Abstract
The Bacteroidetes are numerically abundant Gram-negative organisms of the distal human gut with a greatly expanded capacity to degrade complex glycans. A subset of these are adept at scavenging host glycans within this environment, including mucin O-linked glycans, N-linked glycoproteins and highly sulfated glycosaminoglycans (GAGs) such as heparin (Hep) and chondroitin sulfate (CS). Several recent biochemical studies have revealed the specific polysaccharide utilization loci (PULs) within the model symbiont Bacteroides thetaiotaomicron for the deconstruction of these host glycans. Here we discuss the Sus-like paradigm that defines glycan uptake by the Bacteroidetes and the salient details of the PULs that target heparin/heparan sulfate (HS) and chondroitin sulfate/dermatan sulfate (DS)/hyaluronic acid (HA), respectively, in B. thetaiotaomicron. The ability of the Bacteroidetes to target highly sulfated host glycans is key to their success in the gut environment but can lead to inflammation in susceptible hosts. Therefore, our continued understanding of the molecular strategies employed by these bacteria to scavenge carbohydrate nutrition is likely to lead to novel ways to alter their metabolism to promote host health.
Keywords: Bacteroides, gut microbiota, heparin, mucin, N-linked glycans
Introduction
Competition for nutrients among resident bacteria of the gastrointestinal tract is fierce, with carbohydrates contributing the most abundant source of energy (Makki et al. 2018; Wilson et al. 2020). Both dietary polysaccharides and host-derived glycans are available food sources. Some gut symbionts are specialists and only metabolize select polysaccharides, while others are generalists and utilize a vast array of these molecules (Cockburn and Koropatkin 2016). The three dominant phyla in the human gut include the Bacteroidetes, Firmicutes and Actinobacteria (Human Microbiome Project Consortium 2012). Within these phyla there is profound diversity in both the glycan preferences and degradation strategies employed by these organisms (Ndeh and Gilbert 2018). The Bacteroides are an abundant genus within the dominant Gram-negative Bacteroidetes phylum (Ding and Schloss 2014). These bacteria have a profound capacity for complex carbohydrate degradation due to the vast repertoire of carbohydrate-active enzymes (CAZymes) in their genomes (El Kaoutari et al. 2013; McNulty et al. 2013). This metabolic flexibility enhances the ability of these organisms to persist in the gut despite changes in the host diet (Rogers et al. 2013; Pudlo et al. 2015; Tuncil et al. 2017).
A consistent source of carbohydrate nutrition for all gut bacteria is host-derived glycans including mucin O-linked glycans, glycosaminoglycans (GAGs) like chondroitin sulfate (CS) and heparan sulfate (HS) and cell surface N-linked glycans (Porter and Martens 2017). As highly adept carbohydrate foragers, many Bacteroides either specialize in host glycan utilization or can switch between dietary and host glycans as their nutritional needs dictate (Rogers et al. 2013; David et al. 2014; Pudlo et al. 2015; Tuncil et al. 2017). The ability of individual Bacteroides species to forage upon this milieu of host glycans dictates their in vivo fitness within the host environment and can have profound effects on host biology including inflammatory processes and susceptibility to pathogens (Martens et al. 2008; Bloom et al. 2011; Hickey et al. 2015; Desai et al. 2016). In this review, we cover some of the known molecular strategies employed by the model human gut symbiont B. thetaiotaomicron to attack these host glycans, as well as the broader biological implications of this metabolism. For detailed descriptions of host glycan degradation by commensal and pathogenic members of the Gram-positive Firmicutes, please see reviews by Nathalie Juge, Wade Abbott and Alisdair Boraston within this issue.
The Bacteroidetes Sus-like paradigm
The Bacteroidetes encode machinery necessary for plant-, host- and microbially derived glycan degradation in discrete polysaccharide utilization loci, or PULs (Martens et al. 2008; Martens et al. 2009; Martens et al. 2011; Koropatkin et al. 2012). Typically, one PUL targets a distinct polysaccharide or glycan type and contains all the enzymes required for its degradation and import. As discussed in detail later, the case for complex host-derived glycan consumption is more complicated (Grondin et al. 2017; Porter and Martens 2017; Luis and Martens 2018). These PULs contain a suite of extracellular and periplasmic glycoside hydrolases (GHs) and/or polysaccharide lyases (PLs) to attack glycosidic linkages (Cartmell et al. 2017; Briliūtė et al. 2019; Ndeh et al. 2020). Also encoded are the requisite cell surface proteins for capture and import of discrete glycan fragments. Generally speaking, even highly decorated glycans appear to be minimally degraded at the cell surface, with most of the specialized enzymatic activity within the cell, perhaps as a way to prevent excessive cross-feeding to bacterial competitors (Ndeh et al. 2017; Luis et al. 2018; Briliūtė et al. 2019).
The archetypal PUL, the Starch utilization system (Sus), from the model bacterium B. thetaiotaomicron (B. theta) was first characterized by Abigail Salyers and colleagues (D’Elia and Salyers 1996; Cho and Salyers 2001). Sus contains a TonB-dependent transporter (TBDT) SusC that requires a partner cell surface lipoprotein SusD for oligosaccharide import (Cho and Salyers 2001). Two surface glycan-binding proteins (SGBPs), SusE and SusF, assist in glycan capture and import with the SusCD complex (Anderson and Salyers 1989; Reeves et al. 1997; Cameron et al. 2014; Foley et al. 2018). Recent structures of other Sus-like systems reveal that proteins akin to SusCDEF likely form a discrete complex, with the SusD-like protein acting as a lid over the SusC pore (Glenwright et al. 2017). Starch is degraded at the cell surface into maltooligosaccharides by an α-amylase lipoprotein SusG (Shipman et al. 1999; Koropatkin and Smith 2010). Upon import, limit dextrins are metabolized by SusA, a pullulanase, and SusB, a glucosidase (D’Elia and Salyers 1996). During processing, the maltose generated binds the regulator SusR to upregulate sus expression (D’Elia and Salyers 1996). Sus is one of the simplest examples of the Bacteroidetes glycan uptake paradigm. As the complexity of the polysaccharide increases, in the case of most host-derived glycans, the complexity of the system increases (Grondin et al. 2017; Porter and Martens 2017). Moreover, as a minimal amount of saccharification occurs outside the cell, SusC-like proteins must accommodate branched glycan fragments or those with chemical modifications (Grondin et al. 2017; Porter and Martens 2017). Critical for host glycan utilization is the ability of bacteria to desulfate substrates that decorate GAGs, such as heparin/heparan sulfate and chondroitin sulfate, complex N-glycans and the dominant MUC2 glycoprotein of the mucus layer, among others.
How Bacteroides tackle the sulfation problem within host substrates
The host glycan targeting Sus-like systems within B. theta have been studied in detail in the past several years, affording a unique mechanistic insight into how a model Bacteroidetes has evolved to process several sulfated glycans (Ulmer et al. 2014; Cartmell et al. 2017; Briliūtė et al. 2019; Ndeh et al. 2020). B. theta encodes at least 28 gene clusters, most as distinct PULs, that target host glycans, with 28 sulfatases distributed among these systems (Benjdia et al. 2011) (Table I). Sulfatases require posttranslational modification and activation by the anaerobic sulfatase-maturating enzyme (anSME) (Berteau et al. 2006) BT0238, an essential fitness factor for persistence in mice fed with a simple sugar, Western style diet that forces host glycan scavenging (Benjdia et al. 2011).
Table I.
B. theta gene clusters involved in host glycan degradation
| PUL locus tag | Target glycan | Number of sulfatases | Number of peptidases | CAZymes | Refs |
|---|---|---|---|---|---|
| BT0455–61; BT0506–7 | CNG, HMO, MOG | 0 | 0 | GH2 (2), GH20(4), GH33 | (Tailford et al. 2007; Martens et al. 2008; Marcobal et al. 2011; Park et al. 2013; Briliūtė et al. 2019) |
| BT0752–7 | Host glycan | 1 (S1_7) | 0 | GH2 | (Martens et al. 2008; Ulmer et al. 2014; Hickey et al. 2015) |
| BT1032–53 | CNG, HMO, MOG | 0 | 0 | GH18 (3), GH20, GH92, GH130, GH163 | (Martens et al. 2008; Koropatkin et al. 2009; Zhu et al. 2010; Marcobal et al. 2011; Briliūtė et al. 2019) |
| BT1278–85 | MOG, HMO | 0 | 0 | GH18 (2) | (Martens et al. 2008;Marcobal et al. 2011 ; Pudlo et al. 2015) |
| BT1617–22 | Host glycan | 1 (S1_20) | 0 | GH20 | (Martens et al. 2008; Hickey et al. 2015) |
| BT1623–36 | MOG, CNG | 3 (S1_11, 15, 20) | 0 | GH2, GH18, GH20, GH29 | (Marcobal et al. 2011; Ulmer et al. 2014; Hickey et al. 2015; Briliūtė et al. 2019) |
| BT2618–33 | YM, HMO, MOG | 0 | 0 | GH67, GH76 (2), GH92, GH97, GH125 | (Zhu et al. 2010; Marcobal et al. 2011; Cuskin et al. 2015a |
| BT2818–26 | MOG, HMO | 0 | 0 | GH16, GH18 | (Martens et al. 2008; Marcobal et al. 2011) |
| BT2912–23 | Host glycan | 1 (S1_7) | 0 | GH2, GH43, GH88, GH105, GH146, GH154 | (Martens et al. 2008; Hickey et al. 2015) |
| BT3010–15 | Host glycan | 0 | 1 | Chitobiase | (Martens et al. 2008; Nakjang et al. 2012) |
| BT3092–109 | Host glycan | 6 (3 S1_4; S1_8,15,67) | 1 (SCP,PF00326) | GH2, GH43 (2), GH51 | (Martens et al. 2008; Ulmer et al. 2014; Hickey et al. 2015) |
| BT3172–80 | HMO (partial) | 1 (S1_11) | 0 | GH2, GH20, GH95, | (Marcobal et al. 2011; Hickey et al. 2015) |
| BT1596; BT3324,3328–34, 3348–50; BT4410 | GAG (HA, CS, DS) | 3 (S1_9,15, 27) | 0 | GH88, PL8 (2), PL29, PL33 | (Martens et al. 2008; Shaya et al. 2008; Raghavan et al. 2014; Ulmer et al. 2014; Ndeh et al. 2018; Helbert et al. 2019; Ndeh et al. 2020) |
| BT3461–507 | Host glycan | 4 (2 S1_4; S1_22, 31) | 1 (M23; PF01551) | GH43, GH76 | (Martens et al. 2008; Hickey et al. 2015) |
| BT3748–54 | MOG | 0 | 0 | GH18 | (Pudlo et al. 2015) |
| BT3773–92 | YM, CNG, HMO | 0 | 0 | GH38, GH76 (2), GH92 (2), GH125, GH130 | (Zhu et al. 2010; Marcobal et al. 2011; Cuskin et al. 2015a; Cuskin et al. 2015b |
| BT3796–800 | Host glycan | 2 (S1_16,27) | 0 | GH29, GH36 | (Hickey et al. 2015) |
| BT3853–62 | YM, MOG, HMO, CNG | 0 | 0 | GH92, GH99 | (Zhu et al. 2010; Marcobal et al. 2011; Cuskin et al. 2015a) |
| BT3957–65 | Host glycans | 0 | 1 (Papain-like, PF00648) | GH92 (3), | (Zhu et al. 2010; Marcobal et al. 2011; Thompson et al. 2018) |
| BT3983–94 | MOG, CNG, YM | 0 | 0 | GH18, GH92 (3) | (Martens et al. 2008; Zhu et al. 2010; Cuskin et al. 2015a; Trastoy et al. 2020) |
| BT4132–37 | HMO, MOG | 0 | 0 | GH29, chitobiase | (Marcobal et al. 2011; Pudlo et al. 2015) |
| BT4240–50 | MOG | 0 | 1 (M60) | GH2, GH109 | (Martens et al. 2008; Nakjang et al. 2012; Pudlo et al. 2015; Noach et al. 2017) |
| BT4266–72 | Host glycan | 0 | 1 (M60) | (Nakjang et al. 2012) | |
| BT4294–99 | HMO, MOG | 0 | 0 | Chitobiase | (Marcobal et al. 2011) |
| BT4355–59 | MOG | 0 | 0 | GH89 | (Martens et al. 2008; Pudlo et al. 2015) |
| BT4402–07 | CNG, MOG | 0 | 0 | GH18 | (Pudlo et al. 2015; Briliūtė et al. 2019) |
| BT4631–36 | Host glycan | 1 (S1_15) | 0 | (Hickey et al. 2015) | |
| BT1596, BT4652–75 | GAG (Hep/HS) | 3 (S1_9, 11 Sulfamidase) | 0 | PL15, PL12 (2), GH88, PL13 | (Martens et al. 2008; Ulmer et al. 2014; Cartmell et al. 2017) |
Bold text indicates those loci specifically referred to in the text. The PULHep described in (Cartmell et al. 2017) Figure 1 is in bold italics. For a more extensive list of genes upregulated in B. theta during growth on host glycans, see Martens et al. 2008. MOG, mucin O-glycans; CNG, complex N-glycans; HMO, human milk oligosaccharides; YM, yeast α-mannan. HMO and YM were included because they demonstrate overlap with loci upregulated during growth on CNG and MOG. The classification of sulfatases and peptidases is according to SulfAtlas (Barbeyron et al. 2016) and Pfam (El-Gebali et al. 2019), respectively.
Recent work into the role of sulfatases in GAG consumption has been described in exquisite detail in several key studies (Ulmer et al. 2014; Cartmell et al. 2017; Ndeh et al. 2020). The central goals of this work were to address if desulfation precedes glycan backbone cleavage and if B. theta possessed a suite of nonspecific PLs and GHs that cleave regardless of sulfation status. Cumulatively, this work established the discrete metabolic steps in the deconstruction of several GAGs which are prioritized nutrient sources for some Bacteroides species (Rogers et al. 2013; Tuncil et al. 2017). As examples, we focus on the B. theta heparin and chondroitin sulfate degradation pathways.
HS is prevalent in the extracellular matrix of epithelial cells and likely released during cell turnover, therefore serving as a key nutrient source for colonic bacteria including B. theta (Rogers et al. 2013; Li et al. 2015; Porter and Martens 2017; Tuncil et al. 2017). HS and Hep are comprised of repeating units of glucosamine (GlcN) α1,4 linked to a uronic acid (UA), iduronic acid (IdoA) or glucuronic acid (GlcA), which are α1,4 and β1,4 linked, respectively, to the next GlcN. The UA can be sulfated at O2, whereas GlcN can be N-acetylated, N-sulfated, O6-sulfated and, in some rare cases, O3-sulfated (Gandhi and Mancera 2008; Shriver et al. 2012). Heparin is almost completely sulfated, whereas HS is highly variably. The growth on either substrate upregulates PULHep, from BT4652 to BT4663, as well as the 2O-sulfatase BT1596 and the PL13 BT4675 (Figure 1) (Martens et al. 2008; Cartmell et al. 2017).
Fig. 1.
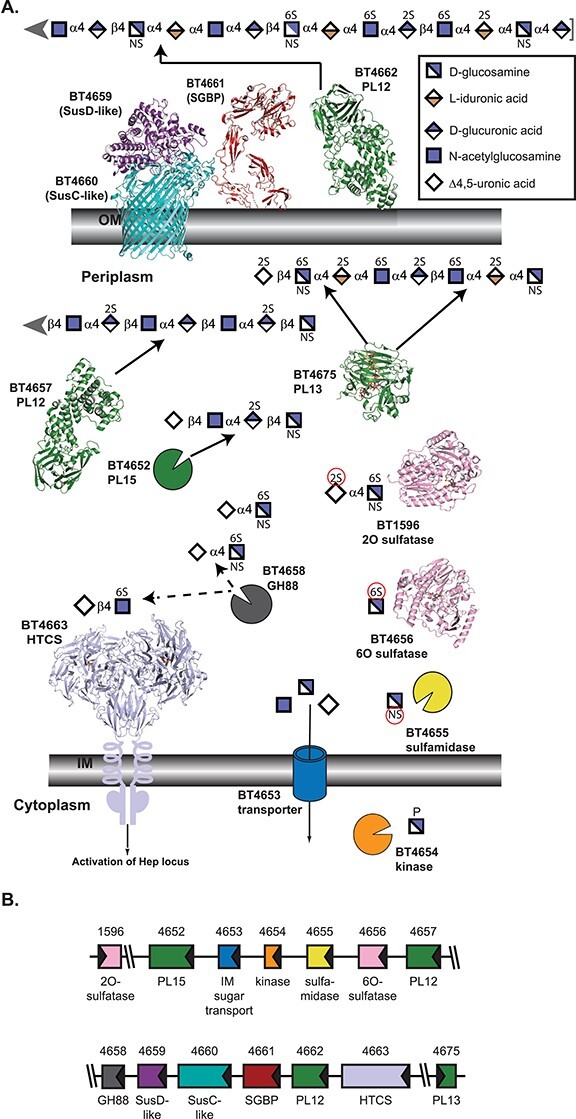
Architecture and activities within B. theta PULHEP, as described by Cartmell et al. 2017. (A) Cartoon representation of the extracellular and periplasmic components of PULHep. Heparin/heparan sulfate is captured at the cell surface, minimally processed by BT4662-PL12, and the oligosaccharides are imported by the SusCD-like complex with assistance from the SGBP BT4661. Once imported, an elaborate network of enzymes reduces the glycan to desulfated monosaccharides. The SusC-like protein is not the Hep SusC-like protein but is the recently determined homolog BT2264 (Glenwright et al. 2017) (PDB 4FQ8). The SusD-like protein is the Hep SusD-like protein (PDB 3IHV). The SGBP structure is a combination of the ligand (Δ4,5UA2S-GlcNS6S-IdoA2S-GlcN6S-IdoA2S-GlcNS6S) from PDB 4AK2 and the full-length protein from PDB 4AK1 (Cartmell et al. 2017). SGBP ligand and all subsequent ligands are colored in dark gray. Unliganded 4662-PL12 is PDB 4FNV (Dong et al. 2012). Unliganded 4657-PL12 is PDB 5JMF (Ulaganathan et al. 2017). 4675-PL13 is shown complexed with a heparin-derived dodecasaccharide and PDB 3INA (Han et al. 2009). The 2O-sulfatase BT1596-S1_9 is complexed with Δ4,5UA2S-GlcNS6S (PDB 5G2T (Cartmell et al. 2017)). The 6O-sulfatase BT4656-S1_11 is complexed with GlcNS6S (PDB 5G2V (Cartmell et al. 2017)). The dimeric periplasmic domain of the HTCS BT4663 is complexed with Δ4,5UA-GlcNAc6S (PDB 4A2M (Lowe et al. 2012)). (B) Architecture of the genetic loci. The gene representations are to scale and colored the same as their protein products.
PULHep has four PL enzymes, but only BT4662-PL12 is found on the cell surface, underscoring the trend that minimal extracellular breakdown occurs (Cartmell et al. 2017). BT4662-PL12 is an endo-acting enzyme that preferentially cleaves low sulfation regions of HS as well as desulfated HS (Dong et al. 2012). The SGBP BT4661 that is central for scavenging liberated glycan fragments can accommodate both sulfated and nonsulfated substrates, as would be found in both Hep and HS (Cartmell et al. 2017).
Due to minimal extracellular processing and a lack of extracellular sulfatases within this gene cluster, the periplasmic enzymatic machinery must be equipped to handle sulfated substrates. The periplasmic BT4657-PL12 performs endo-cleavage on primarily nonsulfated substrates, preferring HS and completely degrading desulfated Hep (Ulaganathan et al. 2017). Both BT4662- and BT4657-PL12 enzymes exhibit some endo-processive character. In contrast, BT4675-PL13 is highly endo-processive and primarily attacks sulfated Hep, consistent with the basicity of its active site (Han et al. 2009). Finally, the exo-processive BT4652-PL15 prefers sulfated substrates but can act on nonsulfated ones, serving as a funnel for the periplasmic enzyme products due to its flexible substrate recognition (Cartmell et al. 2017). The necessity of this PL15 to clean up and capture products with varying sulfation is confirmed by its conservation in other Bacteroides Hep PULs.
BT1596-S1_9 must first remove 2O-sulfates from limit oligosaccharides because BT4658-GH88 cannot tolerate sulfate at this position when digesting Δ4,5-unsaturated disaccharides (Cartmell et al. 2017). BT4658-GH88 competes for substrates with the hybrid two-component system regulator BT4663, modulating upregulation in the process (Lowe et al. 2012). The 6O-sulfatase, BT4656-S1_11, functions primarily on monosaccharides with an absolute requirement for Glc-based substrates (Ulmer et al. 2014). Although it could not be biochemically characterized, deletion of BT4655 from the B. theta genome led to sulfamidated substrate accumulation, suggesting the protein product is a sulfamidase (Cartmell et al. 2017).
The PULHep work revealed that glycan backbone cleavage precedes desulfation. Whereas B. theta relies on significant endo- and exo-processivity and variable sulfation tolerance to harness Hep/HS, the recent work on the B. theta PULCS/DS/HA that targets two types of CS, DS and hyaluronic acid (HA) suggests this is not a conserved feature in sulfated polysaccharide breakdown (Ndeh et al. 2020). These glycans harbor α1,3 IdoA and β1,3 GlcA linkages to the invariantly acetylated amino-sugar and β1,4 linkages between the amino sugar and next UA. HA is comprised of GlcNAc, whereas CS/DS contains GalNAc. GalNAc can be O4- or O6-sulfated and the UA can be O2-sulfated (Figure 2) (Gandhi and Mancera 2008).
Fig. 2.
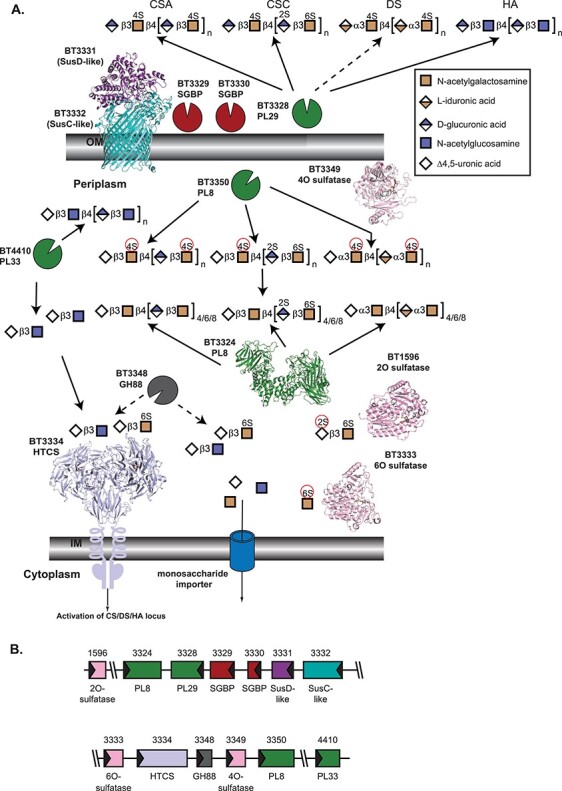
Architecture and activities within B. theta PULCS/DS/HA, as described by Ndeh et al. 2020. (A) Cartoon representation of extracellular and periplasmic components of PULCS/DS/HA. Carbohydrate at the cell surface undergoes some processing by BT3328-PL29, and the oligosaccharides are imported by the SusCD-like complex with help from two SGBP proteins, BT3329 and BT3330. Once imported, a suite of PL enzymes, a GH88, and three sulfatases depolymerize and desulfate all four carbohydrates shown. As in Figure 1A, the SusC-like protein is not the CS/DS/HA SusC-like protein but is the recently determined homolog BT2264 (Glenwright et al. 2017) (PDB 4FQ8). The SusD-like protein is that from PULHEP (PDB 3IHV). Unliganded BT3324-PL8 is PDB 2Q1F (Shaya et al. 2008). The 2O-sulfatase BT1596-S1_9 is complexed with Δ4,5UA2S-GlcNS6S (PDB 5G2T (Cartmell et al. 2017)). The 4O-sulfatase BT3349-S1_27 (PDB 6S21) is complexed with Δ4,5UA-GalNAc4S-GlcA, colored in gray (Ndeh et al. 2020). The 6O-sulfatase BT3333-S1_15 (PDB 6S20) is complexed with GalNAc6S, colored in gray (Ndeh et al. 2020). Instead of the cognate HTCS regulator, BT3334, the dimeric periplasmic domain of the PULHEP BT4663-HTCS is shown complexed with Δ4,5UA-GlcNAc6S (PDB 4A2M (Lowe et al. 2012)). (B) Architecture of the genetic loci. The gene representations are to scale and colored the same as their protein products.
PULCS/DS/HA spans three clusters within BT3324-3350 and two additional genes, the 2O-sulfatase BT1596-S1_9 and PL33 BT4410 that are required for growth on CS (Martens et al. 2011; Raghavan et al. 2014). PULCS/DS/HA encodes a single SusC/D-like pair and a single extracellular enzyme, a PL29-family BT3328, the first identified in this family (Ndeh et al. 2018). CS/DS/HA recognition requires two SGBPs, BT3329 and BT3330, likely due to the greater diversity in monosaccharides and sulfation of these polysaccharides (Ndeh et al. 2020). Both proteins bind all four polysaccharides, albeit with variable preferences.
Once in the periplasm, oligosaccharides encounter two PL8 enzymes and a newly discovered PL33-family enzyme (Helbert et al. 2019). BT3350-PL8 exhibits broad specificity in a primarily endo-fashion but prefers sulfated substrate (Ndeh et al. 2020). Conversely, BT3324-PL8 is exo-active on all four GAGs tested, predominantly releasing disaccharides from O4-sulfated oligos. Substrate binding is predicted to induce active site rearrangements such that the enzyme can accommodate both UA epimers (Shaya et al. 2008). BT4410-PL33 is endo-processive and prefers nonsulfated targets. The strategy adopted here was described as an endo-exo synergy, distinguished from the PULHep processivity (Ndeh et al. 2020). Three sulfatases are deployed, two of which, BT3333-S1_15 and BT3349-S1_27, are encoded in the PUL. The third, BT1596-S1_9 (described above), is active on all O2-sulfated disaccharides. BT3349-S1_27 is an endo-active 4O-sulfatase although the rationale for endo-activity is unclear. BT3333-S1_15 is a 6O-sulfatase and is only active on GalNAc6S. While these two sulfatases contain predicted SPII signal peptides suggesting localization to the outer leaflet of the outer membrane, biochemical evidence reveals they are likely periplasmic, highlighting the importance of validating protein cellular geography (Ndeh et al. 2020). Finally, BT3348-GH88 targets the β1,3 linkages of disaccharides. Analogous to PULHEP, BT3348-GH88 competes for substrate with the HTCS regulator, BT3334, which both target Δ4,5UA-GalNAc6S and Δ4,5UA-GlcNAc (Raghavan et al. 2014). Genomic analyses suggest a variable presence of PULCS/DS/HA among the Bacteroidetes, being most prevalent in Bacteroides (Ndeh et al. 2020). Notably, the growth phenotypes emphasized the importance of orthologous protein machinery and not necessarily conserved PUL organization.
Ensuing complexity: Bacteroides vs. N- and O-linked host glycans
Despite the importance of desulfation for B. theta host glycan consumption and that bacterial sulfatases are significantly upregulated in fiber-free conditions (Benjdia et al. 2011; Hickey et al. 2015; Desai et al. 2016), the degradation of non-GAG substrates has only recently been addressed. O-linked mucin glycans are incredibly complex and harbor additional substituents such as fucose, sialic acid and GlcNAc (Brockhausen and Stanley 2017). Unsurprisingly, transcripts corresponding to enzymes recognizing and hydrolyzing these sugars are upregulated in fiber-free conditions (Desai et al. 2016), some of whose protein products have been characterized (Sakurama et al. 2012; Park et al. 2013). Beyond the Bacteroidetes phylum, fucosidases and N-acetyl-glucosaminidases have been the subject of the recent work in commensal and pathogenic Firmicutes (Pluvinage et al. 2019; Wu et al. 2020). The distribution of these activities highlights the need for a mechanistic understanding of these enzymes as some are targeted by therapeutics to ameliorate diseases related to aberrant mucin maintenance (discussed below).
O-linked and N-linked glycans are often capped by similar modifications (Brockhausen and Stanley 2017; Stanley et al. 2017; Stanley and Cummings 2017); thus, it may not be surprising that B. theta upregulates many of the same loci during growth on each substrate in vitro (Table I). Recent data on the enzymology of mammalian N-glycan processing by B. theta highlight the incredible coordination of multiple enzymes for breakdown of these diverse structures (Briliūtė et al. 2019). It follows that RNA-Seq analysis on cells grown on the model complex N-glycan (CNG) substrate, bovine α1-acid glycoprotein, invoked six loci, including four SusCD-like pairs (Briliūtė et al. 2019). Hydrolysis of the glycan from protein via GH18s, sialic acid removal by BT0455-GH33 (Park et al. 2013) and BT1035-GH163 attack on β-GlcNAc-mannose are required for optimal uptake of oligosaccharides. Periplasmic removal of galactose and fucose enables access of multiple GH20 enzymes to the remaining glycan content (Briliūtė et al. 2019).
Whereas certain N-glycans can be metabolized upon removal from the protein surface by a GH18, mucin O-glycans may be more complicated. Access to this rich source of sugars may be conferred, in part, via cleavage of mucin glycoproteins by M60-like peptidases (Nakjang et al. 2012). One of these, BT4244-M60, degrades the model mucin glycoprotein bovine submaxillary mucin but recognizes the Ser/Thr α-linked GalNAc residue, not a more derivatized glycan chain. This work established that peptidase recognition is driven more by the monosaccharides than the amino acids occupying subsites (Noach et al. 2017). These peptidases, along with the sulfatases, are much less prevalent in gut-associated Firmicutes compared to the Bacteroidetes, possibly contributing to their evolutionary success in inhabiting the human gastrointestinal tract (Berteau et al. 2006; Benjdia et al. 2011; Nakjang et al. 2012). B. theta encodes four predicted M60-like proteases along with 28 sulfatases (Benjdia et al. 2011; Nakjang et al. 2012); for a species considered a microbiota generalist, this is a large amount of protein machinery to maintain.
There is a paucity of mechanistic data regarding how B. theta sulfatases confer access to mucin O-glycans. This may be due, in part, to the lack of biochemical insight into how an extensive PUL repertoire is coordinated to degrade this substrate (Sonnenburg et al. 2005; Martens et al. 2008). Individual enzymes and proteins have been studied, some of which overlap with those required for N-glycan consumption (Koropatkin et al. 2009; Briliūtė et al. 2019; Trastoy et al. 2020). Others have been characterized for their potential as therapeutic targets (Offen et al. 2009; Zhu et al. 2010; Thompson et al. 2012).
Health consequences of host glycan degradation
Foraging of intestinal mucosal glycans by gut species has both positive and negative consequences for both the microbiota and the host. Through the continuous secretion of mucus glycoproteins, the host provides a continuous nutrition source for some bacteria with the ability to breakdown these glycan structures and shields species that can access this niche from starvation despite changes in dietary carbohydrate consumption (Koropatkin et al. 2012). Indeed, some species such as Akkermansia muciniphila, which predominantly consumes mucus, have been linked to improved blood glucose tolerance and therefore less energy harvest as fat storage, though the precise mechanism is unclear (Derrien et al. 2004; Sonnenburg and Bäckhed 2016; Depommier et al. 2019). It has been postulated that in otherwise healthy individuals, microbial consumption of the mucus layer aids in renewal of this protective surface.
Breaches in the integrity of the mucus layer have been linked to inflammatory bowel conditions including Crohn’s disease and ulcerative colitis (Png et al. 2010; Stange and Schroder 2019). In murine models, deficiencies in the production of the core glycan structures 1 and 3 are linked to spontaneous colitis due to thinning of the mucus layer and encroachment of the microbial community close to the epithelial layer (Fu et al. 2011; Bergstrom et al. 2017). However, even without mucus synthesis defects, several key studies have specifically demonstrated that mucosal glycan foraging by Bacteroides can trigger colitis in a susceptible host background. Mucinolytic Bacteroides species including B. theta trigger spontaneous colitis in dnKO mice that are deficient in both IL10 and TGF-β signaling (Bloom et al. 2011). Species of Bacteroides that cannot degrade the mucus layer fail to cause disease, and mutants of B. theta that lack the ability to desulfate mucosal glycans fail to elicit disease (Hickey et al. 2015). In a separate study, formerly germ-free wild-type mice colonized with a synthetic microbiota including the mucin-degrading species B. theta, Bacteroides caccae, Barnesiella intestinihominis and A. muciniphila were placed on diets with different fiber compositions including a diet lacking all dietary fiber (Desai et al. 2016). Significant erosion of the outer mucus layer was noted in animals on the fiber-free diet, with a concomitant increase in those species associated with mucus degradation. While thinning of the mucus layer alone in a wild-type background did not elicit inflammation, these animals were susceptible to infection by Citrobacter rodentium, whereas animals colonized with the same synthetic community and fed a diet with one or more sources of dietary fiber were both protected from mucosal thinning and infection (Desai et al. 2016).
As we learn more about how specific members of the gut microbiota access host glycans, we will find new opportunities to manipulate this critical interaction. It is well understood that we rely on our microbial symbionts to maintain our health and fight disease, but host genetics and environmental factors affect this ongoing dialogue. As we gather molecular data on this interaction, we will have opportunities to change the metabolism of individual gut members toward personalized therapeutic interventions.
Funding
National Institutes of Health (R01 GM118475 to N.M.K.).
Conflict of interest statement
None declared.
References
- Anderson KL, Salyers AA. 1989. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 171:3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeyron T, Brillet-Guéguen L, Carré W, Carrière C, Caron C, Czjzek M, Hoebeke M, Michel G. 2016. Matching the diversity of sulfated biomolecules: Creation of a classification database for sulfatases reflecting their substrate specificity. PLoS One. 11:e0164846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjdia A, Martens EC, Gordon JI, Berteau O. 2011. Sulfatases and a radical S-adenosyl-l-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 286:25973–25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom K, Fu J, Johansson MEV, Liu X, Gao N, Wu Q, Song J, McDaniel JM, McGee S, Chen W, et al. 2017. Core 1- and core 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 10:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteau O, Guillot A, Benjdia A, Rabot S. 2006. A new type of bacterial sulfatase reveals a novel maturation pathway in prokaryotes. J Biol Chem. 281:22464–22470. [DOI] [PubMed] [Google Scholar]
- Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM Jr, Allen PM, Stappenbeck TS. 2011. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 9:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briliūtė J, Urbanowicz PA, Luis AS, Baslé A, Paterson N, Rebello O, Hendel J, Ndeh DA, Lowe EC, Martens EC, et al. 2019. Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat Microbiol. 4:1571–1581. [DOI] [PubMed] [Google Scholar]
- Brockhausen I, Stanley P. 2017. O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology [Internet]. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [Google Scholar]
- Cameron EA, Kwiatkowski KJ, Lee BH, Hamaker BR, Koropatkin NM, Martens EC. 2014. Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. MBio. 5:e01441–e01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell A, Lowe EC, Baslé A, Firbank SJ, Ndeh DA, Murray H, Terrapon N, Lombard V, Henrissat B, Turnbull JE, et al. 2017. How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans. Proc Natl Acad Sci U S A. 114:7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Salyers AA. 2001. Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 183:7224–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn DW, Koropatkin NM. 2016. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol. 428:3230–3252. [DOI] [PubMed] [Google Scholar]
- Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al. 2015a. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 517:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskin F, Baslé A, Ladevèze S, Day AM, Gilbert HJ, Davies GJ, Potocki-Véronèse G, Lowe EC. 2015b. The GH130 family of mannoside phosphorylases contains glycoside hydrolases that target β-1,2-mannosidic linkages in Candida mannan. J Biol Chem. 290:25023–25033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia JN, Salyers AA. 1996. Contribution of a neopullulanase, a pullulanase, and an α-glucosidase to growth of Bacteroides thetaiotaomicron on starch. J Bacteriol. 178:7173–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia JN, Salyers AA. 1996. Effect of regulatory protein levels on utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 178:7180–7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vierira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med. 25:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia muciniphila gen. Nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 54:1469–1476. [DOI] [PubMed] [Google Scholar]
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. 2016. A dietary fiber-derived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 167:1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Schloss PD. 2014. Dynamics and associations of microbial community types across the human body. Nature. 509:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lu W, McKeehan WL, Luo Y, Ye S. 2012. Structural basis of heparan sulfate-specific degradation by heparinase III. Protein Cell. 3:950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, et al. 2019. The Pfam protein families database in 2019. Nucleic Acids Res. 47:D427–D432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. 2013. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 11:497–504. [DOI] [PubMed] [Google Scholar]
- Foley MH, Martens EC, Koropatkin NM. 2018. SusE facilitates starch uptake independent of starch binding in B. thetaiotaomicron. Mol Microbiol. 108:551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wei B, Wen T, Johansson MEV, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al. 2011. Loss of intestinal core 1–derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 121:1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL. 2008. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 72:455–482. [DOI] [PubMed] [Google Scholar]
- Glenwright AJ, Pothula KR, Bhamidimarri SP, Chorev DS, Baslé A, Firbank SJ, Zheng H, Robinson CV, Winterhalter M, Kleinekathöfer U, et al. 2017. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature. 541:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin JM, Tamura K, Déjean G, Abbott DW, Brumer H. 2017. Polysaccharide utilization loci: Fueling microbial communities. J Bacteriol. 199:e00860–e00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YH, Garron ML, Kim HY, Kim WS, Zhang Z, Ryu KS, Shaya D, Xiao Z, Cheong C, Kim YS, et al. 2009. Structural snapshots of heparin depolymerization by heparin lyase I. J Biol Chem. 284:34019–34027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbert W, Poulet L, Drouillard S, Mathieu S, Loiodice M, Couturier M, Lombard V, Terrapon N, Turchetto J, Vincentelli R, et al. 2019. Discovery of novel carbohydrate-active enzymes through the rational exploration of the protein sequences space. Proc Natl Acad Sci U S A. 116:6063–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, et al. 2015. Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe. 17:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium . 2012. Structure, function and diversity of the healthy human microbiome. Nature. 486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 10:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Martens EC, Gordon JI, Smith TJ. 2009. Structure of a SusD homologue, BT1043, involved in mucin O-glycan utilization in a prominent human gut symbiont. Biochemistry. 48:1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Smith TJ. 2010. SusG: A unique cell-membrane-associated α-amylase from a prominent human gut symbiont targets complex starch molecules. Structure. 18:200–215. [DOI] [PubMed] [Google Scholar]
- Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, et al. 2015. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 6:8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EC, Baslé A, Czjzek M, Firbank SJ, Bolam DN. 2012. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc Natl Acad Sci U S A. 109:7298–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Baslé A, Cartmell A, Terrapon N, Stott K, et al. 2018. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat Microbiol. 3:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AS, Martens EC. 2018. Interrogating gut bacterial genomes for the discovery of novel carbohydrate degrading enzymes. Curr Opin Chem Biol. 47:126–133. [DOI] [PubMed] [Google Scholar]
- Makki K, Deehan EC, Walter J, Bäckhed F. 2018. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 23:705–715. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, et al. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 10:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Chiang HC, Gordon JI. 2008. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 4:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Koropatkin NM, Smith TJ, Gordon JI. 2009. Complex glycan catabolism by the human gut microbiota: The Bacteroidetes sus-like paradigm. J Biol Chem. 284:24673–24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, et al. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 9:e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, et al. 2013. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol. 11:e1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakjang S, Ndeh DA, Wipat A, Bolam DN, Hirt RP. 2012. A novel extracellular metallopeptidase domain shared by animal host-associated mutualistic and pathogenic microbes. PLoS One. 7:e30287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeh D, Baslé A, Strahl H, Yates EA, McClurgg UL, Henrissat B, Terrapon N, Cartmell A. 2020. Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus. Nat Commun. 11:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeh D, Gilbert HJ. 2018. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol Rev. 42:146–164. [DOI] [PubMed] [Google Scholar]
- Ndeh D, Munoz JM, Cartmell A, Bulmer D, Wills C, Henrissat B, Gray J. 2018. The human gut microbe Bacteroides thetaiotaomicron encodes the founding member of a novel glycosaminoglycan-degrading polysaccharide lyase family PL29. J Biol Chem. 293:17906–17916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeh D, Rogowski A, Cartmell A, Luis AS, Baslé A, Gray J, Venditto I, Briggs J, Zhang X, Labourel A, et al. 2017. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 544:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach I, Ficko-Blean E, Pluvinage B, Stuart C, Jenkins ML, Brochu D, Buenbrazo N, Wakarchuk W, Burke JE, Gilbert M, et al. 2017. Recognition of protein-linked glycans as a determinant of peptidase activity. Proc Natl Acad Sci U S A. 114:E679–E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen WA, Zechel DL, Withers SG, Gilbert HJ, Davies GJ. 2009. Structure of the Michaelis complex of β-mannosidase, Man2A, provides insight into the conformational itinerary of mannoside hydrolysis. Chem Commun (Camb). 14:2484–2486. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim MG, Ahn HJ, Lee DH, Kim JH, Kim YW, Woo EJ. 2013. Structural and biochemical characterization of the broad substrate specificity of Bacteroides thetaiotaomicron commensal sialidase. Biochim Biophys Acta. 1834:1510–1519. [DOI] [PubMed] [Google Scholar]
- Pluvinage B, Massel PM, Burak K, Borason AB. 2019. Structural and functional analysis of four family 84 glycoside hydrolases from the opportunistic pathogen Clostridium perfringens. Glycobiology 30:49–57.i [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. 2010. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 105:2420–2428. [DOI] [PubMed] [Google Scholar]
- Porter NT, Martens EC. 2017. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol. 71:349–369. [DOI] [PubMed] [Google Scholar]
- Pudlo NA, Urs K, Kumar SS, German JB, Mills DA, Martens EC. 2015. Symbiotic human gut bacteria with variable metabolic priorities for host mucosal glycans. MBio. 6:e01282–e01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V, Lowe EC, Townsend GE 2nd, Bolam DN, Groisman EA. 2014. Tuning transcription of nutrient utilization genes to catabolic rate promotes growth in a gut bacterium. Mol Microbiol. 93:1010–1025. [DOI] [PubMed] [Google Scholar]
- Reeves AR, Wang GR, Salyers AA. 1997. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 179:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TE, Pudlo NA, Koropatkin NM, Bell JSK, Balasch MM, Jasker K, Martens EC. 2013. Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol Microbiol. 88:876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurama H, Tsutsumi E, Ashida H, Katayama T, Yamamoto K, Kumagai H. 2012. Differences in the substrate specificities and active-site structures of two α-l-fucosidases (glycoside hydrolase family 29) from Bacteroides thetaiotaomicron. Biosci Biotechnol Biochem. 76:1022–71024. [DOI] [PubMed] [Google Scholar]
- Shaya D, Hahn BS, Bjerkan TM, Kim WS, Park NY, Sim JS, Kim YS, Cygler M. 2008. Composite active site of chondroitin lyase ABC accepting both epimers of uronic acid. Glycobiology. 18:270–277. [DOI] [PubMed] [Google Scholar]
- Shipman JA, Cho KH, Siegel HA, Salyers AA. 1999. Physiological characterization of SusG, an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 181:7206–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver Z, Capila I, Venkataraman G, Sasisekharan R. 2012. Heparin and heparan sulfate: Analyzing structure and microheterogeneity. Handb Exp Pharmacol. 207:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Bäckhed F. 2016. Diet–microbiota interactions as moderators of human metabolism. Nature. 535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 307:1955–1959. [DOI] [PubMed] [Google Scholar]
- Stange EF, Schroder BO. 2019. Microbiota and mucosal defense in IBD: An update. Expert Rev Gastroenterol Hepatol. 13:963–976. [DOI] [PubMed] [Google Scholar]
- Stanley P, Cummings RD. 2017. Structures Common to Different Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology [Internet]. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Stanley P, Taniguchi N, Aebi M. 2017. N-Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology [Internet]. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [Google Scholar]
- Tailford LE, Money VA, Smith NL, Dumon C, Davies GJ, Gilbert HJ. 2007. Mannose foraging by Bacteroides thetaiotaomicron: Structure and specificty of the beta-mannosidase BtMan2A. J Biol Chem. 282:11291–11299. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Williams RJ, Hakki Z, Alonzi DS, Wennekes T, Gloster TM, Songsrirote K, Thomas-Oates JE, Wrodnigg TM, Spreitz J, et al. 2012. Structural and mechanistic insight into N-glycan processing by endo-α-mannosidase. Proc Natl Acad Sci U S A. 109:781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Spears RJ, Zhu Y, Suits MDL, Williams SJ, Gilbert HJ, Davies GJ. 2018. Bacteroides thetaiotaomicron generates diverse α-mannosidase activities through subtle evolution of a distal substrate-binding motif. Acta Crystallogr D Struct Biol. 74:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trastoy B, Du JJ, Klontz EH, Li C, Cifuente JO, Wang LX, Sundberg EJ, Guerin ME. 2020. Structural basis of mammalian high-mannose N-glycan processing by human gut Bacteroides. Nat Commun. 11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncil YE, Xiao Y, Porter NT, Reuhs BL, Martens EC, Hamaker BR. 2017. Reciprocal prioritization to dietary glycans by gut bacteria in a competitive environment promotes stable coexistence. MBio. 8:e01068–e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaganathan T, Shi R, Yao D, Gu RX, Garron ML, Cherney M, Tieleman DP, Sterner E, Li G, Li L, et al. 2017. Conformational flexibility of PL12 family heparinases: Structure and substrate specificity of heparinase III from Bacteroides thetaiotaomicron (BT4657). Glycobiology. 27:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer JE, Vilén EM, Namburi RB, Benjdia A, Beneteau J, Malleron A, Bonnaffé D, Driguez PA, Descroix K, Lassalle G, et al. 2014. Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J Biol Chem. 289:24289–24303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, Flanagan CA, Sapp FR, Merritt ZT, Bhatti F, et al. 2020. Diet and the human gut microbiome: An international review. Dig Dis Sci. 65:723–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rebello O, Crost EH, Owen CD, Walpole S, Bennati-Granier C, Ndeh D, Monaco S, Hicks T, Colvile A, et al. 2020. Fucosidases from the human gut symbiont Ruminococcus gnavus. Cell Mol Life Sci. doi: 10.1007/s00018-020-03514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Suits MDL, Thompson AJ, Chavan S, Dinev Z, Dumon C, Smith N, Moremen KW, Xiang Y, Siriwardena A, et al. 2010. Mechanistic insights into a Ca2+-dependent family of α-mannosidases in a human gut symbiont. Nat Chem Biol. 6:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]


