The human glycobiome is in constant flux as we transition between states of well-being, acute infection, chronic disease and natural aging. Microbes contribute to these frequent alterations in glycan composition during nutrient acquisition (Bell and Juge 2020; Brown and Koropatkin 2020; Low et al. 2020; Agarwal and Lewis 2021), host molecular mimicry (Patry et al. 2019), microbial warfare (Jank et al. 2015; Nothaft and Szymanski 2019) and activation of host innate and adaptive immune responses (Yang et al. 2018; Garber et al. 2021). Although in many cases, these microbes, both good and bad, are exhibiting primordial requirements for nutrient scavenging, dynamic changes are occurring in all environments that envelop them. In this collection of articles, the contributors discuss the many unique adaptations bacteria have evolved that consequently alter the host glycobiome, particularly at mucosal surfaces.
The motivation for this special issue originated at the Society for Glycobiology (SfG) Annual meeting in New Orleans, Louisiana, November 19–22, 2016. That particular meeting was focused on Glycoscience communities and the impact sugars have on all aspects of life including the ecological habitat that surrounds us, the foods that we ingest and the various organisms that populate our universe, including the microbes within our bodies. It was at that meeting that there were several presentations describing microbial transformation of the host glycobiome. This issue is not an exhaustive review of the excellent work being done in the field, but rather a selection of studies presented at the SfG meetings over the past 5 years that illustrate this process. For example, in this issue, Agarwal and Lewis (2021) describe how vaginal sialoglycan degradation by sialidases produced by Gardnerella vaginalis leads to bacterial vaginosis. The authors emphasize the importance of sialic acid on mucosal surfaces, and the protection provided by the resident anaerobic bacteria and lactobacilli, in the maintenance of a healthy vaginal microbiome. In the next article, Low et al. (2020) review the wide array of glycoside hydrolases (GHs) secreted by Clostridium perfringens. This opportunistic pathogen not only causes necrotic enteritis in the intestinal tract of poultry but also is a significant cause of foodborne diarrheal illness in humans (Wenzel et al. 2020). We learn that one strain of C. perfringens can possess up to 89 different carbohydrate-active enzymes (CAZymes), many of which have been shown to degrade complex host sugars found in the mucosa. These enzymes include sialidases as mentioned above, galactosidases, hexosaminidases, fucosidases, endo- and exoglycosidases, and a new class of zinc metalloproteases (three Zmps) that cleave peptide linkages preceding a sialylated O-glycan residue. A striking feature of the C. perfringens enzyme organization is the multimodularity of many of the GHs and peptidases with 13 that have at least one or as many as six carbohydrate-binding modules (many from CBM family 32 or 51) that tune the specificity and performance of the catalytic domain. Finally, some of these multimodular enzymes such as the GH33 sialidases NanJ and NanH, associate into larger complexes to maximize efficiency of mucin degradation.
Bell and Juge change the focus from microbial pathogens to commensal bacteria such as Akkermansia, Bacteroidetes and Lachnospiraceae that are notorious mucin glycan degraders (Bell and Juge 2020). We learn that microbes that inhabit the gut are ideally suited to occupy this environment and possess an arsenal of systems for nutrient acquisition and degradation. Mucin-degrading bacteria across the three major Phyla found in the gut, namely Bacteroidetes, Firmicutes and Actinobacteria, most commonly possess a range of sulfatases, sialidases, fucosidases and acetylases that target the terminal mucin carbohydrates. Many of these “de-capping” enzymes are localized to the cell surface, facilitating the cross-feeding of both commensal and pathogenic species, and providing access to the core carbohydrate structure for further deconstruction. In the final article, Brown and Koropatkin (2020) describe the model symbiont, Bacteroides thetaiotaomicron, and detail how this common gut commensal not only deconstructs host glycans, but also possesses complex systems for glycan uptake. Complex carbohydrate utilization by gut Bacteroidetes generally proceeds via the limited breakdown of the substrate at the cell surface followed by import of the glycan fragments through a TonB-dependent transporter and then complete saccarification in the periplasm. Using this template, B. thetaiotaomicron catabolizes highly sulfated host glycans such as heparin/heparan sulfate and chondroitin sulfate/dermatan sulfate/hyaluronic acid, providing the microbe with a competitive advantage in the gut, but interestingly has also been shown to lead to inflammation in susceptible hosts. This metabolic strategy of harboring most glycolytic enzymes within the periplasm can limit the potential for glycan sharing as has been observed with xylan degradation by Bacteroides ovatus (Rogowski et al. 2015) and yeast mannan by B. thetaiotaomicron (Cuskin et al. 2015). However, some Bacteroidetes contribute significantly to cross-feeding via cell surface and outer membrane vesicles (Rakoff-Nahoum et al. 2016) that harbor GHs that liberate sialic acid or fucose from mucin for neighboring bacteria (Ndeh and Gilbert 2018) and invasive pathogens (Garber et al. 2020).
Although microbial digestion of host glycans accounts for the majority of the remodeling events observed on host mucosal surfaces, we learned from Yang et al. (2018) in the Marth group that Gram-negative bacterial lipopolysaccharides (LPS) induce host sialidase production through Toll-like receptor 4 (TLR4) activation. Sepsis-induced signaling subsequently stimulates the removal of host innate immune defense enzymes such as alkaline phosphatase, among other glycoproteins, through well-known protein aging and clearance mechanisms including the Ashwell-Morell receptor (Figure 1). Through these studies and others, we are discovering that the host glycobiome provides a rich source of targets for manipulation and nutrients for its resident microbes that in turn stimulate changes to this landscape, both directly and indirectly, through their diverse mechanisms of survival.
Fig. 1.
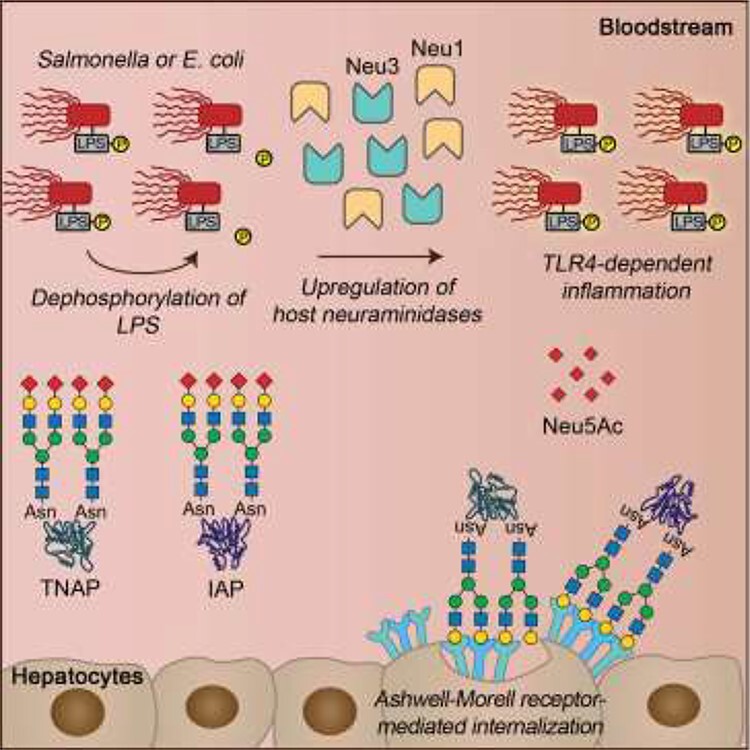
Remodeling of host glycoproteins intensifies sepsis. Gram negative pathogens such as Salmonella species and Escherichia coli stimulate TLR4 to increase production of host neuraminidases, which leads to enhanced clearance of alkaline phosphatase isozymes TNAP and IAP by the Ashwell-Morell receptor. Loss of alkaline phosphatase, which can detoxify LPS, perpetuates inflammation and reduces survival in a murine sepsis model. Reprinted with permission from Yang et al. (2018).
Funding
Department of Defense (CDMRP PR191209) grant to C.M.S.; the National Institutes of Health (R01 GM118475) grant to N.M.K.
Conflicts of interest statement
The authors have no conflicts of interest to declare.
Contributor Information
Christine M Szymanski, Complex Carbohydrate Research Center and Department of Microbiology, University of Georgia, Athens, GA, USA.
Nicole M Koropatkin, Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI, USA.
References
- Agarwal K, Lewis AL. 2021. Vaginal sialoglycan foraging by Gardnerella vaginalis: mucus barriers as a meal for unwelcome guests? Glycobiology. cwab024. doi: 10.1093/glycob/cwab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A, Juge N. 2020. Mucosal glycan degradation of the host by the gut microbiota. Glycobiology. cwaa097. doi: 10.1093/glycob/cwaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HA, Koropatkin NM. 2020. Host glycan utilization within the bacteroidetes Sus-like paradigm. Glycobiology. cwaa054. doi: 10.1093/glycob/cwaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron E, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al. 2015. Human gut bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 517:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber JM, Hennet T, Szymanski CM. 2021. Significance of fucose in intestinal health and disease. Mol Microbiol. 1–8. doi: 10.1111/mmi.14681. [DOI] [PubMed] [Google Scholar]
- Garber JM, Nothaft H, Pluvinage B, Stahl M, Bian X, Porfirio S, Enriquez A, Butcher J, Huang H, Glushka J, et al. 2020. The gastrointestinal pathogen Campylobacter jejuni metabolizes sugars with potential help from commensal Bacteroides vulgatus. Commun Biol. 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank T, Belyi Y, Aktories K. 2015. Bacterial glycosyltransferase toxins. Cell Microbiol. 17:1752–1765. [DOI] [PubMed] [Google Scholar]
- Low KE, Smith SP, Abbott DW, Boraston AB. 2020. The glycoconjugate-degrading enzymes of Clostridium perfringens: tailored catalysts for breaching the intestinal mucus barrier. Glycobiology. cwaa050. [DOI] [PubMed] [Google Scholar]
- Ndeh D, Gilbert HJ. 2018. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol Rev. 42:146–164. [DOI] [PubMed] [Google Scholar]
- Nothaft H, Szymanski CM. 2019. New discoveries in bacterial N-glycosylation to expand the synthetic biology toolbox. Curr Opin Chem Biol. 53:16–24. [DOI] [PubMed] [Google Scholar]
- Patry RT, Stahl M, Perez-Munoz ME, Nothaft H, Wenzel CQ, Sacher JC, Coros C, Walter J, Vallance BA, Szymanski CM. 2019. Bacterial AB5 toxins inhibit the growth of gut bacteria by targeting ganglioside-like glycoconjugates. Nat Commun. 10:1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Foster KR, Comstock LE. 2016. The evolution of cooperation within the gut microbiota. Nature. 533:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Basle A, Morland C, Day AM, Zheng H, et al. 2015. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 6:7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CQ, Mills DC, Dobruchowska JM, Vlach J, Nothaft H, Nation P, Azadi P, Melville SB, Carlson RW, Feldman MF, et al. 2020. An atypical lipoteichoic acid from Clostridium perfringens elicits a broadly cross-reactive and protective immune response. J Biol Chem. 295:9513–9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Heithoff DM, Aziz PV, Haslund-Gourley B, Westman JS, Narisawa S, Pinkerton AB, Millan JL, Nizet V, Mahan MJ, et al. 2018. Accelerated aging and clearance of host anti-inflammatory enzymes by discrete pathogens fuels sepsis. Cell Host Microbe. 24:500, e505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]


