Abstract
Objective
This multilanguage study used simple speech recording and high‐end pattern analysis to provide sensitive and reliable noninvasive biomarkers of prodromal versus manifest α‐synucleinopathy in patients with idiopathic rapid eye movement sleep behavior disorder (iRBD) and early‐stage Parkinson disease (PD).
Methods
We performed a multicenter study across the Czech, English, German, French, and Italian languages at 7 centers in Europe and North America. A total of 448 participants (337 males), including 150 with iRBD (mean duration of iRBD across language groups 0.5–3.4 years), 149 with PD (mean duration of disease across language groups 1.7–2.5 years), and 149 healthy controls were recorded; 350 of the participants completed the 12‐month follow‐up. We developed a fully automated acoustic quantitative assessment approach for the 7 distinctive patterns of hypokinetic dysarthria.
Results
No differences in language that impacted clinical parkinsonian phenotypes were found. Compared with the controls, we found significant abnormalities of an overall acoustic speech severity measure via composite dysarthria index for both iRBD (p = 0.002) and PD (p < 0.001). However, only PD (p < 0.001) was perceptually distinct in a blinded subjective analysis. We found significant group differences between PD and controls for monopitch (p < 0.001), prolonged pauses (p < 0.001), and imprecise consonants (p = 0.03); only monopitch was able to differentiate iRBD patients from controls (p = 0.004). At the 12‐month follow‐up, a slight progression of overall acoustic speech impairment was noted for the iRBD (p = 0.04) and PD (p = 0.03) groups.
Interpretation
Automated speech analysis might provide a useful additional biomarker of parkinsonism for the assessment of disease progression and therapeutic interventions. ANN NEUROL 2021;90:62–75
Given that disease‐modifying treatments aimed at slowing or even halting neurodegeneration in diseases such as Parkinson disease (PD) are currently under development, 1 access to sensitive and reliable quantitative biomarkers of progression to establish the efficacy of such therapies is becoming ever more crucial. The optimal time to introduce disease‐modifying therapies is likely to be the prodromal period. 2 , 3 , 4 Idiopathic or isolated rapid eye movement sleep behavior disorder (iRBD) is a prodromal marker of synucleinopathies that have a high risk (>70–80%) of developing into overt neurodegenerative synucleinopathies, such as PD or dementia with Lewy bodies. 5 , 6 , 7 , 8 , 9 , 10 , 11
Speech represents the most complex quantitative marker of motor function, and it is sensitive to damage of the neural structures. 12 Half a century ago, Darley and colleagues, 13 in their landmark study, noted that the majority of PD patients develop hypokinetic dysarthria, characterized mainly by monopitch and monoloudness (which contribute to reduced stress production), imprecise consonant articulation, inappropriate silences, harsh voice, and deficient speech timing. Recent evidence based on the Czech language showed that subliminal speech abnormalities in patients with iRBD could be detected using objective acoustic analysis. 14 , 15 Furthermore, longitudinal observation based on clinical assessment of subjects with iRBD has shown that speech/bulbar changes are the first motor signs to emerge, developing ≤10 years before the diagnosis of PD and before evident rigidity, gait abnormalities, or limb bradykinesia. 16
Phonemic variability across different languages imposes considerable practical challenges for the development of a unified speech assessment framework. This variability is restricted by a set of universal principles. For instance, English is a Germanic language characterized by frequent consonant clusters, whereas Italian is a Romance language that has a prevalent consonant–vowel syllable structure and fewer consonant clusters. 17 Thus, languages containing more complex consonant clusters might present more challenges for PD patients. The available evidence about speech dysfunction in PD is based mostly on single language assessments, 18 and the extrapolation of findings from existing studies is problematic because of the lack of standardization among speech assessments. Although very high accuracy of acoustic analysis in differentiating PD patients from controls has been demonstrated, 18 the vast majority of existing studies have relied on cross‐sectional data to infer usefulness. Such inferences introduce the risk of overestimating the sensitivity of speech measures. 19 Therefore, confirmation of the stability of speech assessment from longitudinal studies over durations relevant to clinical trial time lines is essential.
This article reports the results of the first multilanguage longitudinal observational study aimed at advancing the understanding of possible language‐specific and pathophysiological differences in motor speech disorders in iRBD and in early manifest PD. We used a protocol designed to mimic a clinical trial, including such aspects as rapid data acquisition, rigorous quality assurance and quality control, and blinded (fully automated) and centralized acoustic speech data analysis. We aimed to assess: (1) in what way motor speech abnormalities might differ across 5 Indo‐European languages, (2) which speech patterns are most sensitive for iRBD and early‐stage PD, and (3) whether speech analyses are able to detect progression at a 12‐month follow‐up assessment.
Subjects and Methods
Study Design and Participants
We examined participants from 7 centers (Medical University of Innsbruck, Innsbruck, Austria; University of Marburg, Marburg, Germany; San Raffaele Hospital, Milano, Italy; Gui‐de‐Chauliac Hospital, Montpellier, France; the Research Institute of the McGill University and the CIUSSS‐NIM Hôpital du Sacré‐Coeur of Montréal, Montreal, QC, Canada; Charles University and General University Hospital, Prague, Czech Republic; and the Mayo Clinic, Rochester, MN, USA) at baseline and after 12 months. Five languages belonging to 3 groups of Indo‐European language families, namely Slavic (Czech), Germanic (English and German) and Romance (French and Italian), were investigated. Each of the participants was a fluent speaker of 1 of the included languages. Our recruitment target for baseline was 90 individuals per language, comprising 30 healthy controls, 30 individuals with iRBD, and 30 individuals with early‐stage PD. We matched the groups by monitoring the group means for age and sex across each individual language as recruitment progressed. The baseline data were collected between May 2017 and March 2019, and follow‐up data were gathered between April 2018 and February 2020.
Patients with iRBD were diagnosed according to the diagnostic criteria of the third edition of the International Classification of Sleep Disorders, including videopolysomnography. 20 The inclusion criteria for iRBD were as follows: (1) onset of rapid eye movement sleep behavior disorder (RBD) after 50 years of age; (2) no history of communication disorders (ie, problems in speech comprehension or expression) that would interfere significantly with the recording protocol, or other significant neurological disorders; and (3) no history of therapy with antiparkinsonian medication. The exclusion criterion was RBD onset within 12 months of introduction of antidepressant treatment. The PD patients were diagnosed based on the Movement Disorder Society clinical diagnostic criteria for PD. 21 All PD patients were investigated in the morning in an off‐medication (OFF) state (ie, after 12 hours without levodopa and 24 hours without dopamine agonists). The inclusion criteria for PD were as follows: (1) Hoehn & Yahr stage 1–2 in the above‐defined OFF state, (2) disease duration from diagnosis ≤5 years, (3) no motor fluctuations or dyskinesias, (4) on a stable dose of medication over the preceding 4 weeks, (5) no history of communication or significant neurological disorders other than PD, and (6) not currently involved in any speech therapy. The inclusion criterion for controls was that participants had no history of neurological disorders or communication disorders and no history of parasomnias or other sleep disorders.
The study was approved by the local responsible ethical committees on human experimentation and was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki. It was registered under reference number NCT03133611 (April 28, 2017) at https://clinicaltrials.gov/. Each participant provided written, informed consent.
Clinical Examination
The clinical evaluation of each subject included the following: (1) their personal and medical history, history of drug and substance intake, and current drug usage; (2) quantitative testing of motor and nonmotor symptoms of PD with the Movement Disorders Society‐Unified Parkinson's Disease Rating Scale, Parts II and III (MDS‐UPDRS); 22 (3) cognitive testing with the Montreal Cognitive Assessment (MoCA); 23 and (4) autonomic testing with the Scales for Outcomes in Parkinson's Disease‐Autonomic Dysfunction scale (SCOPA‐AUT). 24 All diagnoses and evaluations of clinical scales were performed by a neurologist experienced in movement disorders, and all of the scales were administered using versions that had been validated across the tested languages.
Speech Examination
Each center underwent standardized on‐site speech assessment training given by a speech specialist (J.R., M.N., or T.T.) to ensure standardization of the recording process for comparability across sites. Speech recordings were carried out in a quiet room with a low ambient noise level, using a head‐mounted condenser microphone (Shure Beta 53; Shure, Niles, IL, USA) placed approximately 5 cm from the subject's mouth. Speech signals were sampled at 48kHz with 16‐bit resolution. We recorded each subject during a single session in the presence of a trained investigator, and all participants performed 4 vocal tasks, including: (1) sustained phonation of the vowel /a/ in 1 breath for as long and as steadily as possible, (2) fast /pa/−/ta/−/ka/ syllable repetition ≥7 times per 1 breath, (3) reading a short paragraph of standardized text composed of ≥80 words (ranging from 80 to 115 words, depending on language), and (4) a monologue of approximately 90 seconds containing narration of a freely chosen fictional story. The participants each repeated all the tasks (except for the monologue) twice during their session. We chose speaking tasks that can provide most of the information necessary for the objective description and interpretation of motor speech disorders. 12 , 25
Speech Analysis
Based on the landmark work of Darley et al, 13 we selected 7 speech parameters that allow quantitative objective acoustic assessment that corresponds to the perceptual description of the main patterns of hypokinetic dysarthria. These speech parameters were also established in the findings of previous pilot studies on speech disorders in iRBD and de novo PD in Czech. 14 , 15 , 26 We limited the number of acoustic parameters included in the experiment to reduce the probability of a type II error and to reduce potential overfitting for the regression analysis. These 7 chosen acoustic parameters represented different aspects of hypokinetic dysarthria and were found to be only weakly correlated (Pearson: |r| < 0.38).
Harsh voice was assessed using the harmonics‐to‐noise ratio (HNR) via a sustained phonation paradigm. Slow sequential motion rates were assessed using the diadochokinetic rate (DDKR), and imprecise consonants were assessed using the voice onset time (VOT) via the fast syllable repetition paradigm. Monoloudness was assessed through the standard deviation of intensity contour (IntSD), monopitch by the standard deviation of pitch contour (F0SD), and articulation rate through the net speech rate (NSR) via reading passage. Prolonged pauses were assessed using the duration of pause intervals (DPI) via monologue. The reading passage was preferrable for subsequent acoustic analyses because it better reflected the standardization necessary for a multilanguage study. We obtained the prolonged pauses via the monologue, which better reflected both speech motor execution and cognitive linguistic processing. We averaged the final speech values used for the statistical analyses across 2 repetitions to provide greater speech assessment stability. The definitions and graphical demonstration of these 7 acoustic parameters are summarized in Figure 1. Comprehensive algorithmic details on individual acoustics measures have been reported previously. 27 In addition, the testing of the accuracy of algorithms for the identification of temporal intervals, pitch sequences, and glottal cycles has been thoroughly executed in previous studies. 15 , 27 , 28 All analyses were performed in MATLAB (MathWorks, Natick, MA).
FIGURE 1.
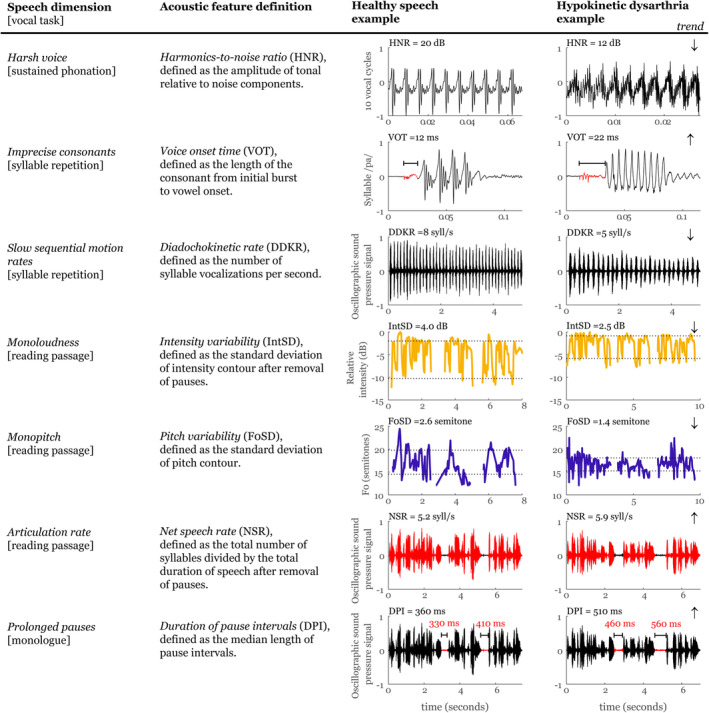
Overview of applied acoustic measurements. [Color figure can be viewed at www.annalsofneurology.org]
Primary and Supporting Endpoints
For analysis using the entire dataset consisting of all 5 languages, each of the 7 speech parameters was converted to the z‐score using the mean and standard deviation of the control group separately for each language. The 1‐sample Kolmogorov–Smirnov test did not indicate non‐normally distributed acoustic features, which allows the proper application of z‐score transformation. Therefore, results are described for all 5 languages combined. The primary endpoint was represented by the composite dysarthria index (CDI, representing the overall severity of speech impairment), which was generated by combining of the 7 acoustic speech parameters associated with hypokinetic dysarthria in PD. For those measures in which lower raw scores were associated with greater severity in speech abnormalities (ie, HNR, DDKR, IntSD, and F0SD), we reversed the z‐scores for purposes of statistical analysis; therefore, in all cases, higher z‐scores indicate more speech impairment. The directionality (ie, increase/decrease of each speech parameter) was based on the previous landmark studies using subjective evaluation, 12 , 13 and objective acoustic findings with regard to hypokinetic dysarthria in PD. 26 , 29 We estimated CDI as the mean value from the 7 calculated z‐scores. The longitudinal test–retest correlation for the CDI between baseline and follow‐up speech assessment was strong for all groups: controls (r = 0.70, p < 0.001), iRBD (r = 0.77, p < 0.001), and PD (r = 0.78, p < 0.001).
We created an additional supporting endpoint to capture elements of speech that might not be captured by the 7 acoustic parameters. This consisted of a subjective perceptual dysarthria score (PDS), which was assessed by 3 speech specialists with >10 years of experience in motor speech disorders (J.R., M.N., and T.T.) and by 3 Parkinson specialists with >10 years of experience in movement disorders (P.D., J.M., and O.U.). The perceptual assessment was performed blindly on randomized audio data from all 3 participant groups and from both the baseline and follow‐up data using vocal paradigms of vowel prolongation, diadochokinetic task, and monologue. The perceptual criteria for dysarthria outlined by Darley et al 13 were used to judge the presence and severity of speech abnormalities. The PDS was ranked as follows: 0 = normal, 1 = slight abnormal signs with ≥1 distinctive speech dimension affected, 2 = mild dysarthria, 3 = moderate dysarthria, and 4 = severe dysarthria. We estimated the inter‐rater reliability using the 2‐way mixed single score, and intraclass correlation reached a value of 0.52 for speech specialists and 0.47 for Parkinson specialists; thus, the final consensus PDS was calculated as the median value of 3 perceptual ratings.
Statistical Analysis
Given the large effect size (Cohen's f of >0.4) observed for overall severity of speech impairment between the iRBD and control groups in a pilot study, 14 and considering an error probability of α set at 0.05 and a false‐negative rate of β set at 0.2 (ie, power of 0.8) for the CDI, the power analysis indicated a recommended minimum overall sample size of 66 for 3 groups (22 × controls, 22 × iRBD, and 22 × PD) for each language (ie, 330 research subjects for 5 languages). 30
We used general least‐squares linear models to assess group differences, language differences, and their interactions; group per language interaction was used to assess whether certain languages were more or less suitable for differentiation between groups. To assess the associations between speech parameters and specific clinical changes, we calculated the partial correlation. All analyses were controlled for age, sex, and study site (covariates). We addressed multiple comparisons via the Bonferroni adjustment and determined estimates of p < 0.05 for the CDI (and the PDS), p < 0.0071 (0.05/7) for individual speech parameters, and p < 0.0125 (0.05/4) for clinical outcomes (MDS‐UPDRS parts II and III, MoCA, and SCOPA‐AUT) and their correlations with the CDI.
Informed by primary hypothesis results, we performed a binary logistic regression followed by a leave‐1‐subject‐out cross‐validation to assess the ability of a combination of acoustic features to distinguish between groups (ie, sensitivity/specificity). An overall indication of diagnostic accuracy was reported as the area under the curve (AUC), which we obtained from the receiver operating characteristic curve.
Results
Baseline: Participants
A total of 488 potential participants were screened, and 40 were subsequently excluded: 9 because they did not meet inclusion criteria, 16 owing to sex and age match‐up exclusions, 7 owing to inadequate quality of the recording, and a further 8 withdrew their consent. The final sample comprised 448 participants, including 149 controls, 150 iRBD patients, and 149 early‐stage PD patients. Participants were equally distributed between Czech, English, French, German, and Italian languages (Table 1; Fig 2).
TABLE 1.
Demographic and Clinical Data of Participants at Baseline
| Baseline Data | Group | Men | Age, | Disease Duration, | MDS‐ | MDS‐ | MoCA | SCOPA‐AUT | Antidepressant Therapy | Benzodiazepine Therapy | Presence of RBD a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| yr | yr | UPDRS | UPDRS | ||||||||
| II | III | ||||||||||
| Czech | HC | 25 | 64.5/6.7 | ‐ | 1.0/1.4 | 2.9/3.0 | 25.7/1.9 | 6.8/4.1 | 0 | 0 | 0 |
| (n = 90) | (83%) | (52–75) | ‐ | (0–5) | (0–13) | (19–29) | (1–21) | (0%) | (0%) | (0%) | |
| iRBD | 25 | 66.3/6.2 | 0.5/0.8 | 2.4/4.9 | 5.4/4.6 | 24.7/2.3 | 11.6/8.8 | 10 | 4 | 0 | |
| (83%) | (52–78) | (0–3) | (0–26) | (0–18) | (16–28) | (2–35) | (33%) | (13%) | (0%) | ||
| PD | 25 | 65.8/7.1 | 2.0/1.2 | 8.1/5.4 | 27.3/12.6 | 25.6/2.4 | 13.3/6.1 | 4 | 3 | 9 | |
| (83%) | (52–77) | (0–5) | (0–22) | (10–72) | (21–29) | (5–26) | (13%) | (10%) | (30%) | ||
| English | HC | 18 | 67.8/8.1 | ‐ | 0.6/1.4 | 0.7/1.1 | 26.5/3.1 | 6.9/5.3 | 2 | 0 | 0 |
| (n = 90) | (60%) | (52–81) | ‐ | (0–7) | (0–4) | (18–30) | (0–22) | (7%) | (0%) | (0%) | |
| iRBD | 21 | 68.3/6.5 | 2.4/2.5 | 1.6/3.3 | 3.1/3.8 | 26.5/2.9 | 11.1/5.7 | 2 | 3 | 0 | |
| (70%) | (56–81) | (0–9) | (0–18) | (0–15) | (14–30) | (1–25) | (7%) | (10%) | (0%) | ||
| PD | 21 | 69.2/5.5 | 2.4/1.3 | 8.2/5.0 | 19.6/12.3 | 25.7/3.2 | 10.6/5.6 | 0 | 1 | 3 | |
| (70%) | (57–85) | (0–5) | (1–20) | (3–56) | (21–30) | (1–23) | (0%) | (3%) | (10%) | ||
| German | HC | 21 | 69.7/8.3 | ‐ | 0.6/1.2 | 1.0/2.1 | 27.0/2.4 | 7.2/5.5 | 3 | 2 | 0 |
| (n = 88) | (72%) | (50–82) | ‐ | (0–5) | (0–10) | (22–30) | (0–24) | (10%) | (7%) | (0%) | |
| iRBD | 25 | 69.4/7.0 | 3.4/3.7 | 1.9/2.8 | 3.7/3.2 | 26.4/3.6 | 12.7/7.9 | 6 | 13 | 0 | |
| (83%) | (58–85) | (0–12) | (0–13) | (0–11) | (15–30) | (0–33) | (20%) | (43%) | (0%) | ||
| PD | 21 | 66.5/7.0 | 1.7/1.6 | 3.3/2.3 | 16.6/7.8 | 28.0/1.7 | 10.2/6.1 | 7 | 4 | 3 | |
| (72%) | (52–79) | (0–5) | (0–9) | (6–41) | (24–30) | (2–26) | (23%) | (13%) | (10%) | ||
| French | HC | 24 | 69.0/6.6 | ‐ | 0.7/1.2 | 2.2/2.6 | 27.7/1.7 | 10.3/8.7 | 3 | 0 | 0 |
| (n = 90) | (80%) | (53–80) | ‐ | (0–5) | (0–10) | (23–30) | (1–35) | (10%) | (0%) | (0%) | |
| iRBD | 25 | 68.2/6.9 | 3.0/2.6 | 1.8/2.2 | 4.2/3.6 | 26.3/2.4 | 10.9/5.4 | 8 | 4 | 0 | |
| (83%) | (53–85) | (0–12) | (0–8) | (0–11) | (19–30) | (3–30) | (27%) | (13%) | (0%) | ||
| PD | 23 | 66.0/7.9 | 1.7/1.4 | 7.8/4.9 | 20.8/9.5 | 25.8/3.2 | 13.7/7.8 | 2 | 0 | 5 | |
| (77%) | (52–78) | (0–5) | (2–19) | (8–43) | (16–30) | (1–30) | (7%) | (0%) | (17%) | ||
| Italian | HC | 20 | 70.7/9.7 | ‐ | 0.2/0.5 | 0.3/1.0 | 24.4/2.4 | 2.9/4.8 | 0 | 2 | 0 |
| (n = 90) | (67%) | (50–94) | ‐ | (0–2) | (0–4) | (21–30) | (0–18) | (0%) | (7%) | (0%) | |
| iRBD | 23 | 70.9/6.0 | 2.1/3.7 | 0.5/1.3 | 1.9/3.3 | 22.0/4.4 | 7.9/6.8 | 6 | 14 | 0 | |
| (77%) | (54–79) | (0–13) | (0–6) | (0–13) | (12–30) | (0–25) | (20%) | (47%) | (0%) | ||
| PD | 20 | 69.7/8.1 | 2.5/2.0 | 10.5/7.8 | 22.4/14.0 | 22.0/4.0 | 11.6/9.2 | 6 | 7 | 6 | |
| (67%) | (50–83) | (0–5) | (1–33) | (3–46) | (14–29) | (1–32) | (20%) | (23%) | (20%) | ||
| All languages | HC | 108 | 68.3/8.1 | ‐ | 0.6/1.2 | 1.4/2.3 | 26.3/2.6 | 6.8/6.3 | 8 | 4 | 0 |
| (n = 448) | (73%) | (50–94) | ‐ | (0–7) | (0–13) | (18–30) | (0–35) | (5%) | (3%) | (0%) | |
| iRBD | 119 | 68.6/6.6 | 2.3/3.0 | 1.6/3.2 | 3.7/3.8 | 25.2/3.6 | 10.8/7.1 | 32 | 38 | 0 | |
| (79%) | (52–85) | (0–13) | (0–26) | (0–18) | (12–30) | (0–35) | (21%) | (25%) | (0%) | ||
| PD | 110 | 67.4/7.3 | 2.1/1.5 | 7.5/5.7 | 21.4/11.8 | 25.4/3.6 | 11.9/7.1 | 19 | 15 | 26 | |
| (74%) | (50–85) | (0–5) | (0–33) | (3–72) | (14–30) | (1–32) | (13%) | (10%) | (17%) | ||
| All languages | HC | 88 | 68.0/8.1 | ‐ | 0.71/1.3 | 1.6/2.4 | 26.5/2.6 | 7.3/6.4 | 7 | 2 | 0 |
| contributing | (75%) | (50–89) | ‐ | (0–7) | (0–13) | (18–30) | (0–35) | (6%) | (2%) | (0%) | |
| data at | iRBD | 103 | 68.7/6.7 | 2.3/3.0 | 1.8/3.4 | 3.7/3.7 | 25.7/3.1 | 10.8/6.9 | 24 | 30 | 0 |
| 12‐mo | (83%) | (53–85) | (0–13) | (0–26) | (0–18) | (12–30) | (0–35) | (19%) | (24%) | (0%) | |
| follow‐up | PD | 85 | 66.9/7.3 | 2.0/1.4 | 7.1/5.5 | 20.9/11.5 | 25.9/3.0 | 11.5/7.0 | 14 | 11 | 21 |
| (n = 350) | (78%) | (51–85) | (0–5) | (0–33) | (3–72) | (16–30) | (1–32) | (13%) | (10%) | (19%) |
Data are the mean/SD (range) or the number (%).
Presence of RBD was diagnosed by videopolysomnography.
HC = healthy controls; iRBD = idiopathic rapid eye movement sleep behavior; MDS‐UPDRS = Movement Disorder Society Unified Parkinson's Disease Rating Scale; MoCA = Montreal Cognitive Assessment; PD = Parkinson disease; SCOPA‐AUT = Scales for Outcomes in Parkinson's Disease – Autonomic Dysfunction.
FIGURE 2.
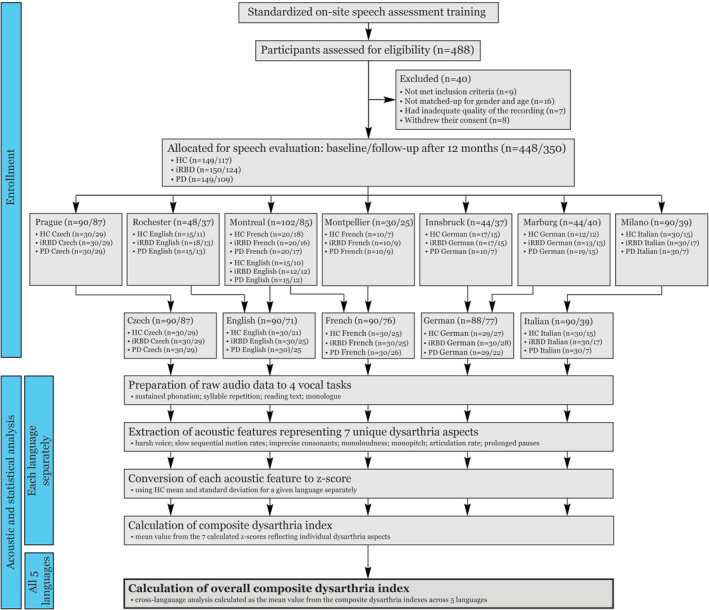
Scheme depicting enrollment of research subjects and process of acoustic and statistical analysis of speech data. HC = healthy controls; iRBD = idiopathic rapid eye movement sleep behavior disorder; PD = Parkinson disease. [Color figure can be viewed at www.annalsofneurology.org]
Baseline: Lingual Differences
No group × language interaction or effect of language was detected for the severity of speech impairment for either the acoustic (CDI) or the perceptual (PDS) analysis (Fig 3). Furthermore, no group × language interaction was seen for any individual speech dimension (Fig 4). Across all diagnostic groups, we did find an effect of language mainly for dimensions representing voice quality, consonant articulation, sequential motion rates, articulation rate (each p < 0.001), and loudness variability (p = 0.006). Increased voice hoarseness was distinctive only for the Italian language, and loudness variability differed only between Czech and English and between German and Italian. Although consonant duration varied across all investigated languages, we found that slower sequential motion rates and articulation rate were specific to the Germanic languages.
FIGURE 3.
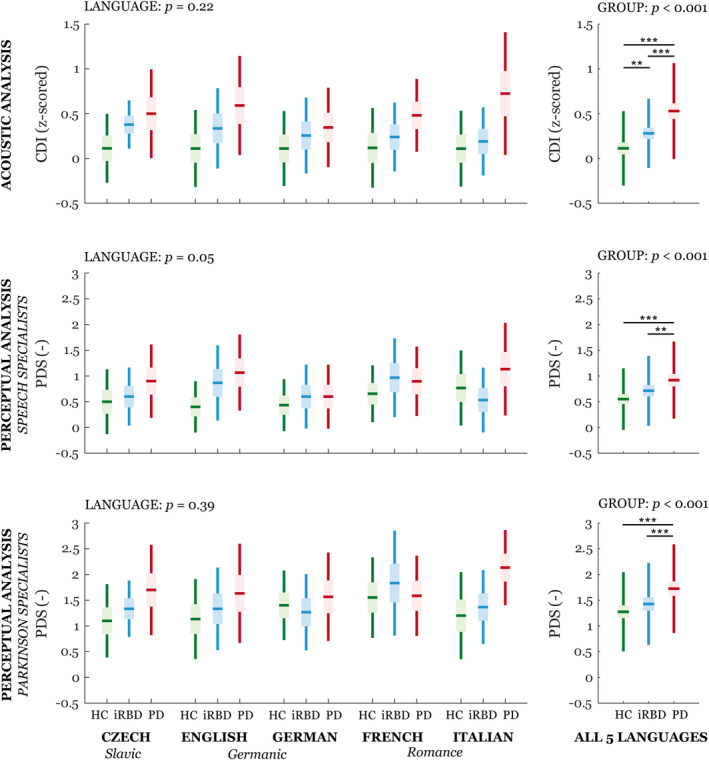
Boxplots of acoustic and perceptual severity of speech impairment across 5 languages. Horizontal lines represent the means, boxes represent 95% confidence interval, and whiskers represent the standard deviation. GROUP and LANGUAGE represent main effects; no GROUP × LANGUAGE interaction was detected. Statistically significant differences between groups or languages after Bonferroni adjustment were: *p < 0.05, **p < 0.01, ***p < 0.001. All results are adjusted for age, sex, and study site. CDI = composite dysarthria index; HC = healthy controls; iRBD = idiopathic rapid eye movement sleep behavior disorder; PD = Parkinson disease; PDS = perceptual dysarthria score. [Color figure can be viewed at www.annalsofneurology.org]
FIGURE 4.
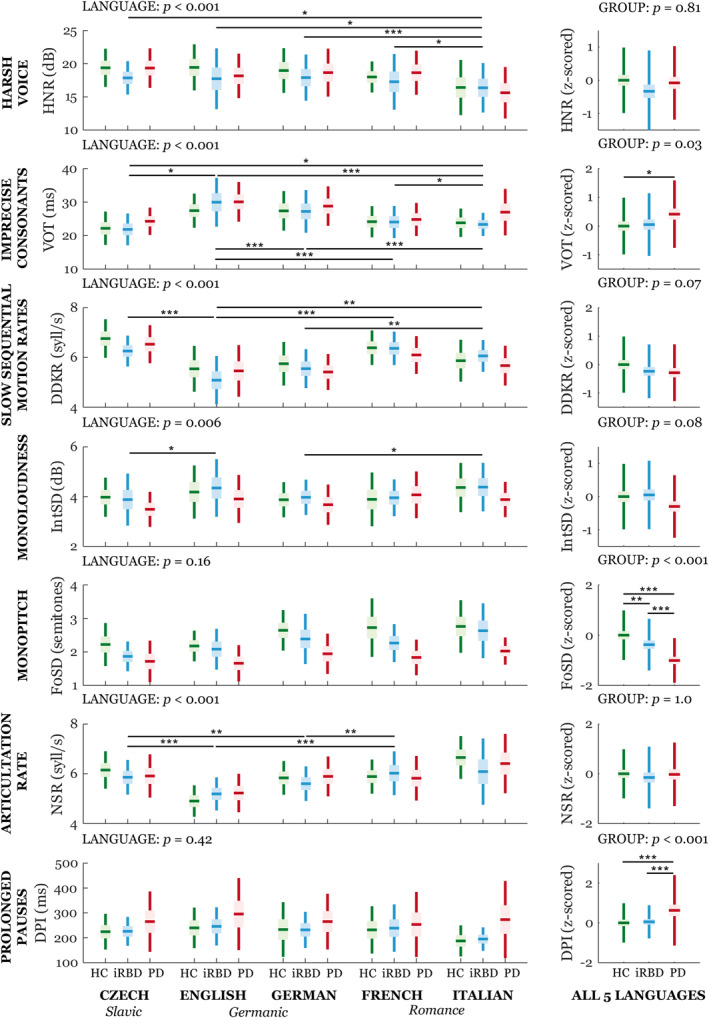
Boxplots of individual acoustic speech dimensions across 5 languages. Horizontal lines represent the means, boxes represent 95% confidence interval, and whiskers represent the standard deviation. GROUP and LANGUAGE represent main effects; no GROUP × LANGUAGE interaction was detected. Statistically significant differences between groups or languages after Bonferroni adjustment were: *p < 0.05, **p < 0.01, ***p < 0.001. All results are adjusted for age, sex, and study site. DDKR = diadochokinetic rate; DPI = duration of pause intervals; F0SD = fundamental frequency variability; HC = healthy controls; HNR = harmonics‐to‐noise ratio; IntSD = intensity variability; iRBD = idiopathic rapid eye movement sleep behavior disorder; NSR = net speech rate; PD = Parkinson disease; VOT = voice onset time. [Color figure can be viewed at www.annalsofneurology.org]
Baseline: Group Differences
Differences between diagnostic groups in overall severity of speech impairment was detected for both the acoustic and perceptual assessments (p < 0.001) (Fig 3). Differentiation between the iRBD patients and controls was possible only with the acoustic analysis (p = 0.002), whereas differences between the PD patients and controls were significant for the acoustic and both perceptual analyses (p < 0.001). We detected a relationship between acoustic and perceptual analysis of speech specialists (r = 0.51, p < 0.001) and Parkinson specialists (r = 0.46, p < 0.001) and between perceptual analyses of both expert groups (r = 0.61, p < 0.001). We did not find a correlation between the severity of speech impairment and clinical scales in PD or iRBD patients. Looking at individual speech dimensions, there were significant group differences for monopitch (p < 0.001), prolonged pauses (p < 0.001), and imprecise consonants (p = 0.03) in the PD group compared with the controls (Fig 4). Only the monopitch analysis showed significant differentiation between iRBD patients and controls (p = 0.004; see Fig 4).
Baseline: Speech Sensitivity Analysis
The acoustic speech patterns led to an overall AUC of 0.80 between PD patients and controls, an AUC of 0.65 between iRBD patients and controls, and an AUC of 0.72 between the iRBD and PD patients for all 5 languages (Fig 5). For the PD group, the French language had the highest AUC (0.84) compared with controls, but the score between the languages was generally consistent, with AUC ranging between 0.78 and 0.84. When the iRBD group was compared with the controls, the Czech language had the highest AUC (0.78) compared with the other languages, with similar AUCs, which ranged between 0.66 and 0.69. When comparing iRBD with PD, the Italian language had the highest AUC (0.83), with the AUC of the other languages ranging between 0.72 and 0.79. The acoustic features that contributed to the best sensitivity/specificity were generally consistent across languages and included monopitch, prolonged pauses, and imprecise consonants for differentiating PD patients and controls; harsh voice, monopitch, and articulation rate for differentiating iRBD patients and controls; and harsh voice, monopitch, articulation rate, and prolonged pauses for differentiating iRBD and PD. On categorical analysis using the 95th percentile of the performance by the control group, monopitch was the most prevalent feature in the PD group, whereas harsh voice was most prevalent in the iRBD group. Harsh voice was also more prevalent in the iRBD group than in PD. With regard to the individual vocal tasks, the reading passage showed the highest accuracy for discrimination, with an AUC of 0.78 between PD patients and controls and an AUC of 0.64 between iRBD and controls.
FIGURE 5.
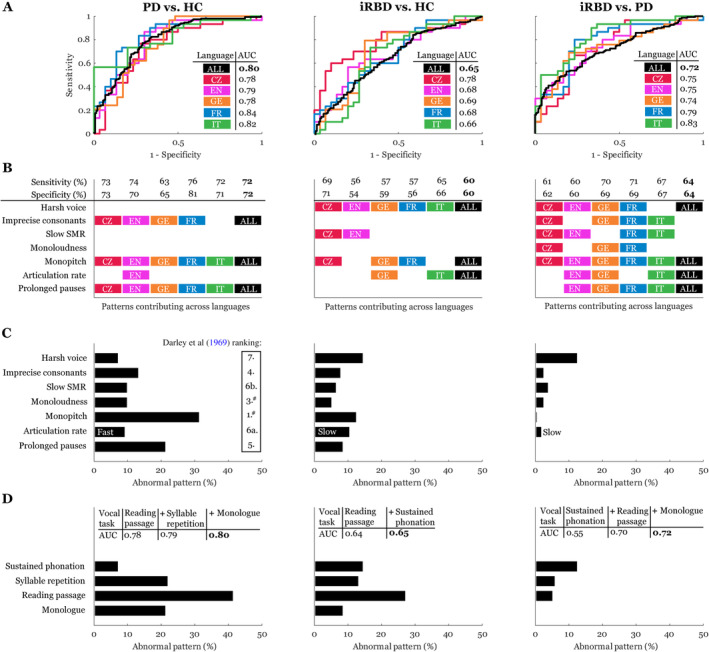
Sensitivity analysis across 5 languages. The left panels are for PD versus HC, the middle are for iRBD versus HC, and the right are for iRBD versus PD. (A) Receiver operating characteristic curves with the listed AUC. (B) Speech patterns contributing to the best sensitivity/specificity across languages. (C) Percentage of abnormal patterns according to individual dysarthric components across all 5 languages based on comparison to the 95th percentile of healthy (left and middle panels) or PD (right panel) speech performance. (D) Percentage of abnormal patterns according to individual vocal tasks across all 5 languages based on comparison to the 95th percentile of healthy (left and middle panels) or PD (right panel) speech performance. The tables list cumulative AUCs across different numbers of vocal tasks. #The second most distinctive speech dimension of reduced stress can hardly be measured by automated acoustic analysis; however, monopitch and monoloudness contribute substantially to perceived reduced stress in PD. ALL = all 5 languages; AUC = area under the curve; CZ = Czech; EN = English; FR = French; GE = German; iRBD = idiopathic rapid eye movement sleep behavior disorder; IT = Italian; PD = Parkinson disease; SMR = sequential motion rate. [Color figure can be viewed at www.annalsofneurology.org]
Follow‐Up
Given that no group × language interaction was observed for speech characteristics at baseline, we performed a follow‐up analysis across all 5 languages using the z‐score normalization based on the control mean for each respective language. A total of 350 participants (78%) completed the 12‐month follow‐up assessment (11.9 ± 1.3 [range 8–18] months). Thirty‐two of 149 (21%) controls, 26 of 150 (17%) iRBD participants, and 40 of 149 (27%) PD participants did not complete the follow‐up assessment. The lower number of completed follow‐ups did not materially alter the distribution of the variables (Table 1; Fig 2).
The only significant clinical change detected was in the MDS‐UPDRS part III for both the PD group (mean increase from baseline to follow‐up: 2.8, p < 0.001) and the iRBD group (mean increase: 1.2, p = 0.001) (Fig 6). On the summary CDI, we detected a significant progression of speech impairment in both the PD group (mean z‐score increase: 0.08, p = 0.03) and the iRBD group (mean z‐score increase: 0.06, p = 0.03), but not in controls (mean z‐score increase: 0.01, p = 0.64). We also observed a worsening of harsh voice in iRBD participants (mean HNR z‐score increase: 0.12, p = 0.04). There was no significant correlation between annual change in severity of speech impairment by acoustic analysis and change in any clinical variables.
FIGURE 6.
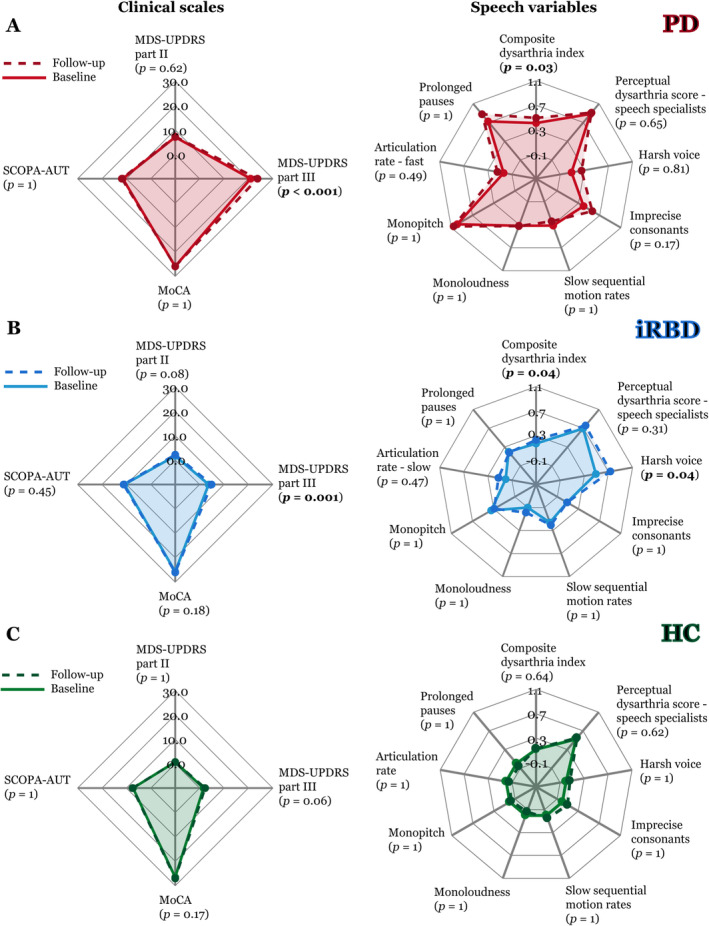
Speech progression analysis from baseline (continuous line) to follow‐up (dashed line) across all 5 languages depicted using a spider plot for: (A) PD, (B) iRBD, and (C) controls. The lines demonstrate the mean values for clinical scales (left panels) and mean z‐scored values of speech variables (right panels); a higher number indicates more vocal impairment. All p‐values between follow‐up and baseline are listed after Bonferroni adjustment. HC = healthy controls; iRBD = idiopathic rapid eye movement sleep behavior disorder; MDS‐UPDRS = Movement Disorders Society‐Unified Parkinson's Disease Rating Scale; MoCA = Montreal Cognitive Assessment; PD = Parkinson disease; SCOPA‐AUT = Scales for Outcomes in Parkinson's Disease‐Autonomic Dysfunction. [Color figure can be viewed at www.annalsofneurology.org]
Discussion
This study provides the first multicenter, multilingual standardized speech assessment of prodromal and clinical synucleinopathy, including a 12‐month follow‐up. The composite dysarthria score confirmed the presence of distinctive speech impairments in iRBD and early‐stage PD that progressed over a 12‐month interval, suggesting that our method of speech assessment could be useful as a potential biomarker of diagnosis and progression in α‐synuclein‐related diseases. Speech impairment in iRBD was detectable through objective acoustic analysis, but not through subjective perceptual analysis by experts. The acoustic approach was effective across multiple languages, which has implications for future use in multicenter clinical trials. Vocal assessment offers intriguing potential advances because it is inexpensive, noninvasive, and recordings can be made digitally, even by smartphone, 31 facilitating future scalability to a larger population. Therefore, our findings support the possibility of deploying a completely automated, objective, and validated speech signal processing approach in future clinical trials.
Differences between Languages
We did not find any lingual‐specific differences among measures. This is consistent with previous studies that revealed largely similar profiles of hypokinetic dysarthria across languages. 32 , 33 In the overall severity of speech impairment and in the majority of specific speech dimensions, the trends of speech change attributable to hypokinetic dysarthria were generally consistent across languages among iRBD patients. All languages showed similar discriminatory power between the PD and control groups, with AUCs ranging between 0.78 and 0.84. The only exception was greater speech impairment for the Czech iRBD group, who had an AUC of 0.78 between the iRBD and control groups compared with the AUC for other languages (0.66–0.69).
Among all groups (regardless of diagnosis), we found differences for voice quality, consonant articulation, and speaking rate. Interestingly, we found that increased hoarseness was evident only for the Italian cohort. The varying lingual‐dependent duration of consonants seen in the present study is well in accordance with differing voicing contrast among languages, 34 which contributes to the distinctive perceptual sound of stop consonant articulation. For example, the point of constriction on the tongue is well represented by the syllable /ta/, where /t/ is apical–dental for French, whereas the matching English consonant is apical–alveolar. 35 Such differences might also be the reason for significant between‐language variations in speaking rate, 36 which could be particularly responsible for the slower oral diadochokinesis and articulation rate we observed in the Germanic language speakers. These findings might have implications for potential future clinical trials, in which cross‐language analysis to increase sample size should be performed carefully, ideally by normalizing individual performance to a set norm that is based on healthy speech performance within specific languages.
Speech Biomarkers
Given that this is the largest study ever performed that looks specifically at speech disorder in iRBD and early‐stage PD, it is likely to provide the most precise estimates of early patterns of hypokinetic dysarthria. In early‐stage PD, the most highly affected dimensions of speech were monopitch, prolonged pauses, and imprecise consonants, which, for this cohort, falls well within the 5 most distinctive aspects of parkinsonian dysarthria originally reported in the perception‐based landmark study by Darley et al. 13 Although monopitch appears to be an early and consistent sign of hypokinetic dysarthria that becomes evident in the prodromal stages (ie, iRBD), changes of loudness and the subjective perception of hypokinetic dysarthria may develop later, in parallel with continuous PD progression. The lack of a relationship between the severity of dysarthria and general motor performance supports the assumption that alterations of voice and speech in PD might be attributable, at least in part, to nondopaminergic mechanisms. 37 This also implies that motor and speech performance could each be used as nonoverlapping/complementary markers of disease severity.
Other than monopitch, it is notable that the 2 features that best distinguished the iRBD and control groups (ie, harsh voice and slow rate) were not significantly different between early‐stage PD and control groups. Harsh voice occurred more frequently in iRBD than in PD. The further decline of voice quality from baseline to follow‐up was the main factor contributing to the progression of speech impairment in iRBD. Noting that the majority (ie, 83%) of our PD patients did not have RBD, this might suggest that patients with iRBD may present with a speech phenotype distinct from PD. The presence of RBD within PD represents a subtype of disease, with more akinetic–rigid disease, gait dysfunction, autonomic dysfunction, and cognitive impairment. 38 , 39 , 40 Other research has suggested similar results; a recent smartphone‐based study found that decreased voice quality was the most distinguishing factor between the iRBD and control groups, but not between the PD and control groups. 41 Another reason for the similarity of voice quality in PD patients and controls might be that hoarseness in PD patients responds to dopaminergic therapy, whereas other features do not. 42 Interestingly, although we did not detect any significant differences in articulation rate between iRBD and controls at baseline, slow articulation rate is typically a speech change in mild cognitive impairment 43 and has been reported in dementia with Lewy bodies. 44 Therefore, the tendency toward slower articulation rate in our iRBD patients might reflect a greater degree of cognitive impairment, with a higher risk of dementia.
Strengths and Limitations
Based on high‐end pattern automated acoustic speech analysis, we systematized characteristic perceptual vocal changes in PD. 13 In particular, we defined 7 robust speech features with well‐defined pathophysiology that are both distinctive for hypokinetic dysarthria and only minimally related to aging. The solution offered here does not require large sample sizes in order to train the model for a specific language of interest, and therefore, it is ready to be used as a potential quantitative digital biomarker of synucleinopathies in clinical trials. Currently, the fully automated system used in this study remains under development, but the free beta version is already available. 45 The 7 primary speech features can also be analyzed using widely used, freely available Praat software, 46 although hand labelling or additional user control of the analysis might be required for some features. We should point out, however, that use of different analytic approaches or algorithms to quantify these speech features (for instance, harsh voice or monoloudness) might result in different interferences.
Our aim was not to train the model to reach the best classification accuracy for distinguishing between PD or iRBD and controls. Instead, we strived to provide robust and reliable estimates of cross‐language speech patterns based on a simple but consistent linear separation of the data. Based on the 7 features we investigated here, the AUC of 0.80 that we detected for our early‐stage PD patients appears to be reasonable, especially given the mild severity of changes on our subjective ratings (ie, a mean perceptual score of 0.9). Furthermore, this value seems to be compatible with a perceptual study reporting that 89% of 200 PD patients with varying disease severity experienced voice disorders. 47 However, we believe the accuracy of detection could be improved substantially by increasing the number of speech features and the use of a more robust classifier with the nonlinear kernel. A recent study revealed that motor markers evaluated through smartphone were highly effective in discriminating PD and healthy control populations, with mean sensitivity and specificity values of ≤88% based on 998 features extracted. 41 The disadvantage, however, of the very many features is the potential for overfitting, and for lack of standardization when using multiple tasks across multiple centers. This might argue for the use of a standardized reading passage if a simplified data collection protocol for future multicenter multilanguage clinical trials is required.
Given that the longitudinal analysis of changes in speech features is based on data from only 1 follow‐up visit, the inferences drawn from longitudinal analysis are rather limited. Future research to elaborate which speech and voice features will best predict phenoconversion from iRBD to established parkinsonism or dementia is warranted. Given that speech disturbances could have utility as outcome measures in a future neuroprotective drug trial, we chose initially to focus on the Indo‐European languages most frequently used in countries where drug trials for registration and approval by the United States Food and Drug Administration and/or European Medicine Agency are conducted. However, the evidence we have generated supports the portability and adaptability of our methods to other language families. A previous study explored hypokinetic dysarthria characteristics in a tonal Chinese language with Cantonese dialect based on the criteria defined by Darley and colleagues 13 (ie, the same criteria used as a foundation for the present study); the perceptual profile for Cantonese was largely similar to profiles for English PD patients, with the most severely affected dimensions of harsh voice, monopitch, monoloudness, and imprecise consonants. 48 In addition, the acoustic features applied in the present study have previously been used successfully to describe dysarthria attributable to drug‐induced parkinsonism in the Georgian language, which belongs to the Kartvelian language family. 49
In conclusion, this study has revealed that speech parameters appear to be a potential biomarker of early PD. We have identified clinical speech endpoints that are sensitive both to the prodromal disease stage and to disease progression over 12 months in iRBD and early‐stage PD. The results of our automated speech analyses obtained from 7 clinical sites and 5 different languages support the addition of automated speech assessment to the batteries of biomarkers currently used in clinical trials.
Author Contributions
J.R., J.H., M.N., T.T., Y.D., B.H., W.O., E.K.St.L., L.F.‐S., E.R., R.B.P., and K.Š. contributed to the conception and design of the study; all authors contributed to the acquisition and analysis of the data; J.R. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Acknowledgments
This study was supported by the Michael J. Fox Foundation for Parkinson's Research (grant 12546) and the Czech Health Research Council (NV19‐04‐00120 and NU20‐08‐00445, J.R., M.N., T.T., and P.D.). The Montreal cohort is supported by the Canadian Institutes of Health Research. J.‐F.G. holds a Canada Research Chair in Cognitive Decline in Pathological Aging. W.O. is Hertie‐Senior‐Research Professor, supported by the Charitable Hertie Foundation, Frankfurt/Main, Germany. Recruitment and phenotyping of subjects with REM Sleep behavior disorder (RBD) was supported by the ParkinsonFonds Deutschland (W.O. and A.J.). Research support for the Mayo Clinic REM Sleep Behavior Disorder Patient Registry was provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (1 UL1 RR024150‐01, E.K.S.) and from NIH/NIA (R34AG056639 [NAPS], E.K.S.). No funding source had any role in the writing of the manuscript or the decision to submit it for publication. No authors have been paid to write this article by a pharmaceutical company or other agency.
We are obliged to Parkinson specialists Jana Mašková and Olga Ulmanová for perceptual rankings of speech data.
References
- 1. Dawson VL, Dawson TM. Promising disease‐modifying therapies for Parkinson's disease. Sci Transl Med 2019;11:eaba1659. [DOI] [PubMed] [Google Scholar]
- 2. Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16‐year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 3. Postuma RB, Gagnon J‐F, Bertrand J‐A, et al. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Videnovic A, Ju YS, Arnulf I, et al. Clinical trials in REM sleep behavioural disorder: challenges and opportunities. J Neurol Neurosurg Psychiatry 2020;91:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid‐eye‐movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. [DOI] [PubMed] [Google Scholar]
- 6. Schenck CH, Montplaisir JY, Frausher B, et al. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy: a consensus statement from the international rapid eye movement sleep behavior disorder study group. Sleep Med 2013;14:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dauvilliers Y, Schenck CH, Postuma RB, et al. REM sleep behaviour disorder. Nat Rev Dis Primers 2018;4:19. [DOI] [PubMed] [Google Scholar]
- 8. Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration ‐ an update. Nat Rev Neurol 2018;14:40–55. [DOI] [PubMed] [Google Scholar]
- 9. Kogan RV, Janzen A, Meles SK, et al. Four‐year follow‐up of [18F]Fluorodeoxyglucose positron emission tomography–based Parkinson's disease–related pattern expression in 20 patients with isolated rapid eye movement sleep behavior disorder shows prodromal progression. Mov Disord 2021;36:230–235. 10.1002/mds.28260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fereshtehnejad S‐M, Yao C, Pelletier A, et al. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain 2019;142:2051–2067. [DOI] [PubMed] [Google Scholar]
- 11. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffy JR. Motor speech disorders: substrates, differential diagnosis and management. 3rd ed. St. Louis, MO: Mosby, 2013. [Google Scholar]
- 13. Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res 1969;12:246–269. [DOI] [PubMed] [Google Scholar]
- 14. Rusz J, Hlavnicka J, Tykalova T, et al. Quantitative assessment of motor speech abnormalities in idiopathic REM sleep behaviour disorder. Sleep Med 2016;19:141–147. [DOI] [PubMed] [Google Scholar]
- 15. Hlavnicka J, Cmejla R, Tykalova T, et al. Automated analysis of connected speech reveals early biomarkers of Parkinson's disease in patients with rapid eye movement sleep behaviour disorder. Sci Rep 2017;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Postuma RB, Lang AE, Gagnon JF, et al. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860–1870. [DOI] [PubMed] [Google Scholar]
- 17. Haspelmath MDM, Gil D, Comrie B. The world atlas of language structures. Oxford: Oxford University Press, 2005. [Google Scholar]
- 18. Brabenec L, Mekyska J, Galaz Z, Rektorova I. Speech disorders in Parkinson's disease: early diagnostics and effects of medication and brain stimulation. J Neural Transm (Vienna) 2017;124:303–334. [DOI] [PubMed] [Google Scholar]
- 19. Stegmann GM, Hahn S, Liss J, et al. Repeatability of commonly used speech and language features for clinical applications. Digit Biomark 2020;4:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Academy of Sleep Medicine . International classification of sleep disorders, third edition: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 21. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 22. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement disorder society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 24. Visser M, Marinus J, Stiggelbout A, Van Hilten JJV. Assessment of autonomic dysfuntion in Parkinson's disease: the SCOPA‐AUT. Mov Disord 2004;19:1306–1312. [DOI] [PubMed] [Google Scholar]
- 25. Rusz J, Tykalova T, Ramig LO, Tripoliti E. Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Mov Disord 2021;36:803–814. 10.1002/mds.28465. [DOI] [PubMed] [Google Scholar]
- 26. Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson's disease. J Acoust Soc Am 2011;129:350–637. [DOI] [PubMed] [Google Scholar]
- 27. Hlavnicka J. Automated analysis of speech disorders in neurodegenerative diseases. PhD thesis. Faculty of Electrical Engineering, Czech Technical University, Prague, Czechia, 2018.
- 28. Hlavnicka J, Cmejla R, Klempir J, et al. Acoustic tracking of pitch, modal and subharmonic vibrations of vocal folds in Parkinson's disease and Parkinsonism. IEEE Access 2019;7:150339–150354. [Google Scholar]
- 29. Tykalova T, Rusz J, Klempir J, et al. Distinct patterns of consonant articulation among Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Brain Lang 2017;165:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 31. Rusz J, Hlavnicka J, Tykalova T, et al. Smartphone allows capture of speech abnormalities associated with high risk of developing Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng 2018;26:1495–1507. [DOI] [PubMed] [Google Scholar]
- 32. Whitehill TL. Studies of Chinese speakers with dysarthria: informing theoretical models. Folia Phoniatr Logop 2010;62:92–96. [DOI] [PubMed] [Google Scholar]
- 33. Orozco‐Arroyave JR, Honig F, Arias‐Londono JD, et al. Automatic detection of Parkinson's disease in running speech spoken in three different languages. J Acoust Soc Am 2016;139:481–500. [DOI] [PubMed] [Google Scholar]
- 34. Cho T, Whalen DH, Docherty G. Voice onset time and beyond: exploring laryngeal contrast in 19 languages. J Phon 2019;72:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dart SN. Comparing French and English coronal consonant articulation. J Phon 1998;26:71–94. [Google Scholar]
- 36. Icht M, Ben‐David B. Oral‐diadochokinesis rates across languages: english and Hebrew norms. J Commun Disord 2014;48:27–37. [DOI] [PubMed] [Google Scholar]
- 37. Skodda S, Gronheit W, Mancinelli N, Schlegel U. Progression of voice and speech impairment in the course of Parkinson's disease: a longitudinal study. Parkinsons Dis 2013;2013:389195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romenets SR, Gagnon JF, Latreille V, et al. Rapid eye movement sleep behaviour disorder and subtypes of Parkinson's disease. Mov Disord 2012;27:996–1003. [DOI] [PubMed] [Google Scholar]
- 39. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain 2017;140:1959–1976. [DOI] [PubMed] [Google Scholar]
- 40. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson's disease. Sleep 2017;40:zsx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arora S, Baig F, Lo C, et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 2018;91:e1528–e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rusz J, Cmejla R, Ruzickova H, et al. Evaluation of speech impairment in early stages of Parkinson's disease: a prospective study with the role of pharmacotherapy. J Neural Transm 2013;120:319–329. [DOI] [PubMed] [Google Scholar]
- 43. De Looze C, Kelly F, Crosby L, et al. Changes in speech chunking in Reading aloud is a marker of mild cognitive impairment and mild‐to‐moderate Alzheimer's disease. Curr Alzheimer Res 2018;15:828–847. [DOI] [PubMed] [Google Scholar]
- 44. Ash S, McMillan C, Gross RG, et al. Impairments of speech fluency in Lewy body spectrum disorder. Brain Lang 2012;120:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hlavnicka J, Ruzickova H, Tykalova T, et al. Dysarthria analyzer [computer program]. Beta version, 2021. Available at: http://www.dysan.cz/.
- 46. Boersma P, Weenink D. Praat: doing phonetics by computer [Computer program]. Version 6.1.32, 2020. Available at: http://www.praat.org/.
- 47. Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and coocurence of vocal tract dysfunction in the speech of a large sample of Parkinson patients. J Speech Hear Disord 1978;43:47–57. [DOI] [PubMed] [Google Scholar]
- 48. Whitehill TL, Ma JK, Lee AS. Perceptual characteristics of Cantonese hypokinetic dysarthria. Clin Linguist Phon 2003;17:265–271. [DOI] [PubMed] [Google Scholar]
- 49. Rusz J, Megrelishvili M, Bonnet C, et al. A distinct variant of mixed dysarthria reflects parkinsonism and dystonia due to ephedrone abuse. J Neural Transm 2014;121:655–664. [DOI] [PubMed] [Google Scholar]


