In a large cohort of persons receiving dialysis who had evidence of SARS-CoV-2 infection, the persistence over time of SARS-CoV-2 receptor-binding domain IgG was determined, and variation by age group, sex, race/ethnicity, and diabetes status was analyzed.
Visual Abstract. SARS-CoV-2 Immune Response in Dialysis.
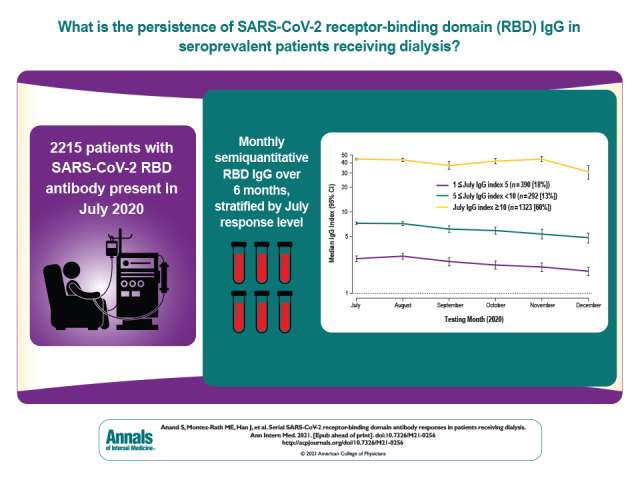
In a large cohort of persons receiving dialysis who had evidence of SARS-CoV-2 infection, the persistence over time of SARS-CoV-2 receptor-binding domain IgG was determined, and variation by age group, sex, race/ethnicity, and diabetes status was analyzed.
Abstract
Background:
Assessing the evolution of SARS-CoV-2 immune response among patients receiving dialysis can define its durability in a highly clinically relevant context because patients receiving dialysis share the characteristics of persons most susceptible to SARS-CoV-2 infection.
Objective:
To evaluate the persistence of SARS-CoV-2 receptor-binding domain (RBD) IgG in seroprevalent patients receiving dialysis.
Design:
Prospective.
Setting:
Nationwide sample from dialysis facilities.
Patients:
2215 patients receiving dialysis who had evidence of SARS-CoV-2 infection as of July 2020.
Measurements:
Remainder plasma from routine monthly laboratories was used to measure semiquantitative RBD IgG index value over 6 months.
Results:
A total of 2063 (93%) seroprevalent patients reached an assay detectable response (IgG index value ≥1). Most (n = 1323, 60%) had responses in July with index values classified as high (IgG ≥10); 1003 (76%) remained within this stratum. Adjusted median index values declined slowly but continuously (July vs. December values were 21 vs. 13; P < 0.001). The trajectory of the response did not vary by age group, sex, race/ethnicity, or diabetes status. Patients without an assay detectable response (n = 137) were more likely to be White and in the younger (18 to 44 years) or older (≥80 years) age groups and less likely to have diabetes and hypoalbuminemia.
Limitation:
Lack of data on symptoms or reverse transcriptase polymerase chain reaction diagnosis, cohort of persons who survived infection, and use of a semiquantitative assay.
Conclusion:
Despite impaired immunity, most seropositive patients receiving dialysis maintained RBD antibody levels over 6 months. A slow and continual decline in median antibody levels over time was seen, but no indication that subgroups with impaired immunity had a shorter-lived humoral response was found.
Primary Funding Source:
Ascend Clinical Laboratories.
Recent studies have begun to delineate breadth and duration of the adaptive immune response to SARS-CoV-2 infection (1–6). Among the antibodies to a set of SARS-CoV-2 antigens—nucleocapsid, receptor-binding domain (RBD) of the spike protein, and S2 domain of the spike protein—IgG responses to the spike protein antigens are among the most durable (3, 5, 6). Studies have also correlated titers of RBD IgG with the ability to neutralize SARS-CoV-2 (5, 7, 8).
Longitudinal studies tracking response to SARS-CoV-2 infection to date have been limited by modest sample sizes (<200) (9, 10), with 1 exception from a homogeneous population from Iceland (6). Early reports emphasized relatively rapid decline or complete disappearance of detectable antibody levels among patients with known infection (1, 4). On the other hand, Wajnberg and colleagues (5) reported high-level and persistent responses for up to 5 months after infection. Although this study included a cross-sectional assessment of 30 082 persons, longitudinal responses were followed among 121 volunteers. In the larger study from Iceland, 1263 persons with SARS-CoV-2 infection were followed for more than 4 months; the authors concluded that antiviral antibodies plateaued after an initial peak near month 2 (6). These studies, and others (2, 3), have been confined to healthy populations or have not separately examined response among subgroups known to have blunted or shortened adaptive immune responses.
In July 2020, we did a survey of a large nationwide sample of patients receiving dialysis in the United States. We showed that patients receiving dialysis can serve as a sentinel population for SARS-CoV-2 seroepidemiology because they are broadly representative of susceptible groups, including older persons, persons of color, and persons with substantial comorbidity (11). Moreover, they have blood drawn monthly, facilitating follow-up testing for antibody responses.
Here, we present 6 months of longitudinal data on the evolution of RBD antibodies in the patients receiving dialysis who were seropositive (n = 2215) in July. Using a semiquantitative commercially available assay, we examine the persistence of antibodies stratified by antibody response level, age group, sex, race/ethnicity, and diabetes status. We also report the characteristics of persons whose antibody response persistently remains below the detectable assay range.
Methods
In July 2020, we tested remainder plasma from 28 503 patients receiving hemodialysis for the presence of total RBD antibody. The patients tested were from 1200 dialysis facilities throughout the United States. Our sampling strategy and testing methods are described in detail elsewhere (11). The mean age, sex, geographic region, and race/ethnicity distribution of our sampled patients matched that of the overall U.S. dialysis population.
Assay Characteristics
The initial antibody test done in July, the Siemens total RBD immunoglobulin assay, measures IgG and IgM antibodies and has a manufacturer-reported sensitivity of 100% and specificity of 99.8% (12). To assess serologic response, we retested all positive samples with sufficient remainder plasma (n = 2215) using a semiquantitative Siemens RBD IgG assay in July 2020 and monthly thereafter (Appendix Figure, available at Annals.org). The Siemens RBD IgG assay is a semiquantitative, 2-step sandwich, indirect, chemiluminescent assay with a manufacturer-reported sensitivity of 96% (95% CI, 93% to 99%) and specificity of 99.9% (CI, 99.6% to 99.9%) for tests done 21 days or more after positive results on reverse transcriptase polymerase chain reaction (RT-PCR). A set of calibration samples processed with each run is used to calculate the numerical index value reported by the instrument. A lot-specific master curve establishes the relationship between relative light units measured in the sample and the index value of the standards to generate an index value for the sample. An index value of 1.0 or greater is considered reactive; as formulated at the time by the manufacturer, an index value of 44.43 was the upper limit of quantification.
Appendix Figure. CONSORT diagram.
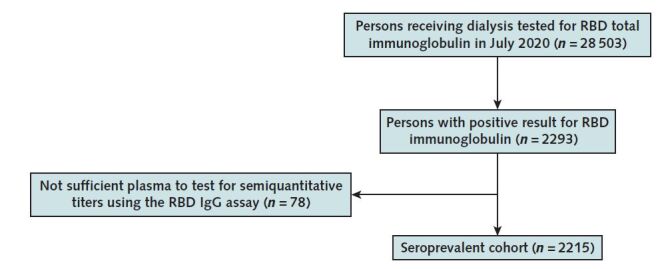
CONSORT = Consolidated Standards of Reporting Trials; RBD = receptor-binding domain.
Stratification of Antibody Responses
We stratified the results as high if the index value was 10 or greater, moderate if between 5 and 10, low if between 1 and 5, and below assay range when less than 1. We chose 10 as the high-value cut point on the basis of data showing that index values of 10 or greater corresponded with pseudovirus neutralization titers (13) greater than 1:500 in a study of 26 patients tested by Siemens. Transfer of convalescent purified IgG with pseudovirus neutralization titers well below this threshold was protective against SARS-CoV-2 challenge in an animal model (14). An additional study (n = 74) evaluating correlation with plaque reduction neutralization test reported that index values of 10 or greater had a positive predictive value of 100% for plaque reduction neutralization test50 greater than 1:80 (Supplement Table) (15, 16).
Correlates
We extracted electronic health record data on age, sex, self-reported race/ethnicity, patient residence (ZIP code), and SARS-CoV-2 RT-PCR result where available. We used ZIP code data to ascertain neighborhood (ZIP code tabulation area level) race/ethnicity composition and the proportion of residents with incomes below the federal poverty threshold. We computed regional COVID-19 case and death rates using data from the American Community Survey (17) and the Center for Systems Science and Engineering at Johns Hopkins University (18), respectively. In laboratory protocols within multiple dialysis networks, routine hemoglobin A1c tests are ordered on a quarterly cycle for patients with diabetes (19). Thus, we used the presence of a hemoglobin A1c test in the preceding 3 months as a proxy for diabetes status.
Statistical Analysis
We provided demographic data and laboratory values using proportions, mean (SD) or median, and 25th to 75th percentile, as applicable.
Among patients with a July index value indicating a reactive test (index ≥1), we estimated unadjusted and adjusted monthly median index values, when appropriate, for age, sex, and neighborhood composition (majority-minority vs. other). We used quantile regression with robust SEs to account for the multiple observations per patient (20), as implemented in Stata qreg and margins commands. In this longitudinal data analysis, model parameters have a population-average interpretation (21). We used quantile regression, and in particular, the median, to describe the data because it is invariant to the data truncation and estimable in all of the analyses presented. Because plasma is collected monthly as part of routine care for patients receiving dialysis, for most of the patients, testing was done within a 28- to 35-day interval (25th to 75th percentile of interval between laboratory tests), allowing us to assume equal spacing between observations and to analyze time as a categorical variable indicating the month of the test. We presented results with or without stratifying patients by their initial antibody index value in July. We also further stratified by age group, sex, diabetes status, and race/ethnicity. The race/ethnicity variable had substantial missingness (about 50%) on self-report. We used neighborhood racial and ethnic composition (Hispanic and/or Black, non-Hispanic White, and integrated) as the primary exposure and presented data stratified by self-reported race/ethnicity as a companion analysis in the Supplement. To present the entire range of index values seen in our antibody quantitation data, we included box plots in the Supplement.
Finally, we described the demographic, community, and health status characteristics of patients from the cohort who did not have assay detectable IgG responses throughout follow-up despite testing positive on the total immunoglobulin test.
Missing Data
For semiquantitative index value results, 80% of the patients with a reactive test in July had complete data for all of the follow-up months. Among the 400 patients missing at least 1 month, 104 had a single (July only) index value. A few patients with missing data returned in subsequent months, whereas others were lost to follow-up (35 vs. 365, respectively). In the main text, we reported results from a complete case analysis where all patients are included but missing records are dropped. Sensitivity analyses restricted to patients with complete data and under missing-at-random assumptions using multiple imputation yielded similar results (Missing Data section of Supplement and Supplement Figure 1).
We assumed statistical significance at an α level of 0.05. All statistical analyses were done with Stata/MP, version 16.1 (StataCorp).
Role of Funding Source
Ascend Clinical Laboratory funded the assays done for this study. Coauthors employed by Ascend Clinical Laboratory (L.C., P.H., R.K., and P.B.) selected the assay, undertook sample processing, and prepared anonymized results for independent analysis and interpretation by Stanford University researchers.
Results
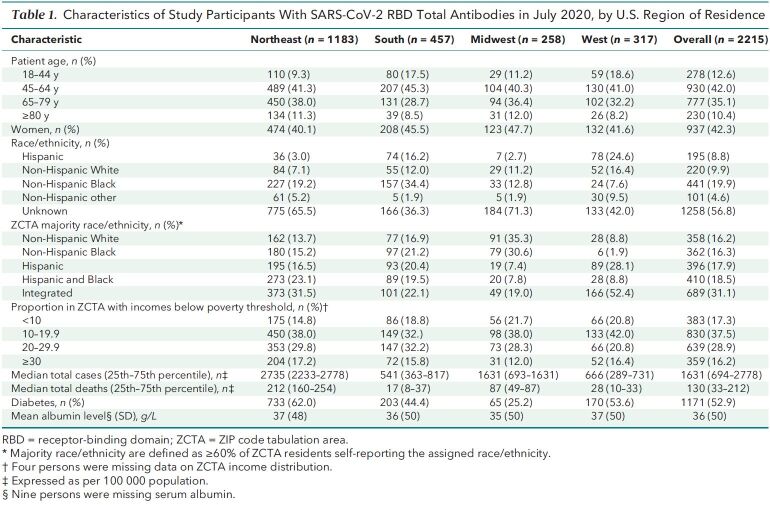
Most (53%) of our patients with total RBD antibodies in July were residents of the U.S. Northeast region, which also had the highest antecedent cumulative case and death rates (Table 1). Fifty-three percent lived in majority-minority neighborhoods; 44% lived in neighborhoods where 20% or more residents had incomes below the federal poverty threshold. About half of the cohort had diabetes, and 30% had a low serum albumin level (<35 g/L).
Table 1. Characteristics of Study Participants With SARS-CoV-2 RBD Total Antibodies in July 2020, by U.S. Region of Residence.
In these seropositive patients with SARS-CoV-2 RBD total antibodies in July, the percentage with RBD IgG index values that were below assay range (<1), low (1 to <5), moderate (5 to <10), and high (≥10) were 9%, 18%, 13%, and 60%, respectively. Among the small subset of patients with a RT-PCR test result available before the July antibody test (n = 46), most (78%) had high IgG index values; 2 (4%) were below assay range.
Longitudinal follow-up showed a small and continuous decline in the median values (Figure 1 and Supplement Figure 1). Among persons with a reactive IgG index value, adjusted median values declined from 21 to 13 from July to December (linear trend test P < 0.001). Overall, 2063 (93%) patients in the cohort had a RBD IgG index value of 1 or greater during follow-up. Of the 210 (9%) patients who had values below assay range in July, 142 (68%) remained below assay range. Of the patients with low index values in July, 84 (22%) reverted to below assay range, compared with 12 (0.7%) patients with moderate to high index values.
Figure 1. The RBD IgG response in a seroprevalent cohort of patients receiving dialysis.
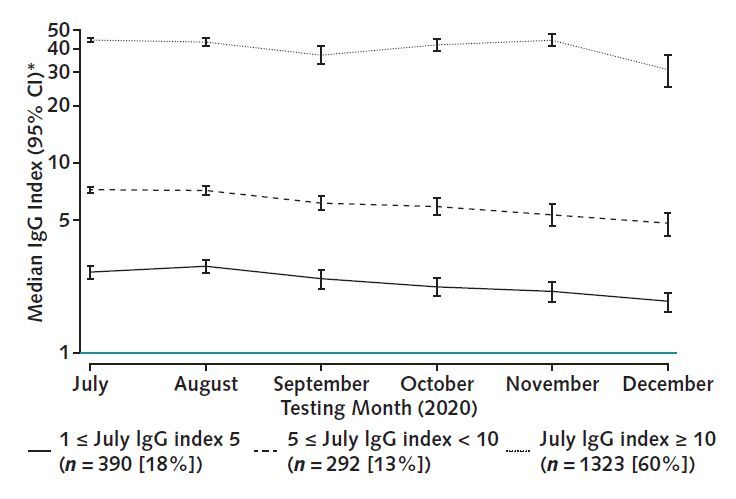
The figure displays adjusted median RBD index values, stratified by response level in July. Most patients (60%) mounted and maintained a high-level semiquantitative index value during the 6 mo of follow-up, with a slow decline over time across all categories of response. Median values account for age, sex, and residence in a majority-minority neighborhood, defined as a majority Hispanic, Black, or Hispanic and Black neighborhood. A total of 210 persons (9%) who had an index value <1 (below assay) in July are not depicted. RBD = receptor-binding domain.
* Plotted on log scale.
In examining the response by demographic, clinical, and geographic characteristics, older patients (aged ≥80 years) had slightly higher median RBD IgG index values in July (median, 16 [25th to 75th percentile, 4 to 44]) than those aged 18 to 44 years (median, 12 [25th to 75th percentile, 2 to 41]). There was no difference in median index values in July between men (median, 16 [25th to 75th percentile, 4 to 44]) and women (median, 17 [25th to 75th percentile, 4 to 44]). Patients with diabetes had higher median values in July (median, 20 [25th to 75th percentile, 5 to 44]) than those without diabetes (median, 13 [25th to 75th percentile, 3 to 44]).
Adjusted median values were similar by region in July (range, 19 to 22 [P = 0.94]) (Figure 2). Over subsequent follow-up, adjusted median values of patients living in the Northeast and Midwest consistently declined from July to December (linear trend test P < 0.001 and P = 0.002, respectively), whereas adjusted median values of patients living in the West and South peaked in August 2020 (Supplement Figures 2 and 3) and declined thereafter.
Figure 2. The RBD IgG response by region.
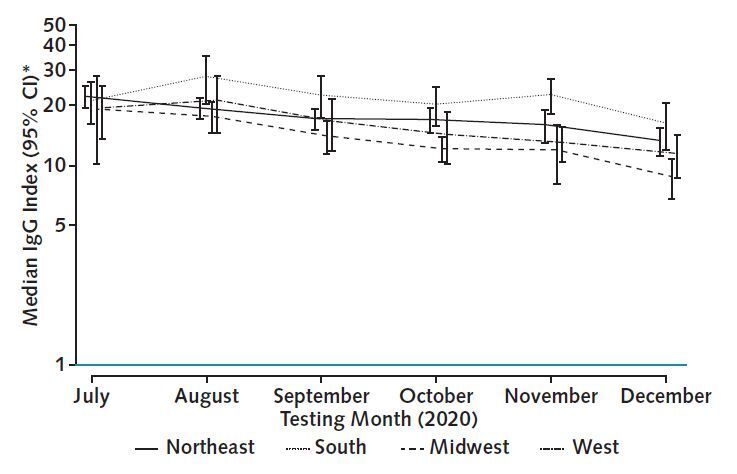
The figure displays overall adjusted median RBD index values, stratified by region. Adjusted medians were similar by region in July. Regions with higher antecedent burden of COVID-19 cases and death (Northeast and Midwest) had a slow, steady decline in index values. In the South and West, the peak occurred in August, indicating proportionally more recent infections in these regions. Median values account for age, sex, and residence in a majority-minority neighborhood, defined as a majority Hispanic, Black, or Hispanic and Black neighborhood. RBD = receptor-binding domain.
* Plotted on log scale.
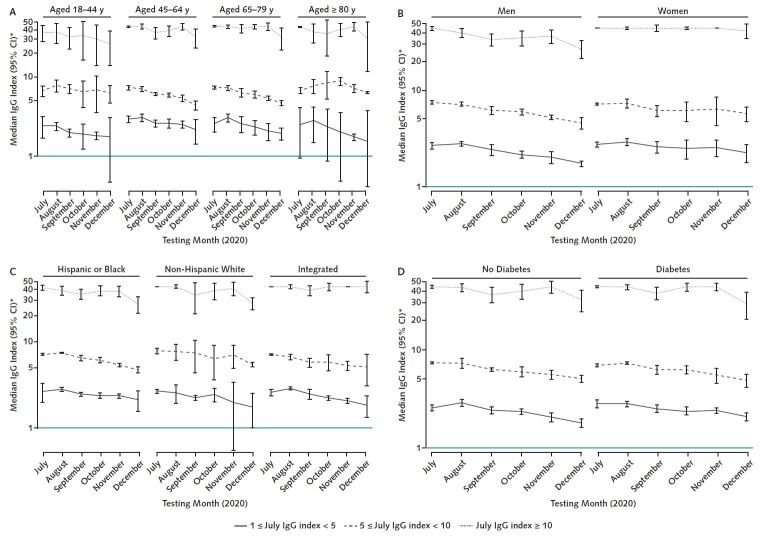
After stratification by initial July response level, there were no major differences in antibody trajectory by age, neighborhood race/ethnicity composition, or diabetes status (Figure 3). A slow decline in adjusted median values was seen for nearly all subgroups. For example, among elderly patients (aged ≥80 years) with moderate index values in July, the adjusted median declined slightly from 7 to 6 between July and December; the index value change in persons aged 18 to 44 years was the same (interaction test P = 0.71). Similarly, among persons living in majority-minority neighborhoods with high index values in July, adjusted median values declined from 41 to 28 between July and December (linear trend test P = 0.006); the corresponding change in index value among persons living in majority-White neighborhoods was 44 to 28 (linear trend test P < 0.001; interaction test P = 0.72). Women with high index values in July did not have substantial decline in adjusted median values between July and December (44 to 42; linear trend test P = 1.0), whereas adjusted median values for men declined from 44 to 26 (linear trend test P < 0.001) (Figure 3, B). However the adjusted 25th percentiles were very similar by sex over the follow-up time (decline from 22 to 13 [linear trend test P < 0.001] for women and 21 to 11 [linear trend test P < 0.001] for men; interaction test P = 0.59), indicating that most women and men stayed within the high index value category during the follow-up period.
Figure 3. The RBD IgG response by age, sex, neighborhood composition, and diabetes status.
All subgroups had a slow decline in RBD IgG over 6 mo of follow-up, with most remaining within their July response level category. Median values account for age, sex, and residence in a majority-minority neighborhood, defined as a majority Hispanic, Black, or Hispanic and Black neighborhood (as appropriate). RBD = receptor-binding domain. A. The lower bound of the 95% CI was truncated at 0.4 for plotting reasons. C. The lower bound of the 95% CI was truncated at 0.7 for plotting reasons.
* Plotted on log scale.
In evaluating unadjusted responses by box plots, we saw a widening of the response range over time without clinically significant differences by subgroups (Supplement Figures 4 to 8).
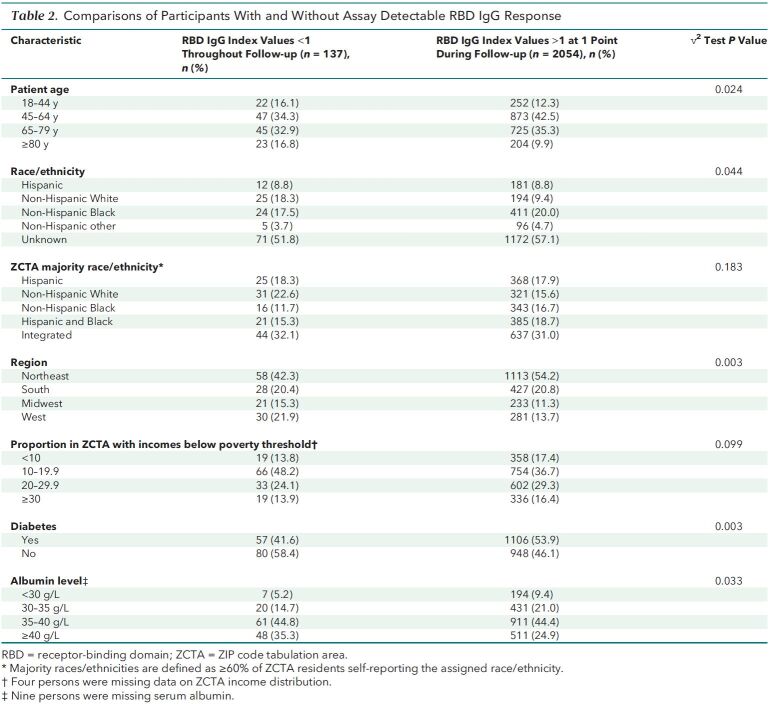
Patients with IgG index values persistently below assay range throughout follow-up were more likely to be in the youngest (18 to 44 years) or oldest (≥80 years) age groups than those with detectable values (Table 2). They were also more likely to be White or living in majority-White neighborhoods, to reside in the U.S. West region, and to live in neighborhoods with lower proportions of residents living in poverty. In terms of health status, patients with IgG index values below assay range were more likely to have an albumin level of 40 g/L or more and less likely to have diabetes.
Table 2. Comparisons of Participants With and Without Assay Detectable RBD IgG Response.
Discussion
In this cohort of patients receiving maintenance dialysis who were seropositive for total SARS-CoV-2 antibodies in July 2020, most demonstrated an RBD IgG response that persisted for at least 6 months after infection. Our cohort provides data on persons with high likelihood of impaired immune responses because more than 40% of persons included are older than 65 years, more than half have diabetes, and all are receiving dialysis. Although we saw a widening in the distribution of these responses, the trajectory of the response did not vary by demographic (for example, older age) or clinical (for example, diabetes) strata that may have been expected to attenuate the adaptive immune response.
Longitudinal data on persistence of antibody response after SARS-CoV-2 infection are conflicting (9), with some studies reporting “rapid” decline (1, 4, 22) and others reporting stability or plateauing of antibody levels (3, 5, 6). In one of the largest analyses of nearly 1300 persons with confirmed SARS-CoV-2 infection and with 4 months of follow-up data, the authors used a commercial RBD IgG assay as well and concluded that there was stability in antibody titers after a peak at 2 months after infection (6). Our analyses confirm persistence of antibodies among a large majority (>90%) of persons over 6 months, but we do not have strong evidence for a plateau in RBD IgG response. Rather, the response seems to show an overall slow and continual decline of the median. In the Northeast, for example, where most patients were likely infected in April (18), we saw a 47% decline in median values between July and December.
Our study also addresses identified gaps in data on differences in humoral immune response among elderly and comorbid populations (10). Data indicating high prevalence of anergy to tuberculin skin tests (23) and impaired response to influenza and hepatitis B vaccination suggest that patients receiving dialysis have impaired humoral immunity (24). In influenza vaccination studies, one (25) focused on H1N1 strain alone and one (26) focused on trivalent vaccine, including H1N1 strain, and reported that fewer than 60% of patients receiving dialysis mounted sufficient titers to be considered immune to H1N1 at 4 weeks, compared with 90% or more of healthy volunteers. In prospective studies of hepatitis B vaccine, only 60% to 70% of patients receiving dialysis mounted a sufficient response and, of these, 40% lost immunity within 1 to 3 years (27, 28). In our characterization of their response to natural SARS-CoV-2 infection, however, we did not find evidence of a shorter-lived humoral immune response compared with the general population. Antibody titers may decline more rapidly among men (29), and our data also suggest a slightly faster decline among men. However, we found no differences in the durability of the response between men and women, and by other clinically significant strata. Comparably, postvaccination data show relative equivalence in efficacy by age and sex (30), although older persons mounted a lower quantitative RBD IgG response in phase 1 data from 1 (31) but not the other (32) mRNA platform vaccine.
Severe SARS-CoV-2 infection elicits higher-level antibody responses than mild infection (4, 7, 9, 33). We have no symptom data on our study sample and, because patients receiving dialysis who are hospitalized with COVID-19 have a high mortality rate (34, 35), a sizeable fraction of patients with the most severe illness may not have survived long enough to be discharged and to have resumed maintenance dialysis. Nevertheless, we found that persons with persistently low or negative IgG responses in our study fell into the groups that are believed to have milder disease—that is, younger persons or persons without diabetes. We also found, however, that persons aged 80 years or older were also somewhat more likely to be in the persistently low or negative category. It is unclear whether this implies a blunted response in a specific subset of the older age group or if it reflects survivor bias (36). Overall, most—204 of 227 persons aged 80 years or older included in our study—did mount a detectable response.
How the level and duration of antibody response informs protection from SARS-CoV-2 reinfection remains unclear. Antibody titers are only 1 marker of immunity, and even persons with low-level or undetectable antibody response can mount a subsequent protective response on reinfection that abrogates symptomatic disease (9, 37, 38). Studies on coronaviruses report a waning of immunity and vulnerability to reinfection at 1 year after infection (39, 40), with modest evidence to suggest susceptibility to infection in persons with lower antibody titers. Among 15 healthy volunteers infected with coronavirus 229E, Callow and colleagues (39) noted that persons with lower preinoculation IgG concentrations were more likely to manifest an infection. On rechallenge 1 year later, 11 of 14 volunteers had reinfection, although only 1 had symptoms. On the basis of these and other data on influenza-like illnesses, experts predict that SARS-CoV-2 may continue to reemerge seasonally (40, 41). Longer follow-up of this cohort of patients receiving dialysis with natural SARS-CoV-2 infection and additional data on status of their vaccination and reinfection will critically advance knowledge about SARS-CoV-2 immunity.
Our analysis is limited by lack of data on several key comorbid conditions and therapeutics (for example, heart failure, chronic obstructive pulmonary disease, chronic liver disease, use of immunosuppressant medications, or use of in-center vs. home dialysis), which could modify the humoral response to infection and modify the competing risk for non–COVID-19–related death. We also did not know the patient's vital status in case of dropout. Moreover, we lack data on SARS-CoV-2 infection by RT-PCR testing or on COVID-19 symptoms. Thus, it is possible that a portion of persons in our study with RBD IgG index below assay had false-positive results on the screening total RBD antibody assay. However, the first assay has been well validated in external studies, including one of 994 prepandemic samples done by Public Health England (42) where its specificity was 99.9% (yielding an expected false-positive rate of 29 samples in our study). Conversely, Irsara and colleagues (43) suggest that lower index value cutoffs could be applied to the Siemens semiquantitative assay to improve its sensitivity. This implies that the patients with “negative” results may have mounted a low-level response and that rather than false-positive total immunoglobulin, we are witnessing false-negative IgG index values. We note also that the index values used with the Siemens RBD IgG assay are considered semiquantitative and do not necessarily have a linear relation to antibody concentrations over the range of values measured. We lack data on dates of infection; thus, our results reflect a lower bound estimate for persistence of antibodies because it is likely that persons residing in the Northeast may have been infected in April during the peak of the regional epidemic. Finally, we measured serial responses to a single antigen; thus, we cannot characterize the breadth of the adaptive immune response.
Our study is the largest to describe longitudinal humoral response in a population that reflects groups most affected by SARS-CoV-2 infection. Furthermore, we are able to assess differences in response among subgroups with highest likelihood of impaired immunity (for example, older persons and persons with diabetes) (10, 44). Measurement of RBD IgG, as opposed to the nucleocapsid immunoglobulins, ensures that our study captures response to natural infection within the context of oncoming vaccines, the early effectiveness of which is being evaluated in part by spike protein RBD IgG response (32). Because we used a commercially available assay, our study can provide reference ranges for clinicians who may assess infection or vaccine response.
In conclusion, nearly all seroprevalent patients in our study had evidence of an assay detectable RBD IgG response through the 6-month follow-up. Most met our assay criteria for a high-level response. We saw a slow and continual decline in median antibody levels over time but found no indication that subgroups with impaired immunity had a shorter-lived humoral response. Our study describes the evolution of SARS-CoV-2 immune response in a large sample of patients receiving dialysis and provides a benchmark for clinicians and researchers assessing humoral response after infection or vaccination in susceptible populations.
Supplementary Material
Footnotes
This article was published at Annals.org on 18 May 2021.
References
- 1. Ibarrondo FJ , Fulcher JA , Goodman-Meza D , et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19 [Letter]. N Engl J Med. 2020;383:1085-1087. [PMID: ] doi: 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seow J , Graham C , Merrick B , et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598-1607. [PMID: ] doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ripperger TJ , Uhrlaub JL , Watanabe M , et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925-933.e4. [PMID: ] doi: 10.1016/j.immuni.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long QX , Tang XJ , Shi QL , et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200-1204. [PMID: ] doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 5. Wajnberg A , Amanat F , Firpo A , et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227-1230. [PMID: ] doi: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gudbjartsson DF , Norddahl GL , Melsted P , et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724-1734. [PMID: ] doi: 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Röltgen K , Powell AE , Wirz OF , et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5. [PMID: ] doi: 10.1126/sciimmunol.abe0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iyer AS , Jones FK , Nodoushani A , et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5. [PMID: ] doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grigoryan L , Pulendran B . The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol. 2020;50:101422. [PMID: ] doi: 10.1016/j.smim.2020.101422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poland GA , Ovsyannikova IG , Kennedy RB . SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595-1606. [PMID: ] doi: 10.1016/S0140-6736(20)32137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anand S , Montez-Rath M , Han J , et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020. [PMID: ] doi: 10.1016/S0140-6736(20)32009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration. EUA authorized serology test performance. Accessed at www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance on 18 March 2021.
- 13. Legros V , Denolly S , Vogrig M , et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318-327. [PMID: ] doi: 10.1038/s41423-020-00588-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMahan K , Yu J , Mercado NB , et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630-634. [PMID: ] doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee WT , Girardin RC , Dupuis AP , et al. Neutralizing antibody responses in COVID-19 convalescent sera. J Infect Dis. 2021;223:47-55. [PMID: ] doi: 10.1093/infdis/jiaa673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Addetia A , Crawford KHD , Dingens A , et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58. [PMID: ] doi: 10.1128/JCM.02107-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Census Bureau American Community Survey. 2018 American Community Survey 5 year estimates, tables B03002, S1701, and B01003. Accessed at https://data.census.gov/cedsci/ on 10 July 2020.
- 18. Dong E , Du H , Gardner L . An interactive web-based dashboard to track COVID-19 in real time [Letter]. Lancet Infect Dis. 2020;20:533-534. [PMID: ] doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Medicare & Medicaid Services. National coverage determination (NCD) for glycated hemoglobin/glycated protein (190.21). Accessed at www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDid=100 on 15 December 2020.
- 20. Hao L, Naiman DQ. Quantile Regression. Sage; 2007.
- 21. Marino MF, Farcomeni A. Linear quantile regression models for longitudinal experiments: an overview. METRON. 2015;73:229-247. doi:10.1007/s40300-015-0072-5
- 22. Ma H , Zhao D , Zeng W , et al. Decline of SARS-CoV-2-specific IgG, IgM and IgA in convalescent COVID-19 patients within 100 days after hospital discharge [Letter]. Sci China Life Sci. 2020. [PMID: ] doi: 10.1007/s11427-020-1805-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang HC , Chou KJ , Chen CL , et al. Tuberculin skin test and anergy in dialysis patients of a tuberculosis-endemic area. Nephron. 2002;91:682-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24. Kato S , Chmielewski M , Honda H , et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526-33. [PMID: ] doi: 10.2215/CJN.00950208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Broeders NE , Hombrouck A , Lemy A , et al. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: a cohort study. Clin J Am Soc Nephrol. 2011;6:2573-8. [PMID: ] doi: 10.2215/CJN.04670511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogtländer NP , Brown A , Valentijn RM , et al. Impaired response rates, but satisfying protection rates to influenza vaccination in dialysis patients. Vaccine. 2004;22:2199-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 27. Peces R , de la Torre M , Alcázar R , et al. Prospective analysis of the factors influencing the antibody response to hepatitis B vaccine in hemodialysis patients. Am J Kidney Dis. 1997;29:239-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 28. Buti M , Viladomiu L , Jardi R , et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am J Nephrol. 1992;12:144-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29. Grzelak L, Velay A, Madec Y, et al. Sex differences in the decline of neutralizing antibodies to SARS-CoV-2. medRxiv. Preprint posted online 15 November 2020. doi:10.1101/2020.11.12.20230466
- 30. Polack FP , Thomas SJ , Kitchin N , et al; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. [PMID: ] doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walsh EE , Frenck RW Jr , Falsey AR , et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439-2450. [PMID: ] doi: 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson EJ , Rouphael NG , Widge AT , et al; mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. [PMID: ] doi: 10.1056/NEJMoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X , Pan Z , Yue S , et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. 2020;5:180. [PMID: ] doi: 10.1038/s41392-020-00301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ng JH , Hirsch JS , Wanchoo R , et al; Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530-1539. [PMID: ] doi: 10.1016/j.kint.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flythe JE , Assimon MM , Tugman MJ , et al; STOP-COVID Investigators. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77:190-203.e1. [PMID: ] doi: 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williamson EJ , Walker AJ , Bhaskaran K , et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [PMID: ] doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumgarth N , Nikolich-Žugich J , Lee FE , et al. Antibody responses to SARS-CoV-2: let's stick to known knowns. J Immunol. 2020;205:2342-2350. [PMID: ] doi: 10.4049/jimmunol.2000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodda LB , Netland J , Shehata L , et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169-183.e17. [PMID: ] doi: 10.1016/j.cell.2020.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Callow KA , Parry HF , Sergeant M , et al. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435-46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edridge AWD , Kaczorowska J , Hoste ACR , et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691-1693. [PMID: ] doi: 10.1038/s41591-020-1083-1 [DOI] [PubMed] [Google Scholar]
- 41. Kissler SM , Tedijanto C , Goldstein E , et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860-868. [PMID: ] doi: 10.1126/science.abb5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Public Health England. Evaluation of sensitivity and specificity of 4 commercially available SARS-CoV-2 antibody immunoassays. Accessed at www.gov.uk/government/publications/covid-19-head-to-head-laboratory-evaluation-of-4-commercial-serological-assays on 8 July 2020.
- 43. Irsara C , Egger AE , Prokop W , et al. Evaluation of four commercial, fully automated SARS-CoV-2 antibody tests suggests a revision of the Siemens SARS-CoV-2 IgG assay. Clin Chem Lab Med. 2021. [PMID: ] doi: 10.1515/cclm-2020-1758 [DOI] [PubMed] [Google Scholar]
- 44. Lipsitch M , Dean NE . Understanding COVID-19 vaccine efficacy. Science. 2020;370:763-765. [PMID: ] doi: 10.1126/science.abe5938 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.