Abstract
Bacterial vaginosis (BV) is a condition of the vaginal microbiome in which there are few lactobacilli and abundant anaerobic bacteria. Members of the genus Gardnerella are often one of the most abundant bacteria in BV. BV is associated with a wide variety of poor health outcomes for women. It has been recognized since the 1980s that women with BV have detectable and sometimes markedly elevated levels of sialidase activity in vaginal fluids and that bacteria associated with this condition produce this activity in culture. Mounting evidence collected using diverse methodologies points to the conclusion that BV is associated with a reduction in intact sialoglycans in cervicovaginal secretions. Here we review evidence for the contributions of vaginal bacteria, especially Gardnerella, in the processes of mucosal sialoglycan degradation, uptake, metabolism and depletion. Our understanding of the impacts of vaginal sialoglycan degradation is still limited. However, the potential implications of sialic acid depletion are discussed in light of our current understanding of the roles played by sialoglycans in vaginal physiology.
Keywords: bacterial vaginosis, Gardnerella, microbiome, sialic acid, sialidase
Introduction to bacterial vaginosis
Bacterial vaginosis (BV) is a condition characterized by low levels of lactic acid-producing bacteria in the vagina. Instead there are higher levels of diverse taxa that are often strict or facultative anaerobic bacteria. BV is associated with increased risks of sexually transmitted infections (Wiesenfeld et al. 2003; Brotman et al. 2010), endometritis (Watts et al. 1990; Wiesenfeld et al. 2002) and pelvic inflammatory disease (Sweet 1995). In pregnancy, BV has been associated with complications such as preterm birth (Hillier et al. 1988; Holst et al. 1994; McGregor et al. 1994; Hillier et al. 1995; Leitich and Kiss 2007), late pregnancy loss (Leitich et al. 2003; Leitich and Kiss 2007), preterm premature rupture of membranes (McGregor et al. 1994), delivery of a low birth weight infant (Holst et al. 1994; Hillier et al. 1995; Svare et al. 2006) and infections of the placenta and amniotic fluid (Silver et al. 1989; Hitti et al. 2001; Svare et al. 2006; Leitich and Kiss 2007; Rezeberga et al. 2008). Importantly, many BV-associated bacterial species have been detected in invasive infections of the placenta and amniotic fluid (Berardi-Grassias et al. 1988; Hillier et al. 1988; Silver et al. 1989; Watts et al. 1990; Holst et al. 1994; DiGiulio et al. 2010; DiGiulio 2012).
In the clinic, a woman is diagnosed with BV if she has three of the four Amsel’s criteria: thin consistency of vaginal fluids, fishy odor upon potassium hydroxide treatment, elevated pH (>4.5) and >20% of the exfoliated epithelial cells being studded with bacteria (“clue cells”) in wet mounts (Gardner and Dukes 1955; Amsel et al. 1983). In the laboratory, BV is determined by the Nugent system of scoring Gram-stained vaginal smears. Briefly, the Nugent scoring scale is from zero to ten; lower scores (0–3) indicate normal vaginal microbiome (No BV) with abundant Gram-positive (purple) elongated rods. Higher scores (7–10) indicate BV with few lactobacilli, abundant Gram-negative/variable bacteria and often the presence of curved rods (Mobiluncus and other bacteria) (Nugent et al. 1991; Hillier et al. 1993; Holst et al. 1994) (Figure 1). DNA sequencing technologies and other molecular tools have provided finer resolution of the diversity and longitudinal variability of vaginal bacterial communities (Srinivasan et al. 2010; Ravel et al. 2011; Gajer et al. 2012). However, the mechanisms linking BV to adverse reproductive outcomes are largely unknown.
Fig. 1.
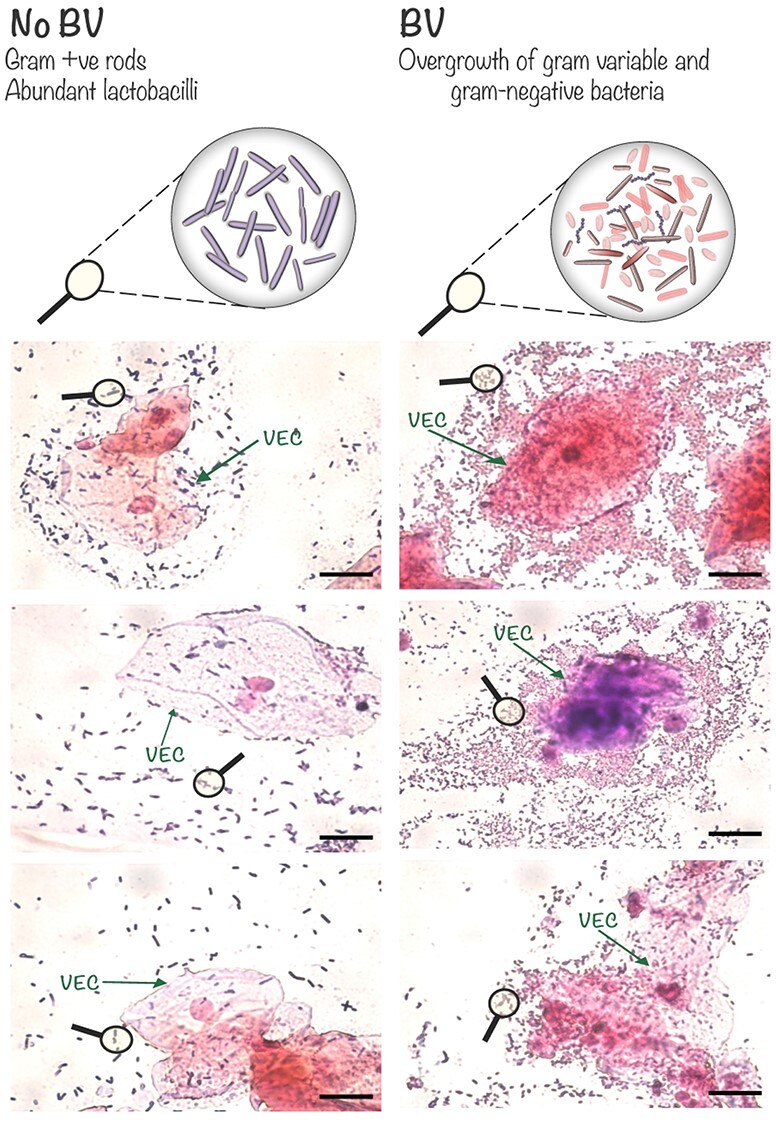
Images of Gram-stained vaginal smears depict some of the microscopic features of BV. Top: schematic magnification illustrating features of the Nugent scoring system (Nugent et al. 1991), in which an abundance of large Gram-positive (purple) rods contributes to a low score (0–3, No BV, left). In contrast, diverse morphotypes of Gram-negative (pink) and Gram-variable bacteria with low levels of long Gram-positive rods contributes to a high Nugent score (7–10, BV, right). Note the higher numbers of bacteria in BV specimens and their tendency to be concentrated around vaginal epithelial cells (VECs). Scale bars = 20 μm.
A cadre of taxa has been associated with BV including one particularly abundant microbe, Gardnerella vaginalis. G. vaginalis was first identified as the causative agent of BV (Gardner and Dukes 1955); however, its role as the primary etiological agent of vaginosis has been argued and remains elusive (Hickey and Forney 2014; Schwebke et al. 2014; Swidsinski et al. 2014). Several studies that have evaluated the fundamental yet ambiguous roles of G. vaginalis in BV were reviewed recently (Schellenberg et al. 2017; Morrill et al. 2020) and will not be discussed extensively here. We here focus on sialidase enzymes present in BV and their possible roles in the pathophysiology of the condition. Emphasis is placed on sialoglycan foraging and sialidases encoded by G. vaginalis as these are the best studied, but we also touch on sialidases, sialic acid transport and catabolic machinery that have been studied in other vaginal bacteria (see Table I).
Table I.
Sialidase activity and predicted sialic acid transport and catabolic machinery among vaginal bacteria
NK, none known to us.
*Four clades have been defined for G. vaginalis based on four sub-groups (A–D) that are defined by sequencing of a region of the chaperonin-60 (cpn60) gene: clade 1 corresponds to subgroup C, clade 2 corresponds to subgroup B, clade 3 corresponds to subgroup D, clade 4 corresponds to subgroup A (Schellenberg et al. 2016).
Sialidases in vaginal fluids during bacterial vaginosis
Women with BV were first reported to have elevated levels of sialidase activity in vaginal fluids compared to women without the condition in a 1992 publication (Briselden et al. 1992). This is a reproducible finding in human specimens (Howe et al. 1999; Smayevsky et al. 2001; Cauci et al. 2003; Lewis et al. 2012) and it was later shown can be recapitulated in mice upon experimental vaginal colonization by G. vaginalis (Gilbert et al. 2013). Isolation and identification of bacterial strains from BV vaginal specimens has demonstrated that certain species displayed sialidase activity in vitro, including isolates of Prevotella, Bacteroides and Gardnerella, but not the tested isolates of Mobiluncus (curtisii or mulieris) or Peptostreptococcus (asaccharolyticus, anaerobius, magnus or prevotii) (Briselden et al. 1992). Indeed, an earlier study reported purification and biochemical characterization of a sialidase from G. vaginalis (von Nicolai et al. 1984). Further studies have linked sialidase enzyme activity in vaginal fluids with increased likelihood of adverse outcomes including premature rupture of membranes and placental infection (Zhang et al. 2002), miscarriage and late pregnancy losses (Cauci and Culhane 2011), preterm birth (Zhang et al. 2002), as well as BV recurrence (McGregor et al. 1994). Many other studies have demonstrated that sialidase activity is not only associated with BV, but can be used as a diagnostic biochemical indicator of the condition (Smayevsky et al. 2001; Myziuk et al. 2003; Bradshaw et al. 2005; Wu et al. 2019).
Multifaceted sources of sialidase activity in vaginal specimens
Sources that yield the sialidase activity detected in the vaginal fluids of women with BV have not been fully characterized. Early studies of sialidase activity produced by vaginal bacterial isolates, from women with BV, concluded that sialidase is microbial in origin. A study by Briselden et al. showed that women with sialidase-positive vaginal fluids harbor multiple sialidase-producing bacteria including Prevotella bivia, P. oralis, P. loeschii, Bacteroides fragilis and G. vaginalis (Briselden et al. 1992). Early studies showed that all strains of P. bivia, but only a subset of G. vaginalis isolates produce sialidase activity; later studies have largely confirmed these findings (Moncla et al. 1990; Santiago et al. 2011; Moncla et al. 2016). However, the production of sialidase activity by Gardnerella and Prevotella in culture does not rule out the participation of sialidases from other potential origins in vivo. Early studies seem to conclude that because all Prevotella isolates produced sialidase in vitro, whereas only a fraction of Gardnerella isolates did, the former must be the main source of sialidase activity in women (Moncla et al. 1990). Recent studies in mouse models, which reflect some but not all features of BV in women, show that Gardnerella and Prevotella colonization both result in an increased sialidase activity compared to a mock-infected control group. However, Prevotella seemed to require 100-fold higher bacterial levels to result in similar levels of sialidase activity as seen in Gardnerella colonized C57BL/6 mice (Gilbert et al. 2019). These data suggest that there may be other factors such as different expression levels of sialidase among bacterial strains/species that in some cases may be more important than the levels of bacteria themselves. Future studies would benefit from the development of genetic tools to make mutants in Gardnerella, as would the entire field of Gardnerella biology. Studies have shown that multiple strains of Gardnerella can concurrently occupy the vagina (Balashov et al. 2014; Hilbert et al. 2017; Hill et al. 2019; Shipitsyna et al. 2019) and may therefore also contribute to the heterogeneity of sialidase sources in individual samples (Schellenberg et al. 2016). Finally, it has not (to our knowledge) been studied whether host sialidases (at least four are known) (Miyagi and Yamaguchi 2012) might also contribute to the enzyme activity seen in BV.
Endogenous microbiota-derived sialidase activity has also been reported in laboratory mice (C57BL/6) from specific vendors (e.g. Charles River/NCI, Envigo, but not Jackson) (Gilbert et al. 2013; Agarwal et al. 2020). In these studies, sialidase-positive colonies of Bacteroides spp. or Enterococcus gallinarum were isolated from vaginal washes of Envigo mice (202-A Indianapolis facility, IN, USA) (Agarwal et al. 2020), and bacteria of Eubacteria consortium or Enterococcus spp. were isolated from Charles River/NCI mice with vaginal sialidase activity (Gilbert et al. 2013). In the presence of potential sialidase-producing microbiotas, another consideration is that the addition of BV bacteria to existing microbial ecosystems could itself trigger changes in the naturally occurring sialidase producers, which could also influence sialidase levels. An example of this was shown in a recent study in which (sialidase-negative) Fusobacterium nucleatum addition to ex vivo cultures of mouse, as well as human, vaginal microbiotas led to marked increases in sialidase activity (Agarwal et al. 2020). As with other models of mucosal colonization/infection using conventionally raised animals, indirect effects of exogenously added microbe(s) on the endogenous microbiota may contribute to sialidase activity. The use of antibodies, proteomics, bacterial genetic tools to make mutants in fastidious vaginal anaerobes and/or gnotobiotic models may help further clarify the sources of sialidase activity in vivo.
Vaginal pH may also play an important role in determining which bacterial sialidases contribute to sialidase activity in BV or which targets they act on. The human vaginal microbiome is unique among mammals studied to date, with Lactobacillus dominance often contributing to a low pH (<4.5) (Miller et al. 2016). A recent study measured an even lower vaginal pH in women with Lactobacillus dominated microbiota, utilizing methods to maintain physiologically relevant hypoxic and high CO2 conditions, estimating an average pH of 3.5 when equilibrated at 5% CO2 (O'Hanlon et al. 2013). A higher pH is observed in women with BV that may be closer to the pH optima of many sialidases (~pH 5.5) (von Nicolai et al. 1984; Yamamoto et al. 2018). In vitro studies suggest that the pH optimum for purified Gardnerella sialidase using glycoprotein substrates (von Nicolai et al. 1984; Robinson et al. 2019) and recombinant B. fragilis (Yamamoto et al. 2018) sialidase using a small molecule fluorogenic substrate is between 5.0 to 5.5. However, the pH optimum may also depend on the substrate; for example—the sialidase purified from B. fragilis (same strain as the above study, SBT3182) was reported to have optimal activity at pH 6.1 with colominic acid (α2–8-linked polymer) (Tanaka et al. 1992). Thus, vaginal pH might help determine which vaginal bacteria contribute to sialidase activity and/or which potential sialoglycoprotein substrates may become targets; this requires further study.
Can G. vaginalis trigger features of BV?
The evidence for G. vaginalis as a trigger of the characteristic features of BV following vaginal inoculation in women, non-human primates and animal models has been recently reviewed (Morrill et al. 2020) and therefore will not be extensively covered here. The causal role of Gardnerella in triggering features of BV in mice (including sialidase activity in vaginal fluids) and the potential metabolic functions of sialidase action were studied in two 2013 papers (Gilbert et al. 2013; Lewis et al. 2013). In these studies, G. vaginalis strain JCP8151B was introduced into β-estradiol treated C57BL/6 mice (Charles River/NCI) reproducing BV features, including vaginal sialidase activity (Figure 2A), mucus sialoglycan degradation and depletion (Figure 2B and C), increased numbers of shed epithelial cells in vaginal washes (Figure 2D), Gardnerella adhering to shed epithelial cells (Figure 2E), and no evidence of inflammation by histopathology (Gilbert et al. 2013, Lewis et al. 2013). As discussed below, two of the prominent phenotypes commonly seen in BV—the increase in pH and a fishy amine odor—are believed to be due to organisms other than G. vaginalis in BV (Wolrath et al. 2001; Nelson et al. 2015; Srinivasan et al. 2015) and were not studied in the mouse model.
Fig. 2.
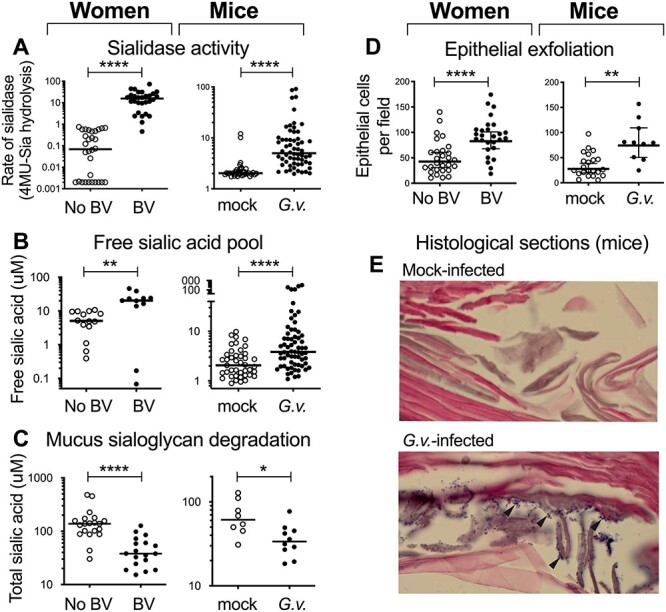
Mouse model of Gardnerella infection replicates features of BV. Representative data from multiple publications directly compare some of the phenotypes of G. vaginalis colonized mice to features of BV in women. (A) Sialidase activity in vaginal fluids measured with the 4-Methylumbelliferone (4MU)-Sia assay. (B-C) Free and total sialic acids measured by fluorescent derivatization and HPLC resolution. (D) Epithelial cells in vaginal washes counted by blinded observers. (E) Hematoxylin and eosin (H&E)-stained vaginal sections reveal bacteria (purple puncta indicated by arrowheads) on the epithelium (pink) of Gardnerella infected mice. *P < 0.05, **P < 0.01, ****P < 0.0001. Adapted from data previously published (Gilbert et al. 2013; Lewis et al. 2013).
First, the acidity of the human vagina (a human-specific trait) is likely produced by dominant lactobacilli, which produce lactic acid. When lactobacilli are in short supply, as in women with BV, there is typically a higher pH due to lower levels of lactic acid. Studies suggest that vaginal microbiotas in mice are rarely dominated by lactobacilli (Jasarevic et al. 2017; Vrbanac et al. 2018; Agarwal et al. 2020). For example, in studies by Vrbanac et al. (2018) only 1 out of 20 mice was found to have a Lactobacillus-dominant microbiome at day 0, which by day 6 had transitioned to a Staphylococcus dominated vaginal microbiota. It is not known whether the rare instance of a Lactobacillus-dominant mouse vaginal microbiome coincides with a low vaginal pH. From these data, we conclude that the pH shift observed in BV is not a feature that can be modeled starting with the endogenous microbiota in mice.
A second feature of BV not reproducible in mice through introduction of Gardnerella alone is the characteristic fishy amine odor in some women with BV. A bioinformatic analysis of vaginal taxa revealed that only a few members of the vaginal microbiome encode the predicted pathways for synthesizing the amines, including putrescine, cadaverine and trimethylamine (TMA), associated with “fishy” odor (Nelson et al. 2015). For example, Escherichia coli 83972, P. mirabilis and Janthinobacterium spp. were the only taxa found encoding the pathway for cadaverine biosynthesis, whereas Prevotella, Dialister, Veillonella spp. and a few others were found to encode pathways for putrescine production. Although, prior studies in women have revealed an association between a positive whiff test and the presence of Gardnerella (in addition to other organisms) (Srinivasan et al. 2012), Gardnerella has not been shown to encode the predicted pathways for biosynthesis of these amines (Nelson et al. 2015), nor produce these compounds in culture (Chen et al. 1979). Further studies are required to determine roles and inter-relationships of vaginal bacteria in reproductive health problems associated with BV physiology.
Genetic and biochemical basis for sialidase activity in BV bacteria
More recently, studies have begun to explore the genes underlying sialidase activity in bacterial species associated with BV, including members of the genera Bacteroides, Prevotella and Gardnerella. Although the genes for sialidase activity among species and strains of Bacteroides and Prevotella from the vagina have not been studied to our knowledge, many studies have investigated the genes (as well as the biochemical and biological functions of sialidases) for sialidase activity from members of the Bacteroidetes that reside in the gut (Ng et al. 2013; Juge et al. 2016; Robinson et al. 2017). Indeed, the sialidase gene from Bacteroides fragilis was first identified more than three decades ago by heterologous expression studies in E. coli (Russo et al. 1990). Recently, Yamamoto et al. (2018) have also characterized a sialidase from B. fragilis YCH46 that preferentially hydrolyzes α2–8-linked sialic acids. Sialidase activity in vaginal strains of Gardnerella has been under investigation for approximately three decades. However, the genetic machinery underlying sialidase activity in laboratory-cultured Gardnerella was not understood until 2019 when recombinant sialidase homologs from G. vaginalis were investigated for their biochemical activity and substrate specificity (Robinson et al. 2019).
To date, three sialidase homologs have been reported in G. vaginalis, namely NanH1 (also known as sialidase A) (Janulaitiene et al. 2018), NanH2 and NanH3 (Robinson et al. 2019). Sialidases NanH2 and NanH3 from G. vaginalis were recently identified and named based on their homology to the NanH2 Bifidobacterium longum sialidase, to which they share ~60% identity. Gardnerella NanH1 is more closely related to Bifidobacterium NanH1 than to NanH2. The genome of G. vaginalis strain JCP8151B contains all three homologs in different regions. NanH2 and HanH3 are 49% identical, while NanH1 is 30% identical to both NanH2 and NanH3, with closest homology at the active domain (Robinson et al. 2019). Recombinant NanH1 had little to no activity against most of the tested substrates, while NanH2/NanH3 cleaved sialic acid from nearly all tested substrates (Robinson et al. 2019).
Similar to bifidobacteria (Sela et al. 2008; Kiyohara et al. 2011; Sela et al. 2011), strains of Gardnerella can access sialic acid from different linkages and a wide range of substrates. Recombinant NanH1 from gut-associated Bifidobacterium longum subspecies infantis had 140-fold lower turnover rate (kcat = 1.0 ± 0.1 s−1, i.e. one molecule of sialic acid generated per molecule of enzyme per second) than NanH2 (kcat = (1.4 ± 0.1) × 102 s−1) for substrate containing α2–3-linked sialic acid, and 175-fold lower turnover on α2–6-linked substrate (Sela et al. 2011). As with B. longum NanH1, Gardnerella NanH1 had little activity when tested on a wide variety of plausible mucosal substrates. Consistent with a prior report that NanH1 (sialidase A) had activity when 200 mM of 4MU-N-acetylneuraminic acid (Neu5Ac) was used as substrate (Janulaitiene et al. 2018), recombinant NanH1 was found to release small amounts of Neu5Ac from 4-MU-Neu5Ac used at lower substrate concentrations (250 μM) (Robinson et al. 2019). NanH1 also released small amounts of Neu5Ac from bovine submaxillary mucin (BSM), and colostrum IgA. However, it was completely unable to access Neu5Ac from α2–3- or α2–6-linked sialyllactose, nor was it able to access 7-O- or 9-O-acetylated sialic acids from BSM under the conditions tested. In contrast, recombinant NanH2 and NanH3 were able to cleave sialic acids in a variety of contexts (e.g. α2–3- and α2–6-linked sialic acids as well as N- and O-linked sialoglycans found on secreted IgA and mucin) (Robinson et al. 2019). Similarly, strains of G. vaginalis encoding NanH2 or NanH3 were also able to deplete α2–3- or α2–6-linked sialyllactose added to culture media (Robinson et al. 2019).
Prior studies observed that the sialidase activity present in human clinical specimens could access a broad range of exogenously provided sialoglycan substrates relevant to the mammalian mucosa (Lewis et al. 2012). Recombinant NanH2 and NanH3, as well as cultured strains of Gardnerella, also cleave sialic acid from the same range of substrates as accessed by sialidases in human vaginal samples (Robinson et al. 2019). These broad and overlapping substrate preferences suggest that the sialidase activity observed in human vaginal specimens is plausibly derived from Gardnerella sialidases NanH2 and NanH3.
Molecular characterization of the three G. vaginalis sialidase homologs showed that sialidase activity in cultured G. vaginalis has been misattributed to the gene encoding “sialidase A” (sldA, renamed nanH1) (Robinson et al. 2019). This study showed substantial levels of NanH1 were successfully expressed (validated by Coomassie and anti-His Western), and the need for metal cations was also ruled out. However, it remains possible that NanH1 was incorrectly folded for optimal activity or that it acts by a mechanism or on a substrate that has not yet been tested. We note that the multiple sialidases of Streptococcus pneumoniae yield different sialic acid products (Xu et al. 2011); however, this has not been studied extensively for members of the Bifidobacteriacea. NanH1 was originally believed to encode the Gardnerella sialidase since it bore homology to other sialidases, predicted catalytic residues were conserved (Robinson et al. 2019), and is encoded immediately adjacent to the predicted sialic acid catabolic gene cluster in Gardnerella vaginalis strain JCP8151B (Lewis et al. 2013). All of these facts indicate its central role in sialic acid biology. However, nanH1 is often present in strains of Gardnerella that do not have detectable sialidase activity in culture. Some Gardnerella strains encode all three nanH homologs and some encode none; most of the strains encode either encode only nanH1 or they encode nanH1 along with either nanH2 or nanH3 (Robinson et al. 2019). The presence of a predicted sialic acid catabolic pathway has only been evaluated in a limited number of Gardnerella strains, therefore it is not clear if nanH2 or nanH3 are encoded only in strains that have nanH1 and the sialic acid catabolic pathway (and vice versa). Although nanH1 appears to be present in all sialidase-positive G. vaginalis strains, at least three different studies have reported that many strains have the nanH1 gene while being negative for sialidase activity in culture (Pleckaityte et al. 2012; Schellenberg et al. 2016; Hardy et al. 2017). In one strain set (Schellenberg et al. 2016), less than 50% of 77 nanH1 positive strains produced sialidase activity that could be detected in culture. In another set of 34 divergent Gardnerella strains, sialidase activity corresponded exactly with the presence of nanH2 or nanH3 by polymerase chain reaction (PCR, Robinson et al. 2019). In contrast, 16 of 19 sialidase-negative strains of Gardnerella were PCR positive for nanH1, but not nanH2 or nanH3. Conversely, Gardnerella strains encoding only nanH1 had no detectable sialidase activity in culture. In conclusion, NanH1 appears unlikely to contribute significantly to the sialidase activity seen in Gardnerella cultures. These data strongly suggest that “sialidase A” is a misnomer, at least as it refers to the sialidase activity measured in laboratory cultures of Gardnerella.
If the primary role of NanH1 (aka sialidase A; sldA) is not as a sialidase, then what is its function?
Although NanH1 does not appear to be responsible for the sialidase activity seen in Gardnerella cultures, several studies nevertheless point toward carbohydrate or sialic acid-related roles of this protein in the biology of Gardnerella-host interactions. Gardnerella NanH1 appears to be an ortholog of the NanH1 protein encoded by B. longum (note that both Gardnerella and Bifidobacterium belong to the Bifidobacteriales). NanH1 in B. longum and G. vaginalis share the following features: 1) very low but detectable levels of sialidase activity when expressed as recombinant proteins (Sela et al. 2011; Robinson et al. 2019), 2) an N-terminal putative lectin domain, 3) proximity to putative sialic acid catabolic operons, 4) lack of canonical signal sequences (for secretion by the Sec apparatus) or membrane anchoring regions and 5) a C-terminal CBM40/GH33 sialidase domain with apparently intact active site residues. Interestingly, recent studies have shown that some CBM40-containing sialidase domains can bind to sialic acid residues of glycans, promoting adherence to the mucosa. For example, a CBM40 containing Ruminococcus gnavus sialidase (RgNanH) was recently shown to bind broadly to sialoglycans with a preference for α2–3-linked sialic acids on sialoglycans and to mediate interactions with intestinal mucus (Owen et al. 2017). Sialic acid binding was also demonstrated for the CBM40-containing sialidase of Vibrio cholerae (Moustafa et al. 2004). Additionally, the extracellular sialidase from Bifidobacterium bifidum, SiaBb2, was found to bind α2,6-linked sialic acids to facilitate sialoglycan foraging (Nishiyama et al. 2017). Together, these features suggest that G. vaginalis NanH1 may be involved in bacterial adherence to sialoglycans on mucus or epithelial cells; however, this requires further investigation.
Several translational studies have tested associations between clinical parameters and the presence/absence (contingency, two studies) or abundance of the nanH1 gene (quantitative, two studies). Two small studies evaluated whether presence/absence of the nanH1 gene (based on the PCR detection) was associated with the BV status of women from whom the strains had been isolated, with neither finding a positive association (Castro et al. 2015; Mohammadzadeh et al. 2019). However, another larger study went beyond presence/absence of nanH1 to measure the “genomic load” of nanH1 in relation to BV. This study identified a strong association between a high nanH1 load and BV diagnosis. There was also a strong correlation between high levels of nanH1 and the presence of adhesive sheets of bacteria dominated by Gardnerella as shown by fluorescence in situ hybridization (Hardy et al. 2017). This finding further supports the idea that NanH1 might afford adherence traits. Another study investigated the relationship between Gardnerella nanH1 gene abundance and the persistence of high risk human papillomavirus (HPV), showing a strong correlation between high levels of the nanH1 gene and HPV persistence (as opposed to clearance) (Di Paola et al. 2017). Since nanH1 is not found among all G. vaginalis strains, the association with HPV persistence may indicate relationships with specific subtypes of Gardnerella.
Gardnerella sialidase: tethered to the cell surface, excreted or both?
Sialidase activity can be measured in the cell-free supernatant of G. vaginalis cultures in significant quantities. However, most of the G. vaginalis sialidase activity appears to be cell associated (Lewis et al. 2012; Lewis et al. 2013). When G. vaginalis cells were incubated with secreted IgA (sIgA, containing the highly glycosylated secretory component), free sialic acid was liberated into the extracellular environment followed by apparent uptake and catabolism (as evidenced by the disappearance of free sialic acid from the supernatant) (Figure 3A). This is perhaps the best piece of biochemical evidence that the sialidase is tethered to the bacterial surface facing outward. At present, it is unclear if G. vaginalis actively secretes the sialidase enzyme(s) observed in culture supernatants or if this activity is released upon bacterial lysis or proteolytic cleavage from the cell surface. The discovery of Gardnerella NanH2 and NanH3, which bear homology to Bifidobacterium sialidases, revealed predicted transmembrane α-helices at their C termini (Robinson et al. 2019). Studies with recombinant G. vaginalis sialidases also provide insight into the plausible cellular location of these enzymes. When constructs encoded a C-terminal His-tag just after the predicted transmembrane regions, recombinant protein could not be detected in soluble fractions or supernatants of E. coli lysates using anti-His western blot, even though sialidase enzyme activity could be detected in both (Robinson et al. 2019). These data suggested that the full-length proteins were insoluble due to hydrophobic α-helices. Moreover, the presence of sialidase activity in culture supernatant and soluble lysates, together with its absence in identically processed fractions from E. coli containing the empty vector, suggested that some sialidase activity was being released by cell death or proteolysis into soluble and secreted fractions. In contrast, truncation of both nanH2 and nanH3 to remove the predicted transmembrane regions, but still containing the C-terminal His-tag resulted in soluble proteins that could be readily purified using the intact His-tag. Together these and other published observations suggest that NanH2 and NanH3 are embedded in the G. vaginalis cell surface but may be released into the supernatant following cell death or proteolytic cleavage. Likewise, the cell surface sialidase of S. pneumoniae (NanA) can also be solubilized by proteolysis and released without substantial loss of activity (Lock et al. 1988). In some bacteria hydrolytic enzymes are also carried in spherical membranous structures that are released from the outer membrane, commonly known as outer membrane vesicles (OMVs), and provide a protective environment for dissemination of the encapsulated cargo (Caruana and Walper 2020). For example, B. fragilis, sialidase activity has been reported in the OMVs (Elhenawy et al. 2014). While membrane vesicles have recently been reported for Gardnerella (Shishpal et al. 2020), it is unclear if sialidase is present in these because the study used a sialidase-negative type strain.
Fig. 3.
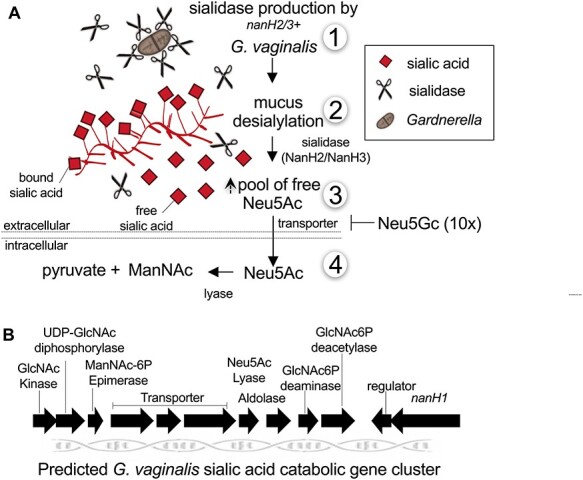
A model of Gardnerella sialoglycan foraging and depletion. (A) Model based on biochemical evidence for G. vaginalis sialoglycan degradation and foraging in the vagina. 1) G. vaginalis strains with nanH2 or nanH3 in their genomes produce sialidase activity in culture. 2) Free sialic acid is released by sialidases NanH2 or NanH3 from mucosal sialo-glycoconjugates such as mucin and secretory IgA. 3) Extracellular free Neu5Ac can be transported into the bacteria, depleting them from the culture or assay supernatant. In G. vaginalis the transport of Neu5Ac is inhibited by presence of high concentrations of Neu5Gc. 4) Intracellular sialic acid (Neu5Ac) is converted to N-acetyl mannosamine (ManNAc) and pyruvate by a sialic acid lyase. (B) The predicted sialic acid catabolic gene cluster of G. vaginalis. Predictions are based on homology and have not been functionally tested. Note that the sialidases of G. vaginalis, NanH2 and NanH3 are encoded elsewhere in the genome. There are some strains that encode putative catabolic machinery for sialic acid without encoding NanH2 or NanH3. Note that multiple BV bacteria encode sialidases and/or sialic acid catabolic machinery (Lewis et al. 2013).
Sialoglycan foraging by Gardnerella and other vaginal bacteria
Sialidase activity is linked with the ability of Gardnerella to liberate sialic acids from bound sources (mucus sialoglycans) (Lewis et al. 2012, Lewis et al. 2013). The incubation of Gardnerella with exogenous sialoglycan substrates revealed that sialidase action occurs extracellularly, liberating an increased pool of free sialic acid, which is then depleted from the extracellular milieu (Figure 3A). The predicted sialic acid catabolic gene cluster in G. vaginalis ATCC14019, contains both transporters and an N-acetyl neuraminate lyase that are required for the uptake and utilization of sialic acid (Lewis et al. 2013) (Figure 3B). Gardnerella are capable of cleaving both Neu5Ac and N-glycolylneuraminic acid (Neu5Gc) from sialoglycans such as BSM, although Neu5Ac was cleaved preferentially (Lewis et al. 2013; Robinson et al. 2019). The depletion of Neu5Gc by G. vaginalis cultures is interesting in that Neu5Gc is not synthesized by humans, though it can be found in various human tissues due to metabolic incorporation from other sources (Dhar et al. 2019). Despite the capacity of G. vaginalis sialidase to liberate Neu5Gc, the nonhuman sialic acid had an inhibitory effect at high concentrations on the ability of Gardnerella to take up Neu5Ac. In contrast, neither sialidase activity nor Neu5Ac lyase activity were affected by excess Neu5Gc (Lewis et al. 2013) (Figure 3A). This appears to be consistent with the observation that Gardnerella are not generally found at high levels in other nonhuman primates tested, which could have higher levels of Neu5Gc in the reproductive tract.
The broad substrate preferences of Gardnerella sialidases (NanH2 and NanH3) suggest that their main functional difference is the improved capacity of NanH2 compared to NanH3 to cleave 9-O-acetylated sialic acid substrates (Robinson et al. 2019). It has not been systematically studied whether the female reproductive tract in mammals contains O-acetylated sialic acids. Analyses of the rapidly expanding number of Gardnerella genomes has revealed wide genetic diversity among the strains so far isolated and suggest that there are multiple species yet to be adequately classified or studied. Only a subset of the sialidase-positive strains of Gardnerella encode nanH2. Moreover, foraging studies revealed that the presence of nanH2 in the genome predicted a greater capacity to forage on 9-O-acetylated sialic acids compared to strains with only nanH3 (Robinson et al. 2019). In the human colon, 9-O-acetylation of sialic acids are highly abundant and these modifications restrict the action of many of the sialidases ubiquitously found in the colonic microenvironment (Corfield et al. 1992; Robinson et al. 2017). Although Gardnerella has been considered to be a vaginal bacterium, studies of anal swabs have shown that the bacterium is also present in the distal gastrointestinal tracts of men, women and young children (Myhre et al. 2002; Marrazzo et al. 2012; Cox et al. 2017). If NanH2 confers increased capacity for growing on 9-O-acetylated sialic acids, this may allow strains encoding this sialidase to colonize the colon. However this possibility has not been investigated.
Sialoglycan foraging in the vagina may further extend to potential pathogens that do not encode a sialidase but may derive benefits from sialidase producers like G. vaginalis (Figure 4). For example, with assistance from exogenous sialidases produced by other bacteria, F. nucleatum can uptake and utilize free sialic acid for growth under nutrient limiting conditions, a behavior that promoted persistence within a sialidase-positive vaginal niche (Agarwal et al. 2020). Similarly, in vitro studies have shown that sialidases from B. fragilis and B. thetaiotaomicron can facilitate sialic acid utilization by E. coli that does not encode its own functional sialidase and cannot access sialic acid bound to glycoconjugates (Robinson et al. 2017). Although B. thetaiotaomicron only encodes sialidase, B. fragilis encodes both a sialidase and an O-acetyl esterase (EstA) that can convert 9-O-acetylated sialic acids to non-acetylated sialic acids. This is important because O-acetylated sialic acids are often resistant to sialidases and therefore O-acetylation limits the scavenging of this carbohydrate residue by microbes. Sialic acid O-acetyl esterase activity facilitates foraging of O-acetylated sialoglycans that were otherwise inaccessible to the sialic acid consumers. It is plausible that similar metabolic interactions may occur among bacteria in the vaginal microbiota. For example, presence of B. fragilis in the vaginal microbial community may provide access to 9-O-acetylated sialic acids to G. vaginalis strains that do not encode nanH2 or other sialidase-producing bacteria. Though the presence of O-acetylated sialic acids has not been documented in the reproductive tract, Gardnerella might also benefit from the presence of esterase-producing bacteria when colonizing other niches such as the gastrointestinal tract (Myhre et al. 2002, Marrazzo et al. 2012, Cox et al. 2017). Indeed, cross-feeding of host-derived sialic acids has been shown to support persistent colonization of polymicrobial communities associated with dysbiosis in the gastrointestinal and reproductive tracts (Ali et al. 2014; Huang et al. 2015; Agarwal et al. 2020). Mutualistic and antagonistic interactions among vaginal bacteria involving other metabolic pathways have been reviewed earlier (Pybus and Onderdonk 1997; Pybus and Onderdonk 1999).
Fig. 4.
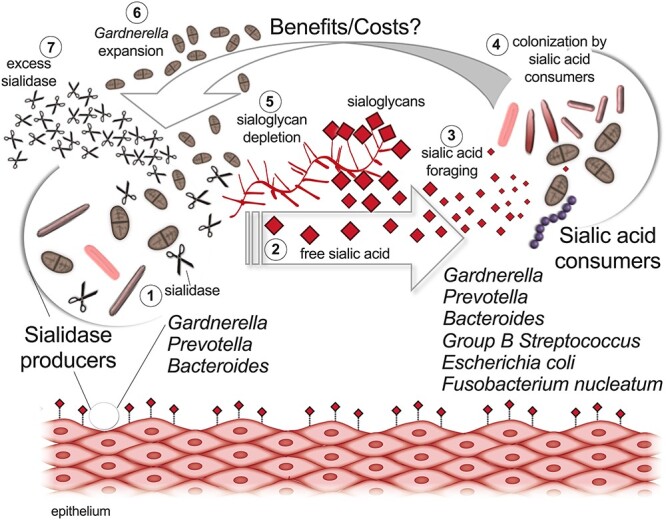
A model of sialoglycan degradation and foraging by vaginal bacteria. 1) Sialidase-producing bacteria including members of the Gardnerella, Prevotella and Bacteroides express sialidases, leading to sialidase activity in vaginal fluids, and 2) creating higher levels of free sialic acid. 3) Free sialic acid is taken up and catabolized by not only sialidase-producing bacteria, but taxonomic groups that do not encode their own sialidases, such as group B Streptococcus, E. coli and Fusobacterium nucleatum. 4) Improved growth or colonization by sialic acid consumers can in turn lead to 5) Sialoglycan depletion and 6) benefits or costs for other community members (e.g. expansion of Gardnerella) and 7) increase in sialidase (Agarwal et al. 2020).
Evidence that bacterial sialidase acting on host glycans leads to mucosal sialic acid depletion in BV
Several studies have provided experimental evidence that significant degradation of sialoglycans occurs in vaginal specimens from women with BV compared to women with normal Lactobacillus-dominant vaginal microbiomes. The first of these studies measured levels of free (already preliberated within the sample) and total (released by mild acid hydrolysis) sialic acids fluorescently derivatized with 1,2-diamino-4,5-methylenedioxybenzene (DMB) for high-performance liquid chromatography (HPLC) separation and measurement (Lewis et al. 2013) (Figure 2). Compared to women without BV, women with the condition had ~3-fold lower levels of total sialic acids and 3.5-fold higher levels of free/liberated sialic acid. These data suggest that sialidase enzymes present in BV specimens catalyze the degradation of sialoglycans, leading to the liberation of free sialic acid and the simultaneous depletion of intact sialoglycans. Consistent with these findings, another group used metabolomics to compare small molecule metabolites present within the vaginal microbiome in the setting of Lactobacillus-dominant microbiotas or diverse BV microbiotas (Srinivasan et al. 2015). This study confirmed the earlier finding that women with BV have higher levels of free/liberated sialic acid compared to women with Lactobacillus-dominant microbiotas.
Finally, lectins (carbohydrate-binding proteins) have been used to evaluate differences in the presence and context of intact sialoglycans present within vaginal secretions in women with and without BV. These lectin binding studies were done utilizing samples from the same cohort of women, collected in two different ways: (i) cervical vaginal fluids were collected by inserting a softcup to base of cervix (Moncla et al. 2016) and (ii) cervicovaginal lavages (CVLs) were collected by washing the ectocervix and vaginal vault with saline (Moncla et al. 2015; Wang et al. 2015). In the cervical cup samples from women with BV, there was an increase in sialidase activity compared to women without BV and a reduction in binding of Maackia amurensis lectin (MAL-II) and Sambucus nigra agglutinin (SNA), which interact with α2-3- and α2-6-linked sialic acids respectively (Moncla et al. 2016). These data are consistent with biochemical data showing that sialidase enzymes in BV specimens can efficiently access sialic acids bound by both these linkages (Lewis et al. 2012). In an earlier study using CVLs, Moncla et al. (2015) observed a significant reduction in binding of SNA (α2-6), but not MAL-II (α2-3), in BV positive women. Similarly, in a glycomic analysis (using lectin microarray) of CVLs, reduced binding was observed for SNA in BV samples, but no significant changes were observed with MAL-I (another variant of Maackia amurensis that also binds to α2-3-linked sialic acids) (Wang et al. 2015). Although both variants of Maackia amurensis lectin (MAL-I and MAL-II) selectively recognize α2–3-linked sialic acids, they can also bind to sulphated galactose residues in different contexts (Geisler and Jarvis 2011). Therefore, different results observed in binding of MAL (α2–3-linked sialic acids) in the above studies remains ambiguous and need to be verified using other biochemical approaches. Moncla et al. (2015, 2016) note that cervical cup samples had lower overall glycosidase activity as compared to CVL; however, these sample types were not directly compared in either study. The authors noted this was consistent with the additional unpublished observation that cervical cup samples have lower concentrations of bacteria compared to bacterial levels previously reported in CVL. It is noteworthy that in spite of the differences in the observed range of glycosidase activity and bacterial concentrations, depletion of α2-6-linked sialic acids was similar in both types of specimens in women with BV. Mucins are heavily glycosylated proteins and one of the most prominent sources of sialoglycans in the reproductive tract. Interestingly, in the cervical cup samples sialic acid-containing glycans were depleted in spite of a reported increase in mucins that are expressed in the reproductive tract, including MUC1, MUC4, MUC5AC and MUC7.
Implications of sialic acid depletion and sialidases in vaginal secretions
Negatively charged sialic acid residues present at the terminus of carbohydrate chains of mucin provide a rigid conformation to these molecules and ionic interactions between the carbohydrate chains allows for a specific arrangement of these glycoproteins in the mucus layer (Elstein 1978). The presence of sialic acids at the terminal ends of glycan chains has also been proposed to protect underlying glycans from other glycosidase activities (Moncla et al. 2015), that would result in successive deglycosylation of cervicovaginal glycoproteins in BV (Lewis et al. 2012), and consequently protects the underlying protein backbone from proteolysis (Lewis et al. 2012). For example, higher levels of terminal galactose and N-acetylgalactosamine were evident on glycans within BV vaginal secretions using lectin probes (Wang et al. 2015). In addition to higher levels of sialidase in BV, it has been shown that women with BV have higher levels of other glycosidase activities (including β-galactosidase) (Olmsted et al. 2003; Moncla et al. 2015; Moncla et al. 2016). Also, metabolic profiling revealed increased levels of free galactose in women with BV, further supporting the action of β-galactosidas(s) on host glycans (Srinivasan et al. 2015). Studies by Deman et al. (1973) suggested that removal of charged sialic acid residues from cervicovaginal mucins impacts the arrangement of mucin molecules in the presence of EDTA when pH is titrated below 3.0 (Rabouille et al. 1989). As such, it is not surprising the viscosity of the mucus gel is significantly reduced in BV (Olmsted et al. 2003; Chappell et al. 2014). Thus, changes in physical properties of mucus during BV might lead to greater susceptibility to invading genital tract pathogens.
Studies suggest that sialidase could be important in BV pathophysiology by enabling further breakdown of mucus components. One study used BV vaginal specimens as a source of enzyme activity, incubating them along with a heavily glycosylated model protein (the secretory component of IgA) to better understand the relationship of deglycosylation and proteolysis in BV (Lewis et al. 2012). Incubation of exogenous sIgA in BV specimens resulted in lower molecular weight products that were recognized by the mannose-binding lectin ConA, similar to patterns observed when adding 3 exogenous enzymes: sialidase, β-galactosidase and hexosaminidase as a positive control. In contrast, after incubation of secretory component with vaginal specimens of women without BV, significantly lower levels of ConA reactivity were observed compared to BV specimens. These results suggest that enzymes found in the vaginal milieu during BV are engaging in processive deglycosylation of N-glycans, revealing underlying mannose residues within these glycans. In further biochemical experiments, it was shown that partial N-deglycosylation of secretory component led to enhanced bacterial proteolysis of secretory component.
Among other Gram-positive pathogens, S. pneumoniae shares two features in common with Gardnerella: it encodes multiple sialidases and can also catabolize sialic acids. In addition to this, S. pneumoniae is able to bind to underlying carbohydrate residues, once exposed by sialidase (Blanchette et al. 2016). It is possible Gardnerella could similarly benefit from the exposure of cryptic receptors. Gardnerella sialidase could also modify the properties of mucus secretions, leading to greater capacity for bacteria to gain proximity to the epithelium or invade the upper reproductive tract. Indeed, several lines of investigation suggest that sialic acids may be an important driver of niche specificity and pathogenic potential for Gardnerella. In addition to potential metabolic benefits of sialic acid catabolism (Lewis et al. 2013), the addition of sialidase inhibitors reduced (by half) G. vaginalis attachment and invasion of HeLa cells in vitro (Govinden et al. 2018). Preliminary studies by Cook et al. (1989) revealed that G. vaginalis is most frequently found adhering to clue cells present in vaginal fluid of women with BV. More recently, investigation of vaginal biopsy specimens show that G. vaginalis forms adherent biofilms on the vaginal epithelium, along with some other BV associated bacteria (Swidsinski et al. 2005; Swidsinski et al. 2014). So far, it is unclear how G. vaginalis attaches to the vaginal epithelial cells and the mechanisms underlying epithelial cell damage remain unknown. Future studies in this direction may evaluate the role of sialidases and carbohydrates in attachment to vaginal epithelial cells by different strains of G. vaginalis. This will provide new avenues for development of therapeutics such as carbohydrate analogs, which may act as substrate decoys or competitive inhibitors of bacterial attachment to vaginal epithelium, for BV.
Several sialic acid-binding proteins known for their immune-modulatory functions have been described in the female reproductive tract, in particular, sialic acid binding immunoglobulin-like lectins (Siglecs) have been reported on amniotic membranes, the cervical endothelium and on immune cells throughout the urogenital system (Brinkman-Van der Linden et al. 2007; Ali et al. 2014; Patras et al. 2017; Tecle et al. 2019). The removal of sialic acids within cervicovaginal secretions suggests that these immune-modulating receptors might be regulated inappropriately. This could have myriad effects on bacterial-host interactions in an array of different cell types and might help explain the overgrowth of particular subsets of bacteria, as well as the enhanced inflammatory potential described in women with a diverse BV-like microbiota (Farcasanu and Kwon 2018). In fact, sialidases have been shown in other contexts to regulate immune states through Siglec receptors (Chang et al. 2012; Chen et al. 2014).
The widespread roles of sialic acids and sialoglycans in human reproduction include aspects of sperm migration in the female reproductive tract (through mucus), formation of the sperm oviductal reservoir, sperm capacitation- a required process after ejaculation for sperm to become capable of fertilization (Tecle et al. 2019), as well as fertilization itself (Lassalle and Testart 1994). One of the most well-known sialoglycans, glycodelin, affects embryo implantation, placental development and immune regulation. This topic has been recently reviewed (Lee et al. 2016), with recent studies suggesting a role for Siglec-6 in glycodelin-mediated fetal trophoblast invasion into the maternal decidua (Lam et al. 2011).
In conclusion, the female reproductive tract has a wide array of important functions that might be disrupted in the setting of BV-associated sialoglycan depletion of vaginal secretions. More study is needed to understand the key functions of sialoglycans and sialic acid binding receptors in the female reproductive tract and to understand how microbes endanger reproductive health by interfering with these functions.
Supplementary Material
Acknowledgement
We thank Dr. Wandy Beatty for assistance with imaging of Gram-stained slides of vaginal specimens.
Contributor Information
Kavita Agarwal, Department of Obstetrics, Gynecology and Reproductive Sciences, Glycobiology Research and Training Center, University of California San Diego, 9500 Gilman Drive, La Jolla CA 92093, USA.
Amanda L Lewis, Department of Obstetrics, Gynecology and Reproductive Sciences, Glycobiology Research and Training Center, University of California San Diego, 9500 Gilman Drive, La Jolla CA 92093, USA.
Funding
We thank the National Institute of Allergy and Infectious Diseases (R01 AI114635) and the Burroughs Wellcome Fund (preterm birth initiative) for supporting this work.
References
- Agarwal K, Robinson LS, Aggarwal S, Foster LR, Hernandez-Leyva A, Lin H, Tortelli BA, O'Brien VP, Miller L, Kau AL, et al. 2020. Glycan cross-feeding supports mutualism between Fusobacterium and the vaginal microbiota. PLoS Biol. 18:e3000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, Areschoug T, Parast M, Varki N, Murray J, et al. 2014. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 211:1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 74:14–22. [DOI] [PubMed] [Google Scholar]
- Balashov SV, Mordechai E, Adelson ME, Gygax SE. 2014. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J Med Microbiol. 63:162–175. [DOI] [PubMed] [Google Scholar]
- Berardi-Grassias L, Roy O, Berardi JC, Furioli J. 1988. Neonatal meningitis due to Gardnerella vaginalis. Eur J Clin Microbiol Infect Dis. 7:406–407. [DOI] [PubMed] [Google Scholar]
- Blanchette KA, Shenoy AT, Milner J 2nd, Gilley RP, McClure E, Hinojosa CA, Kumar N, Daugherty SC, Tallon LJ, Ott S, et al. 2016. Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun. 84:2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. 2005. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 43:1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham C, Caughlan R, Gallegos R, Dallas MB, Godoy VG, Malamy MH. 2009. Sialic acid (N-acetyl neuraminic acid) utilization by Bacteroides fragilis requires a novel N-acetyl mannosamine epimerase. J Bacteriol. 191:3629–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, Varki N. 2007. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 17:922–931. [DOI] [PubMed] [Google Scholar]
- Briselden AM, Moncla BJ, Stevens CE, Hillier SL. 1992. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 30:663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, Schwebke JR. 2010. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 202:1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana JC, Walper SA. 2020. Bacterial membrane vesicles as mediators of microbe - microbe and microbe - host community interactions. Front Microbiol. 11:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Alves P, Sousa C, Cereija T, Franca A, Jefferson KK, Cerca N. 2015. Using an in vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci Rep. 5:11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauci S, Culhane JF. 2011. High sialidase levels increase preterm birth risk among women who are bacterial vaginosis-positive in early gestation. Am J Obstet Gynecol. 204:142 e141–142 e149. [DOI] [PubMed] [Google Scholar]
- Cauci S, Thorsen P, Schendel DE, Bremmelgaard A, Quadrifoglio F, Guaschino S. 2003. Determination of immunoglobulin A against Gardnerella vaginalis hemolysin, sialidase, and prolidase activities in vaginal fluid: Implications for adverse pregnancy outcomes. J Clin Microbiol. 41:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Uchiyama S, Varki A, Nizet V. 2012. Leukocyte inflammatory responses provoked by pneumococcal sialidase. MBio. 3:e00220–e00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, Hillier SL. 2014. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am J Obstet Gynecol. 211:226 e221–226 e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Brown NK, Wu W, Khedri Z, Yu H, Chen X, van de Vlekkert D, D'Azzo A, Zheng P, Liu Y. 2014. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife. 3:e04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Forsyth PS, Buchanan TM, Holmes KK. 1979. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J Clin Invest. 63:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Reid G, Pond DG, Schmitt CA, Sobel JD. 1989. Clue cells in bacterial vaginosis: Immunofluorescent identification of the adherent gram-negative bacteria as Gardnerella vaginalis. J Infect Dis. 160:490–496. [DOI] [PubMed] [Google Scholar]
- Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. 1992. Mucin degradation in the human colon: Production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 60:3971–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C, Watt AP, McKenna JP, Coyle PV. 2017. Gardnerella vaginalis and Mollicute detection in rectal swabs from men who have sex with men. Int J STD AIDS. 28:708–714. [DOI] [PubMed] [Google Scholar]
- Deman J, Mareel M, Bruyneel E. 1973. Effects of calcium and bound sialic acid on the viscosity of mucin. Biochim Biophys Acta. 297:486–490. [DOI] [PubMed] [Google Scholar]
- Dhar C, Sasmal A, Varki A. 2019. From ``serum sickness'' to ``Xenosialitis'': Past, present, and future significance of the non-human sialic acid Neu5Gc. Front Immunol. 10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, Tanturli M, Rivero D, Cozzolino F, Cavalieri D, et al. 2017. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 7:10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiulio DB. 2012. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 17:2–11. [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, et al. 2010. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhenawy W, Debelyy MO, Feldman MF. 2014. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. MBio. 5:e00909–e00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstein M. 1978. Functions and physical properties of mucus in the female genital tract. Br Med Bull. 34:83–88. [DOI] [PubMed] [Google Scholar]
- Farcasanu M, Kwon DS. 2018. The influence of cervicovaginal microbiota on mucosal immunity and prophylaxis in the battle against HIV. Curr HIV/AIDS Rep. 15:30–38. [DOI] [PubMed] [Google Scholar]
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, et al. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 4:132–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner HL, Dukes CD. 1955. Haemophilus vaginalis vaginitis: A newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol. 69:962–976. [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. 2011. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology. 21:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert NM, Lewis WG, Lewis AL. 2013. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 8:e59539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert NM, Lewis WG, Li G, Sojka DK, Lubin JB, Lewis AL. 2019. Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. J Infect Dis. 220:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govinden G, Parker JL, Naylor KL, Frey AM, Anumba DOC, Stafford GP. 2018. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch Microbiol. 200:1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines-Menges BL, Whitaker WB, Lubin JB, Boyd EF. 2015. Host sialic acids: A delicacy for the pathogen with discerning taste. Microbiol Spectr. 3:10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2017. The presence of the putative Gardnerella vaginalis sialidase a gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One. 12:e0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey RJ, Forney LJ. 2014. Gardnerella vaginalis does not always cause bacterial vaginosis. J Infect Dis. 210:1682–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert DW, Schuyler JA, Adelson ME, Mordechai E, Sobel JD, Gygax SE. 2017. Gardnerella vaginalis population dynamics in bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 36:1269–1278. [DOI] [PubMed] [Google Scholar]
- Hill JE, AYK A, VOGUE Research Group . 2019. Resolution and cooccurrence patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii, and G. vaginalis within the vaginal microbiome. Infect Immun. 87:e00532–e00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 16(Suppl 4):S273–S281. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. 1988. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 319:972–978. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG 2nd, Rao AV, et al. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The vaginal infections and prematurity study group. N Engl J Med. 333:1737–1742. [DOI] [PubMed] [Google Scholar]
- Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA. 2001. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 97:211–219. [DOI] [PubMed] [Google Scholar]
- Holst E, Goffeng AR, Andersch B. 1994. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 32:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L, Wiggins R, Soothill PW, Millar MR, Horner PJ, Corfield AP. 1999. Mucinase and sialidase activity of the vaginal microflora: Implications for the pathogenesis of preterm labour. Int J STD AIDS. 10:442–447. [DOI] [PubMed] [Google Scholar]
- Huang YL, Chassard C, Hausmann M, von Itzstein M, Hennet T. 2015. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat Commun. 6:8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulaitiene M, Gegzna V, Baranauskiene L, Bulavaite A, Simanavicius M, Pleckaityte M. 2018. Phenotypic characterization of Gardnerella vaginalis subgroups suggests differences in their virulence potential. PLoS One. 13:e0200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Howard CD, Misic AM, Beiting DP, Bale TL. 2017. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 7:44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Tailford L, Owen CD. 2016. Sialidases from gut bacteria: A mini-review. Biochem Soc Trans. 44:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivoda KA, Steenbergen SM, Vimr ER. 2013. Control of the Escherichia coli sialoregulon by transcriptional repressor NanR. J Bacteriol. 195:4689–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivoda KA, Steenbergen SM, Vimr ER, Plumbridge J. 2003. Regulation of sialic acid catabolism by the DNA binding protein NanR in Escherichia coli. J Bacteriol. 185:4806–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara M, Tanigawa K, Chaiwangsri T, Katayama T, Ashida H, Yamamoto K. 2011. An exo-alpha-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology. 21:437–447. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Rao H, Nayak V, Ramaswamy S. 2018. Crystal structures and kinetics of N-acetylneuraminate lyase from Fusobacterium nucleatum. Acta Crystallogr F Struct Biol Commun. 74:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KK, Chiu PC, Lee CL, Pang RT, Leung CO, Koistinen H, Seppala M, Ho PC, Yeung WS. 2011. Glycodelin-a protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J Biol Chem. 286:37118–37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle B, Testart J. 1994. Human zona pellucida recognition associated with removal of sialic acid from human sperm surface. J Reprod Fertil. 101:703–711. [DOI] [PubMed] [Google Scholar]
- Lee CL, Lam KK, Vijayan M, Koistinen H, Seppala M, Ng EH, Yeung WS, Chiu PC. 2016. The pleiotropic effect of Glycodelin-a in early pregnancy. Am J Reprod Immunol. 75:290–297. [DOI] [PubMed] [Google Scholar]
- Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. 2003. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol. 189:139–147. [DOI] [PubMed] [Google Scholar]
- Leitich H, Kiss H. 2007. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 21:375–390. [DOI] [PubMed] [Google Scholar]
- Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. 2013. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem. 288:12067–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WG, Robinson LS, Perry J, Bick JL, Peipert JF, Allsworth JE, Lewis AL. 2012. Hydrolysis of secreted sialoglycoprotein immunoglobulin a (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem. 287:2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock RA, Paton JC, Hansman D. 1988. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb Pathog. 4:33–43. [DOI] [PubMed] [Google Scholar]
- Marrazzo JM, Fiedler TL, Srinivasan S, Thomas KK, Liu C, Ko D, Xie H, Saracino M, Fredricks DN. 2012. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 205:1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ND, Lubin JB, Chowdhury N, Boyd EF. 2016. Host-derived sialic acids are an important nutrient source required for optimal bacterial fitness in vivo. MBio. 7:e02237–e02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor JA, French JI, Jones W, Milligan K, McKinney PJ, Patterson E, Parker R. 1994. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: Results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol. 170:1048–1059 discussion 1059-1060. [DOI] [PubMed] [Google Scholar]
- Miller EA, Beasley DE, Dunn RR, Archie EA. 2016. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front Microbiol. 7:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Yamaguchi K. 2012. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology. 22:880–896. [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh R, Sadeghi Kalani B, Kashanian M, Oshaghi M, Amirmozafari N. 2019. Prevalence of vaginolysin, sialidase and phospholipase genes in Gardnerella vaginalis isolates between bacterial vaginosis and healthy individuals. Med J Islam Repub Iran. 33:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Braham P, Hillier SL. 1990. Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J Clin Microbiol. 28:422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Chappell CA, Debo BM, Meyn LA. 2016. The effects of hormones and vaginal microflora on the glycome of the female genital tract: Cervical-vaginal fluid. PLoS One. 11:e0158687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Chappell CA, Mahal LK, Debo BM, Meyn LA, Hillier SL. 2015. Impact of bacterial vaginosis, as assessed by Nugent criteria and hormonal status on glycosidases and lectin binding in cervicovaginal lavage samples. PLoS One. 10:e0127091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill S, Gilbert NM, Lewis AL. 2020. Gardnerella vaginalis as a cause of bacterial vaginosis: Appraisal of the evidence from in vivo models. Front Cell Infect Microbiol. 10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, von Itzstein M, Taylor G. 2004. Sialic acid recognition by Vibrio cholerae neuraminidase. J Biol Chem. 279:40819–40826. [DOI] [PubMed] [Google Scholar]
- Myhre AK, Bevanger LS, Berntzen K, Bratlid D. 2002. Anogenital bacteriology in non-abused preschool children: A descriptive study of the aerobic genital flora and the isolation of anogenital Gardnerella vaginalis. Acta Paediatr. 91:885–891. [DOI] [PubMed] [Google Scholar]
- Myziuk L, Romanowski B, Johnson SC. 2003. BVBlue test for diagnosis of bacterial vaginosis. J Clin Microbiol. 41:1925–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TM, Borgogna JL, Brotman RM, Ravel J, Walk ST, Yeoman CJ. 2015. Vaginal biogenic amines: Biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol. 6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, et al. 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 502:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, Mukai T. 2017. Bifidobacterium bifidum extracellular Sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. MBio. 8:e00928–e00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon DE, Moench TR, Cone RA. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 8:e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted SS, Meyn LA, Rohan LC, Hillier SL. 2003. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex Transm Dis. 30:257–261. [DOI] [PubMed] [Google Scholar]
- Owen CD, Tailford LE, Monaco S, Suligoj T, Vaux L, Lallement R, Khedri Z, Yu H, Lecointe K, Walshaw J, et al. 2017. Unravelling the specificity and mechanism of sialic acid recognition by the gut symbiont Ruminococcus gnavus. Nat Commun. 8:2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patras KA, Coady A, Olson J, Ali SR, Ramachandra Rao SP, Kumar S, Varki A, Nizet V. 2017. Tamm-Horsfall glycoprotein engages human Siglec-9 to modulate neutrophil activation in the urinary tract. Immunol Cell Biol. 95:960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzicoli A, Ruggiero P, Amerighi F, Telford JL, Soriani M. 2012. Exogenous sialic acid transport contributes to group B Streptococcus infection of mucosal surfaces. J Infect Dis. 206:924–931. [DOI] [PubMed] [Google Scholar]
- Pleckaityte M, Janulaitiene M, Lasickiene R, Zvirbliene A. 2012. Genetic and biochemical diversity of Gardnerella vaginalis strains isolated from women with bacterial vaginosis. FEMS Immunol Med Microbiol. 65:69–77. [DOI] [PubMed] [Google Scholar]
- Pybus V, Onderdonk AB. 1997. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: Potential significance for bacterial vaginosis. J Infect Dis. 175:406–413. [DOI] [PubMed] [Google Scholar]
- Pybus V, Onderdonk AB. 1999. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1:285–292. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Aon MA, Thomas D. 1989. Interactions involved in ovomucin gel-forming properties: A rheological-biochemical approach. Arch Biochem Biophys. 270:495–503. [DOI] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 108(Suppl 1):4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezeberga D, Lazdane G, Kroica J, Sokolova L, Donders GG. 2008. Placental histological inflammation and reproductive tract infections in a low risk pregnant population in Latvia. Acta Obstet Gynecol Scand. 87:360–365. [DOI] [PubMed] [Google Scholar]
- Robinson LS, Lewis WG, Lewis AL. 2017. The sialate O-acetylesterase EstA from gut Bacteroidetes species enables sialidase-mediated cross-species foraging of 9-O-acetylated sialoglycans. J Biol Chem. 292:11861–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LS, Schwebke J, Lewis WG, Lewis AL. 2019. Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. J Biol Chem. 294:5230–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Thompson JS, Godoy VG, Malamy MH. 1990. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 172:2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago GL, Deschaght P, Aila N, Kiama TN, Verstraelen H, Jefferson KK, Temmerman M, Vaneechoutte M. 2011. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am J Obstet Gynecol. 204:450 e451–450 e457. [DOI] [PubMed] [Google Scholar]
- Schellenberg JJ, Paramel Jayaprakash T, Withana Gamage N, Patterson MH, Vaneechoutte M, Hill JE. 2016. Gardnerella vaginalis subgroups defined by cpn60 sequencing and Sialidase activity in isolates from Canada, Belgium and Kenya. PLoS One. 11:e0146510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg JJ, Patterson MH, Hill JE. 2017. Gardnerella vaginalis diversity and ecology in relation to vaginal symptoms. Res Microbiol. 168:837–844. [DOI] [PubMed] [Google Scholar]
- Schwebke JR, Muzny CA, Josey WE. 2014. Reply to Hickey and Forney. J Infect Dis. 210:1683–1684. [DOI] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 105:18964–18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, Chen X, Lebrilla CB, Mills DA. 2011. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J Biol Chem. 286:11909–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsyna E, Krysanova A, Khayrullina G, Shalepo K, Savicheva A, Guschin A, Unemo M. 2019. Quantitation of all four Gardnerella vaginalis clades detects abnormal vaginal microbiota characteristic of bacterial vaginosis more accurately than Putative G. vaginalis Sialidase a gene count. Mol Diagn Ther. 23:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishpal P, Kasarpalkar N, Singh D, Bhor VM. 2020. Characterization of Gardnerella vaginalis membrane vesicles reveals a role in inducing cytotoxicity in vaginal epithelial cells. Anaerobe. 61:102090. [DOI] [PubMed] [Google Scholar]
- Silver HM, Sperling RS, St Clair PJ, Gibbs RS. 1989. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 161:808–812. [DOI] [PubMed] [Google Scholar]
- Smayevsky J, Canigia LF, Lanza A, Bianchini H. 2001. Vaginal microflora associated with bacterial vaginosis in nonpregnant women: Reliability of sialidase detection. Infect Dis Obstet Gynecol. 9:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, et al. 2012. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN. 2010. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. 2015. Metabolic signatures of bacterial vaginosis. MBio. 6:e00204–e00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svare JA, Schmidt H, Hansen BB, Lose G. 2006. Bacterial vaginosis in a cohort of Danish pregnant women: Prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG. 113:1419–1425. [DOI] [PubMed] [Google Scholar]
- Sweet RL. 1995. Role of bacterial vaginosis in pelvic inflammatory disease. Clin Infect Dis. 20(Suppl 2):S271–S275. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Loening-Baucke V, Mendling W, Dorffel Y, Schilling J, Halwani Z, Jiang XF, Verstraelen H, Swidsinski S. 2014. Infection through structured polymicrobial Gardnerella biofilms (StPM-GB). Histol Histopathol. 29:567–587. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, Lochs H. 2005. Adherent biofilms in bacterial vaginosis. Obstet Gynecol. 106:1013–1023. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ito F, Iwasaki T. 1992. Purification and characterization of a sialidase from Bacteroides fragilis SBT3182. Biochem Biophys Res Commun. 189:524–529. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ito F, Iwasaki T. 1994. Two sialidases which preferentially hydrolyze sialyl alpha 2-8 linkage from Bacteroides fragilis SBT3182. J Biochem. 115:318–321. [DOI] [PubMed] [Google Scholar]
- Tecle E, Reynoso HS, Wang R, Gagneux P. 2019. The female reproductive tract contains multiple innate sialic acid-binding immunoglobulin-like lectins (Siglecs) that facilitate sperm survival. J Biol Chem. 294:11910–11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Troy FA. 1985a. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 164:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Troy FA. 1985b. Regulation of sialic acid metabolism in Escherichia coli: Role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 164:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nicolai H, Hammann R, Salehnia S, Zilliken F. 1984. A newly discovered sialidase from Gardnerella vaginalis. Zentralbl Bakteriol Mikrobiol Hyg A. 258:20–26. [DOI] [PubMed] [Google Scholar]
- Vrbanac A, Riestra AM, Coady A, Knight R, Nizet V, Patras KA. 2018. The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus. BMC Microbiol. 18:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Koppolu S, Chappell C, Moncla BJ, Hillier SL, Mahal LK. 2015. Studying the effects of reproductive hormones and bacterial vaginosis on the glycome of lavage samples from the cervicovaginal cavity. PLoS One. 10:e0127021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. 1990. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 75:52–58. [PubMed] [Google Scholar]
- Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, Sweet RL. 2002. Lower genital tract infection and endometritis: Insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 100:456–463. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. 2003. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 36:663–668. [DOI] [PubMed] [Google Scholar]
- Wolrath H, Forsum U, Larsson PG, Boren H. 2001. Analysis of bacterial vaginosis-related amines in vaginal fluid by gas chromatography and mass spectrometry. J Clin Microbiol. 39:4026–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Lin X, Hui KM, Yang S, Wu X, Tan Y, Li M, Qin AQ, Wang Q, Zhao Q, et al. 2019. A biochemiluminescent Sialidase assay for diagnosis of bacterial vaginosis. Sci Rep. 9:20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kiefel MJ, Wilson JC, Andrew PW, Oggioni MR, Taylor GL. 2011. Three Streptococcus pneumoniae sialidases: Three different products. J Am Chem Soc. 133:1718–1721. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hirose Y, Nakata M, Uchiyama S, Yamaguchi Y, Goto K, Sumitomo T, Lewis AL, Kawabata S, Nizet V. 2016. Evolutionary inactivation of a sialidase in group B Streptococcus. Sci Rep. 6:28852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ugai H, Nakayama-Imaohji H, Tada A, Elahi M, Houchi H, Kuwahara T. 2018. Characterization of a recombinant Bacteroides fragilis sialidase expressed in Escherichia coli. Anaerobe. 50:69–75. [DOI] [PubMed] [Google Scholar]
- Yoneda S, Loeser B, Feng J, Dmytryk J, Qi F, Merritt J. 2014. Ubiquitous sialometabolism present among oral fusobacteria. PLoS One. 9:e99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W, Egert M, Bassett SA, Bibiloni R. 2015. Detection of sialic acid-utilising bacteria in a caecal community batch culture using RNA-based stable isotope probing. Nutrients. 7:2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu X, Li J, Li N, Yan T, Ju X. 2002. Relationship between vaginal sialidase bacteria vaginosis and chorioammionitis. Zhonghua Fu Chan Ke Za Zhi. 37:588–590. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


