Abstract
Purpose
Both emmetropic and myopic eyes elongate throughout childhood. The goals of this study were to compare axial elongation among untreated progressing myopes, progressing myopes treated with a myopia control contact lens and emmetropes, in order to place axial elongation in the context of normal eye growth in emmetropic children, and to consider whether normal physiological eye growth places limits on what might be achieved with myopia control.
Methods
Axial elongation data were taken from the 3‐year randomised clinical trial of a myopia control dual‐focus (MiSight® 1 day) contact lens. These were compared with data for myopic and emmetropic children in two large cohort studies: the Orinda Longitudinal Study of Myopia (OLSM) and the Singapore Cohort Study of the Risk Factors for Myopia (SCORM). Each study's published equations were used to calculate annual axial elongation. Four virtual cohorts—myopic and emmetropic for each model—were created, each with the same age distribution as the MiSight clinical trial subjects and the predicted cumulative elongation calculated at years 1, 2 and 3 for myopes and emmetropes using both the OLSM and SCORM models.
Results
The untreated control myopes in the MiSight clinical trial showed mean axial elongation over 3 years (0.62 mm) similar to the virtual cohorts based on the OLSM (0.70 mm) and SCORM (0.65 mm) models. The predicted 3‐year axial elongation for the virtual cohorts of emmetropes was 0.24 mm for both the OLSM and SCORM models—similar to the mean 3‐year elongation in MiSight‐treated myopes (0.30 mm).
Conclusions
The 3‐year elongation in MiSight‐treated myopes approached that of virtual cohorts of emmetropes with the same age distribution. It is hypothesised that myopic axial elongation is superimposed on an underlying physiological axial elongation observed in emmetropic eyes, which reflects increases in body stature. We speculate that optically based myopia control treatments may minimise the myopic axial elongation but retain the underlying physiological elongation observed in emmetropic eyes.
Keywords: axial length, children, contact lenses, myopia, myopia control
Introduction
Myopia is quantified by the dioptric distance of the eye's far point. This definition of myopia has important historical value in that it specifies the power of the optical lens required to correct it, but it belies the fact that the primary origin of the majority of myopia does not lie with the optical components of the anterior eye, but rather in excessive axial elongation of the globe. Indeed, the distribution of corneal power among different refractive groups is very similar. 1 Although axial length is highly correlated with adult spherical refractive error, 2 a large range of axial lengths are observed for any single refractive error, 1 indicating that axial length is not uniquely associated with spherical equivalent refractive errors and eye size differences can exist without generating myopia. Larger emmetropic adult eyes also have longer focal lengths due to larger radii of the cornea and lens, with increasing lens radii (surface flattening) playing the major role in maintaining emmetropia. 3 Furthermore, the relationship between axial length and refractive error may be nonlinear and vary with age, eye size or both.4, 5
The eye and its components grow from birth through childhood. Corneal power reaches an average power of 43 to 44 D by 9 months of age and remains stable throughout early 6 and later childhood. 7 In contrast, all eyes elongate steadily throughout childhood. 8 Vitreous chamber depth is the largest single component of overall axial length, which elongates from an average of 12 mm at 3 months6, 9 to a final average value of around 16 mm in emmetropic eyes. 7 The rate and time course of axial elongation and ultimate axial length varies across refractive groups.8, 10, 11 In myopes, axial length follows a similar rate of growth to emmetropes up to a few years before myopia onset. 8 Thereafter, at the ages when axial elongation is steadily slowing in emmetropes it continues, and even accelerates.8, 11 Indeed, the peak rate of axial elongation occurs in the 2 to 3 years before myopia onset. 8 Refractive error changes are larger before. as well as after, the onset of myopia compared to eyes that remain emmetropic. 8 Thereafter, the rate of myopia progression 12 and axial elongation 13 decreases steadily, likely following an exponential decay.13, 14 The crystalline lens undergoes age‐related flattening from birth to 10‐12 years of age,7, 15, 16 although lens power decreases beyond this age.7, 15 Power changes of the lens compensate for ongoing axial elongation, which maintains emmetropia. Although the lens flattens at a higher rate in myopic eyes, it is insufficient to compensate for the more rapid axial elongation.11, 16, 17
In a recent summary report, the increased precision of axial length measurements, combined with the observation that future pathologies in highly myopic eyes are associated with excessive axial elongation of young eyes, prompted a conclusion that, “the goal of all clinical trials for myopia control should be the reduction of axial elongation.” 18 Indeed, increasing levels of myopia are strongly associated with a range of diseases,19, 20 including myopic maculopathy, 21 open angle glaucoma, 22 posterior subcapsular cataract 23 and retinal detachment. 24 Furthermore, visual impairment is more strongly associated with axial length than refractive error. 25 It is not surprising, therefore, that axial elongation has become an important outcome measure in clinical trials of interventions to slow the progression of myopia.18, 26 Most,27, 28 though not all,29, 30 widely adopted interventions show concurrent slowing of myopia progression and axial elongation. Three‐year clinical trials of myopia control are rare,31, 32, 33 but the MiSight® 1 day dual‐focus soft contact lens demonstrates a slowing of axial elongation across all 3 years of treatment 34 and 6‐year findings have been presented recently. 35
Myopia routinely develops between the ages of 5 and 15 years, 36 and therefore its time course overlaps that of the coordinated eye growth that results in and maintains emmetropia. This raises the issue of whether the increases in axial length among children with progressing myopia reflect only abnormal myopic elongation, or some combination of normal and abnormal eye growth. Here, the trajectory of axial elongation in treated and untreated myopic eyes are compared with published models of eye growth in progressing myopes and emmetropes.7, 37 The goals of the analysis were:
To compare eye growth, in terms of axial elongation among untreated progressing myopes, treated progressing myopes and emmetropes;
To place the axial elongation in treated and untreated myopes in the context of normal eye growth in emmetropic children;
To consider whether normal physiological eye growth places limits on what might be achieved in myopia control.
Methods
The current study analysed axial elongation data obtained during a 3‐year randomised controlled clinical trial of a myopia control dual‐focus contact lens (MiSight® 1 day). 34 In brief, the study enrolled 144 myopic children aged 8 to 12 years with no prior contact lens experience in a 3‐year, double‐masked, randomised clinical trial at four investigational sites in Canada, England, Portugal and Singapore. Around half of the participants were of European descent and a quarter were East Asian. Subjects with –0.75 to –4.00 D of myopia and <1.00 D of astigmatism were randomised to either a MiSight contact lens (treated) or Proclear® 1 day single vision lens (untreated), both manufactured in omafilcon A (CooperVision, Inc., coopervision.com) and worn on a daily disposable basis. Compliance was high, with mean reported wearing time at the end of the study over 13 hours per weekday and over 12 hours per weekend day. Primary outcome measures were cycloplegic auto‐refraction measured using the Grand Seiko Binocular Auto‐refractor/ Keratometer WR‐5100K or WAM‐5500 (Grand Seiko Co., grandseiko.com) and axial length measured using the IOLMaster (Carl Zeiss Meditec, zeiss.com). Of the subjects who were dispensed lenses, 81% (109/135) completed the 3‐year clinical trial (53 treated, 56 untreated). Unadjusted mean myopia progression was 0.73 D lower in the treated group than the untreated group (–0.51 ± 0.64 D vs –1.24 ± 0.61 D, p < 0.001). Mean axial elongation was 0.32 mm less in the treated group than the untreated group (0.30 ± 0.27 vs 0.62 ± 0.30 mm, p < 0.001). Changes in refractive error and axial length were highly correlated (r = –0.90, p < 0.001).
The above longitudinal axial length data were compared with data for myopic and emmetropic children in two large cohort studies: the Orinda Longitudinal Study of Myopia (OLSM) in the United States 38 and the Singapore Cohort Study of the Risk Factors for Myopia (SCORM). 39 Both studies used cycloplegic auto‐refraction for refractive error and contact ultrasound for axial length and have published equations for the growth of ocular components in children.7, 37
The OLSM included children between 6 and 14 years of age who were required to attend at least three annual visits to be included in the analysis. Of the 1504 children recruited, 737 were eligible for the analysis. Of these, 247 children were classified as myopic (178 white and 59 Asian, with at least –0.75 D in both meridians), of whom 76% were emmetropic or hyperopic at baseline, and 194 persistent emmetropes (170 white and 16 Asian). 7 SCORM included children aged between 6 and 12 years and, again, at least three visits were required to be eligible for the analysis. Of the 1979 children recruited, 1775 were eligible of whom 616 were progressing myopes (at least –0.50 D spherical equivalent) and 369 were emmetropes. This population was predominantly Chinese in ethnicity. 37
The OLSM and SCORM published equations for axial length7, 37 that were used to compare with the MiSight clinical trial data. The OLSM paper presents two separate equations for children younger or older than 10.5 years. 7 The SCORM paper presents a quadratic model for axial elongation fitted for ages up to 12 years. 37 Unfortunately, their model asymptotes at age 13 years and thereafter predicts shrinking of eye length. Given that comparisons were to be made for children through the age of 15 years, their equations for vitreous chamber depth, which do not suffer from the same limitations, were substituted. Their reported longitudinal changes in anterior chamber depth and lens thickness cancel each other, so vitreous chamber depth is a reasonable surrogate for axial length.
In summary, the following equations as a function of age were used.
OLSM 7 :
Myopes up to 10.5 years:
Myopes after 10.5 years:
Emmetropes up to 10.5 years:
Emmetropes after 10.5 years:
SCORM 37 :
Myopes:
Emmetropes:
ln = natural logarithm.
Using the above equations, axial lengths (or vitreous chamber depth) at ages 8 through 15 years were calculated. Thereafter, annual elongation was calculated.
Finally, four virtual cohorts were created, each with the same age distribution as the MiSight clinical trial subjects at baseline: 14% of 8 year olds, 27% of 9 year olds, 16% of 10 year olds, 23% of 11 year olds and 20% of 12 year olds (mean = 10.1 years). The predicted elongation was calculated at years 1, 2 and 3 for myopes and emmetropes using both the OLSM and SCORM models.
Results
Table 1 shows the annual axial elongation rates (mm/year) predicted by the OLSM and SCORM models in myopes and emmetropes. As expected, annual axial elongation is higher in myopes than in emmetropes, and annual axial elongation decreases with age.
Table 1.
Annual axial elongation (mm/year) as a function of age predicted by the OLSM 7 and SCORM 37 models in myopes and emmetropes
| Age | Myopes | Emmetropes | ||
|---|---|---|---|---|
| OLSM | SCORM† | OLSM | SCORM | |
| 8 | 0.28 | 0.39 | 0.15 | 0.14 |
| 9 | 0.25 | 0.31 | 0.13 | 0.11 |
| 10 | 0.30 | 0.25 | 0.09 | 0.09 |
| 11 | 0.22 | 0.20 | 0.07 | 0.08 |
| 12 | 0.20 | 0.17 | 0.06 | 0.06 |
| 13 | 0.19 | 0.14 | 0.06 | 0.06 |
| 14 | 0.18 | 0.12 | 0.05 | 0.05 |
All values are in mm. OLSM, Orinda Longitudinal Study of Myopia; SCORM, Singapore Cohort Study of the Risk Factors for Myopia.
For the SCORM cohorts, equations for vitreous chamber depth elongation were used
Table 2 and Figure 1 show the observed cumulative axial elongation at years 1, 2 and 3 of the MiSight clinical trial for both treated and control groups. Also shown are elongation rates for myopes and emmetropes as predicted using both the OLSM and SCORM models for virtual cohorts.
Table 2.
Predicted cumulative elongation at years 1, 2 and 3 for myopes and emmetropes using both the OLSM 7 and SCORM 37 models for a virtual cohort with the same age distribution as the MiSight clinical trial subjects
| Duration | “Virtual Cohort” myopes | MiSight trial myopes | “Virtual Cohort” emmetropes | |||
|---|---|---|---|---|---|---|
| OLSM | SCORM | Control | Treated | OLSM | SCORM | |
| 1‐year | 0.25 | 0.26 | 0.24 | 0.09 | 0.10 | 0.09 |
| 2‐year | 0.48 | 0.46 | 0.45 | 0.21 | 0.18 | 0.17 |
| 3‐year | 0.70 | 0.63 | 0.62 | 0.30 | 0.24 | 0.24 |
Also shown are the values for cumulative axial elongation from the MiSight clinical trial. 34 All values are in mm. OLSM, Orinda Longitudinal Study of Myopia; SCORM: Singapore Cohort Study of the Risk Factors for Myopia.
Figure 1.
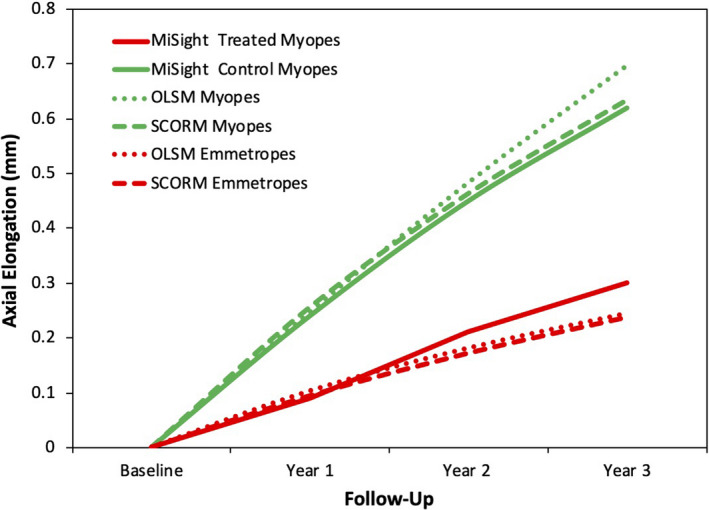
Cumulative axial elongation (mm) for treated and control myopes in the MiSight clinical trial are compared to the virtual myopic and emmetropic cohorts developed using both the OLSM 7 and SCORM 37 models (see Methods). OLSM: Orinda Longitudinal Study of Myopia; SCORM: Singapore Cohort Study of the Risk Factors for Myopia.
The untreated control myopes in the MiSight clinical trial show similar cumulative axial elongation over 3 years (0.62 mm) to those predicted for the virtual cohorts of myopic children based on both the OLSM (0.70 mm) and SCORM (0.63 mm) models (Table 2). In contrast, the mean 3‐year elongation in MiSight treated myopes is significantly lower (0.30) mm, and not substantially different from the predicted axial elongation rates for the virtual cohorts of emmetropes (0.24 mm, for both the OLSM and SCORM models).
Discussion
The mean axial elongation in children wearing Proclear single vision soft contact lenses in the MiSight clinical trial was 0.62 mm over 3 years, 34 which is very similar to that predicted for age‐matched virtual cohorts of myopes using the growth models from OLSM and SCORM (0.70 and 0.63 mm, respectively). Other studies in the USA have reported similar mean 3‐year elongation in cohorts with a comparable age distribution.40, 41 In contrast, the mean axial elongation in children wearing the dual‐focus MiSight soft contact lenses was 0.30 mm over 3 years, 34 which is less than half that for the untreated control subjects and similar in magnitude to that predicted for age‐matched virtual cohorts of emmetropes using the growth models from OLSM and SCORM (both 0.24 mm).7, 37
It is noteworthy that emmetropic eyes in OLSM and SCORM continued elongating throughout childhood, albeit at a slower rate than myopic eyes.7, 37 Axial elongation continues into the late teens in both myopes 42 and emmetropes, 43 although only around 0.06 and 0.03 mm/year, respectively. In emmetropes, the ongoing axial elongation is compensated by crystalline lens flattening and thinning, such that refractive error does not change to a meaningful degree,7, 15, 16 although the rate of crystalline lens change may not differ significantly between emmetropes and myopes 7 and some, 44 though not all, 45 studies have reported adult myopes to have thinner crystalline lenses than adult emmetropes. However, it is likely that crystalline lens thinning in myopes is simply insufficient to compensate for their more rapid axial elongation. Presumably ongoing eye growth in emmetropes reflects increases in body stature before, during and after puberty. Cross‐sectional46, 47 and longitudinal studies 48 demonstrate an association between axial length and height, with taller emmetropic children having longer eyes. A recent longitudinal study demonstrated a high correlation between 2‐year changes in height and axial length in emmetropes over two consecutive periods (r = 0.71 and 0.63), while myopes show no consistent association. 48 This leads to two hypotheses. First, myopic axial elongation is superimposed on this underlying physiological axial elongation. Second, optically based myopia control treatments may minimise the myopic axial elongation but may not be able to prevent the underlying physiological elongation. Based on the OLSM and SCORM growth curves, on average, emmetropic eyes elongate at 30% to 40% the rate of myopic eyes (Table 2, Figure 1).7, 37 The aforementioned hypotheses would thus predict that myopic control methods that slow progression by more than 60% to 70% in a population may be challenging to attain. In other words, the slowing of axial elongation in the MiSight clinical trial approaches the population limits placed upon therapy by physiological eye growth. It is noteworthy that no 2‐ or 3‐year clinical trials of optical myopia control modalities exceed this value. 27
Since this work was first presented, 49 the notion of underlying physiological growth has been embraced by others. Some presenters have also begun to adjust and re‐quantify the efficacy of myopia control studies to claim higher percentage treatment effects based on this possible physiological eye growth. 50 There are, however, two reasons not to adopt this generalised approach of applying an arbitrary correction factor based on current understanding of eye growth. First, there is currently no evidence for two mechanisms (pathological and physiological) driving axial elongation. It is the total eye growth that is crucial to ocular health, 25 so distinctions between mechanisms of action may have no clinical ramifications. Second, any reports of percentage treatment efficacy are confounded by age, underlying progression rate and likely ethnicity. 26 Adjusting these rates for, as yet, unproven physiological growth would further obfuscate comparisons among myopia control therapies.
Although these data suggest that the MiSight dual‐focus contact lens is able to slow eye growth to non‐myopic levels, studies using daily dosing with higher concentrations of atropine (1%) have stopped eye growth. For example, the Atropine for the Treatment of Childhood Myopia (ATOM) Study reported a reduction in axial length (–0.14 ± 0.28 mm) in the first year for 166 eyes treated unilaterally with 1% atropine, followed by a + 0.12 mm elongation in the second year. 51 More recently, Yi et al. 52 reported virtually no change (–0.03 ± 0.07 mm) in 66 children treated bilaterally with 1% atropine for 1 year. These data suggest that, in the short‐term, high concentrations of atropine may arrest axial elongation, although the mean elongation in the second year of the ATOM study is identical to that of treated eyes in the MiSight clinical trial data used for this study (Table 2). Unfortunately, atropine‐treated eyes show a dramatic acceleration of myopia progression and axial elongation following cessation of treatment. The atropine‐treated eyes in the ATOM study elongated by over 0.30 mm in the year following discontinuation of treatment, compared with around 0.15 mm in the untreated eyes. 53 Furthermore, concentrations as high as 0.5% have far less influence on axial elongation. For example, in a 2‐year clinical trial, axial length increased by 0.27, 0.28 and 0.41 mm in subjects treated with 0.5%, 0.1% and 0.01%, respectively. 30
The literature on optical interventions in animals has the potential to contribute to our understanding. Unfortunately, these experiments are typically conducted on neonatal animals, undergoing a rapid period of eye growth. For example, Hung et al. 54 demonstrated that +3 or +6 D lenses imposed in front of one eye of infant rhesus monkeys ranging from 72 to 113 days induced hyperopic anisometropia and a relative slowing of axial elongation in the treated eye. Nonetheless, while the treated eyes showed up to 0.4 mm less relative elongation, they still elongated by 1 to 2 mm. In other words, imposition of plus lenses did not completely arrest growth of infant eyes.
The comparison of axial elongation among untreated progressing myopes, treated progressing myopes and emmetropes has some limitations. The predicted axial elongation for age‐matched virtual cohorts based on the growth curves published by OLSM and SCORM agree well with the reported mean axial elongation for the untreated myopes in the MiSight clinical trial. The ethnic composition of the children in the MiSight clinical trial includes a minority of Asian eyes, and is similar to the ethnic distribution within the OLSM cohort, although the studies were conducted over 20 years apart. For further comparison, the 3‐year mean axial elongation for children wearing single vision spectacles in the Correction of Myopia Evaluation Trial (COMET) was 0.75 mm, but their mean age was younger by a year (9.3 ± 1.3 years). 31 Likewise, a clinical trial of myopic US children with a mean age of 10.4 ± 1.1 years at baseline reported mean 3‐year axial elongation of 0.63 and 0.59 mm in those randomised to soft contact lenses (n = 247) and spectacles (n = 237), respectively. 40 More recently, another study of US children (n = 97) with an average age of 10.3 ± 1.1 years randomised to single vision contact lenses in a myopia control clinical trial found a 3‐year progression of 0.62 mm. 41 In summary, the mean axial elongation of the untreated myopes in the MiSight clinical trial is consistent with other similar aged cohorts.
Inspection of Table 1 demonstrates that the predicated elongation based on the SCORM model is higher than that for OLSM for 8 and 9 year olds, but the opposite is true for older children. As described earlier, the use of vitreous chamber depth was necessary and this may have underestimated axial elongation, given that increases in vitreous chamber depth account for around 90% of axial elongation.6, 55 Furthermore, the SCORM models are based on children no older than 12 years, and this may limit the accuracy of the growth curves at older ages.
While the SCORM model only represents data from children who are myopic at all timepoints, 37 the OLSM model includes both consistent and incident myopes. 7 As discussed in the introduction, axial elongation accelerates over a few years prior to myopia onset and is fastest in the 2 to 3 years immediately before onset. Thus, axial elongation in the SCORM model includes myopes in various phases of their refractive history. This can be partially quantified by comparing axial elongation rates from the SCORM model for both progressing and incident myopes (Table 3). At younger ages, the progressing myopes elongate faster, but by 10 or 11 years of age there is little difference.
Table 3.
Annual vitreous chamber elongation as a function of age (in years) predicted by the SCORM 37 models for progressing and incident myopes
| Age | Progressing myopes | Incident myopes |
|---|---|---|
| 8 | 0.39 | 0.32 |
| 9 | 0.31 | 0.27 |
| 10 | 0.25 | 0.23 |
| 11 | 0.20 | 0.19 |
| 12 | 0.17 | 0.16 |
| 13 | 0.14 | 0.14 |
| 14 | 0.12 | 0.12 |
All values are in mm.
SCORM, Singapore Cohort Study of the Risk Factors for Myopia.
As described in the Methods, there are differences between the OLSM and SCORM cohorts and the criteria adopted by the respective authors when developing their growth models. These may have contributed to discrepancies between the virtual cohorts derived from the models. OLSM followed children between 6 and 14 years, while SCORM studied children between 6 and 12 years. In OLSM, myopia was defined as at least –0.75 D in both meridians, whereas in SCORM the definition was at least –0.50 D spherical equivalent. Likewise, emmetropia was defined differently: –0.25 to +1.00 D in both meridians and –0.50 to +1.00 D spherical equivalent, respectively. OLSM used 1% tropicamide for cycloplegia, but SCORM used 1% cyclopentolate, although any differences attributed to these choices should be minimal. 56 Finally, among the children contributing to the OLSM models, 33.5% were myopic, compared with 68.6% of those for the SCORM models. It should also be noted that the MiSight clinical trial excluded children with astigmatism greater than 0.75 D, whereas both the OLSM and SCORM models appear to have included myopes regardless of astigmatism. Given the association of myopia with astigmatism,57, 58 this may have influenced our findings, but it is unclear in which direction.
A curious feature of the axial elongation rate of the predominantly Singaporean Chinese myopes in SCORM is that it is similar in magnitude to the rate in OLSM. It would be expected that the axial elongation for myopes in the SCORM model would be higher given that the rates of myopic progression 59 and axial elongation 13 are 50% higher among myopes in Asia compared to children of European descent. Both OLSM and SCORM used ultrasound to measure axial length, while the MiSight clinical trial used the IOLMaster. While the latter technique has superior repeatability, any systematic differences between the methods is small and invariant with axial length. Such small differences are very unlikely to affect the applicability of models of axial elongation based on one technique to measures made with the other.60, 61
When comparing axial elongation among refractive groups, it must be acknowledged that emmetropic eyes are, on average, shorter than myopic eyes. Thus, the comparisons between myopes undergoing myopia control treatment in the MiSight clinical trial with emmetropes might be improved by matching the groups for baseline axial length. The mean baseline axial length of the MiSight treated myopes was 24.42 mm, whereas an age‐matched virtual cohort of emmetropes would be 23.66 and 23.32 mm, based on the OLSM and SCORM models, respectively. As shown in Table 3, the SCORM authors publish separate growth curves based on both 616 progressing myopes (used herein) and 601 incident myopes, i.e., emmetropes who become myopes. As would be expected the modelled axial length is around 0.08 mm shorter in the incident myopes compared to the progressing myopes, but the predicted rate of axial elongation (Table 3) differs by no more than 0.02 mm per year beyond 10 years of age. Furthermore, neither the OLSM nor SCORM models factor in baseline eye size. However, there was no correlation between baseline axial length and 3‐year elongation in the MiSight clinical trial, although this and other trials show that axial elongation is slower in those who were older at baseline.34, 40 Indeed, data from this MiSight study shows that when the control group illustrated in this analysis were switched to the MiSight lens at an average age of 13.1 years, the subsequent 2‐year axial elongation was equivalent to that of the original MiSight group continuing in treatment, despite the fact that the original control group commenced the 2‐year assessment period with greater refractive error and longer eyes, as a result of the treatment effect in the first 3 years of the trial.
Finally, this analysis represents average change across a population of children. Individuals within a population display great variation; for example, the standard deviations for axial elongation for the 3‐year MiSight clinical trial were 0.27 and 0.30 mm in the treated and control groups, respectively. 34 The same is true for refractive error with some treated children showing myopia progression similar to the mean progression in untreated children. Conversely, some treated children and very few untreated children show almost no progression. For example, in the MiSight clinical trial, 41% of treated eyes showed −0.25 D or less progression over 3 years compared with 4% of the eyes in the control group. Future research may explain why some subjects progress less than others, and the factors that affect the variation in treatment effect. This may lead to improved treatments, as the search for optimal myopia control modalities is still in its early stages.
In summary, the untreated myopes in the MiSight clinical trial show 3‐year axial elongation similar to control myopes in other clinical trials31, 40 and similar to that predicted from models of eye growth based on large cohorts.7, 37 The myopes treated with the MiSight lens show similar 3‐year axial elongation to that of emmetropes predicted by models of eye growth. It is proposed that axial elongation in patients undergoing myopia control be considered in the context of both normal myopic and emmetropic eye growth and expectations be set accordingly. Nonetheless, arbitrary correction factors to account for the impact of physiological eye growth should not be applied when reporting myopia control levels, until this area has been better understood.
Conflict of interest
Paul Chamberlain, Percy Lazon and Baskar Arumugam are employees of CooperVision. Mark Bullimore is a consultant for Alcon Research, Inc., Apellis, Inc., Arctic Vision, Inc., Ascpleix, Inc., CooperVision, Inc., Corneagen, Inc., Essilor International S.A.., Eyenovia, Inc., Genentech, Inc., Johnson & Johnson Vision, Inc., Presbia, Inc., and is the sole owner of Ridgevue Publishing, LLC, Ridgevue Technologies LLC, and Ridgevue Vision LLC. Preparation of this paper was supported by CooperVision, Inc.
Author contributions
Paul Chamberlain: Conceptualization (equal); Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Percy Lazon: Writing‐review & editing (equal). Baskar Arumugam: Writing‐review & editing (equal). Mark Bullimore: Formal analysis (equal); Methodology (equal); Writing‐original draft (lead).
Chamberlain P, Lazon de la Jara P, Arumugam B, & Bullimore MA. Axial length targets for myopia control. Ophthalmic Physiol Opt. 2021; 41: 523–531. 10.1111/opo.12812
Presented in part at the 16th Biennial International Myopia Conference (IMC) in Birmingham, September 2017.
References
- 1. Grosvenor T & Goss DA. Role of the cornea in emmetropia and myopia. Optom Vis Sci 1998; 75: 132–145. [DOI] [PubMed] [Google Scholar]
- 2. Grosvenor T & Scott R. Role of the axial length/corneal radius ratio in determining the refractive state of the eye. [See Comments] Optom Vis Sci 1994; 71: 573–579. [DOI] [PubMed] [Google Scholar]
- 3. Rozema JJ, Atchison DA & Tassignon MJ. Comparing methods to estimate the human lens power. Invest Ophthalmol Vis Sci 2011; 52: 7937–7942. [DOI] [PubMed] [Google Scholar]
- 4. Cruickshank FE & Logan NS. Optical 'Dampening' of the refractive error to axial length ratio: implications for outcome measures in myopia control studies. Ophthalmic Physiol Opt 2018; 38: 290–297. [DOI] [PubMed] [Google Scholar]
- 5. Tideman JWL, Polling JR, Vingerling JR et al. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol 2018; 96: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mutti DO, Sinnott LT, Mitchell GL et al. Ocular component development during infancy and early childhood. Optom Vis Sci 2018; 95: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones LA, Mitchell GL, Mutti DO et al. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci 2005; 46: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 8. Mutti DO, Hayes JR, Mitchell GL et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 2007; 48: 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutti DO, Mitchell GL, Jones LA et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 2005; 46: 3074–3080. [DOI] [PubMed] [Google Scholar]
- 10. Xiang F, He M & Morgan IG. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology 2012; 119: 1478–1484. [DOI] [PubMed] [Google Scholar]
- 11. Rozema J, Dankert S, Iribarren R et al. Axial growth and lens power loss at myopia onset in Singaporean children. Invest Ophthalmol Vis Sci 2019; 60: 3091–3099. [DOI] [PubMed] [Google Scholar]
- 12. Chua SY, Sabanayagam C, Cheung YB et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt 2016; 36: 388–394. [DOI] [PubMed] [Google Scholar]
- 13. Brennan N, Cheng X, Toubouti Y & Bullimore M. Influence of age and race on axial elongation in myopic children. Optom Vis Sci 2018; 95: E‐abstract 180072. [Google Scholar]
- 14. COMET Group . Myopia stabilization and associated factors among participants in the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci 2013; 54: 7871–7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mutti DO, Zadnik K, Fusaro RE et al. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci 1998; 39: 120–133. [PubMed] [Google Scholar]
- 16. Mutti DO, Mitchell GL, Sinnott LT et al. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci 2012; 89: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zadnik K, Mutti DO, Fusaro RE & Adams AJ. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci 1995; 36: 1581–1587. [PubMed] [Google Scholar]
- 18. Wolffsohn JS, Kollbaum PS, Berntsen DA et al. IMI ‐ Clinical myopia control trials and instrumentation report. Invest Ophthalmol Vis Sci 2019; 60: M132–M160. [DOI] [PubMed] [Google Scholar]
- 19. Bullimore MA & Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci 2019; 96: 463–465. [DOI] [PubMed] [Google Scholar]
- 20. Haarman AEG, Enthoven CA, Tideman JWL et al. The complications of myopia: a review and meta‐analysis. Invest Ophthalmol Vis Sci 2020; 61: ARVO E‐Abstract 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vongphanit J, Mitchell P & Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology 2002; 109: 704–711. [DOI] [PubMed] [Google Scholar]
- 22. Ramakrishnan R, Nirmalan PK, Krishnadas R et al. Glaucoma in a rural population of Southern India: the Aravind comprehensive eye survey. Ophthalmology 2003; 110: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 23. Wong TY, Foster PJ, Johnson GJ & Seah SK. Refractive errors, axial ocular dimensions, and age‐related cataracts: the Tanjong Pagar survey. Invest Ophthalmol Vis Sci 2003; 44: 1479–1485. [DOI] [PubMed] [Google Scholar]
- 24. Risk factors for idiopathic rhegmatogenous retinal detachment. The Eye Disease Case‐Control Study Group. Am J Epidemiol 1993; 137: 749–757. [PubMed] [Google Scholar]
- 25. Tideman JW, Snabel MC, Tedja MS et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol 2016; 134: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 26. Brennan NA, Toubouti YM, Cheng X & Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res 2020; 27: 100923. 10.1016/j.preteyeres.2020.100923. Accessed 19 Mar 2021. Epub ahead of print. PMID: 33253901. [DOI] [PubMed] [Google Scholar]
- 27. Wildsoet CF, Chia A, Cho P et al. IMI ‐ Interventions myopia Institute: interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci 2019; 60: M106–M131. [DOI] [PubMed] [Google Scholar]
- 28. Bullimore MA & Richdale K. Myopia control 2020: where are we and where are we heading? Ophthalmic Physiol Opt 2020; 40: 254–270. [DOI] [PubMed] [Google Scholar]
- 29. Bullimore MA & Berntsen DA. Low‐dose atropine for myopia control: considering all the data. JAMA Ophthalmol 2018; 136: 303. [DOI] [PubMed] [Google Scholar]
- 30. Chia A, Chua WH, Cheung YB et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012; 119: 347–354. [DOI] [PubMed] [Google Scholar]
- 31. Gwiazda J, Hyman L, Hussein M et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci 2003; 44: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 32. Walline JJ, Jones LA, Mutti DO & Zadnik K. A randomized trial of the effects of rigid contact lenses on myopia progression. Arch Ophthalmol 2004; 122: 1760–1766. [DOI] [PubMed] [Google Scholar]
- 33. Cheng D, Woo GC, Drobe B & Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three‐year results of a randomized clinical trial. JAMA Ophthalmol 2014; 132: 258–264. [DOI] [PubMed] [Google Scholar]
- 34. Chamberlain P, Peixoto‐de‐Matos SC, Logan NS et al. A 3‐year randomized clinical trial of misight lenses for myopia control. Optom Vis Sci 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 35. Chamberlain P, Arumugam B, Jones D et al. Myopia progression in children wearing dual‐focus contact lenses: 6‐year findings. Optom Vis Sci 2020; 97: E‐abstract 200038. [DOI] [PubMed] [Google Scholar]
- 36. Kleinstein RN, Sinnott LT, Jones‐Jordan LA et al. New cases of myopia in children. Arch Ophthalmol 2012; 130: 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong HB, Machin D, Tan SB et al. Ocular component growth curves among singaporean children with different refractive error status. Invest Ophthalmol Vis Sci 2010; 51: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 38. Zadnik K, Mutti DO, Friedman NE & Adams AJ. Initial cross‐sectional results from the Orinda longitudinal study of myopia. Optom Vis Sci 1993; 70: 750–758. [DOI] [PubMed] [Google Scholar]
- 39. Saw SM, Tong L, Chua WH et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci 2005; 46: 51–57. [DOI] [PubMed] [Google Scholar]
- 40. Walline JJ, Jones LA, Sinnott L et al. A randomized trial of the effect of soft contact lenses on myopia progression in children. Invest Ophthalmol Vis Sci 2008; 49: 4702–4706. [DOI] [PubMed] [Google Scholar]
- 41. Walline JJ, Walker MK, Mutti DO et al. Effect of high add power, medium add power, or single‐vision contact lenses on myopia progression in children: the blink randomized clinical trial. JAMA 2020; 324: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hou W, Norton TT, Hyman L et al. Axial elongation in myopic children and its association with myopia progression in the correction of myopia evaluation trial. Eye Contact Lens 2018; 44: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hagen LA, Gilson SJ, Akram MN & Baraas RC. Emmetropia is maintained despite continued eye growth from 16 to 18 years of age. Invest Ophthalmol Vis Sci 2019; 60: 4178–4186. [DOI] [PubMed] [Google Scholar]
- 44. McBrien NA & Millodot M. A biometric investigation of late onset myopic eyes. Acta Ophthalmol (Copenh) 1987; 65: 461–468. [DOI] [PubMed] [Google Scholar]
- 45. Bullimore MA, Gilmartin B & Royston JM. Steady‐state accommodation and ocular biometry in late‐onset myopia. Doc Ophthalmol 1992; 80: 143–155. [DOI] [PubMed] [Google Scholar]
- 46. Saw SM, Chua WH, Hong CY et al. Height and its relationship to refraction and biometry parameters in Singapore Chinese children. Invest Ophthalmol Vis Sci 2002; 43: 1408–1413. [PubMed] [Google Scholar]
- 47. Wang D, Ding X, Liu B et al. Longitudinal changes of axial length and height are associated and concomitant in children. Invest Ophthalmol Vis Sci 2011; 52: 7949–7953. [DOI] [PubMed] [Google Scholar]
- 48. Kearney S, Strang NC, Cagnolati B & Gray LS. Change in body height, axial length and refractive status over a four‐year period in Caucasian children and young adults. J Optom 2020; 13: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Logan N. Conference report: the 16th International myopia conference. Ophthalmic Physiol Opt 2018; 38: 215–216. [DOI] [PubMed] [Google Scholar]
- 50. Rappon J, Neitz J, Neitz M & Chalberg T. A new method for determining an age‐independent axial length percentage reduction for myopia therapies. Optom Vis Sci 2020;97:E‐abstract 205349. [Google Scholar]
- 51. Chua WH, Balakrishnan V, Chan YH et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006; 113: 2285–2291. [DOI] [PubMed] [Google Scholar]
- 52. Yi S, Huang Y, Yu SZ et al. Therapeutic effect of atropine 1% in children with low myopia. J AAPOS 2015; 19: 426–429. [DOI] [PubMed] [Google Scholar]
- 53. Tong L, Huang XL, Koh AL et al. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology 2009; 116: 572–579. [DOI] [PubMed] [Google Scholar]
- 54. Hung LF, Crawford ML & Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med 1995; 1: 761–765. [DOI] [PubMed] [Google Scholar]
- 55. Hyman L, Gwiazda J, Hussein M et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol 2005; 123: 977–987. [DOI] [PubMed] [Google Scholar]
- 56. Mutti DO, Zadnik K, Egashira S et al. The effect of cycloplegia on measurement of the ocular components. Invest Ophthalmol Vis Sci 1994; 35: 515–527. [PubMed] [Google Scholar]
- 57. Tong L, Saw SM, Carkeet A et al. Prevalence rates and epidemiological risk factors for astigmatism in Singapore school children. Optom Vis Sci 2002; 79: 606–613. [DOI] [PubMed] [Google Scholar]
- 58. O'Donoghue L, Rudnicka AR, McClelland JF et al. Refractive and corneal astigmatism in white school children in Northern Ireland. Invest Ophthalmol Vis Sci 2011; 52: 4048–4053. [DOI] [PubMed] [Google Scholar]
- 59. Donovan L, Sankaridurg P, Ho A et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carkeet A, Saw SM, Gazzard G et al. Repeatability of Iolmaster biometry in children. Optom Vis Sci 2004; 81: 829–834. [DOI] [PubMed] [Google Scholar]
- 61. Sheng H, Bottjer CA & Bullimore MA. Ocular component measurement using the Zeiss Iolmaster. Optom Vis Sci 2004; 81: 27–34. [DOI] [PubMed] [Google Scholar]


