Abstract
Background: Studies of closed-loop control (CLC) in patients with type 1 diabetes (T1D) consistently demonstrate improvements in glycemic control as measured by increased time-in-range (TIR) 70–180 mg/dL. However, clinical predictors of TIR in users of CLC systems are needed.
Materials and Methods: We analyzed data from 100 children aged 6–13 years with T1D using the Tandem Control-IQ CLC system during a randomized trial or subsequent extension phase. Continuous glucose monitor data were collected at baseline and during 12–16 weeks of CLC use. Participants were stratified into quartiles of TIR on CLC to compare clinical characteristics.
Results: TIR for those in the first, second, third, and fourth quartiles was 54%, 65%, 71%, and 78%, respectively. Lower baseline TIR was associated with lower TIR on CLC (r = 0.69, P < 0.001). However, lower baseline TIR was also associated with greater improvement in TIR on CLC (r = −0.81, P < 0.001). During CLC, participants in the highest versus lowest TIR-quartile administered more user-initiated boluses daily (8.5 ± 2.8 vs. 5.8 ± 2.6, P < 0.001) and received fewer automated boluses (3.5 ± 1.0 vs. 6.0 ± 1.6, P < 0.001). Participants in the lowest (vs. the highest) TIR-quartile received more insulin per body weight (1.13 ± 0.27 vs. 0.87 ± 0.20 U/kg/d, P = 0.008). However, in a multivariate model adjusting for baseline TIR, user-initiated boluses and insulin-per-body-weight were no longer significant.
Conclusions: Higher baseline TIR is the strongest predictor of TIR on CLC in children with T1D. However, lower baseline TIR is associated with the greatest improvement in TIR. As with open-loop systems, user engagement is important for optimal glycemic control.
Keywords: Closed-loop systems, Type 1 diabetes, Insulin pump, Time-in-range
Introduction
Management of type 1 diabetes (T1D) in childhood is challenging, and only a small percentage of children achieve the recommended glycemic targets.1 Before the advent of newer diabetes technologies, the primary modifiable predictor of glycemic control as measured by HbA1c was the frequency of blood glucose monitoring.2
Over the past several years, use of continuous glucose monitoring (CGM) has increased rapidly in children with T1D.1 As expected, CGM use in children is associated with improved glycemic outcomes regardless of the modality of insulin delivery used.1,3 With increased use of CGM technology, metrics of glycemic control have shifted to CGM-based outcomes, primarily time-in-range (TIR), defined by a target of 70–180 mg/dL. Recently established CGM-based glycemic targets recommend TIR >70% with additional out-of-range targets of <5% above 250 mg/dL, <25% above 180 mg/dL, <4% under 70 mg/dL, and <1% under 54 mg/dL.4
Closed-loop control (CLC) systems (also referred to as artificial pancreas or automated insulin delivery systems), which utilize CGM data along with a predictive algorithm to automatically adjust basal insulin delivery and in some instances give automated correction boluses, have consistently been shown to increase TIR in children and adults with T1D.5–8 Published trials of Food and Drug Administration (FDA)-approved hybrid CLC systems have reported achieving a mean TIR just above the goal of >70% in users.5,8 However, not all users benefit from CLC equally and are able to meet this metric. The clinical characteristics of users who are able to achieve the highest (and lowest) TIR on CLC are of significant interest to clinicians hoping to optimize this new treatment modality for individuals with T1D.
We conducted a 16-week randomized clinical trial (RCT) comparing the Tandem t:slim X2 Control-IQ hybrid Control-IQ CLC system versus a sensor-augmented pump (SAP) in 6–13-year-olds and found this CLC system to be safe and effective.9 Following the RCT, the SAP group used the CLC system for 12 weeks.10 We utilized the data from this study to determine predictors of TIR while using this CLC system. We hypothesized that children with higher TIR using CLC would have better glycemic control at baseline, spend more time in CLC, and demonstrate higher levels of user engagement with a greater number of carbohydrate boluses per day.
Materials and Methods
The Diabetes Closed Loop—Protocol 5 (DCLP5) RCT was conducted at four pediatric diabetes centers in the United States (clinicaltrials.gov registration NCT03844789). The protocol was approved by a central Institutional Review Board (Tampa, FL), written informed consent was obtained from the parent or guardian of each participant, and assent was obtained from each participant when applicable. An Investigational Device Exemption was approved by the U.S. Food and Drug Administration. The protocol has previously been reported in detail9 and is available at NEJM.org. Major inclusion criteria were age 6–<14 years, T1D diagnosed for at least 1 year, use of insulin for at least 6 months, and a total daily insulin dose of at least 10 U.
In brief, between June 6, 2019 and March 20, 2020, 101 children 6–13 years old were randomized in a 3:1 ratio to use CLC on a Tandem t:slim X2 insulin pump with Control-IQ Technology and a DexCom G6 CGM (n = 78) or SAP (n = 22) for 16 weeks. This randomization phase was followed by a 12-week extension phase, during which the SAP group transitioned to CLC and the CLC group continued on the system.10 Participants' pump, CGM, glucose meter, and ketone meter data were downloaded and reviewed multiple times throughout the course of the study. HbA1c was measured at a central laboratory at enrollment, the end of the randomization phase, and the end of the extension phase.
Statistical analysis
For the purposes of this study “baseline” refers to the time period before initiating CLC, which was during the prerandomization phase for those randomized to CLC at study onset and during the randomization phase for those who were in the SAP group who subsequently transitioned to CLC. For the group randomized to CLC at study onset, baseline CGM metrics were calculated from either the 2 weeks before enrollment or from a 2-week run-in phase (required for CGM or insulin pump naive participants), and CLC data were from the 16-week randomization phase. For the group randomized to SAP, baseline CGM metrics were calculated from the randomized trial phase and the CLC TIR data from the 12-week extension phase.10 Both randomization groups showed a similar increase in TIR on CLC compared with baseline,9 thus, the CLC TIR data for both of these groups were combined for the current analysis.
Participants were categorized into four groups based on quartiles of TIR while using CLC. These groups were created for the purpose of tabulating the data only. All models were based on TIR as a continuous variable.
To assess the association of demographic, clinical, and system use characteristics with TIR while using CLC, univariate linear regression models were fit with continuous TIR at follow-up as the dependent variable and the characteristic as the predictor. In addition, a multivariate linear regression model was fit with continuous TIR at follow-up as the dependent variable and all characteristics included as predictors. To avoid multicollinearity, some characteristics were not included in the multivariate model. P-values are two-sided and have been adjusted for multiple comparisons to control the false discovery rate using the adaptive Benjamini-Hochberg procedure.11 Analyses were conducted with SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Of the 101 children in the clinical trial, one participant in the SAP group dropped out during the randomization phase and 100 completed the study. Seventy-eight participants first used the CLC system during the randomization phase and the remaining 22 in the SAP group went on to use the CLC system during the extension phase.10 Age range at the time of initiation of CLC was 6.5–14.3 years (mean ± SD 11.2 ± 2.0), duration of T1D was 1.2–13.0 years (mean 5.3 ± 2.9), and baseline HbA1c was 5.6%–10.0% (mean 7.6% ± 1.0%). Baseline characteristics of the entire cohort by intervention group have been previously published.9
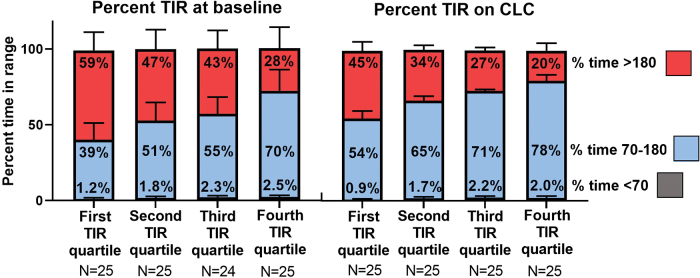
The cohort was divided into quartiles based on TIR while on CLC using the following cut points: 59%, 69%, and 73%. TIR according to quartile is shown in Figure 1. Mean TIR on CLC for those in the first, second, third, and fourth quartiles was 54%, 65%, 71%, and 78%, respectively. Time above 180 mg/dL was higher for the lowest TIR quartile compared with the highest quartile (mean 45% vs. 20%) (Table 1). Time below 70 mg/dL was low for all groups but was higher in the highest TIR quartile compared with the lowest (median 1.66% vs. 0.80%).
FIG. 1.
Mean TIR at baseline and on CLC by quartile of TIR on CLC. Red represents percent time >180 mg/dL. Blue represents percent time 70–180 mg/dL. Gray represents percent time <70 mg/dL. CLC, closed-loop control; TIR, time-in-target range. Color images are available online.
Table 1.
Glycemic Outcomes by Time-in-Range During Closed-Loop Control
| Time-in-range 70–180 mg/dL during CLCa |
Univariate, Pb | ||||
|---|---|---|---|---|---|
| First quartile (n = 25) | Second quartile (n = 25) | Third quartile (n = 25) | Forth quartile (n = 25) | ||
| Baseline glycemic measures | |||||
| Time-in-range 70–180 mg/dL | 39% ± 11% | 51% ± 12% | 55% ± 11% | 70% ± 14% | <0.001 |
| Mean glucose (mg/dL) | 212 ± 27 | 187 ± 25 | 178 ± 21 | 152 ± 23 | <0.001 |
| Time >180 mg/dL | 59% ± 12% | 47% ± 13% | 43% ± 12% | 28% ± 14% | <0.001 |
| Time <70 mg/dL | 0.73% (0.08%, 1.40%) | 1.78% (0.57%, 2.56%) | 1.46% (0.75%, 3.17%) | 1.90% (0.98%, 2.86%) | 0.008 |
| HbA1c (%) | 8.6 ± 0.7 | 7.6 ± 0.6 | 7.5 ± 0.7 | 6.8 ± 0.7 | <0.001 |
| Glycemic measures during CLC | |||||
| Time-in-range 70–180 mg/dL | 54% ± 5% | 65% ± 3% | 71% ± 1% | 78% ± 4% | NA |
| Mean Glucose (mg/dL) | 188 ± 12 | 166 ± 6 | 154 ± 4 | 144 ± 8 | <0.001 |
| Time >180 mg/dL | 45% ± 6% | 34% ± 3% | 27% ± 2% | 20% ± 5% | <0.001 |
| Time <70 mg/dL | 0.80% (0.37%, 1.01%) | 1.67% (0.99%, 2.23%) | 2.13% (1.21%, 2.90%) | 1.66% (1.16%, 2.38%) | <0.001 |
| HbA1c (%) | 7.9 ± 0.8 | 7.3 ± 0.4 | 6.8 ± 0.4 | 6.5 ± 0.4 | <0.001 |
Data are mean ± SD or median (IQR).
Groups based on quartiles are for display only. Analysis was based on time-in-range 70–180 mg/dL as a continuous variable.
Univariate P-values are from linear regression models with continuous time-in-range 70–180 mg/dL at follow-up as the dependent variable and the other glycemic measure as the predictor. P-values have been adjusted to control the false discovery rate.
CLC, closed-loop control; IQR, interquartile range; NA, not applicable; SD, standard deviation.
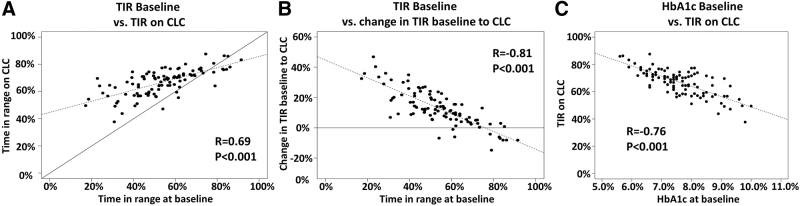
There was a strong correlation between TIR at baseline and TIR on CLC (r = 0.69, P < 0.001, Fig. 2A). However, those with lower TIR at baseline had greater improvement in TIR after the initiation of CLC (r = −0.81, P < 0.001, Fig. 2B). Those with lower HbA1c at baseline tended toward more TIR on CLC (Fig. 2C).
FIG. 2.
Correlation between TIR at baseline, on CLC, and change in TIR between these periods. Correlations are provided for (A) TIR 70–180 mg/dL at baseline versus on CLC, (B) TIR at baseline versus change in TIR between baseline and on CLC, and (C) HbA1c baseline versus TIR on CLC. Dashed line represents the regression line; solid line represents the line of identity in (A) and no change in (B).
The TIR on CLC quartile groups did not differ with respect to age, duration of diabetes, BMI percentile, weight, or family income at baseline (Table 2). Fewer participants were CGM users before enrollment in the lowest quartile compared to the highest quartile (88% vs. 100%; P = 0.02).
Table 2.
Demographic, Clinical, and System Use Characteristics by Time-in-Range During Closed-Loop Control
| Time-in-range 70–180 mg/dL during CLCb |
Univariate, Pc,e | Multivariate, Pd,e | ||||
|---|---|---|---|---|---|---|
| First quartile (n = 25) | Second quartile (n = 25) | Third quartile (n = 25) | Forth quartile (n = 25) | |||
| Baseline glycemic measures | ||||||
| Time-in-range 70–180 mg/dL | 39% ± 11% | 51% ± 12% | 55% ± 11% | 70% ± 14% | <0.001 | <0.001 |
| Baseline demographic and clinical characteristics | ||||||
| Age (years) | 11.4 ± 2.1 | 11.1 ± 2.0 | 10.8 ± 2.1 | 11.7 ± 1.9 | 0.83 | 0.08 |
| Sex: female | 15 (60%) | 11 (44%) | 9 (36%) | 14 (56%) | 0.76 | 0.03 |
| Race/ethnicity: non-Hispanic White | 20 (80%) | 19 (76%) | 22 (88%) | 20 (80%) | 0.75 | 0.48 |
| Weight (kg) | 46 ± 16 | 44 ± 16 | 40 ± 12 | 45 ± 13 | 0.50 | N/A |
| BMI percentile | 63 ± 30 | 63 ± 29 | 62 ± 27 | 60 ± 25 | 0.64 | 0.13 |
| Duration of diabetes (years) | 4.8 ± 2.6 | 6.5 ± 2.7 | 5.2 ± 2.9 | 4.6 ± 3.0 | 0.92 | 0.57 |
| CGM user before baseline | 22 (88%) | 24 (96%) | 23 (92%) | 25 (100%) | 0.02 | 0.13 |
| Insulin pump before baseline | 22 (88%) | 18 (72%) | 23 (92%) | 21 (84%) | 0.93 | 0.69 |
| Detectable C-peptidea | 8 (32%) | 2 (8%) | 7 (28%) | 8 (32%) | 0.37 | 0.06 |
| Highest parent education | 0.14 | 0.06 | ||||
| <Bachelor's degree | 1 (4%) | 3 (12%) | 2 (8%) | 2 (8%) | ||
| Bachelor's degree | 13 (52%) | 13 (52%) | 11 (44%) | 4 (16%) | ||
| ≥Master's degree | 11 (44%) | 9 (36%) | 12 (48%) | 19 (76%) | ||
| Annual household income | 0.10 | 0.21 | ||||
| <$100,000 | 7 (32%) | 10 (40%) | 5 (22%) | 5 (21%) | ||
| $100,000–<$200,000 | 7 (32%) | 7 (28%) | 9 (39%) | 12 (50%) | ||
| ≥$200,000 | 8 (36%) | 8 (32%) | 9 (39%) | 7 (29%) | ||
| CLC system use | ||||||
| CGM use | 96% (93%, 97%) | 98% (96%, 98%) | 96% (93%, 98%) | 98% (97%, 98%) | 0.001 | NA |
| Closed-loop mode use | 92% (88%, 93%) | 94% (91%, 95%) | 94% (91%, 96%) | 94% (92%, 96%) | <0.001 | 0.13 |
| Closed-loop mode use <90% | 9 (36%) | 4 (16%) | 4 (16%) | 0 (0%) | <0.001 | NA |
| CLC system interaction | ||||||
| Total daily insulin per kg | 1.13 ± 0.27 | 0.95 ± 0.27 | 0.88 ± 0.27 | 0.87 ± 0.20 | 0.008 | 0.12 |
| Total daily basal insulin per kg | 0.55 ± 0.15 | 0.47 ± 0.13 | 0.37 ± 0.12 | 0.37 ± 0.11 | <0.001 | NA |
| Average insulin to carb ratio during the daytime (6 am–8 pm) | 10.7 ± 6.3 | 13.7 ± 6.6 | 13.3 ± 5.5 | 11.8 ± 4.1 | 0.99 | NA |
| Average insulin correction factor during the daytime (6 am–8 pm) | 60 ± 42 | 77 ± 48 | 74 ± 41 | 67 ± 33 | 0.83 | NA |
| No. bolus doses per day | 11.8 ± 2.8 | 11.6 ± 2.5 | 11.3 ± 2.6 | 12.0 ± 2.7 | 0.72 | NA |
| No. automatic bolus doses per day | 6.0 ± 1.6 | 4.6 ± 1.7 | 4.0 ± 1.2 | 3.5 ± 1.0 | <0.001 | <0.001 |
| No. user-initiated bolus doses per day | 5.8 ± 2.6 | 6.9 ± 3.1 | 7.3 ± 2.6 | 8.5 ± 2.8 | <0.001 | 0.46 |
| No. user-initiated bolus doses with carb entry per day | 4.2 ± 2.0 | 5.8 ± 3.0 | 6.0 ± 1.7 | 5.8 ± 2.4 | <0.001 | NA |
| No. user-initiated bolus doses with carb entry <3 per day | 6 (24%) | 2 (8%) | 1 (4%) | 2 (8%) | 0.01 | NA |
| Carbohydrate entered per day (g) | 165 ± 67 | 199 ± 81 | 216 ± 70 | 206 ± 84 | 0.009 | NA |
Data are mean ± SD, median (IQR), or n (%).
The detection limit of the assay was 0.003 nmol/L.
Groups based on quartiles are for display only. Analysis was based on time-in-range 70–180 mg/dL as a continuous variable.
Univariate P-values are from linear regression models with continuous time-in-range 70–180 mg/dL at follow-up as the dependent variable and the participant characteristics as the predictor.
Multivariate P-values are from a single linear regression model with continuous time-in-range 70–180 mg/dL at follow-up as the dependent variable and the characteristics as predictors. To avoid multicollinearity, some factors were not included in the multivariate model. These variables are indicated by NA.
P-values have been adjusted to control the false discovery rate.
BMI, body mass index; CGM, continuous glucose monitoring; CLC, closed-loop control.
Those with the lowest TIR on CLC had lower CGM use and closed-loop mode use (Table 2). In the lowest quartile, 36% participants were in closed-loop mode <90% of time compared to none in the highest quartile (P < 0.001).
In univariate analyses, children with lower TIR on CLC required greater amounts of daily insulin per kg (P = 0.008). The number of total boluses (user-initiated and automatic) per day was not associated with TIR on CLC (P = 0.72), with the lowest quartile receiving 11.8 ± 2.8 boluses per day compared to 12.0 ± 2.7 boluses per day for the highest quartile. However, in univariate analyses, lower TIR on CLC was associated with more automatic boluses (6.0 ± 1.6 vs. 3.5 ± 1.0 in the lowest and highest quartiles, respectively; P < 0.001), fewer user-initiated boluses (5.8 ± 2.6 vs. 8.5 ± 2.8; P < 0.001), fewer user-initiated boluses with carbohydrate entry (4.2 ± 2.0 vs. 5.8 ± 2.4; P < 0.001), and overall less total grams of carbohydrates entered each day (165 ± 67 vs. 206 ± 84; P = 0.009). There also was an association between the proportion of participants in each group receiving <3 boluses for carbohydrates per day and TIR (lowest TIR quartile 24%, highest TIR quartile 8%; P = 0.01).
After adjusting for baseline TIR in a multivariate model, user-initiated bolus doses and total daily insulin per kg were no longer significantly associated with TIR on CLC (Table 2).
Discussion
With the availability of two FDA-approved hybrid CLC systems for clinical use, attention is likely to shift from whether CLC systems improve glycemia in long-term use to how individual users can improve glycemic outcomes further while on a CLC system.
In this study of 12–16 weeks of use, we found that children who achieved greater TIR on the CLC system (compared to those with lower TIR) had more active involvement in their T1D management while on the system. This was evidenced by a greater number of user-initiated boluses, including more boluses for carbohydrate intake. However, these factors also were associated with baseline TIR before initiating CLC, and in a multivariate model, none of these factors remains significant after adjusting for baseline TIR. This suggests that even though CLC systems are designed to require less user input, individuals who are more attentive to administering boluses for carbohydrates, for example, are likely to achieve better outcomes.
It is notable that participants in the highest TIR quartile while using CLC already had good glycemic control at baseline, with 70% TIR and HbA1c of 6.8%—both of which nonetheless improved on the CLC system. While on CLC, this group initiated 2.7 noncarbohydrate boluses per day, suggesting that the participant or a family member continued to provide boluses for high blood glucose correction rather than rely on automated corrections, which are programmed to deliver 60% of the insulin calculated by the individual's correction factor and occur only if the most recent user-initiated bolus was delivered two or more hours prior. As such, these user-initiated correction boluses appeared to obviate the need for the CLC system to deliver automated boluses, which were lower overall in the highest versus lowest TIR quartile (3.5 vs. 6.0 daily).
Compared with those in the lowest TIR quartile, those in the highest TIR quartile also had greater time <70 mg/dL (1.66% vs. 0.80%), which was not significantly different from baseline (1.90% vs. 0.73%) and remains within the recommendation of <4% time <70 mg/dL.
The lowest TIR quartile while using CLC appeared to have omitted boluses of insulin for some amount of carbohydrate intake, delivering 4.2 boluses daily for a total of 165 grams compared with 5.8 boluses for a total of 206 g in the highest TIR group. The CLC system appeared, in part, to compensate for this by providing additional automated correction boluses (6.0 vs. 3.5 in the lowest vs. highest TIR group). Indeed, this CLC system has been shown to reduce hyperglycemia following unannounced carbohydrate ingestion, although not without some hyperglycemia excursion.12 Of note, participants in the lowest TIR quartile using CLC required more insulin per body weight (1.13 vs. 0.87 U/kg/day in the highest TIR group), suggesting a greater amount of insulin resistance, although the reason for this in unclear. It is possible that increased exposure to hyperglycemia contributed to this increased insulin resistance.13
Although users with high TIR at baseline were able to achieve the highest TIR on CLC, those starting with a low TIR tended to improve the most. An analysis from the initial report of the RCT from the current study showed that individuals with baseline HbA1c ≥8.0% (compared with those with HbA1c <8.0%) experienced larger increases in TIR on CLC (19.5% vs. 10.8%), also reflective of their having had lower TIR at baseline (41% vs. 60%).9 This remains an important consideration regarding which patients may benefit the most from a CLC system.
Our study benefited from a large cohort with only 1% drop-out. Limitations include the composition of the cohort, which was overall well controlled, with mean baseline HbA1c values below 8% for each of the quartiles. This may limit the generalizability of the findings from this study, particularly given that the T1D exchange reported that mean HbA1c nationally in this age range was 8.5%.1 In addition, the families represented were relatively affluent and well-educated, with 70% of families having a yearly income >$100,000 and 45% of families having at least a Master's degree.
Additional limitations of the current analysis include its observational nature; therefore, some of the differences we observed between TIR quartiles may have merely been associations and not causative of the variation in TIR. In addition, we lack information on the true amount of carbohydrates consumed, limiting our ability to understand the impact of the differences in the amount of carbohydrates entered and the number of boluses between groups. Finally, this analysis combined baseline data of variable durations, although CGM metrics were similar between groups as previously published.9
In conclusion, we found that among children 6–14 years old who used the Control-IQ hybrid CLC system for up to 16 weeks in a clinical trial, TIR was most closely linked to baseline glycemic control and to the degree of engagement of the family with the system. Those with the lowest TIR at baseline were the ones who improved TIR the most, but ultimately those with the highest TIR at baseline were those who had the highest TIR on CLC. The data on system engagement serve as a reminder that hybrid CLC systems are likely to provide greater TIR with greater user interaction, including user-initiated boluses for carbohydrate intake and correction of elevated blood sugar. Further observational studies will be instructive regarding TIR achievements among cohorts with poorer baseline control and lower system engagement.
Acknowledgments
iDCL Trial Research Group: University of Virginia, Center for Diabetes Technology, Charlottesville, VA: Melissa Schoelwer (PI), Marc Breton (Grant PI), Mark DeBoer (I), Linda Gonder-Frederick (I), Daniel Cherñavvsky (I), Jessica Robic, Emma Emory, Mary Voelmle, Katie Conschafter, Kimberly Morris, Charlotte Barnett, Kelly Carr, Jacob Hellmann, Matthew Kime, Mary Oliveri; Barbara Davis Center for Diabetes, University of Colorado, Anschutz Medical Campus, Aurora, CO: R. Paul Wadwa (PI), Greg Forlenza (I), G. Todd Alonso (I), Robert Slover (I), Laurel Messer (I), Erin Cobry (I), Emily Jost, Cari Berget, Lindsey Towers, Samantha Lange; Department of Pediatrics, Division of Pediatric Endocrinology and Diabetes, Stanford University School of Medicine: Bruce Buckingham (PI), David Maahs (I), Rayhan Lal (I), Laya Ekhlaspour (I), Lisa Norlander (I), Korey Hood (I), Marissa Town, Christine Weir, Kerren Smith, Liana Hsu, Deanna Shinksy, Julia Viana; Yale University: Eda Cengiz (PI), Stuart Weinzimer (I), Kate Weyman (I), Lori Carria, Melinda Zgorski; Jaeb Center for Health Research: Katrina Ruedy, Roy Beck, Sarah Borgman, Jessica Rusnak, Lauren Kanapka, Craig Kollman, Carlos Murphy; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): Guillermo Arreza-Rubin (Project Scientist), Neal Green (Project Manager); iDCL Steering Committee Members: Boris Kovatchev, Sue Brown, Stacey Anderson, Marc Breton, Lori Laffel, Jordan Pinsker, Carol Levy, Yogish C. Kudva, R. Paul Wadwa, Bruce Buckingham, Francis Doyle III, Eric Renard, Claudio Cobelli, Yves Reznik, Guillermo Arreza-Rubin, John Lum, Roy Beck, Katrina Ruedy; Central Laboratory—University of Minnesota Advanced Research and Diagnostic Laboratory: Robert Janicek, Deanna Gabrielson.
Contributor Information
Collaborators: for the iDCL Trial Research Group, Linda Gonder-Frederick, Jessica Robic, Mary Voelmle, Katie Conschafter, Kimberly Morris, Charlotte Barnett, Kelly Carr, Jacob Hellmann, Matthew Kime, G. Todd Alonso, Robert Slover, Cari Berget, Lindsey Towers, Samantha Lange, Bruce Buckingham, David Maahs, Rayhan Lal, Laya Ekhlaspour, Lisa Norlander, Korey Hood, Marissa Town, Christine Weir, Kerren Smith, Liana Hsu, Deanna Shinksy, Julia Viana, Eda Cengiz, Stuart Weinzimer, Kate Weyman, Lori Carria, Melinda Zgorski, Katrina Ruedy, Roy Beck, Sarah Borgman, Jessica Rusnak, Lauren Kanapka, Craig Kollman, Carlos Murphy, Guillermo Arreza-Rubin, Neal Green, Boris Kovatchev, Sue Brown, Stacey Anderson, Marc Breton, Lori Laffel, Jordan Pinsker, Carol Levy, Yogish C. Kudva, R. Paul Wadwa, Bruce Buckingham, Francis Doyle III, Eric Renard, Claudio Cobelli, Yves Reznik, Guillermo Arreza-Rubin, John Lum, Roy Beck, Katrina Ruedy, Robert Janicek, and Deanna Gabrielson
Authors' Contributions
M.J.S. and M.D.D. developed the concept for the article. L.G.K. analyzed the data. M.J.S., L.G.K., and M.D.D. wrote the article. All authors were responsible for reviewing and revising this article and assume responsibility and accountability for the results.
Author Disclosure Statement
M.J.S. received grant support, paid to her institution from Medtronic, Insulet and Tandem. L.G.K. has no disclosures to report. R.P.W. reports receiving grant support, consulting fees, and supplies, provided to his institution, from DexCom, advisory fees from Medtronic, and grant support, provided to his institution, from Tandem Diabetes Care and Bigfoot Biomedical, grant support, paid to his institution, advisory board fees, and supplies, provided to his institution, from Eli Lilly, and grant support, paid to his institution, and supplies, provided to his institution, from MannKind and Novo Nordisk. M.D.B. has no disclosures to report. K.J.R. has no disclosures to report. L.E. reports receiving consultancy fees from Tandem Diabetes Care. E.C. has no disclosures to report. E.J. has no disclosures to report. G.P.F. reports receiving grants support and lecture fees from Medtronic, MiniMed, Insulet, and Tandem, grant support from Abbott, and grant support and consulting fees from Eli Lilly. E.C.C. has no disclosures to report. L.H.M. has received speaking/consulting honoraria from Tandem Diabetes and DexCom, Inc., and also consults for Clinical Sensors and Capillary Biomedical; her institution receives research grants from Medtronic, Tandem Diabetes, DexCom, Abbott, and Insulet Corp. E.C. reports speaker and consultancy fees from Novo Nordisk, MannKind, Lexicon, and Arecor outside the submitted work. E.J. has no disclosures to report. L.C. has no disclosures to report. E.E. has no disclosures to report. L.J.H. has no disclosures to report. S.A.W. reports speaker and consultancy fees from Medtronic, Insulet, Zealand, and Sanofi outside the submitted work. B.A.B. reports receiving grant support and advisory board fees from Medtronic Diabetes and ConvaTec, grant support and presentation fees from Insulet, advisory board fees from Novo Nordisk and Profusa, grant support from Eli Lilly, grant support and equipment from DexCom, and holding patent 61197230 on a hypoglycemia prediction algorithm. R.A.L. has consulted for Abbott Diabetes Care, Biolinq, Capillary Biomedical, Morgan Stanley and Tidepool. M.O. has no disclosures to report. C.K. has no disclosures to report. B.B.D. has no disclosures to report. D.R.C. was a part time assistant professor of UVA at the time of the trial since then he transitioned to be a full-time employee of DexCom, Inc. R.W.B. reports receiving consulting fees, paid to his institution, from Insulet, Bigfoot Biomedical, and Eli Lilly, grant support and supplies, provided to his institution, from Tandem and DexCom, and supplies from Ascenia and Roche. M.D.D. received grant support, paid to his institution from Medtronic and Tandem.
Funding Information
Study funded by Tandem Diabetes, Inc., and the National Institute of Diabetes and Digestive and Kidney Diseases. Tandem Diabetes Care provided the experimental closed-loop systems used in the trial, system-related supplies, including the DexCom CGM and Roche glucometer, and technical expertise. Tandem Diabetes Care was not involved in data analysis and was provided a copy of the article for review before publication.
References
- 1. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine BS, Anderson BJ, Butler DA, et al. : Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr 2001;139:197–203 [DOI] [PubMed] [Google Scholar]
- 3. Laffel LM, Kanapka LG, Beck RW, et al. : Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thabit H, Tauschmann M, Allen JM, et al. : Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russell SJ, El-Khatib FH, Sinha M, et al. : Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 9. Breton MD, Kanapka LG, Beck RW, et al. : A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanapka LG, Wadwa RP, Breton MD, et al. : Extended use of the control-IQ closed loop control system in children with type 1 diabetes. Diabetes Care 2021;44:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benjamini Y, Hochberg Y: Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300 [Google Scholar]
- 12. Cherñavvsky DR, DeBoer MD, Keith-Hynes P, et al. : Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes 2016;17:28–35 [DOI] [PubMed] [Google Scholar]
- 13. Tomas E, Lin YS, Dagher Z, et al. : Hyperglycemia and insulin resistance: possible mechanisms. Ann N Y Acad Sci 2002;967:43–51 [DOI] [PubMed] [Google Scholar]