Abstract
G protein‐coupled receptors (GPCRs) are a large class of cell‐surface receptor involved in cellular signaling that are currently the target of over one third of all clinically approved therapeutics. Classically, an agonist‐bound, active GPCR couples to and activates G proteins through the receptor intracellular core. To attenuate G protein signaling, the GPCR is phosphorylated at its C‐terminal tail and/or relevant intracellular loops, allowing for the recruitment of β‐arrestins (βarrs). βarrs then couple to the receptor intracellular core in order to mediate receptor desensitization and internalization. However, our laboratory and others have observed that some GPCRs are capable of continuously signaling through G protein even after internalization. This mode of sustained signaling stands in contrast with our previous understanding of GPCR signaling, and its molecular mechanism is still not well understood. Recently, we have solved the structure of a GPCR–G protein–βarr megacomplex by cryo‐electron microscopy. This ‘megaplex’ structure illustrates the independent and simultaneous coupling of a G protein to the receptor intracellular core, and binding of a βarr to a phosphorylated receptor C‐terminal tail, with all three components maintaining their respective canonically active conformations. The structure provides evidence for the ability of a GPCR to activate G protein even while being bound to and internalized by βarr. It also reveals that the binding of G protein and βarr to the same GPCR is not mutually exclusive, and raises a number of future questions to be answered regarding the mechanism of sustained signaling.
Keywords: cryo‐electron microscopy, G protein, G protein‐coupled receptors, structure, sustained endosomal signaling, β‐arrestin
A cryo‐electron microscopy structure of a G protein‐coupled receptor (GPCR)–G protein–beta‐arrestin megacomplex reveals simultaneous engagement of two transducer to the same GPCR and provides a potential mechanism for endosomal G protein signaling.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- GPCRs
G protein‐coupled receptors
- GRK
G protein‐coupled receptor kinase
- ICL
intracellular loop
- Nb32
nanobody 32
- Nb35
nanobody 35
- TM
transmembrane helix
- V2R
vasopressin receptor type 2
- V2T
V2R tail
- β2AR
β2 adrenergic receptor
- βarr
beta‐arrestin
Introduction
G protein‐coupled receptors (GPCRs), also known as seven transmembrane receptors, are the largest family of cell‐surface receptors involved in the signaling and regulation of many physiological processes [1, 2, 3]. They are also highly dynamic proteins capable of sampling a number of inactive and active conformations at basal conditions [4]. GPCRs are capable of binding an array of ligands, from small molecules to polypeptides, which shifts their conformational equilibria toward active states. The shift toward active receptor conformations favors the interaction of signal transducers such as heterotrimeric G proteins (Gαβγ) to the intracellular core of the receptor (Fig. 1) [4]. Upon coupling to the receptor, a guanosine diphosphate‐bound G protein heterotrimer undergoes nucleotide exchange to GTP, causing activation and dissociation of the GTP‐bound Gα subunit from the Gβγ subunit (Fig. 1) [1, 4]. G protein activation allows for the Gα subunit to interact with enzymes such as adenylyl cyclase, spurring the generation of second messenger molecules such as cyclic AMP (cAMP) (Fig. 1). These second messenger molecules continue a cascade that eventually leads to physiological responses.
Fig 1.
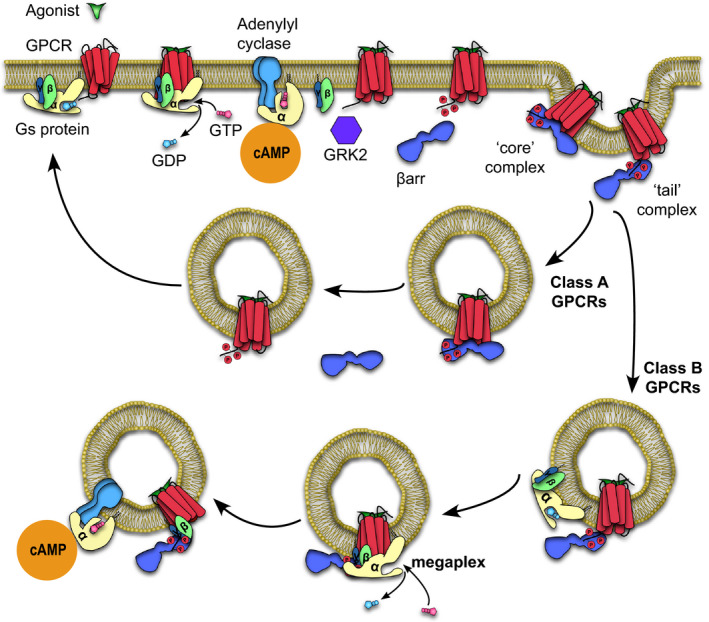
Pathway detailing the activation and interaction of a GPCR with transducers, leading to sustained signaling within endosomes through the formation of megaplexes.
To prevent overstimulation, a GPCR kinase (GRK) phosphorylates serine and threonine residues on the receptor, most often within its C‐terminal tail or intracellular loop (ICL; Fig. 1) [2, 3]. This facilitates binding of the adapter protein beta‐arrestin (βarr), which competes with G protein to bind to the receptor intracellular core, thus blocking further G protein coupling and attenuating G protein signaling [5, 6]. Subsequently, through scaffolding of endocytic proteins such as adapter protein 2 and clathrin, βarr initiates the internalization of the receptor‐βarr complex (Fig. 1) [3, 7]. After internalization, the GPCR–βarr complex is either (a) quickly recycled back to the plasma membrane for GPCRs that interact transiently with βarr (class A GPCRs) or (b) remains internalized in endosomes and is subsequently degraded, for GPCRs that interact strongly with βarr (class B GPCRs; Fig. 1) [8, 9, 10]. Notably, the abundance of serine/threonine clusters within a class B GPCR C‐terminal tail or relevant ICL allows for a stronger interaction with βarr, whereas class A GPCRs have less abundant serine/threonine clusters in these regions, leading to a more transient GPCR–βarr interaction [8, 9]. βarr is a signaling molecule in its own right, capable of activating molecular pathways that are distinct from those associated with G protein, most notably through scaffolding of numerous mitogen‐activated protein kinase cascades [2, 3].
For many decades, this ‘classical’ view of GPCR signaling implies that (a) G protein signaling occur primarily at the plasma membrane and (b) βarr and G protein binding to a receptor is mutually exclusive. To add another layer of complexity, a number of GPCRs have been reported to engage in sustained G protein signaling even after receptor internalization [11, 12, 13, 14]. This is inconsistent with our current understanding of desensitization, which proposes that βarr coupling to a receptor blocks further G protein coupling, therefore attenuating further G protein signaling. Through real‐time cellular cAMP measurements within cells, we and others further report that some GPCRs are indeed capable of continuously producing second messenger molecules in a sustained fashion even after receptor internalization into endosomes. More specifically, recent reports indicate that this sustained phase of cAMP production is enhanced by βarr [13, 15]. This view starkly contrasts with the abovementioned ‘classical’ view, which indicates that βarr primarily acts as a desensitizer of G protein signaling as well as an independent signaling molecule [2, 5, 6].
Recently, we have shown that βarr can adopt two overall conformations when bound to a GPCR: (a) a ‘tail’ conformation whereby the βarr only attaches to the receptor phosphorylated tail and (b) a ‘core’ conformation whereby βarr additionally engages the receptor intracellular core via its finger loop (Fig. 1) [16]. Additionally, we and others have also shown that a βarr in the tail conformation is fully capable of performing its canonical functions (i.e., signaling and receptor internalization), with the exception of desensitization of G protein signaling, which is performed exclusively by the receptor core‐engaged βarr [17, 18]. Taken together, we posit that the receptor core would still be unoccupied in the tail conformation of a GPCR–βarr complex and thus could additionally accommodate the coupling of a G protein, forming a GPCR–G protein–βarr megacomplex (Fig. 1). Such a ‘megaplex’ would provide a biophysical explanation for (a) the ability of an internalized GPCR–βarr complex in the tail conformation to continue to signal from within endosomes, (b) the ability of a receptor to signal in a sustained fashion which is somehow enhanced by the presence of βarr, and (c) the fact that a large majority of receptors that have been observed to signal from within endosomes are class B GPCRs [12, 13, 15, 19, 20].
Using bioluminescent resonance energy transfer (BRET), cellular imaging, and in vitro assays, we have recently shown that a GPCR–Gs protein–βarr megaplex does indeed form with the prototypical class B vasopressin receptor type 2 (V2R), as well as the β2V2R, a chimeric GPCR with the first 341 residues of the β2 adrenergic receptor (β2AR) combined with the last 29 amino acids of the V2R C‐terminal tail (V2T) [21]. The β2V2R maintains the pharmacological properties of the wild‐type β2AR and interacts strongly with βarrs and signals comparably to the class B V2R. We additionally showed that a G protein within a megaplex could be activated and could still undergo nucleotide exchange in vitro [21]. However, the structure of such a megaplex was still unknown, and thus raised a number of questions: (a) Are the conformations of each megaplex component (i.e., the GPCR, G protein, and βarr) different from their canonically active ones and (b) are there additional contacts between βarr and G protein that were previously unappreciated? To answer these questions, and to obtain higher resolution information regarding the architecture of the megaplex, we sought to obtain its structure using cryo‐electron microscopy.
Megaplex purification and structure solution
In order to form a stable megaplex in vitro amenable to structural determination, we sought to first form the β2V2R–βarr complex. Initially, we coexpress the β2V2R, βarr1, and a prenylated GRK2 (GRK2‐CAAX) in Spodoptera frugiperda 9 (sf9) cells (Fig. 2) [16]. Upon stimulation of the β2V2R by the high‐affinity β2AR agonist BI‐167107 (BI), GRK2‐CAAX facilitates the phosphorylation of the V2T, leading to recruitment of βarr1. As the cells are lysed, a conformation‐specific antibody fragment, Fab30, is added to stabilize the β2V2R–βarr1 complex (Fig. 2). Fab30 binds specifically to an active βarr1 bound to the phosphorylated V2T and has been previously shown to be crucial in stabilizing the β2V2R–βarr1 complex [16, 22]. The stabilized complex is then solubilized by the detergent lauryl maltose neopentyl glycol (LMNG), after which it is additionally purified by coimmunoprecipitation of a Flag‐tag on the β2V2R and subjected to size exclusion chromatography [16]. Finally, the heterotrimeric Gs protein and two stabilizing nanobodies, nanobody 32 (Nb32) and nanobody 35 (Nb35), are added to the purified β2V2R–βarr1 complex, followed by an additional round of Flag coimmunoprecipitation, to arrive at a stabilized β2V2R–Gs protein–βarr1 megaplex (Fig. 2).
Fig 2.
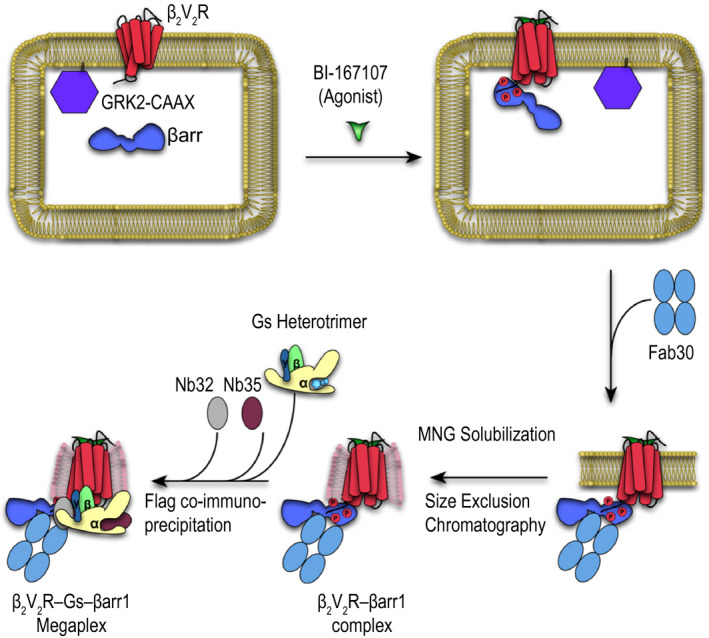
In vitro formation and purification of the megaplex.
The entire complex could not be refined past 7 Å, owing to the relative flexibility of megaplex components [23]. Further analysis of this low‐resolution map reveals that the Nb35‐stabilized Gs heterotrimer indeed couples to the intracellular core of the β2V2R, with the βarr1 binding to the flexible V2T (Fig. 3A and center). As the Fab30 and Nb32 bind to this flexible V2T, a significant portion of this complex is flexible compared with the β2V2R–Gs protein–Nb32 portion. To circumvent this flexibility, we computationally analyzed the β2V2R–Gs protein–Nb32 and Nb32–V2T– βarr1–Fab30 subcomplexes separately, which led to two separate reconstructions at 3.8 and 4.0 Å, respectively [23]. We then aligned these two reconstructions to the overall complex reconstruction, allowing us to analyze all relevant megaplex interactions (Fig. 3A and center).
Fig 3.
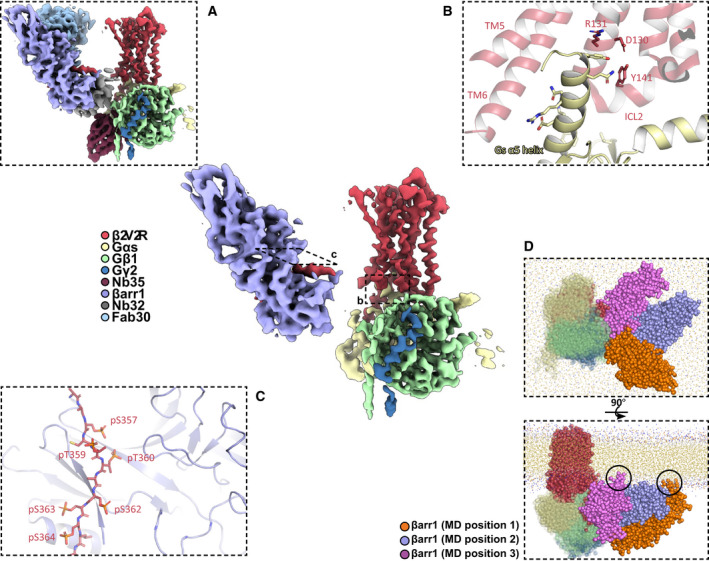
(center) Structure of the β2V2R–Gs protein–βarr1 megaplex with all stabilizing protein components removed. (A) Same as in center, but with all stabilizing proteins shown. (B) Interaction between the Gs α5 helix of Gs protein and the β2V2R. Critical receptor residues within the DRY motif and ICL2 are labeled. (C) Binding interface between the phosphorylated β2V2R tail (V2T) and βarr1, with phosphorylated V2T residues labeled. (D) Orthogonal views of the final frame of a coarse‐grained molecular dynamics simulation of a megaplex with three coarse‐grained models that each differ in their βarr1 position relative to the β2V2R–Gs protein portion of the megaplex. Circles indicate contacts observed between the βarr1 C‐edge loops with the membrane.
Architecture of the megaplex
The receptor in the megaplex adopts a canonically active conformation, and the Gs protein engages the intracellular core of the β2V2R in a similar manner to the previously reported β2AR–Gs crystal structure, primarily due to contacts between the Gs α5 helix with transmembrane helix (TM) 3, TM5, and TM6 of the receptor (Fig. 3B) [23, 24]. Gs also stabilizes the flexible ICL 2 into an alpha helix, and together with residues of the Gs α5 helix, ICL2 interacts with the highly conserved DRY motif of the receptor, which leads to the stabilization of the β2V2R in an active conformation (Fig. 3B) [23, 24]. It is interesting to note that the active conformation of the β2V2R, Gs, and their interactions within the megaplex are not changed compared with a β2AR–Gs crystal structure, which suggests that G protein is being activated in a canonical fashion by a megaplex GPCR.
The βarr1–V2T region of the megaplex is additionally stabilized by Fab30 and Nb32, and displays βarr1 in the active conformation, as evident by a 20° rotation of its N‐terminal and C‐terminal lobes (Fig. 3A). From our structure, we identified six phosphorylated residues on the V2T: pS357, pT359, pT360, pS362, pS363, and pS364. All of these residues, with the exception of pT359, form electrostatic interactions with various lysines and arginines on βarr1 (Fig. 3C) [23]. Interestingly, only the first four residues (pS357, pT359, pT360, and pS362) were phosphorylated upon agonist stimulation, which suggests that their phosphorylation may be GRK2‐dependent and contribute significantly to the recruitment and activation of βarr1 [23]. In order to simulate how a megaplex behaves in a membranous environment, we employed coarse‐grained molecular dynamics (MD) simulation of a β2V2R–Gs protein–βarr1 megaplex in a membrane composed of dipalmitoylphosphatidylcholine [23]. The MD analysis utilizes three possible starting positions of a βarr1 bound to the tail of a β2V2R that is also simultaneously coupled to a Gs protein. Fig. 3D illustrates two orthogonal views of a final frame of the MD simulation, showing three separate coarse‐grained models that have each been aligned by the β2V2R–Gs portion of the megaplex in order to show the three different positions of βarr1. The analysis reveals that βarr1 moves freely in the lateral direction in relation to the membrane, with its vertical movement limited by the actual membrane itself [23]. Importantly, we observe that the C‐edge loops of βarr1, a series of flexible loops located at the edge of the C‐terminal lobe of βarr1, form transient interactions with the membrane (Fig. 3D) [23]. The βarr C‐edge loop has been shown to form interactions with the membrane while bound to a GPCR and is observed to be a critical element in maintaining the ‘core’ interaction of GPCR–arrestin complexes [25, 26, 27]. However, our molecular dynamics evidence shows that a GPCR tail‐bound βarr1 in a megaplex can also form such membrane interactions [23]. This raises interesting questions regarding the role of βarr tethering within endosomal compartments and also raises the possibility that an active βarr1 can potentially associate with the membrane without interacting with the GPCR core.
Finally, our structure highlights that a single receptor can modularly and simultaneously activate G protein and βarr1. The activation of one transducer does not in any way hinder the activation of the other, as each transducer binds to a different motif on the same GPCR. To further illustrate this point, we show using βarr CRISPR knockout cells that β2V2R–βarr1 fusion proteins perform sustained G protein signaling almost identically to a β2V2R [23]. This experiment suggests that the close proximity of βarr1 through covalent linkage does not significantly hinder the ability of class B GPCRs to perform sustained signaling.
Concluding remarks and perspective
While recent evidence has highlighted the importance of endosomal signaling for an ever‐increasing number of GPCRs, its full molecular mechanism remains to be elucidated. Our megaplex structure presented herein illustrates that the binding of G protein and βarr to a receptor is not mutually exclusive and provides a biophysical explanation for the ability of a GPCR to continue signaling through G protein while being internalized by βarr. The megaplex and the phenomenon of sustained signaling raise a number of interesting questions: (a) What is the signaling function of a βarr in the context of a megaplex and (b) what is the physiological consequence of sustained G protein signaling?
Previous studies presented above collectively illustrate that βarr may enhance the sustained phase of G protein signaling for some GPCRs. Is βarr acting as a trafficking molecule, extending the receptor's ability to activate G protein by keeping the receptor internalized within endosomes? Or is βarr extending G protein signaling through another mechanism? Some evidence suggests that a receptor‐bound βarr is enhancing G protein signaling by binding to the Gβγ subunit after it dissociates from the Gα subunit, forming a GPCR–Gβγ–βarr complex. In this complex, it is theorized that βarr tethers Gβγ nearby in order to regenerate a competent G protein heterotrimer to hasten second messenger generation in endosomes [15]. Indeed, we have previously shown that that purified Gβγ interacts with the β2V2R–βarr1 complex and that the β2V2R–βarr1 complex interacts more strongly with Gβγ from Gs heterotrimers after separation between the Gαs and Gβγ was induced by a nonhydrolyzable GTP analog [21]. The formation of a megaplex and a GPCR–Gβγ–βarr complex highlights the codependency between G protein and βarr signaling. Equally as interesting is the question of whether a megaplex βarr can perform its own G protein‐independent signaling. We and others have discovered that the tail conformation of a GPCR–βarr complex is capable of signaling through scaffolding MAP kinases [17, 18]. It is tempting to speculate whether an active βarr within a megaplex can also scaffold additional effectors and function much like an active, receptor‐bound βarr.
The regulation of sustained, endosomal G protein signaling remains an important area of future investigations. For example, how does the acidic lumen of endosomes influence GPCR‐mediated signaling? What role does adenylyl cyclase subtypes play in cAMP generation? And what is the eventual fate of megaplexes after endocytosis? It has been hypothesized that for endosomal signaling to occur, the receptor–ligand complex must be of sufficiently high affinity to withstand the acidic lumen of endosomes. Indeed, one study show that for the class B parathyroid hormone receptor (PTHR), acidification of endosomal lumina promotes dissociation of parathyroid hormone from the PTHR, leading to gradual attenuation of endosomal G protein signaling. Inhibition of vacuolar ATPases responsible for intraluminal acidification of endosomes led to an increase in the duration of cAMP generation [28]. Given that many class B GPCRs such as the V2R are known to recycle slowly, it is possible that lysosomal degradation of megaplexes play a role in signaling termination [29, 30]. However, some data implicate the recycling machinery in attenuating endosomal signaling [13].
Much remains to be discovered regarding the role of adenylyl cyclase in sustained signaling. A recent study has shown that endosomal cAMP may potentially arise from only certain subtypes of adenylyl cyclases that have been selectively trafficked to endosomes [31]. Subtypes of adenylyl cyclases have been known to be differentially compartmentalized, and it is tempting to speculate that these subtypes may play a different role or lead to different cellular responses with respect to compartmentalized G protein signaling.
Finally, emerging data suggest that the second messenger molecules generated during this late phase of G protein signaling lead to different physiological consequences than those generated during the early phase. A prominent example of this difference has been shown for the parathyroid hormone receptor: modulation of this receptor by short‐acting or long‐acting parathyroid hormones leads to acute or sustained cAMP generation, leading to either an acute or prolonged hypercalcemic state in mice [32, 33]. Sustained signaling has also been shown to be responsible for pain sensation and analgesia through the neurokinin 1 receptor and δ‐opiod receptors [34,35]. As additional physiological consequences for sustained signaling within endosomes are discovered, one can already envision that specifically targeting this late phase of G protein signaling may potentially impart spatial and temporal ‘bias’ to the types of responses elicited. To fully realize this goal, we need now to characterize at a molecular level how sustained signaling is mediated through additional structural/cellular investigations, and our structure of the megaplex will hopefully serve as an important first step.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
AHN and RJL both wrote and revised the manuscript.
Acknowledgements
We thank Dr. Alex Thomsen for a critical reading of this manuscript. This work is supported by NIH grants F30HL149213 to AHN and R01HL016037 to RJL RJL is an investigator of the Howard Hughes Medical Institute.
References
- 1. Lefkowitz RJ, Stadel JM & Caron MG (1983) Adenylate cyclase‐coupled beta‐adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem 52, 159–186. [DOI] [PubMed] [Google Scholar]
- 2. Pierce KL & Lefkowitz RJ (2001) Classical and new roles of beta‐arrestins in the regulation of G‐protein‐coupled receptors. Nat Rev Neurosci 2, 727–733. [DOI] [PubMed] [Google Scholar]
- 3. Reiter E, Ahn S, Shukla AK & Lefkowitz RJ (2012) Molecular mechanism of beta‐arrestin‐biased agonism at seven‐transmembrane receptors. Annu Rev Pharmacol Toxicol 52, 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weis WI & Kobilka BK (2018) The Molecular basis of G protein‐coupled receptor activation. Annu Rev Biochem 87, 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lohse MJ, Benovic JL, Codina J, Caron MG & Lefkowitz RJ (1990) beta‐Arrestin: a protein that regulates beta‐adrenergic receptor function. Science 248, 1547–1550. [DOI] [PubMed] [Google Scholar]
- 6. Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG & Lefkowitz RJ (1992) Receptor‐specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta‐arrestin and arrestin in the beta 2‐adrenergic receptor and rhodopsin systems. J Biol Chem 267, 8558–8564. [PubMed] [Google Scholar]
- 7. Laporte SA, Oakley RH, Holt JA, Barak LS & Caron MG (2000) The interaction of beta‐arrestin with the AP‐2 adaptor is required for the clustering of beta 2‐adrenergic receptor into clathrin‐coated pits. J Biol Chem 275, 23120–23126. [DOI] [PubMed] [Google Scholar]
- 8. Oakley RH, Laporte SA, Holt JA, Barak LS & Caron MG (1999) Association of beta‐arrestin with G protein‐coupled receptors during clathrin‐mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274, 32248–32257. [DOI] [PubMed] [Google Scholar]
- 9. Oakley RH, Laporte SA, Holt JA, Barak LS & Caron MG (2001) Molecular determinants underlying the formation of stable intracellular G protein‐coupled receptor‐beta‐arrestin complexes after receptor endocytosis*. J Biol Chem 276, 19452–19460. [DOI] [PubMed] [Google Scholar]
- 10. Oakley RH, Laporte SA, Holt JA, Caron MG & Barak LS (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein‐coupled receptors delineate two major classes of receptors. J Biol Chem 275, 17201–17210. [DOI] [PubMed] [Google Scholar]
- 11. Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L & Lohse MJ (2009) Persistent cAMP‐signals triggered by internalized G‐protein‐coupled receptors. PLoS Biol 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D & Seuwen K (2009) Persistent signaling induced by FTY720‐phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 5, 428–434. [DOI] [PubMed] [Google Scholar]
- 13. Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Hallows KR, Brown D, Bouley R & Vilardaga J‐P (2013) Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem 288, 27849–27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ & Vilardaga J‐P (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wehbi VL, Stevenson HP, Feinstein TN, Calero G, Romero G & Vilardaga JP (2013) Noncanonical GPCR signaling arising from a PTH receptor‐arrestin‐Gbetagamma complex. Proc Natl Acad Sci USA 110, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shukla Arun K, Westfield Gerwin H, Kunhong X, Reis Rosana I, Li‐Yin H, Prachi T‐S, Jiang Q, Sheng Li, Adi B, Oleskie Austin N et al. (2014) Visualization of arrestin recruitment by a G‐protein‐coupled receptor. Nature 512, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ et al. (2017) Distinct conformations of GPCR‐beta‐arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci USA 114, 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumari P, Srivastava A, Banerjee R, Ghosh E, Gupta P, Ranjan R, Chen X, Gupta B, Gupta C, Jaiman D et al. (2016) Functional competence of a partially engaged GPCR‐beta‐arrestin complex. Nat Commun 7, 13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotowski SJ, Hopf FW, Seif T, Bonci A & von Zastrow M (2011) Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jimenez‐Vargas NN, Pattison LA, Zhao P, Lieu TM, Latorre R, Jensen DD, Castro J, Aurelio L, Le GT, Flynn B et al. (2018) Protease‐activated receptor‐2 in endosomes signals persistent pain of irritable bowel syndrome. Proc Natl Acad Sci USA 115, E7438–E7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomsen ARB, Plouffe B, Cahill TJ, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP et al. (2016) GPCR‐G Protein‐beta‐arrestin super‐complex mediates sustained G Protein signaling. Cell 166, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY et al. (2013) Structure of active beta‐arrestin‐1 bound to a G‐protein‐coupled receptor phosphopeptide. Nature 497, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen AH, Thomsen ARB, Cahill TJ 3rd, Huang R, Huang LY, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben‐Hail D et al. (2019) Structure of an endosomal signaling GPCR‐G protein‐beta‐arrestin megacomplex. Nat Struct Mol Biol 26, 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D et al. (2011) Crystal structure of the beta2 adrenergic receptor‐Gs protein complex. Nature 477, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lally CC, Bauer B, Selent J & Sommer ME (2017) C‐edge loops of arrestin function as a membrane anchor. Nat Commun 8, 14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA et al. (2017) Identification of phosphorylation codes for arrestin recruitment by G protein‐coupled receptors. Cell 170, 457–469 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staus DP, Hu H, Robertson MJ, Kleinhenz ALW, Wingler LM, Capel WD, Latorraca NR, Lefkowitz RJ, Skiniotis G et al. (2020) Structure of the M2 muscarinic receptor‐beta‐arrestin complex in a lipid nanodisc. Nature 579, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gidon A, Al‐Bataineh MM, Jean‐Alphonse FG, Stevenson HP, Watanabe T, Louet C, Khatri A, Calero G, Pastor‐Soler NM, Gardella TJ et al. (2014) Endosomal GPCR signaling turned off by negative feedback actions of PKA and v‐ATPase. Nat Chem Biol 10, 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Innamorati G, Sadeghi HM, Tran NT & Birnbaumer M (1998) A serine cluster prevents recycling of the V2 vasopressin receptor. Proc Natl Acad Sci USA 95, 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lutz W, Sanders M, Salisbury J & Kumar R (1990) Internalization of vasopressin analogs in kidney and smooth muscle cells: evidence for receptor‐mediated endocytosis in cells with V2 or V1 receptors. Proc Natl Acad Sci USA 87, 6507–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazar AM, Irannejad R, Baldwin TA, Sundaram AB, Gutkind JS, Inoue A, Dessauer CW & Von Zastrow M (2020) G protein‐regulated endocytic trafficking of adenylyl cyclase type 9. Elife 9, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheloha RW, Gellman SH, Vilardaga JP & Gardella TJ (2015) PTH receptor‐1 signalling‐mechanistic insights and therapeutic prospects. Nat Rev Endocrinol 11, 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vilardaga JP, Jean‐Alphonse FG & Gardella TJ (2014) Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol 10, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen DD, Lieu T, Halls ML, Veldhuis NA, Imlach WL, Mai QN, Poole DP, Quach T, Aurelio L, Conner J et al. (2017) Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jimenez‐Vargas NN, Gong J, Wisdom MJ, Jensen DD, Latorre R, Hegron A, Teng S, DiCello JJ, Rajasekhar P, Veldhuis NA et al. (2020) Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc Natl Acad Sci USA 117, 15281–15292. [DOI] [PMC free article] [PubMed] [Google Scholar]


