otitis media, which suggests its pathogenic potentiality.1 10 T. otitidis is rarely identified in the clinical samples due to difficulty in phenotypically distinguishing from other coryneform bacteria which are mostly considered as contaminants in culture. It is also difficult to identify T. otitidis biochemically as no such specific tests are established yet. There is no available literature regarding the identification of T. otitidis with PCR using species-specific primers.11 Hence, T. otitidis can be identified with VITEK or matrix-assisted laser desorption/ionisation-time of flight-mass spectrometry following culture and next-generation sequencing. In our case, we have identified T. otitidis with VITEK after observing the growth of bacterial colonies on blood agar plates obtained from corneal scrapings.
T. otitidis is primarily susceptible to ß-lactams, vancomycin and fluoroquinolone antibiotics.12 Graevenitz et al found T. otitidis to be susceptible to many antimicrobials, with surprisingly low MIC 90 values for penicillin, cephalosporin, carbapenem, chloramphenicol, ciprofloxacin, aminoglycosides, rifampicin, tetracyclines, linezolid, teicoplanin and vancomycin; the only exceptions being clindamycin and erythromycin.13 Corynebacterium spp isolated from endophthalmitis or corneal infections have been found susceptible to vancomycin.14 In our case, the child sustained an accidental alkali for which he underwent multiple surgeries to restore the ocular surface. He was systemically fit without any evidence of ear infection. In this case, despite several attempts to restore some useful vision, the eye presented subsequently with multiple episodes of corneal perforations and finally with microbial keratitis. In the last visit, a tectonically stable eye was achieved with the application of cyanoacrylate glue.
In this report, the isolated T. otitidis was found susceptible to amikacin, ciprofloxacin, gatifloxacin, moxifloxacin, ofloxacin, cefazolin with the disc diffusion method. As per the history and clinical examination, the presence of an ear infection was ruled out. We presume that the broken eyelash from preexisting entropion, found embedded at the perforated site might be the probable source for keratitis from such an unusual bacterium. Apart from the poor ocular surface, the use of topical steroids for combating inflammation and multiple surgical interventions also may be responsible for the suppression of local immunity, and the growth of such an opportunistic bacterium.
This is the first case reported in the literature of T. otitidis causing microbial keratitis to the best of our knowledge. The pathogenicity of T. otitidis remains controversial and only very few reports of the organism causing bacteraemia have been published so far. T. otitidis is not a common pathogen. We assume that any commensal present for a long time (eyelash) in a poor ocular surface (multiple surgeries) which is also locally immunosuppressed (topical steroids) has a potential to behave as pathogenic. Thus, it is an alarm for the microbiologist not to consider all coryneform bacterium as contaminants. The Vitek 2 Compact system is used for both identification and calculating the minimum inhibitory concentration (MIC) value for different antibiotics in respect to the identified bacteria. It uses fluorescence-based methods by detecting the metabolic changes for identification of a bacteria and a turbidimetric method for calculating the MIC value. VITEK was found suitable for the identification of T. otitidis in the case of microbial keratitis with bacterial aetiology. Hence, VITEK can be useful to identify rare bacteria. Despite being an uncommon pathogen, it was susceptible to the most commonly used antibiotics. At the same time, it is also important to do culture and antibiotic susceptibility to ensure appropriate treatment and rule out resistant strains.
Patient’s perspective.
My son suffered a chemical injury to his eyes a while back. He experienced a lot of pain and a drop in vision subsequently. He underwent several surgeries for the same at this hospital. The doctors here have taken good care of him. We have been explained about all the procedures and their risks in detail. While I know that his vision will not be the same as before, he is comfortable at present.
Learning points.
Possible risk of microbial keratitis in the setting of multiple episodes of ocular surface interventions followed by use of prolonged topical steroids.
Turicella is commonly seen in ear infections, but rare cases of ocular infestation may be possible.
VITEK is an efficient tool for detection of rare bacteria in diagnostic dilemmas.
Acknowledgments
We acknowledge Aparajita Mallick and Shilpa Priyadarshini (technical staffs of the microbiology department in our hospital), for their work in microbiological studies.
Footnotes
Contributors: HSB: microbiological analysis, manuscript writing. SRP: patient care, editing. SS: patient care. AD: manuscript editing and patient care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
References
- 1.Funke G, Stubbs S, Altwegg M, et al. Turicella otitidis gen. nov., sp. nov., a coryneform bacterium isolated from patients with otitis media. Int J Syst Bacteriol 1994;44:270–3. 10.1099/00207713-44-2-270 [DOI] [PubMed] [Google Scholar]
- 2.Brinkrolf K, Schneider J, Knecht M, et al. Draft genome sequence of Turicella otitidis ATCC 51513, isolated from middle ear fluid from a child with otitis media. J Bacteriol 2012;194:5968–9. 10.1128/JB.01412-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds SJ, Behr M, McDonald J. Turicella otitidis as an unusual agent causing a posterior auricular abscess. J Clin Microbiol 2001;39:1672–3. 10.1128/JCM.39.4.1672-1673.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa Martínez L, González Velasco C, Gaona Álvarez CE, et al. [External otitis due Turicella otitidis: two case reports]. Rev Esp Quimioter 2017;30:474–5. [PubMed] [Google Scholar]
- 5.Loïez C, Wallet F, Fruchart A, et al. Turicella otitidis in a bacteremic child with acute lymphoblastic leukemia. Clin Microbiol Infect 2002;8:758–9. 10.1046/j.1469-0691.2002.00474.x [DOI] [PubMed] [Google Scholar]
- 6.Bacteremia Caused by Turicella otitidis in a Patient with Diffuse Large B-Cell Lymphoma. Clin Lab 2020;66. 10.7754/Clin.Lab.2019.190641 [DOI] [PubMed] [Google Scholar]
- 7.Bîrlutiu V, Mihaila RG, Bîrlutiu RM. Bacteremia with Turicella otitidis in an institutionalized elderly patient with multiple hospital admissions: a case report. Biomed. Res 2017;28:2196–8. [Google Scholar]
- 8.Koumaki D, Koumaki V, Boumpoucheropoulos S, et al. Turicella otitidis as an Unusual Agent Causing Palmoplantar Eczema: An Emerging Pathogen. Eur J Case Rep Intern Med 2020;7:001458. 10.12890/2020_001458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammo DA, Watson D, Armbrust KR. Post-intravitreal injection endophthalmitis secondary to Turicella otitidis: a case report. BMC Ophthalmol 2020;20:142. 10.1186/s12886-020-01412-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faden H, Dryja D. Recovery of a unique bacterial organism in human middle ear fluid and its possible role in chronic otitis media. J Clin Microbiol 1989;27:2488–91. 10.1128/jcm.27.11.2488-2491.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lappan R, Jamieson SE, Peacock CS. Reviewing the Pathogenic Potential of the Otitis-Associated Bacteria Alloiococcus otitidis and Turicella otitidis. Front Cell Infect Microbiol 2020;10:51. 10.3389/fcimb.2020.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Garces JL, Alhambra A, Alos JI, et al. Acute and chronic otitis media and Turicella otitidis: a controversial association. Clin Microbiol Infect 2004;10:854–7. 10.1111/j.1198-743X.2004.00965.x [DOI] [PubMed] [Google Scholar]
- 13.von Graevenitz A, Funke G. Turicella otitidis and Corynebacterium auris: 20 years on. Infection 2014;42:1–4. 10.1007/s15010-013-0488-x [DOI] [PubMed] [Google Scholar]
- 14.Kuriyan AE, Sridhar J, Flynn HW, et al. Endophthalmitis Caused by Corynebacterium Species: Clinical Features, Antibiotic Susceptibility, and Treatment Outcomes. Ophthalmol Retina 2017;1:200–5. 10.1016/j.oret.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]