Abstract
Dietary factors that may reduce the risk of relapse or prolong remission are an area of interest for patients with cancer. There is little data on the role of omega-3 fatty acids (n-3 FA) in patients diagnosed with lymphoma. This pilot biomarker study evaluated the prognostic significance of n-3 FA levels in patients with diffuse large B cell lymphoma (DLBCL). In this analysis, low n-3 FA may be associated with inferior event free survival at 24 months in this disease. Larger studies are needed to confirm this association and could provide the basis for prospective investigation of whether modifying n-3 levels with diet may improve outcome in lymphoma.
Keywords: Non-hodgkins lymphoma, Biomarkers, Fat/omega-3, omega-6/fish oil, Diet, Micronutrients
Introduction
The identification of modifiable dietary factors that can reduce the risk of malignancy is of interest to patients and clinicians. Epidemiological studies have linked trends in dietary fat and protein intake and risk of non-Hodgkin lymphoma (NHL). (1, 2) Macronutrients may modulate the immune system and tumor microenvironment in a variety of pathways important to lymphomagenesis.
Case-control assessments using dietary questionnaires suggest that diets high in omega-3 (n-3) fatty acids (FA) are inversely associated with lymphoma risk.(3, 4) However, there is little data on the role of blood n-3 FA levels in patients already diagnosed with lymphoma and if these micronutrient levels predict outcome in lymphoma. Serum n-3 levels appear to be altered in patients with NHL compared to age matched controls, and lower n-3 levels in NHL patients may be associated with poorer response to chemotherapy.(5, 6) To our knowledge, no studies have assessed the potential role of n-3 levels as a prognostic biomarker for outcome in patients with NHL, specifically diffuse large B cell lymphoma (DLBCL). We performed this pilot study on the serum of patients with newly diagnosed DLBCL to investigate the potential relevance of plasma n-3 FA levels and prognosis. We hypothesized that patients with low levels of n-3 FA would have a higher likelihood of relapse.
Methods
To assess the whether n-3 levels in patients with DLBCL have prognostic significance, we utilized plasma from patients with previously untreated DLBCL who were prospectively enrolled on the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER). For this pilot study, 25 patients treated with the standard frontline regimen of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) or similar rituximab containing immunochemotherapy who had a pre-treatment plasma sample were included. Collection of blood for analysis occurred at diagnosis, prior to receipt of any treatment. N-3 FA levels, specifically the marine n-3 FAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and the vegetable n-3 FA, alpha linolenic acid (ALA), were assessed on stored research plasma samples using a gas chromatography-mass spectrometry stable isotope dilution method in the Mayo Clinic clinical reference laboratory. Event-free survival (EFS) was defined as the time from diagnosis to relapse, retreatment or death due to any cause. EFS24 was defined using EFS status at 24 months from diagnosis.(7) Twelve samples from patients who achieved EFS24 (favorable outcome) and 13 samples from those who did not (unfavorable outcome) were selected for testing. The groups were matched for clinical characteristics. The laboratory conducting the assay was blinded to which the outcome associated with a given sample. Comparisons of n-3 FA levels between outcome groups were assessed using Wilcoxon rank-sum tests.
Results
Plasma samples for 25 patients were evaluated for n-3 FA and baseline patient characteristics are summarized in Table 1. The median age was 57 years (range 41–84), and 44% were male. International prognostic index (8, 9) was 0–1 in 8 patients, 2 in 11 patients and 3 in 6 patients. Thirteen patients achieved EFS24 and 12 patients failed to achieve EFS24.
TABLE 1:
Patient Characteristics
| Median age at diagnosis (median; range) | 57 (41–84) |
| Male gender, n(%) | 11 (44) |
| International Prognostic Index (IPI), n(%) | |
| 0–1 | 8 (32) |
| 2 | 11 (44) |
| 3 | 6 (24) |
| Achieved EFS24, n(%) | 13 (52) |
Median n-3 levels were as follows: DHA of 128 mcmol/L (reference range: 30–250, standard deviation [SD]=52.6), EPA of 55 mcmol/L (reference range: 14–100 mcmol/L, SD=37.6), and ALA 57 mcmol/L (reference: 50–130 mcmol/L, SD=27.5). In comparison to the clinical reference range, all patients had normal DHA, 5 patients (20%) had elevated EPA, and 6 patients (25%) had low ALA. There were no significant associations between EPA, DHA or ALA and the clinical characteristics age, sex, and IPI (all p>0.10).
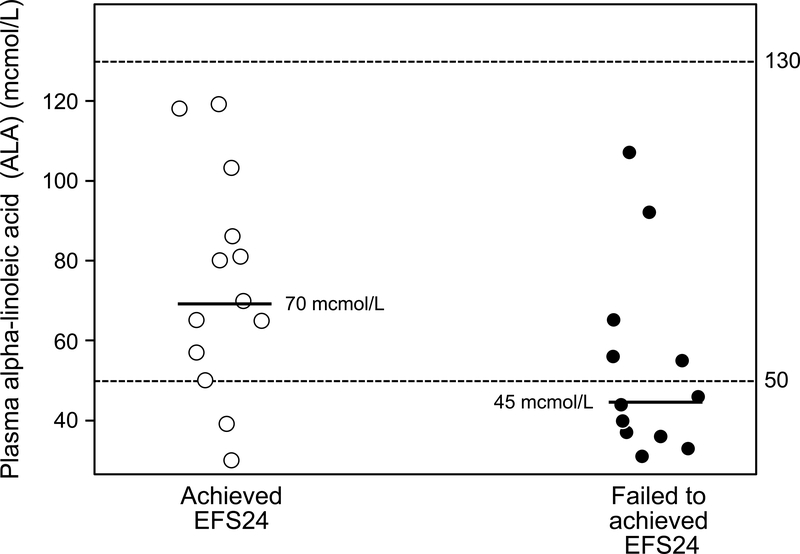
ALA was lower in patients failing to achieve EFS24 (N=13, median ALA of 45 mcmol/L, SD=24.0) compared to patients who achieved EFS 24 (N=12, median ALA of 70 mcmol/L, SD=27.7), and this was of borderline statistical significance (p = 0.053).
Median levels of EPA in patients failing to achieve EFS24 (109 mcmol/L) appeared lower than in those who achieved EFS24 (148 mcmol/L) (p=0.33), and similarly, DHA appeared lower in patients failing to achieve EFS24 than dose who did (median 38 versus 70 mcmol/L) (p=0.17), though these did not meet statistical significance.
Discussion
This pilot study is the first to suggest that plasma n-3 FA levels in patients with DLBCL may have prognostic relevance. Lower n-3 FA levels, specifically ALA, in DLBCL patients may be associated with early relapse as assessed by EFS24. Trends in DHA and EPA in patients in this study also favor an association between lower levels and poorer prognosis in patients with lymphoma, but power was limited. However, these pilot data provide the impetus for further studies of diet and plasma n-3 FA levels in patients with DLBCL. They also provide rationale to study replacement of n-3 FA in patients with low levels. Whether this replacement will be able to alter outcome will require carefully designed prospective trials that include measurements before and after intervention to ensure that the target concentration are indeed normalized.
The exact mechanisms of the role of n-3 in the biology of lymphoma are unclear. Cvetkovic et al have suggested that there may be altered expression of enzymes involved in fatty acid generation and denaturation in NHL cells leading to impaired metabolism on the level of particular FAs(5). It is unknown how altered metabolism may contribute to tumor growth. N-3 FAs have been postulated to have ant-inflammatory and anti-angiogenic effects which play a role in lymphomagenesis(10).
This study suggests that n-3 levels may have prognostic significance in patients with DLBCL and provides the rationale to test n-3 FA levels in a larger group of DLBCL patients to confirm these findings.
Figure 1.
Dot plot of ALA in patients with newly diagnosed DLBCL. Hashed lines delinate normal reference range for the assay. Median bars are shown. Patients who failed to achieve EFS24 (unfavorable outcome, right, median ALA of 45 mcmol/L) had lower ALA levels than those who achieved EFS24 (favorable outcome, left, median ALA 70 mcmol/L) (p = 0.053).
Acknowledgments
Funding Source
This research was supported by the Mayo Clinic / University of Iowa Lymphoma SPORE grant from the National Institute of Health (P50 CA97274-13) and the Predolin Foundation.
Footnotes
Disclosures
The authors have no relevant conflicts of interest to disclose.
References
- 1.Chiu BC, Cerhan JR, Folsom AR, Sellers TA, Kushi LH, Wallace RB, et al. Diet and risk of non-Hodgkin lymphoma in older women. JAMA. 1996;275(17):1315–21. [DOI] [PubMed] [Google Scholar]
- 2.Chang ET, Balter KM, Torrang A, Smedby KE, Melbye M, Sundstrom C, et al. Nutrient intake and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2006;164(12):1222–32. [DOI] [PubMed] [Google Scholar]
- 3.Charbonneau B, O’Connor HM, Wang AH, Liebow M, Thompson CA, Fredericksen ZS, et al. Trans fatty acid intake is associated with increased risk and n3 fatty acid intake with reduced risk of non-hodgkin lymphoma. J Nutr. 2013;143(5):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fritschi L, Ambrosini GL, Kliewer EV, Johnson KC. Dietary fish intake and risk of leukaemia, multiple myeloma, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13(4):532–7. [PubMed] [Google Scholar]
- 5.Cvetkovic Z, Vucic V, Cvetkovic B, Petrovic M, Ristic-Medic D, Tepsic J, et al. Abnormal fatty acid distribution of the serum phospholipids of patients with non-Hodgkin lymphoma. Annals of hematology. 2010;89(8):775–82. [DOI] [PubMed] [Google Scholar]
- 6.Cvetković Z, Vučić V, Cvetković B, Karadžić I, Ranić M, Glibetić M. Distribution of plasma fatty acids is associated with response to chemotherapy in non-Hodgkin’s lymphoma patients. Med Oncol. 2013;30(4):1–7. [DOI] [PubMed] [Google Scholar]
- 7.Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. New England Journal of Medicine. 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 9.Maurer MJ, Jais JP, Ghesquieres H, Witzig TE, Hong F, Haioun C, et al. Personalized risk prediction for event-free survival at 24 months in patients with diffuse large B-cell lymphoma. Am J Hematol. 2016;91(2):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45(12):2077–86. [DOI] [PubMed] [Google Scholar]