Abstract
The delays in the production and delivery of COVID-19 vaccines and the growing number of fatal infections across the globe raised the question whether it would be more advantageous to vaccinate a larger group of individuals with one dose instead of a smaller one with two doses. Through a group of vaccinated healthcare workers, we describe the qualitative and quantitative serological response to a single dose of the BNT162b2 vaccine. We found that, before the second dose inoculation, 95.3 % (182/191) already had anti-SARS-CoV-2 IgG and, half of them, antibodies concentrations against RBD (the key target of neutralizing antibodies) that reached maximum values for the used evaluation immunoassay. In order to improve the execution of vaccination programs, further studies are needed to assess whether there are individuals for whom a single dose of mRNA vaccine or a delay in the inoculation of the second dose, produce a sufficient immune response. Additionally, follow-up studies will help in understanding post-vaccination immunity, how long it lasts and how it relates to infection and reinfection.
Keywords: SARS-CoV-2, mRNA vaccine, BNT162b2, Multiplex imunnosassay
1. Introduction
In December 2019, the World Health Organization (WHO) was informed about an outbreak of pneumonia in Wuhan, Hubei Province, China (Zhu et al., 2019). On January 30, 2020, WHO declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic as a public health emergency.
SARS-CoV-2 belongs to the genus Betacoronavirus in the subfamily and family of Coronavirinae and Coronaviridae, respectively. The genome size of SARS-CoV-2 is approximately 29.9 kb and encodes sixteen non-structural proteins and four structural proteins, namely the nucleoprotein (N), the membrane glycoprotein (M), the envelope glycoprotein (E), and the spike protein (S). Spike protein is composed of two subunits, S1 and S2. S1 contains an exposed receptor-binding domain (RBD) that binds angiotensin-converting enzyme 2 (ACE2) receptors while S2 is necessary for fusion of viral and host membranes (Brian and Baric, 2005; Lu et al., 2020).
Early in the pandemic, S protein was identified as an antigenic target for a SARS-CoV-2 vaccine. This can be explained, first, due to its ability to bind ACE2 on host cells, promote endocytosis and then the fusion of viral and endosomal membranes and consequent release of the viral genome into the cytoplasm; and second, because the antibodies against S protein, especially against RBD, prevent its attachment to the host cell and neutralize the virus (Tortorici and Veesler, 2019; Wrapp et al., 2020; Letko et al., 2020; Wang et al., 2008).
Over the past decade, mRNA vaccines became a promising therapeutic tool, representing an alternative to conventional vaccine approaches because of their high potency, capacity for rapid development and potential for low-cost manufacture and safe administration (Karikó et al., 2008; Kauffman et al., 2016; Guan and Rosenecker, 2017; Thess et al., 2015; Karikó et al., 2011).
On the 27th December 2020, the vaccination plan started across Europe with the SARS-CoV-2 spike mRNA vaccine BNT162b2 (Pfizer-BioNTech), the first one to be approved (Krammer, 2020). BNT162b2 consists of nucleoside-modified mRNA encoding the full-length SARS-CoV-2 spike protein formulated in lipid nanoparticles. The mRNA contains mutations which stabilize the S protein in an antigenically preferred, prefusion conformation. The lipid nanoparticles protect the non-replicating RNA from degradation and allow it to be delivered into host cells after intramuscular injection. Inside cells, the mRNA is translated into S protein and the transient expression of this S antigen induces neutralizing antibody and cellular immune responses (Polack et al., 2020; Walsh et al., 2020; Sahin et al., 2020).
In participants without any previous evidence of COVID-19, phase 3 trials reported high efficacy in preventing symptomatic SARS-CoV-2 infections after two doses of the vaccine, administered 21 days apart (Polack et al., 2020). However, this vaccination plan was put in question by the shortage in the availability of vaccine shots. The reduction in production and the delay in delivery, associated with the growing number of fatal infections worldwide, raised the hypothesis that it could be more advantageous to vaccinate a larger group of people with one dose, instead of a smaller one with two doses. Accordingly, those responsible for the UK vaccination plan recommended that the second dose should be delayed up to 12 weeks after the first one (DHSC – Department of Health and Social Care, 2021). In order to better understand the effects of vaccination, we describe here the qualitative and quantitative serological response to the first dose of the BNT162b2 vaccine in a set of high priority healthcare workers (HCW).
2. Material and methods
Monitoring of the anti-SARS-CoV-2 IgG antibodies against the Pfizer-BioNTech vaccine was assessed in 54 healthcare workers from Centro Hospitalar Universitário de São João (CHUSJ). The antibodies production was evaluated from serum samples collected on days 1, 8, 15 and 22 after vaccination. These HCW, included 8 men and 46 women, with an average age of 47.0 (27–65) and 42.4 (26–64) years-old, respectively.
In order to further investigate the qualitative and quantitative production of SARS-CoV-2 IgG before the second dose administration, the serum samples from 144 additional participants were collected after day 21 and up to one day before the second dose of the vaccine (for all participants the second dose was given after 27 days). This second group of HCW, included 20 men and 124 women with an average age of 42.4 (24–62) and 47.1 (20–65) years-old, respectively.
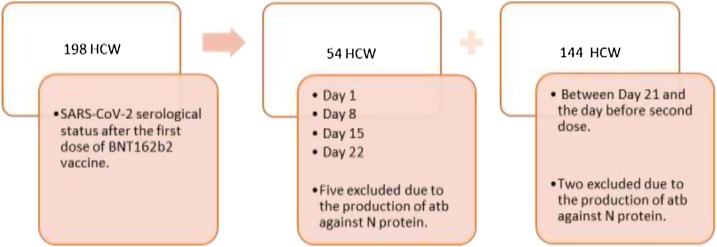
Among the two groups, all participants were white and Latino, regarding to race and ethnicity, respectively. Seventy-nine percent (156/198) completed a requested survey. Among them, 38 % (59/156) reported underlying pathologies. The diseases reported are the following: high blood pressure (22), other cardiovascular disease (3), diabetes mellitus (7), asthma (9), chronic bronchitis (4), cancer being treated (1), immunodeficiency (2), autoimmune disease (10) and others diseases (22), including allergies, hypothyroidism, hereditary anemia and others. The study design is shown in Fig. 1 .
Fig. 1.
Study design of serological response to a single dose of BNT162b2 vaccine. The serological response was assessed using 198 HCW. Among them, 54 were monitored on days 1, 8, 15 and 22. An additional 144 HCW were evaluated after day 21 and up to the day before inoculation of the second dose. The participants’ serum was screened for antibodies against RBD, S1, S2 and N proteins using BioPlex 2200 SARS-CoV-2 IgG Panel immunoassay.
The production of anti-SARS-CoV-2 IgG antibodies was assessed using BioPlex 2200 SARS-CoV-2 IgG Panel (BIO-RAD, Hercules, California, USA), a multiplex immunoassay for the qualitative detection and semi-quantitative differentiation of IgG class antibodies against the following targeted viral antigen: receptor-binding domain (RBD) of SARS-CoV-2 spike protein, S1 domain of the SARS-CoV-2 spike protein, S2 domain of the SARS-CoV-2 spike protein and nucleocapsid (N) protein of SARS-CoV-2. SARS-CoV-2 IgG assay results are expressed as U/mL and as Negative or Positive. Results of < 10U/mL are reported as Negative and results of ≥ 10 U/mL are reported as Positive. The measuring range for the assays is 1–100 U/mL. Results outside of this range are reported as either < 1 U/mL or > 100 U/mL.
At each moment of the sample collection, the percentage of seroconversion was calculated, and the participant’s serological profile characterized at the qualitative and quantitative level.
We must note that only HCW with no prior known SARS-CoV-2 infection were considered for vaccination.
The Ethics Committee of CHUSJ approved this study.
3. Results
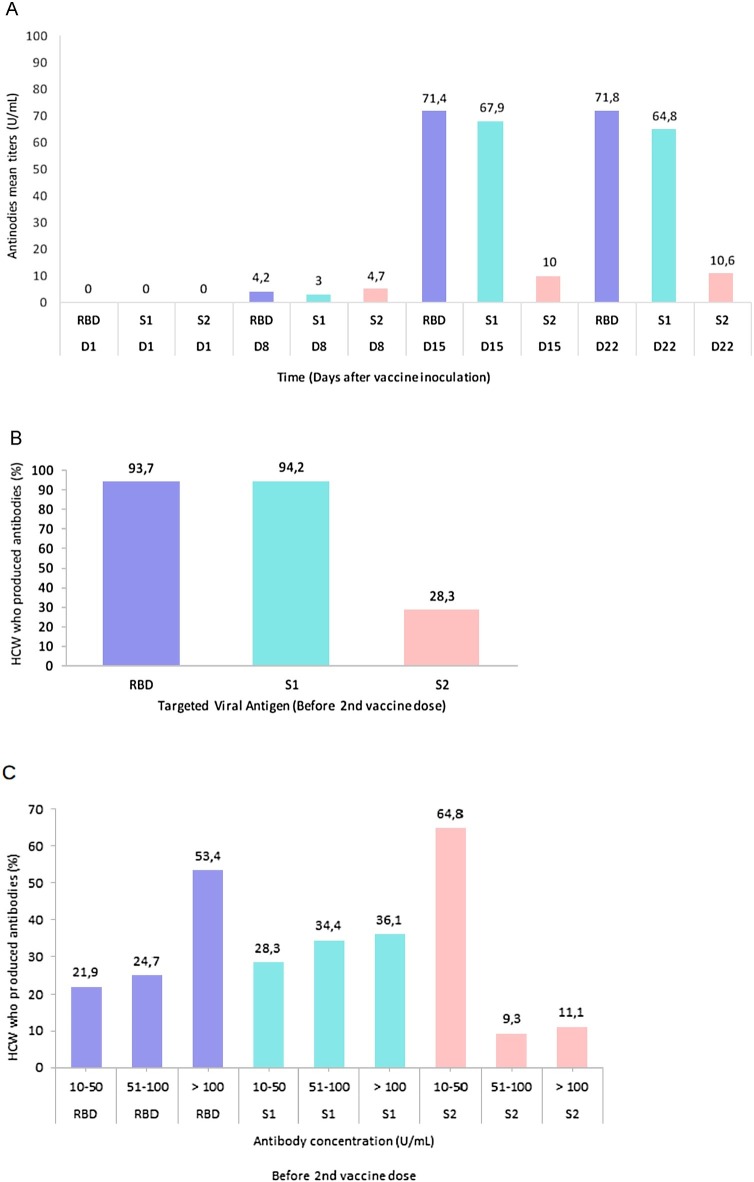
Among the participants evaluated at days 1, 8, 15 and 22 after the first inoculation of the vaccine, we found that by day 15, 98 % (48/49) had already produced anti-SARS-CoV-2 IgG antibodies. Five participants started produced antibodies on day 1, three on day 8, thirty-five on day 15 and one on day 22. The five participants who have already produced antibodies on day 1, produced antibodies against the N protein, which is compatible with an asymptomatic or not reported past infection with SARS-CoV-2. These participants were excluded from the study.
When we considered the largest HCW group monitored, we concluded that 95.3 % (182/191) already had anti-SARS-CoV-2 IgG production before the second dose inoculation. Among the additional participants, we also found two who showed antibodies against the N protein. They were also not considered for the study.
Among the seroconverted participants, we found that 93.2 % and 94.2 % produced antibodies against RBD and S1 proteins, respectively. Additionally, we found that 53.4 % produced >100 U/mL of antibodies against RBD (the maximum threshold), the key target of SARS-CoV-2 neutralizing antibodies (He et al., 2004a, 2004b, 2005).
Out of the nine participants who did not produce anti-SARS-CoV-2 IgG, we could identify two liver-transplanted, one undergoing immunosuppressive therapy and another that had SARS-CoV-2 infection diagnosed after the first shot.
The qualitative and quantitative serological response is shown in Fig. 2 .
Fig. 2.
Serological response of vaccinated healthcare workers. A: The mean titers of the three antibodies types (RBD, S1, S2) are represented across the time. B: The qualitative serological response is shown after day 21 and up to the day before inoculation of the second dose (n=191). The proportion of HCW that produced antibodies against the three SARS-CoV-2 target antigen RBD, S1 and S2 was evaluated. C: The quantitative serological response is shown before inoculation of the second dose of the vaccine. The quantitative response was accessed by calculating the percentage of HCW that produce antibodies against the three target antigen RBD, S1 and S2 within three concentration ranges: 10-50 U/mL, 51-100 U/mL and &100 U/mL.
4. Discussion
These findings suggest that a single dose of Pfizer-BioNTech vaccine induces a rapid immune response with the production of antibodies against the SARS-CoV-2 neutralizing epitopes in more than 95 % of participants. Interestingly, in half of them, the antibodies concentrations against RBD, the main target of neutralizing antibodies, reached maximum values for the used evaluation technique.
The RBD is a small domain that is part of the S1 protein. Ninety-seven percent of the participants simultaneously presented antibodies against RBD and S1. Since several SARS-CoV-2 immunoassays for monitoring the vaccine response are based on S1 subunit, we believe in the importance of highlighting this correlation.
Only 28.3 % of participants produced anti-S2 antibodies. Additionally, the anti-S2 antibodies titers (median = 10.6 U/mL) were much lower than those of anti-RBD and anti-S1, with a median titers of 71.8 U/mL and 64.8 U/mL, respectively. Perhaps this can be explained by the poor accessibility of the S2 subunit, which is found in the spike protein stem, and its consequent lower immunogenicity. However, further studies are needed to clarify this issue.
Monitoring the four target antigens involved in the immune response to SARS-CoV-2 in this “real-world” vaccine seroconversion study has enabled us to distinguish the serological response to the vaccine from the natural infection. In seven participants, the production of antibodies against N protein, which is not encoded by the BNT162b2 vaccine mRNA, was observed. The identification of asymptomatic or unreported infection cases may help to prioritize the vaccines administration more effectively, since a first vaccine dose could act as an immunological boost in previous naturally infected individuals. Our findings are in line with other “real world” studies that showed the effectiveness of the first dose of Pfizer vaccine in producing an immune response in most participants. However, this immune response appears to be greater in pre-infected than in naïve individuals (Bradley et al., 2021; Prendecki et al., 2021; Reynolds et al., 2021; Ebinger et al., 2021; Krammer et al., 2021). Some studies even showed that in pre-infected HCW the antibodies titers are comparable to or exceeds the titers found in naïve participants who received two doses (Ebinger et al., 2021; Krammer et al., 2021).
Further studies will be needed to assess whether there is a group of people, beyond those previously infected, who do not need the two doses of mRNA vaccines, improving the execution of vaccination plans. Possibly, those who produce maximum antibodies titers against RBD are among these candidates to an alternative approach. In addition, only ongoing follow-up studies will show whether these IgG titers are maintained over time.
In conclusion, these preliminary results indicate an efficient qualitative and quantitative serological response to the first dose of Pfizer-BioNTech vaccine. However, we have to note that only accessing the serological response, without considering the cellular response, we are just looking at the tip of the iceberg.
Author statement
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the Journal of Virological Methods.
Research funding
This work was supported by FCT Special Support Research4Covid (Project 186).
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The BioPlex 2200 SARS-CoV-2 IgG Panel tests were supplied by Bio-Rad Laboratories, Inc., Hercules, California, USA. We would also like to thank all CHUSJ health professionals who voluntarily participated in the study.
References
- Bradley T., Grundberg E., Selvarangan R., et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384(May 20):1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. PMID: 15609507; PMCID: PMC7120446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHSC – Department of Health and Social Care . 2021. Optimising the COVID-19 Vaccination Programme for Maximum Short-term Impact.https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvistatement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact Available in: [Google Scholar]
- Ebinger J.E., Fert-Bober J., Printsev I., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27(June 6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(March 3):133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- He Y., Zhou Y., Siddiqui P., Jiang S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem. Biophys. Res. Commun. 2004;325:445–452. doi: 10.1016/j.bbrc.2004.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhou Y., Liu S., et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. Biophys.Res. Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Lu H., Siddiqui P., Zhou Y., Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J. Immunol. 2005;174:4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- Karikó K., Muramatsu H., Welsh F.A., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16(November 11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39(November 21):e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman K.J., Webber M.J., Anderson D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release. 2016;(October 240):227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Krammer F., Srivastava K., Alshammary H., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384(April 14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(April 4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(February 22 (10224)):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendecki M., Clarke C., Brown J., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397(March 10280):1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;(April 30):eabh1282. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(October 7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Thess A., Grund S., Mui B.L., et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23(September 9):1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(December 25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(February 2):290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(March 6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382(February 8):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]