Abstract
Background
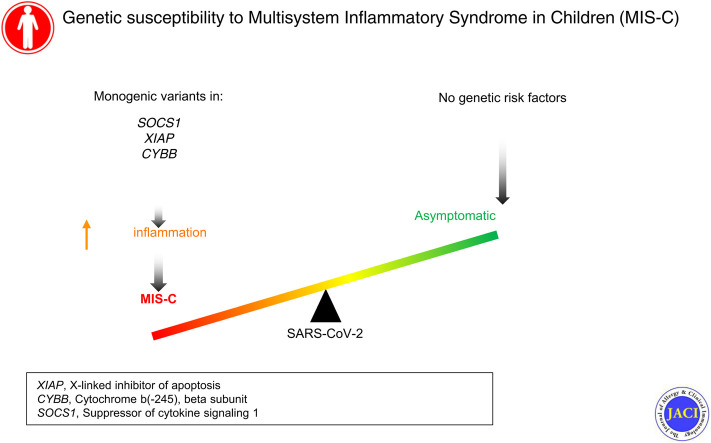
Multisystem inflammatory syndrome in children (MIS-C) is a pediatric complication of severe acute respiratory syndrome coronavirus 2 infection that is characterized by multiorgan inflammation and frequently by cardiovascular dysfunction. It occurs predominantly in otherwise healthy children. We previously reported haploinsufficiency of suppressor of cytokine signaling 1 (SOCS1), a negative regulator of type I and II interferons, as a genetic risk factor for MIS-C.
Objectives
We aimed to identify additional genetic mechanisms underlying susceptibility to severe acute respiratory syndrome coronavirus 2–associated MIS-C.
Methods
In a single-center, prospective cohort study, whole exome sequencing was performed on patients with MIS-C. The impact of candidate variants was tested by using patients’ PBMCs obtained at least 7 months after recovery.
Results
We enrolled 18 patients with MIS-C (median age = 8 years; interquartile range = 5-12.25 years), of whom 89% had no conditions other than obesity. In 2 boys with no significant infection history, we identified and validated hemizygous deleterious defects in XIAP, encoding X-linked inhibitor of apoptosis, and CYBB, encoding cytochrome b-245, beta subunit. Including the previously reported SOCS1 haploinsufficiency, a genetic diagnosis was identified in 3 of 18 patients (17%). In contrast to patients with mild COVID-19, patients with defects in SOCS1, XIAP, or CYBB exhibit an inflammatory immune cell transcriptome with enrichment of differentially expressed genes in pathways downstream of IL-18, oncostatin M, and nuclear factor κB, even after recovery.
Conclusions
Although inflammatory disorders are rare in the general population, our cohort of patients with MIS-C was enriched for monogenic susceptibility to inflammation. Our results support the use of next-generation sequencing in previously healthy children who develop MIS-C.
Key words: Multisystem inflammatory syndrome in children, MIS-C, COVID-19, SARS-CoV-2, whole exome sequencing
Abbreviations used: CGD, Chronic granulomatous disease; COVID-19, Coronavirus disease 2019; CYBB, Cytochrome b-245, beta subunit; HLH, Hemophagocytic lymphohistiocytosis; IVIG, Intravenous immunoglobulin; MIS-C, Multisystem inflammatory syndrome in children; NOD2, Nucleotide-binding oligomerization domain-containing 2; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SOCS1, Suppressor of cytokine signaling 1; TLR, Toll-like receptor; WES, Whole exome sequencing; XIAP, X-linked inhibitor of apoptosis
Graphical abstract
Introduction
Multisystem inflammatory syndrome (MIS-C) is a life-threatening complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure occurring in individuals younger than 21 years of age.1 , 2 As defined by the Centers for Disease Control and Prevention, the diagnostic criteria include fever, elevated inflammatory marker levels, multisystem organ involvement, and SARS-CoV-2 infection or exposure within 4 weeks of symptoms without an alternative diagnosis.3 Because most children with MIS-C have mild or no symptoms at the time of initial infection, MIS-C is thought to be a postinfectious syndrome. Nearly all patients with MIS-C have detectable antibodies to SARS-CoV-2, and many have detectable SARS-CoV-2 virus by RT-PCR testing.1 , 2 The clinical features of MIS-C overlap with those of acute coronavirus disease 2019 (COVID-19) and those of the pediatric vasculitic disease Kawasaki disease.1 , 2 Symptoms include fever, rash, gastrointestinal symptoms, coagulopathy, cardiac dysfunction, and/or shock.1 , 2 Studies of hospitalized adults with severe COVID-19 have identified deleterious genetic variants impairing type I interferon signaling in up to 3.5% of patients.4 , 5 In contrast, MIS-C is not associated with preexisting cardiopulmonary, autoimmune and/or immune, or hematologic diseases, and its genetic basis is largely unknown.1
We previously reported haploinsufficiency of suppressor of cytokine signaling 1 (SOCS1), a negative regulator of type I and II interferons, as a genetic risk factor for MIS-C.6 Here, we present findings from our prospective cohort sequencing study of children and adolescents with MIS-C.
Results and discussion
Whole exome sequencing (WES) was performed on 18 patients with a diagnosis of MIS-C (Table I ). Nearly all of them (89% [n = 16]) had no preexisting medical conditions (other than obesity in 44%). Nine patients required critical care during their hospital stay. This cohort’s median age, predominance of Hispanic ethnicity, lack of other comorbidities, and clinical characteristics are concordant with those of previously published cohorts of patients with MIS-C.1 , 2 In addition to SOCS1 haploinsufficiency,6 we identified defects in X-linked inhibitor of apoptosis (XIAP) and CYBB, amounting to a genetic diagnosis in 3 of 18 patients in our cohort (17%).
Table I.
Summary features of enrolled patients
| Feature | MIS-C, no. (%) (N = 18) |
|---|---|
| Age (y) | |
| Median | 8 |
| Age range | 5- 2.25 |
| Sex, no. (%) | |
| Male | 8 (44) |
| Female | 10 (56) |
| Race and ethnicity, no. (%) | |
| White, non-Hispanic | 2 (11) |
| Black, non-Hispanic | 1 (5.5) |
| Asian, non-Hispanic | 1 (5.5) |
| Other, non-Hispanic | 1 (5.5) |
| White, Hispanic | 5 (28) |
| Black, Hispanic | 1 (5.5) |
| Other, Hispanic | 7 (39) |
| Prior medical diagnoses, no. (%) | |
| None (excluding overweight and obesity)∗ | 16 (89) |
| Body mass index, no. (%) | |
| Normal (<85th percentile for age and sex) | 7 (39) |
| Overweight (85th-95th percentile for age and sex) | 3 (17) |
| Obesity (>95th percentile for age and sex) | 8 (44) |
| SARS-CoV-2 testing result, no. (%) | |
| Positive for SARS-CoV-2 RT-PCR | 8 (44) |
| Positive for SARS-CoV-2 serology† | 18 (100) |
| Hospital care required, no. (%) | |
| Required critical care | 9 (50) |
Of the 2 patients with preexisting medical conditions, 1 had sickle cell anemia and the other had Evans syndrome.
Positive SARS-CoV-2 serology was obtained by using either the Roche Elecsys or Viracor assay.
Patient 1 is an 11-year-old boy with no history of disease other than obesity and resolved pityriasis lichenoides chronica. He developed fever, vomiting, diarrhea, rash, and conjunctivitis. The result of RT-PCR testing for SARS-CoV-2 was negative on the fourth day of illness but positive on the seventh. The patient had neutrophilia; T-cell lymphopenia; increased levels of the T-cell activation marker soluble CD25; and elevated levels of IL-18, IL-6, IL-10, and C-X-C motif chemokine ligand 9 (CXCL9) indicative of type I and II interferon signaling (Table II ). He developed warm cardiogenic shock in the setting of severely depressed systolic cardiac function and third-degree heart block. He was treated with remdesivir, anakinra, methylprednisolone (2 mg/kg twice daily), intravenous immunoglobulin (IVIG) (1 g/kg), and vasopressors. While taking methylprednisolone, he was found to have EBV viremia (4.3 log10 copies/mL). He had undetectable IgM to capsid and early D antigen, consistent with EBV reactivation. His EBV viremia peaked at 6 log10 copies/mL while he was taking prednisone, and it persisted at 3.9 log10 copies/mL for 9 months until it was cleared by 1 dose of rituximab. After he recovered from MIS-C, his natural killer cytotoxicity function was found to be normal.
Table II.
Results of immunologic evaluation of the patients
| Indicator | Patient 1 |
Patient 2 |
Reference value | |
|---|---|---|---|---|
| Day 1 | Day 1 | Day 11 | ||
| Hemogram results | ||||
| White blood cells (103 cells/μL) | 18.0 | 21.7 | 29.4 | 5.52-9.29 |
| Neutrophils (103 cells/μL) | 15.4 | 25.6 | 24.3 | 3.04-6.06 |
| Lymphocytes (103 cells/μL) | 1.27 | 0.69 | 1.12 | 1.17-2.30 |
| Monocytes (103 cells/μL) | 0.60 | 0.24 | 1.55 | 0.19-0.72 |
| Platelets (103 cells/μL) | 173 | 395 | 621 | 189-342 |
| Inflammatory markers | ||||
| C-reactive protein (mg/dL) | 21.9 | 12.8 | 10.8 | ≤0.5 |
| Fibrinogen (mg/dL) | 551 | 528 | 623 | 200-400 |
| Ferritin (ng/mL) | 1138 | 231 | 1116 | 10-80 |
| d-dimer (μg/mL) | 3.1 | 9.1 | 4.2 | ≤0.5 |
| Soluble CD25 (pg/mL) | 14,800 | nd | 1550 | ≤1033 |
| Lymphocyte subsets | ||||
| CD3+ (cells/μL) | 516 | 475 | 883 | 1000-2600 |
| CD3+CD4+ (cells/μL) | 357 | 262 | 443 | 530-1500 |
| Naive (% CD4+) | 64.8 | nd | 62.6 | 21-61.4 |
| Central memory (% CD4+) | 16.7 | nd | 20.6 | 26.8-62.1 |
| Effector memory (% CD4+) | 13.1 | nd | 16.1 | 7.6-25.1 |
| TEMRA (% CD4+) | 5.4 | nd | 0.8 | 0.1-4.0 |
| CD3+CD8+ (cells/μL) | 145 | 196 | 196 | 330-1100 |
| Naive (% CD8+) | 60.3 | nd | 79.6 | 11.4-66.5 |
| Central memory (% CD8+) | 16.7 | nd | 2.4 | 3.7-23.2 |
| Effector memory (% CD8+) | 13.1 | nd | 13.6 | 16.8-54.6 |
| TEMRA (% CD8+) | 18.6 | nd | 4.4 | 5.6-43.9 |
| CD19+ (cells/μL) | 421 | 197 | 387 | 110-570 |
| Naive (% CD19+) | 65.6 | nd | 72.1 | 48.4-79.7 |
| Unswitched memory (% CD19+) | 8.10 | nd | 8.8 | 7.0-23.80 |
| Switched memory (% CD19+) | 21.1 | nd | 14.3 | 8.30-27.8 |
| Plasmablast (% CD19+) | 9.7 | nd | 2.7 | 0.1-2.4 |
| CD3–CD56+ (cells/μL) | 73 | 60 | 81 | 70-480 |
| Immunoglobulin levels | ||||
| IgG (mg/dL) | 1147 | 1423 | 1522 | 639-1344 |
| IgM (mg/dL) | 320 | 90 | 148 | 40-240 |
| IgA (mg/dL) | 169 | nd | 97 | 70-312 |
| Positive titers to pneumococcal subtypes (out of 23 subtypes) | 17 | nd | 8 | >14 |
| Tetanus (IU/mL) | 0.1 | nd | 3.62 | >0.15 |
| Cytokines (pg/mL) | ||||
| IL-2 | 7 | nd | <5 | ≤12 |
| IL-12 | <5 | nd | <5 | ≤6 |
| IL-10 | 33 | nd | 11 | ≤18 |
| IL-6 | 44 | nd | 8 | ≤5 |
| IL-18 | 1427 | nd | nd | 89-540 |
| CXCL9 (induced by type I and type II interferons) | 1575 | nd | nd | <121 |
Bolded values are outside the normal range. At the time of this blood draw, patient 1 had not received any immunomodulatory medications, whereas patient 2 had received methylprednisolone (0.3 mg/kg) treatment for 6 days. IL-12, IFN-γ, IL-4, IL-5, IL-13, IL-17, IL-1β, IL-8, and TNF-α levels were normal in both patients. Neither patient had received IVIG before testing. Naive T cells, CD45RA+CCR7+, Central memory T cells, CD45RA–CCR7+, effector memory T cells, CD45RA–CCR7–, TEMRA, CD45RA+CCR7–. Naive B cells, CD27–IgD+, unswitched memory B cells, CD27+IgD+, switched memory B cellss, CD27+IgD–, plasmablasts CD24lowCD38high.
nd, Not detected; TEMRA, terminally differentiated effector cell.
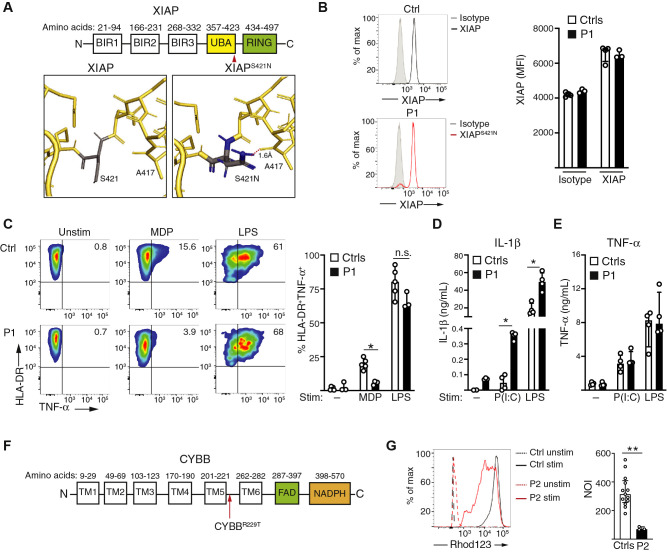
WES identified a novel hemizygous missense variant in XIAP (NP_001158.2: p.Ser421Asn). XIAP is a widely expressed protein that contributes to cellular survival, activation, and negative regulation of the NLRP3 inflammasome.7 This variant has a minor allelic frequency of 1.1 × 10–5 in the Genome Aggregation Database and was predicted to be benign with a Combined Annotation Dependent Depletion score of 4.9. However, structural modeling predicts that the variant creates a hydrogen bond with alanine 417 within the ubiquitin-associated domain, thereby potentially disrupting the domain’s structure and/or capacity for binding ubiquitin (Fig 1 , A). The patient exhibited intact XIAP protein expression (Fig 1, B). The ubiquitin-associated domain is important for TNF-α secretion following nucleotide-binding oligomerization domain–containing 2 (NOD2) activation.7 Compared with the controls, CD14+HLA-DR+ monocytes from patient 1 secreted less TNF-α in response to NOD2 activation with muramyl dipeptide (Fig 1, C), which is indicative of impaired XIAP function. TNF-α secretion after LPS stimulation, which is independent of NOD2 signaling, was intact in the patient (Fig 1, C). Patients with hemizygous loss-of-function variants in XIAP are at risk for virally triggered hemophagocytic lymphohistiocytosis (HLH) and cytokine storm syndromes.8 This has been attributed to loss of XIAP-mediated negative regulation of the NLRP3 inflammasome, which relies on the protein’s ubiquitylation function.7 , 9 Mice lacking either XIAP or XIAP-mediated ubiquitinylation secrete increased IL-1β downstream of Toll-like receptor 3 (TLR3)- or TLR4-mediated activation of the NLRP3 inflammasome.9 In contrast, XIAP is not required for IL-6 or TNF-α secretion after TLR stimulation.10 TLR3 binds to double-stranded viral RNA intermediates, whereas TLR4 binds to SARS-Cov-2 spike protein.4 , 11 Compared with the controls, PBMCs from patient 1 secreted increased IL-1β after TLR3 and TLR4 stimulation (Fig 1, D). As anticipated, TNF-α secretion after TLR3 or TLR4 stimulation was comparable between patient 1 and the controls (Fig 1, E). These findings show the impaired function of XIAPSer421Asn, leading to inflammatory signaling that likely predisposed this patient to MIS-C.
Fig 1.
Genetic risk factors for MIS-C. A, Schematic of XIAP with structural modeling of the ubiquitin-associated (UBA) domain identifies a new hydrogen bond (indicated by the dotted red line) formed between the S421N mutant found in patient 1 and alanine 417. B, Flow cytometric quantification of XIAP protein expression in CD14+ monocytes from a control (Ctrl) and the patient from 2 experiments with 3 controls. C, Quantification of HLA-DR+TNF-α+ monocytes, gated on CD14+ cells, after stimulation with 200 ng/mL of muramyl dipeptide or LPS for 2.5 hours in 2 experiments with 5 controls. D, IL-1β secretion after stimulation of PBMCs from 4 controls and patient 1 with indicated stimuli, pooled from 2 experiments. E, TNF-α secretion after stimulation of PBMCs from 4 controls and patient 1, pooled from 2 experiments. F, Schematic of CYBB. G, Quantification of the neutrophil oxidative burst in the presence and absence of stimulation with phorbol 12–myristate 13–acetate, pooled from 3 independent experiments with 13 controls. max, Maximum; MFI, mean fluorescence intensity; n.s., not significant; NOI, neutrophil oxidative index; stim, stimulated; unstim, unstimulated.
Patient 2 is a 16-year-old male whose clinical course has been recently described.12 He was healthy until 3 weeks before admission, when he developed hematochezia. He had neutrophilia, CD4+ and CD8+ T-cell lymphopenia, procalcitonin and C-reactive protein levels exceeding the upper normal limits by 100- and 30-fold, a mild coagulopathy, and a positive result of IgG testing for SARS-CoV-2 (Table II). Gastrointestinal biopsies revealed duodenitis, patchy colitis, and crypt abscesses consistent with Crohn disease; notably, he also had duodenal submucosal vasculitis atypical for inflammatory bowel disease. Despite treatment with broad-spectrum antibiotics, methylprednisolone (0.3 mg/kg twice daily), vitamin K, and bowel rest, he had persistent episodes of fever, elevated inflammatory marker levels, and worsening hematochezia. After 7 days of hospitalization, he became febrile to 40oC and severely hypotensive. He had persistent T-cell lymphopenia with a predominance of naive CD4+ and CD8+ T cells and an elevated soluble CD25 level (Table II). No secondary infections were identified. As his duodenal vasculitis, coagulopathy, and compensated shock were more consistent with MIS-C than with inflammatory bowel disease, he was given methylprednisolone (increased to 0.5 mg/kg twice daily) and high-dose IVIG. He had rapid improvement of his inflammatory marker levels, hematochezia, and diarrhea. He is currently taking infliximab and is clinically well.
In patient 2, WES identified a novel hemizygous missense variant in CYBB (p. Arg229Thr), which encodes the p91phox subunit of the NADPH oxidase that is essential for the phagocytic oxidative burst. Deleterious variants in CYBB cause chronic granulomatous disease (CGD). Although the crystal structure of the extracellular domain harboring this variant has not yet been identified (Fig 1, F), this variant is predicted to be pathogenic, with a Combined Annotation Dependent Depletion score of 31, and it has a minor allelic frequency of 3.9 × 20–4 in the Genome Aggregation Database. The patient’s neutrophil oxidative burst was impaired but not absent (Fig 1, G). This contrasts with the minimal neutrophil oxidative burst typical of CYBB variants that causes classical CGD, thereby indicating the variant’s hypomorphic effect on p91phox function. Unlike patients with typical inflammatory bowel disease, the majority of patients with CGD experience inflammatory sequelae, including infection-associated cytokine storm syndromes and granulomatous lesions.13 Loss of NADPH oxidase function impairs the generation of reactive oxidant species that inhibit type I interferon signaling, resulting in a proinflammatory macrophage phenotype.13, 14, 15
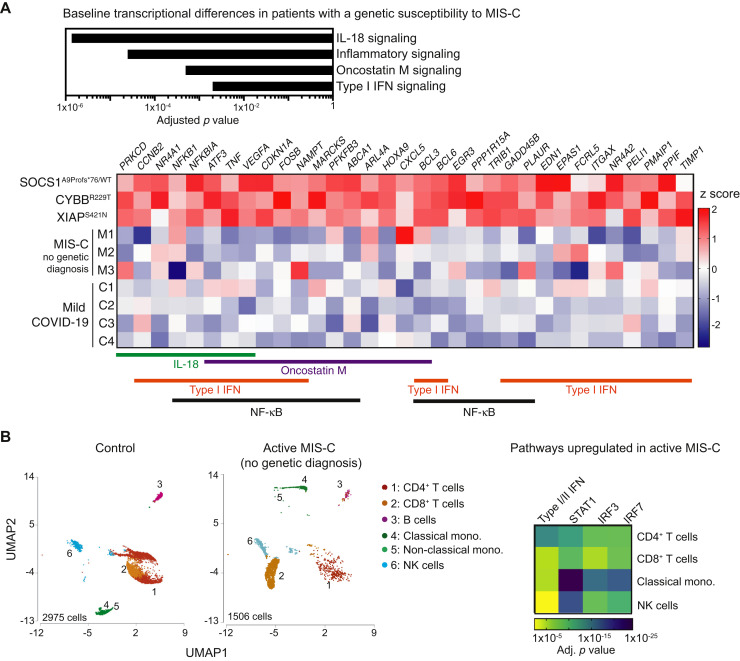
We previously showed that SOCS1 haploinsufficiency in a patient with a history of MIS-C leads to increased tonic type I and/or II interferon signaling in unstimulated PBMCs on account of reduced inhibition of the Janus-activated kinases. Transcriptomic analysis of unstimulated PBMCs obtained at least 7 months after recovery from SARS-CoV-2 infection identified 712 genes with more than a 1.5-fold difference between the 3 patients in this cohort with likely genetic susceptibility to MIS-C and 4 otherwise healthy children with a history of mild COVID-19. Differentially expressed genes were enriched in signaling pathways downstream of the inflammatory response, nuclear factor κB, IL-18, oncostatin M, and type I interferon signaling (Fig 2 , A). Increased level of IL-18, a marker of inflammasome activation, is a known finding in individuals with defects in XIAP, CYBB, or SOCS1.14 , 16 , 17 Oncostatin M, a member of the IL-6 family of cytokines, induces type I interferon–stimulated genes.18 Type I interferon signaling triggers activation of the NLRP3 inflammasome in tissues such as the gastrointestinal and respiratory epithelium and coronary artery endothelium, as well as in myeloid cells.19 , 20 However, recovered patients lacking a genetic diagnosis for MIS-C did not exhibit significantly increased interferon or inflammatory gene expression in their unstimulated PBMCs (Fig 2, A). This contrasts with the robust immune cell activation characteristic of active MIS-C highlighted here by single-cell RNA sequencing of PBMCs from a patient with ongoing MIS-C despite the lack of any identifiable genetic risk factors. Compared with the control cells, the cells of the patient with active MIS-C exhibited enrichment of differentially expressed genes promoting interferon signaling in CD4+ T cells, CD8+ T cells, CD14+CD16low classical monocytes, and natural killer cells (Fig 2, B). Thus, the pathways upregulated in PBMCs from recovered, genetically susceptible patients converge with those also increased during active MIS-C.
Fig 2.
A, Transcriptome analysis of unstimulated PBMCs from patients 1 and 2, as well as those from the patient with SOCS1 haploinsufficiency whom we previously described,6 compared with PBMCs from 4 otherwise healthy individuals who had previously had mild COVID-19. PBMCs were collected at least 7 months after recovery, at which time the individuals had returned to their baseline state of health. ∗P < .05; ∗∗P < .01 by the Mann-Whitney test. B, Uniform Manifold Approximation and Projection (UMAP) plots depict transcriptional clusters generated by single-cell RNA sequencing of PBMCs from a control and a patient with active MIS-C but no identifiable genetic diagnosis. This research sample was taken early in the patient’s course; she had received 1 dose of IVIG but subsequently required additional doses of IVIG and methylprednisolone before clinical improvement occurred. Ingenuity Pathway Analysis of differentially expressed genes indicates upregulation of signaling pathways downstream of type I and/or II interferons, STAT1, IRF3, and IRF7. mono, Monocyte; NF-κB, nuclear factor κB; NK, natural killer.
Patients previously known to have primary immunodeficiencies or autoinflammatory disorders are at increased risk of development of life-threatening COVID-19 rather than MIS-C.21 The cohort that we have presented is unique from those of prior studies in that our patients have hypomorphic variants with mild to no immunologic sequelae before SARS-CoV-2 infection. Therefore, varying degrees of immunologic impairment may result in clinical outcomes distinct from those of SARS-CoV-2 infection. Our report thus provides proof of principle for recent perspectives proposing that genetic risk factors for MIS-C may be incompletely penetrant.22 Future studies with larger cohorts are needed to determine additional genetic risk factors for MIS-C, because the risk of MIS-C may vary among different genetic causes of autoinflammation.
In identifying a genetic variant that affects immunity in 17% of patients, our study suggests that MIS-C may indicate an underlying disorder of immune dysregulation. As clinical-grade WES has become increasingly accessible as a diagnostic tool, this study has clinically relevant implications for the use of WES in identifying inborn errors of immunity in patients with MIS-C.
For detailed methods, please see the Methods section of this article's Online Repository materials (available at www.jacionline.org).
Key messages.
-
•
In this prospective study of 18 patients with MIS-C, 17% of patients were found to have a genetic variant impairing negative regulation of interferon and inflammatory signaling.
-
•
A history of MIS-C should prompt consideration of WES for the identification of variants affecting host immunity in affected children.
Acknowledgments
We are grateful to our patients and their families for their participation in this study. We thank the Perkin family and the Perkin Fund for their support of this research and the Turkel family and the Samara Jan Turkel Clinical Center for their support of these patients’ clinical care.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supported by the National Institute of Allergy and Infectious Diseases (grants R01-AI139633 [to R.S.G.], R01-AI139633-S1 [to R.S.G., J.C., and A.G.R.], R01-AI154470 [to A.G.R.], the Centers for Disease Control and Prevention (grant 75D30120C07725 [to A.G.R. and J.C.]), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R21-HD095228 [to A.G.R.]), the Perkin Fund (to R.S.G.), and the Samara Jan Turkel Center for Autoimmune Diseases (to J.C.), as well as by the Institutional Centers for Clinical and Translational Research and the Children’s Rare Disease Cohorts initiative at Boston Children’s Hospital.
Contributor Information
Taking on COVID-19 Together Study Investigators:
Abduarahman Almutairi, Faris Jaber, Tina Banzon, Jordan Roberts, Olha Halyabar, Mindy Lo, Stacy Kahn, Lauren A. Henderson, Pui Y. Lee, Mary Beth Son, and Leah Cheng
Methods
Study design
This prospective observational study was approved by the Boston Children’s Hospital Institutional Review Board. Informed assent and/or consent was obtained from participants and/or their legal guardians.
WES
WES was performed by GeneDx using IDT xGen probes with an average coverage across the WES of 100× and more than 95% of targets covered at 20×, as previously described.E1 Variant calling and candidate variant analysis were completed by utilizing the Boston Children’s Hospital Genomic Learning System, as previously described.E1 Minor allelic frequencies for the specified variants were identified by using the Genome Aggregation Database.E2
Single-cell RNA sequencing
For each sample, approximately 17,000 PBMCs at a concentration of 1000 cells/μL were input into a 10× Genomics Chromium Controller. Chromium Next GEM Single Cell 3ʹ kits (version 3.1) were used to generate single-cell gene expression libraries, which we subsequently sequenced by using an Illumina NextSeq 500 system with 150-bp paired-end sequencing. Libraries were processed by using CellRanger version 3.1 (10× Genomics, Pleasanton, Calif) and GrCh38 as the reference. Partek Flow was used to analyze the data output from CellRanger (data filtering, log normalization, integration, scaling, dimensionality reduction, and cluster identification). We excluded genes expressed in fewer than 5 cells, as well as those encoding ribosomal structural proteins and noncoding ribosomal RNA.E3 Low-quality cells with more than a 10% mitochondrial gene content or fewer than 200 features were also excluded. Principal component analysis was performed by using the elbow heuristics method to determine the 15 top principal components for subsequent clustering analysis using the Louvain clustering algorithm, followed by Uniform Manifold Approximation and Projection visualization. Pathway analysis of differentially expressed genes within cell types was performed by using Ingenuity Pathway Analysis (Qiagen Bioinformatics, Redwood City, Calif).
Flow cytometry
Protocols for staining of XIAP were performed as previously described.E4 For quantification of intracellular TNF-α, PBMCs were rested overnight in a 48-well plate and nonadherent cells were removed the following day. Adherent PBMCs, consisting primarily of monocytes, were stimulated with lipidated muramyl dipeptide (200 ng/mL, InvivoGen, San Diego) or LPS (200 ng/mL, InvivoGen) for 2.5 hours with brefeldin A, followed by flow cytometric staining for HLA-DR+CD14+TNF-α+ monocytes as previously described.E5 IL-1β secretion downstream was measured by using cytometric bead array (BD Biosciences, Piscataway, NJ) per the manufacturer’s protocol after PBMCs were stimulated with Poly(I:C) (10 μg/mL; InvivoGen, San Diego, Calif) or LPS (100 ng/mL; InvivoGen) for 24 hours.
Transcriptome analysis
mRNA was isolated from PBMCs by using the RNeasy Mini Kit (Qiagen), followed by cDNA synthesis using the SuperScript VILO cDNA Synthesis Kit (ThermoFisher Scientific). The Ion AmpliSeq Transcriptome Human Gene Expression Kit was used to prepare bar-coded libraries and sequenced by using an Ion S5 next-generation sequencer. The AmpliSeqRNA plug-in (ThermoFisher Scientific) was used to calculate differential gene expression analysis. Pathway analysis was performed by using Ingenuity Pathway Analysis and Gene Set Enrichment Analysis (Broad Institute and University of California San Diego)E6, E7 on genes with at least a 1.5-fold difference between the controls and patients (P < .05). Final analyses were performed by using adjusted P values calculated with the Benjamini-Hochberg procedure.
Statistical analysis
All tests were 2 sided, and P values less than .05 were considered significant when using the indicated statistical tests. Statistical analyses were performed by using Prism 8.0 software (GraphPad Software, San Diego, Calif).
References
- 1.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P.Y., Day-Lewis M., Henderson L.A., Friedman K.G., Lo J., Roberts J.E. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC . Centers for Disease Control and Prevention; 2020. Multisystem inflammatory syndrome in children (MIS-C) [Internet]https://www.cdc.gov/mis-c/cases/index.html Available at: [Google Scholar]
- 4.Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19 [abstract] Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee P.Y., Platt C.D., Weeks S., Grace R.F., Maher G., Gauthier K. Immune dysregulation and multisystem inflammatory syndrome in children (MIS-C) in individuals with haploinsufficiency of SOCS1. J Allergy Clin Immunol. 2020;146:1196–1200. doi: 10.1016/j.jaci.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jost P.J., Vucic D. Regulation of cell death and immunity by XIAP. Cold Spring Harb Perspect Biol. 2020;12 doi: 10.1101/cshperspect.a036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh R.A., Madden L., Kitchen B.J., Mody R., McClimon B., Jordan M.B. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabal M., Müller N., Adler H., Knies N., Groß C.J., Damgaard R.B. XIAP Restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh W.-C., Chuang Y.-T., Chiang I.-H., Hsu S.-C., Miaw S.-C., Lai M.-Z. Inability to resolve specific infection generates innate immunodeficiency syndrome in Xiap-/- mice. Blood. 2014;124:2847–2857. doi: 10.1182/blood-2014-03-564609. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y., Kuang M., Li J., Zhu L., Jia Z., Guo X. SARS-CoV-2 spike protein interacts with and activates TLR4. Cell Res. 2021:1–3. doi: 10.1038/s41422-021-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeny K.F., Zhang Y.J., Crume B., Martz C.A., Blessing M.M., Kahn S.A. Inflammatory bowel disease presenting with concurrent COVID-19 multisystem inflammatory syndrome. Pediatrics. 2021;147 doi: 10.1542/peds.2020-027763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner F., Seger R.A., Moshous D., Fischer A., Reichenbach J., Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meda Spaccamela V., Valencia R.G., Pastukhov O., Duppenthaler A., Dettmer M.S., Erb J. High levels of IL-18 and IFN-γ in chronically inflamed tissue in chronic granulomatous disease. Front Immunol. 2019;10:2236. doi: 10.3389/fimmu.2019.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Geer A., Nieto-Patlán A., Kuhns D.B., Tool A.T.J., Arias A.A., Bouaziz M. Inherited p40phox deficiency differs from classic chronic granulomatous disease. J Clin Invest. 2018;128:3957–3975. doi: 10.1172/JCI97116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada T., Kanegane H., Ohta K., Katoh F., Imamura T., Nakazawa Y. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine. 2014;65:74–78. doi: 10.1016/j.cyto.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Thaventhiran J.E.D., Lango Allen H., Burren O.S., Rae W., Greene D., Staples E. Whole-genome sequencing of a sporadic primary immunodeficiency cohort. Nature. 2020;583:90–95. doi: 10.1038/s41586-020-2265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai B., Yang Y., Wang Q., Li M., Tian C., Liu Y. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:1–18. doi: 10.1038/s41419-020-02985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothlichet J., Meunier I., Davis B.K., Ting J.P.-Y., Skamene E., von Messling V. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2020;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancho-Shimizu V., Brodin P., Cobat A., Biggs C.M., Toubiana J., Lucas C.L. SARS-CoV-2–related MIS-C: a key to the viral and genetic causes of Kawasaki disease [abstract]? J Exp Med [Internet] 2021;218 doi: 10.1084/jem.20210446. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Rockowitz S., LeCompte N., Carmack M., Quitadamo A., Wang L., Park M. Children’s rare disease cohorts: an integrative research and clinical genomics initiative. NPJ Genom Med. 2020;5:29. doi: 10.1038/s41525-020-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genome Aggregation Database Consortium. Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A. RPG: the Ribosomal Protein Gene database. Nucleic Acids Res. 2004;32:168D–170D. doi: 10.1093/nar/gkh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R.A., Villanueva J., Zhang K., Snow A.L., Su H.C., Madden L. A rapid flow cytometric screening test for X-linked lymphoproliferative disease due to XIAP deficiency. Cytometry B Clin Cytom. 2009;76:334–344. doi: 10.1002/cyto.b.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann S., Elling R., Gyrd-Hansen M., Dückers G., Bredius R., Burns S.O. A new functional assay for the diagnosis of X-linked inhibitor of apoptosis (XIAP) deficiency. Clin Exp Immunol. 2014;176:394–400. doi: 10.1111/cei.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene Set Enrichment Analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha V.K., Lindgren C.M., Eriksson K.-F., Subramanian A., Sihag S., Lehar J. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]