Abstract
Platelet-bound complement activation products (PC4d) are associated with thrombosis in Systemic Lupus Erythematosus (SLE). This study investigated the effect of PC4d on platelet function, as a mechanistic link to arterial thrombosis. In a cohort of 150 SLE patients, 13 events had occurred within five years of enrollment. Patients with arterial events had higher PC4d levels (13.6 [4.4–24.0] vs. 4.0 [2.5–8.3] net MFI), with PC4d 10 being the optimal cutoff for event detection. The association of arterial events with PC4d remained significant after adjusting for antiphospholipid status, smoking, and prednisone use (p=0.045). PC4d levels correlated with lower platelet counts (r=−0.26, p=0.002), larger platelet volumes (r=0.22, p=0.009) and increased platelet aggregation: the adenosine diphosphate (ADP) concentration to achieve 50% maximal aggregation (EC50) was lower in patients with PC4d 10 compared with PC4d<10 (1.6 vs. 3.7, p=0.038, respectively). These results suggest that PC4d may be a mechanistic marker for vascular disease in SLE.
Keywords: Systemic Lupus Erythematosus, Cardiovascular Disease, Thrombosis, Antiphospholipid Syndrome, Complement Split Products, Platelet, Inflammation, Autoimmune Disease, Biomarker
1. INTRODUCTION:
Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune disease and a leading cause of morbidity and mortality among women 15–44 years old.[1] Vascular thromboses, and especially cardiovascular (CV) events, are a major factor of this increased mortality.[2] Traditional CV risk factors do not fully explain the increased risk, as there is an additional 2.7 fold higher risk beyond the Framingham score prediction.[3] The disease mechanisms that link SLE to accelerated atherosclerosis are not well understood.
Platelets have a well-defined role in arterial thrombosis and increased platelet aggregation is associated with future CV events.[4] Platelets isolated from SLE patients, as compared to healthy controls, aggregate at lower stimulus thresholds and demonstrate increased ability to activate endothelial cells.[5] Immune-complexes isolated from patients with autoimmune conditions activate resting platelets.[6–8] Whether this immune-mediated platelet activation contributes to the thrombogenic risk in SLE patients is unknown.
In SLE, circulating immune complexes (IC) activate complement, resulting in the formation of complement fragments, including C4d. C4d is covalently bound the surface of erythrocytes (EC4d), lymphocytes (BC4d) and platelets (PC4d).[9] While BC4d and EC4d are relevant to SLE diagnosis and monitoring of disease activity, PC4d correlates with thrombosis, all-cause mortality, and presence of antiphospholipid (aPL) antibodies.[10–17] PC4d is present in 15–20% of SLE patients and is associated with venous thrombotic events.[12, 18, 19] However, the association with arterial vascular events has been less consistent. Two larger studies evaluating PC4d and vascular events found that PC4d was associated with ischemic stroke but not coronary events.[15, 20] Meanwhile, in a cross-sectional analysis of 148 SLE patients, adjusted for traditional cardiovascular risk factors, PC4d was not associated with arterial thrombosis.[16]
The current study confirms the association of PC4d with arterial thromboses, and for the first time identifies the association between PC4d and platelet function using platelet volumetric indices and platelet aggregation.
2. MATERIALS and METHODS:
2.1. Study Population.
This was a cross-sectional study of 150 patients, ages >18 years, from the Columbia University Lupus Cohort. All patients met the 1997 American College of Rheumatology (ACR) and/or SLICC classification criteria.[19, 21] Patients were enrolled in the study at the time of their usual care visits; medical history obtained from the patients was confirmed by chart review. Written informed consent was obtained from all patients. The study was approved by the Columbia University Institutional Review Board.
2.2. Clinical and Laboratory Covariates.
Demographics and smoking history were self-reported. History of vascular events was confirmed by chart review. Cerebrovascular accidents (CVA) were defined as acute ischemic central vascular events with characteristic radiographic imaging at time of diagnosis; transient ischemic attacks (TIA) were excluded. Myocardial infarctions (MI) were defined as acute coronary syndromes with elevated troponin levels supporting myocardial injury; myocarditis was not included as an event. SLE disease duration was defined as the duration in years from the date of physician diagnosis. SLE disease activity was scored using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) at study enrollment.[22] Hypertension (HTN) was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg and anti-hypertensive medication use. Diabetes was defined as a glycated hemoglobin (HbA1c) greater than 6.4% or antidiabetic medications use. Body mass index (BMI) was calculated as weight in kilograms divided by the height in meters squared. Serological covariates were measured at the New York Presbyterian Hospital clinical laboratory as ascertained from chart review and confirmed by testing through the Exagen clinical laboratory at the time of enrollment. Whole blood hydroxychloroquine levels were measured at the Exagen clinical laboratory at time of enrollment.
2.3. PC4d Measurement.
Venous blood was collected in ethylenediamine-tetraacetic acid-containing. (EDTA) tubes and shipped overnight to the Exagen clinical laboratory between July 2018 and January 2020. PC4d levels were measured using fluorescence-activated cell sorting (FACS) as described previously and expressed as net mean fluorescence intensity (MFI).[13, 23] Briefly, red blood cells from EDTA whole blood were lysed and platelets were stained using mouse monoclonal antibody against human C4d (Quidel, Sand Diego, California), or alternatively using mouse IgG1 kappa monoclonal isotype control (MPOC-21). After incubation for 30 min at 2–8 degrees Celcius, samples were stained using goat antimouse conjugated to Fluorescein Isothiocyanate (FITC) (30 min at 2–8 degrees Celcius in the dark). A monoclonal antibody against human CD42b conjugated to phycoerythrin (PE) was used to identify platelets. FACS analysis was performed using a Gallios (10-colors) flow cytometer (Beckman Coulter, Brea, California). Light scatter (forward and side) gating parameters were used to isolate the platelet population, followed by secondary gating based on positive CD42b PE staining. Quantification of the non-specific (isotype control) and specific (C4d) fluorescence was determined for the CD42b PE gated platelet cells (5000 events). Net MFI was determined by subtraction of isotype control background MFI results from the specific C4d MFI results on gated platelet cells. Abnormal PC4d status corresponded to levels greater than the 99th percentile of normal healthy group (>20 net MFI)[24].
2.4. Platelet Aggregation.
A randomly selected subset of patients with high and low PC4d levels, evenly stratified by antiphospholipid antibody status, were included in the platelet aggregation studies. Venous blood was collected in 3.2% sodium citrate tubes after the first 2 mL were discarded to avoid platelet activation. The samples were spun at 150 x g for 10 minutes to obtain platelet rich plasma (PRP). The remaining plasma was centrifuged at 1260 x g to obtain platelet poor plasma (PPP). Platelet reactivity was assessed within three hours of phlebotomy using standard light transmittance aggregometry (LTA). LTA was assessed with an 8-channel optical aggregometer (Model PAP-8E, BioData Corporation Horsham, PA). With platelet poor plasma (PPP) as a reference, platelet aggregation was induced by the addition of adenosine diphosphate (ADP, purchased from BioData Corporation). Maximum aggregation was defined as the greatest amplitude of the aggregation response within the first 6 minutes. Using decreasing doses of adenosine diphosphate (ADP, 20uM, 10uM, 5uM, 2.5uM, 1.25uM, and 0.83uM), a dose-response curve for ADP was constructed for each patient as previously described,[25] and the data were expressed as the ADP concentration at which 50% of maximal aggregation was achieved (EC50). A lower EC50 indicates greater platelet sensitivity to the addition of the platelet aggregation agonist.
2.5. Statistical Analysis.
Patient characteristics were summarized using descriptive statistics as mean ± standard error of the mean (SEM) or median [interquartile range, IQR] for continuous variables depending on data distribution. Counts and percentages were reported for categorical variables. Vascular event rates were modeled based on PC4d levels, transformed as natural log ln(PC4d+1) to normalize the positively skewed distribution for multivariable regression analysis. Covariates associated with vascular events (p value ≤0.05) were adjusted for using logistic regression analysis. Aspirin and statin use were not adjusted for as these medications were prescribed after the occurrence of the outcome (CVA or MI).
PC4d was dichotomized and the ability of different cutoff values to discriminate for the history of vascular events was evaluated. Receiver operating characteristics (ROC) analysis was used to characterize the diagnostic performance of different PC4d cutoffs, ranging from 5 to 20 net MFI, on vascular event detection.
The association between log transformed PC4d levels and platelet count and volume indices was analyzed using Pearson’s correlation. Finally, Mann-Whitney U test was used to compare results of median platelet aggregation testing between patients with positive vs. negative PC4d levels. An p value≤0.05 was defined as statistically significant. All statistical calculations were performed in Stata IC v15.1.
3. RESULTS:
3.1. Patient Characteristics.
The demographic and disease characteristics of the 150 SLE patients are summarized in Table 1. The average age was 40±13 years and 91% were women. Thirty three percent of the patients were Hispanic and 26% were African American. All patients had positive ANA titers and 69% had a history of elevated dsDNA titers. Median SLEDAI-2K was 5 [IQR 2–8] (consistent with moderate disease activity); 43% of patients had lupus nephritis, and 38% tested positive for aPL antibodies.
Table 1.
Demographics and Clinical Characteristics of SLE Patients at Enrollment.
| SLE | SLE | |||
|---|---|---|---|---|
| All patients (n=150) | No Vascular Events (n=122) | Arterial Events (n=13) | P-value‡ | |
| Age, years | 40±13 | 40±13 | 41±13 | 0.78 |
| Female, n (%) | 137 (91%) | 112 (92%) | 11 (85%) | 0.33 |
| Race/ethnicity | ||||
| White, n (%) | 40 (27%) | 33 (27%) | 4 (31%) | 0.75 |
| Hispanic, n (%) | 49 (33%) | 40 (33%) | 4 (31%) | 1.00 |
| Black, n (%) | 39 (26%) | 29 (24%) | 4 (31%) | 0.52 |
| Disease duration in years | 11 [5–16] | 11 [6–16] | 14 [3–17] | 0.89 |
| SLEDAI-2K | 5 [2–8] | 5 [2–8] | 4 [2–8] | 0.82 |
| ACR Criteria, total | 6 [5–7] | 6 [5–7] | 6 [5–7] | 0.71 |
| Lupus Nephritis | 65 (43%) | 51 (42%) | 5 (39%) | 1.00 |
| Low C3/C4 (at enrollment) | 47 (31%) | 42 (34%) | 4 (31%) | 1.00 |
| Hydroxychloroquine (blood, ng/mL) | 610±613 | 613±602 | 567±776 | 0.82 |
| Prednisone (or equivalent) dose >7.5mg/day, n (%) | 27 (18%) | 18 (15%) | 6 (46%) | <0.01 |
| Antibodies | ||||
| ANA (ever), n (%) | 150 (100%) | 122 (100%) | 13 (100%) | - |
| ds-DNA antibody (ever), n (%) | 104 (69%) | 80 (66%) | 12 (92%) | 0.06 |
| Sm antibody (ever), n (%) | 55 (34%) | 47 (39%) | 4 (31%) | 0.77 |
| RNP antibody (ever), n (%) | 81 (50%) | 64 (53%) | 9 (69%) | 0.38 |
| aPL (ever), n (%) | 57 (38%) | 40 (33%) | 10 (77%) | <0.01 |
| Cardiovascular Risk Factors | ||||
| Hypertension, n (%) | 44 (29%) | 34 (28%) | 5 (39%) | 0.52 |
| Diabetes, n (%) | 8 (5%) | 7 (6%) | 0 | 1.00 |
| Ever smoker n (%) | 29 (19%) | 18 (15%) | 7 (54%) | <0.01 |
| BMI, kg/m2 | 28±6 | 28±6 | 27±4 | 0.80 |
| Total cholesterol, mg/dL | 173±52 | 175±51 | 147±70 | 0.11 |
| HDL, mg/dL | 56±19 | 56±19 | 51±9 | 0.39 |
| LDL, mg/dL | 93±41 | 95±40 | 76±55 | 0.19 |
| Aspirin use, n (%) | 39 (26%) | 27 (22%) | 7 (54%) | 0.02 |
| Statin use, n (%) | 18 (12%) | 11 (9%) | 5 (39%) | <0.01 |
SLE adults with arterial events compared with SLE adults without vascular events; Characteristics are expressed as n (%), as the mean ± standard deviation, or as the median [interquartile range]. SLE, systemic lupus erythematosus; SLEDAI-2K Systemic Lupus Erythematosus Disease Activity Index 2000; low C3: C3<80mg/dL, low C4: C4<14mg/dL; aPL, antiphospholipid antibodies; BMI, body mass index; HDL, high-density lipoprotein; LDL, low density lipoprotein.
3.2. Vascular Events.
Thirty-one vascular events occurred in 28 patients within five years of study enrollment (Table 1). Three patients had history of both a venous and an arterial event. There were 13 arterial events (8 with CVA and 5 with MI), 18 venous events (12 with deep vein thrombosis, 4 with pulmonary embolism, 1 with portal vein thrombosis, 1 with mesenteric vein thrombosis). Patients with an arterial vascular event were more likely to have a history of smoking (54% vs 15%, p=<0.01) and more likely to test positive for aPL antibodies (77% vs. 33% p<0.01). Age, BMI, cholesterol levels, history of hypertension, diabetes, were not different between patients with arterial events and those without any vascular events. Similarly, SLE disease duration and the SLEDAI-2K score were not associated with arterial events. Participants with a history of arterial events were more likely to be taking prednisone-equivalent ≥7.5mg/day; there was no difference in the use of baseline immunosuppressive medications.
3.3. Higher PC4d Levels are Associated with Vascular Events.
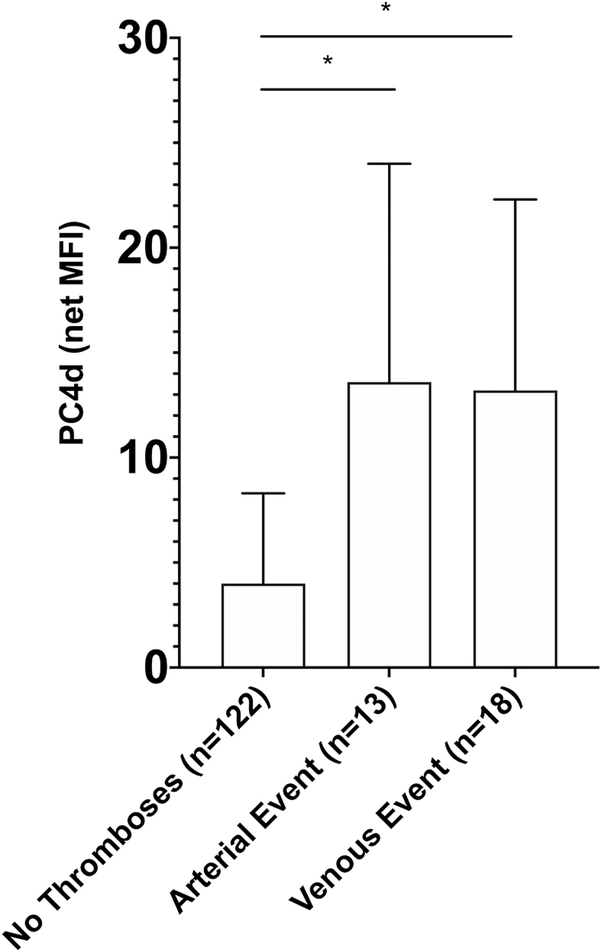
Patients with a five-year history of an arterial event prior to enrollment (n=13), had higher levels of PC4d as compared with patients without any thromboses (13.6 [4.4–24.0] net MFI vs 4.0 [2.5–8.3] net MFI, p=0.01). Additionally, patients with a five-year history of a venous vascular event prior to enrollment (n=18) had higher levels of PC4d as compared with patients without any history of thromboses (13.2 [3.2–22.3] net MFI vs 4.0 [2.5–8.3] net MFI, p=0.03). (Figure 1) In a multivariable model adjusting for presence of aPL antibodies, history of smoking, and prednisone (or equivalent) >7.5mg/day, the association between arterial events and PC4d (natural log transformed) remained significant (OR=1.71 95%CI 1.01–2.89, p=0.045).
Figure 1: PC4d Levels are Higher in SLE Patients with a Five-Year History of a Vascular Event.
PC4d levels (net MFI) are shown as median values with bars showing the interquartile range (IQR). *p<0.05 Mann-Whitney U two-sample test.
3.4. Identifying the Optimal PC4d Cutoff for Vascular Event Detection.
PC4d as an indicator for arterial events resulted in an overall area under the curve (AUC) of 0.71±0.08. The established PC4d cutoff of PC4d≥20 net MFI was defined by the 99th percentile of a control group of healthy adults, thus optimizing biomarker performance for the diagnosis of SLE. We assessed whether a different cutoff may be more discriminating in evaluating the risk of vascular events. We compared test sensitivity and specificity at cutoffs ranging from 5 to 20 net MFI. We identified the optimal cut-off point for vascular thromboses to be 10 net MFI, with sensitivity and specificity for venous and arterial events of 56% and 78%, and 62% and 78%, respectively (Table 2). Importantly, the cutoff at PC4d≥10 net MFI, as compared to PC4d≥20 net MFI, resulted in a greater detection of individuals with vascular events (16/28 vs 11/28 respectively), suggesting it may be more sensitive in identifying at risk individuals in a clinical setting.
Table 2.
Performance of different PC4d cutoffs for vascular event detection.
| Sensitivity | Specificity | Sensitivity | Specificity | ||
|---|---|---|---|---|---|
| Venous Events: | Arterial Events: | ||||
| PC4d≥20 | 44% | 89% | PC4d≥20 | 39% | 89% |
| PC4d≥15 | 44% | 84% | PC4d≥15 | 39% | 84% |
| PC4d≥10 | 56% | 78% | PC4d≥10 | 62% | 78% |
| PC4d≥5 | 67% | 57% | PC4d≥5 | 69% | 57% |
PC4d levels are expressed as net MFI.
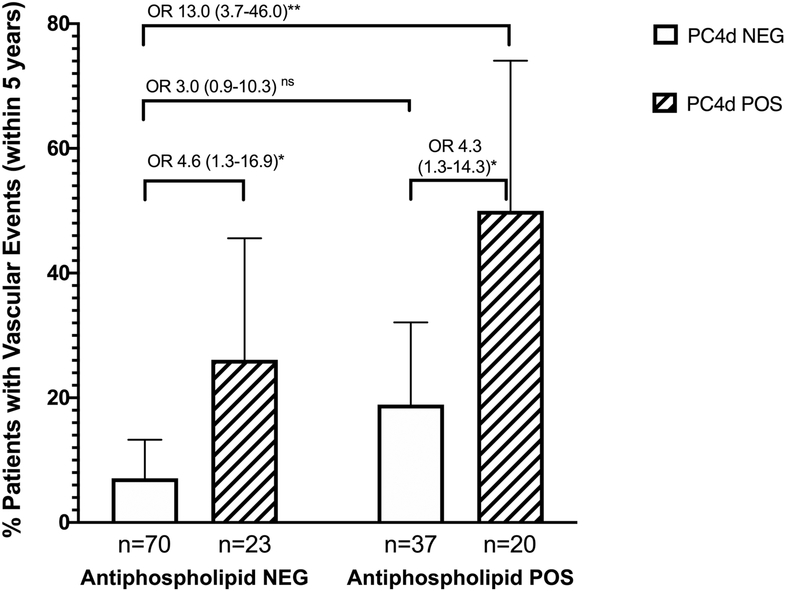
Using a cutoff of PC4d≥10 net MFI, 43 (29%) of our cohort was positive for PC4d. Stratifying by aPL negative status, PC4d≥10 was associated with a history of any vascular events, with a stronger association seen in patients positive for both aPL and PC4d. (OR 4.6 95% CI 1.3–16.9 for PC4d+/aPL− and OR 13.0 95% CI 3.7–46.0 for PC4d+/aPL+ as compared with PC4d−/aPL− patients. (Figure 2).
Figure 2: Vascular Events in SLE Stratified by Antiphospholipid Antibody (aPL) status and PC4d.
Percent patients with a history of a vascular event within five years of study enrollment, stratified by aPL presence (NEG=negative; POS=positive) and PC4d status (NEG=PC4d<10, POS=PC4d≥10). Results are percent patients with events ± 95% confidence interval (CI). Odds ratio (OR) with 95% confidence interval are displayed. *p=0.02, **p<0.0001, ns not significant at α = 0.05. Regression coefficients were compared using the Wald test in a logistic regression model, exponentiated to determine the odds ratio.
3.5. PC4d is Associated with Platelet Count and Volume.
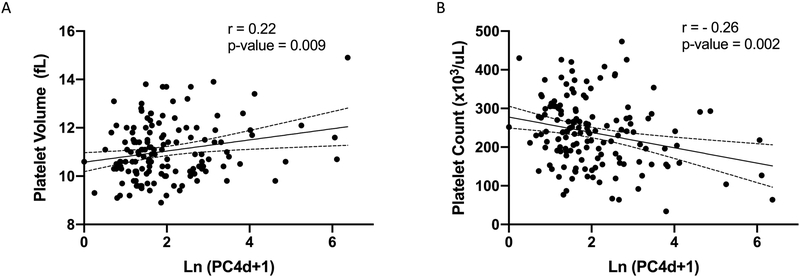
Patients with PC4d≥10 net MFI were more likely to have a history of thrombocytopenia by ACR SLE classification criteria[21] than patients with PC4d<10 net MFI (34.9% vs. 15.9%, p=0.01). We next looked at the association between PC4d levels and platelet measures on the day of study enrollment. PC4d levels, natural log transformed to account for the positive skew of the distribution, showed positive correlation with platelet volume (r = 0.22, p=0.009) and inverse correlation with total platelet number (r = −0.25, p=0.002). (Figure 3). Because presence of aPL antibodies is known to be associated with both PC4d and thrombocytopenia[16, 26], we adjusted for the presence of aPL using linear regression modeling. For every increase in one unit of natural log PC4d, the platelet count was predicted to decrease by 20.8±6.2 ×103/uL (p=0.001) while the mean platelet volume was increased by 0.23±0.09 fL (p=0.008) while adjusting for aPL status.
Figure 3: PC4d levels correlate with mean platelet volume and count.
Correlation between PC4d levels, natural log transformed (Ln), and A, mean platelet volume and B, platelet count. r is the Pearson’s correlation coefficient.
3.6. Platelets Isolated from Patients with Elevated PC4d Are Hyper-Aggregable on Stimulation.
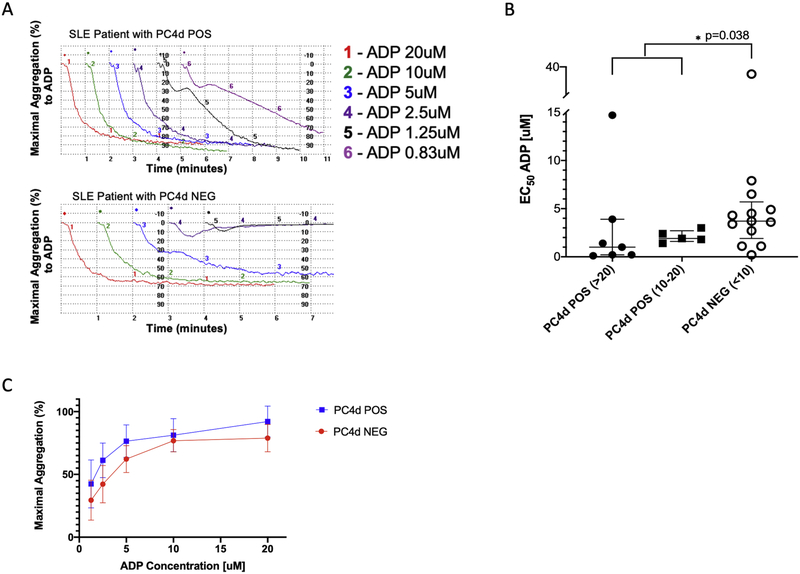
We used light transmittance aggregometry (LTA), the traditional gold standard technique for evaluation of platelet function,[27] to study platelet aggregation in 12 patients with positive PC4d≥ 10 net MFI and 13 patients with negative PC4d<10 net MFI. (Figure 4A) Antiphospholipid antibodies were present in 7/13 and 5/12 of the patients in each group, respectively. Patients on anti-platelet therapy were excluded. EC50 values were significantly lower in patients with PC4d≥10 net MFI compared with PC4d<10 net MFI (1.6 vs. 3.7, p=0.038), with a non-significant trend for lower EC50 in patients with PC4d≥20 net MFI as compared to those with PC4d in the 10–20 net MFI range (Figure 4B). The maximal aggregation response in PC4d positive patients, as compared to PC4d negative patients, trended towards greater aggregation at all concentrations of ADP agonist, approaching statistical significance at the lower concentrations of ADP (5uM, p=0.002 and 2.5uM, p=0.08) (Figure 4C).
Figure 4: PC4d POS (PC4d≥10) platelets, as compared with PC4d NEG (PC4d≥10) platelets, aggregate more in response to addition of ADP agonist.
A, Representative platelet aggregation curves from a PC4d POS patient (top) and a PC4d NEG patient (bottom). Decreasing concentrations of ADP agonist were added to platelet rich plasma and the aggregation response was recorded for six minutes. B, Platelet aggregation was analyzed as EC50, expressed as the ADP concentration [uM] that achieves 50% of the maximal aggregation. A lower EC50 indicates greater platelet sensitivity to the addition of the platelet aggregation agonist. C, Maximal platelet aggregation response after the addition of 20, 10, 5 and 2.5uM ADP agonist. Bars show the median ± interquartile range (IQR); comparisons were analyzed using the Mann-Whitney U two-sample test; Patients on aspirin were excluded.
4. DISCUSSION:
The main finding of our study is that PC4d positive platelets are hyper-aggregable, suggesting a mechanistic link with arterial vascular events. Our study confirms that SLE patients with a history of arterial vascular events had higher PC4d levels as compared with SLE patients with no history of vascular events (13.6 vs 4.0 net MFI p=0.01). The association between all vascular events and PC4d remained significant after adjusting for covariates found to be significant in the univariable analysis (aPL antibodies, smoking history and prednisone use). Higher PC4d levels were associated with lower platelet counts and larger platelet volumes, which are likely consequences of activation and destruction of C4d coated platelets. Indeed, decreased platelet counts and larger MPVs have been described in patients with cardiovascular events, suggesting both may be markers of increased platelet activity.[28, 29]
Activated platelets adhere to the vascular endothelium and release granular contents that are central to the formation of intravascular platelet-rich thrombi.[30] Platelet activation and aggregation are independent predictors of coronary events and mortality.[31, 32] Platelets from SLE patients have increased reactivity, measured by LTA, as compared with healthy control platelets.[5] We now report for the first time the association between PC4d and increased platelet aggregation in SLE. On average, PC4d positive platelets require half the ADP concentration to achieve 50% maximal aggregation response as compared to PC4d negative platelets. The difference in aggregation response was most notable at the lower doses of ADP 5uM and less, implying increased platelet sensitivity to ADP.
Taken together, these findings suggest that PC4d is a biomarker of increased platelet activity and an indicator of thrombotic arterial and/or venous vascular events that have occurred within five years of PC4d measurement. Complement activation at the platelet surface, marked by C4d deposition (PC4d), likely results in downstream immune-mediated mechanisms of platelet priming leading to enhanced aggregation responses following stimulation. The lower platelet counts and larger platelet volumes in PC4d positive patients further suggests that these platelets are experiencing increased activation and turnover compared to PC4d negative platelets. The PC4d biomarker is therefore a readout of both platelet activity and secondary pro-thrombotic risk in SLE patients.
C4d fragments generated during complement activation are bound covalently to cell surfaces and remain attached for the lifespan of the cell.[9] In patients with elevated PC4d levels, all circulating platelets are homogenously coated with C4d.[12] Using the commercial laboratory standard PC4d≥20 net MFI cutoff, elevated levels of PC4d are found in 15–20% of SLE patients and are highly specific for SLE.[9, 13] PC4d ≥20 net MFI cutoff was identified based on optimizing ROC analysis for the diagnosis of SLE. However, a different cutoff may be more appropriate if PC4d is to be used as a biomarker for vascular event risk. Using ROC analysis, we identified a PC4d cutoff of ≥ 10 net MFI to have the optimal sensitivity and specificity for identifying patients with venous and arterial events (56% and 78% and 62% and 78%, respectively). In our cohort, 16% of patients had PC4d≥20 net MFI and 29% had PC4d≥10 net MFI; the lower cutoff identifying a larger proportion of individuals with thrombotic disease and could function more effectively as a biomarker of cardiovascular events.
Although both aPL and PC4d contribute to pro-thrombotic risk, our adjusted data analysis suggests that they each confer an independent risk, with highest risk present when both aPL and PC4d levels are elevated. In patients with autoimmune disease, platelets are activated when immune-complexes bind antigens on the platelet membrane and/or cross-link the platelet surface FcγRIIa receptor.[6, 8] Platelet activation is associated with platelet morphology, including shape changes and a “flip-flop” of platelet membrane phospholipids.[33] In a recent publication, Lonati et al. show that activated platelets express Beta-2 glycoprotein 1 (β2-GP1) and that anti-β2-GP1 antibodies preferentially react to the β2-GP1 on theses platelets, secondarily activating complement and leading to C4d deposition as an identifier on the activated platelet surface.[34] In immune-mediated thrombocytopenia, autologous anti-platelet antibodies may serve a similar role by binding antigens on the platelet membrane, resulting in PC4d formation.[35] We suggest that the association highlighted by our data between aPL antibodies, lower platelet counts, thrombocytopenia and arterial vascular events is further evidence for the role of immune mediated mechanisms in pro-thrombotic risk.
Our study has several limitations. Despite enrolling a multi-ethnic cohort of SLE patients with active disease, the number of arterial events within five years of enrollment was 13, limiting our multivariable modeling to only a few variables at a time to prevent model overfitting. Pooling arterial and venous events into a combined outcome allowed for inclusion of additional covariates in our model but may have obscured differences in the pathogenesis of venous vs. arterial events. However, all PC4d analyses were confirmed for arterial events with adjustment for aPL and CV risk factors. Additionally, while we show an association between PC4d levels and vascular events, temporality and causation cannot be determined from this cross-sectional analysis. At the same time, the observed increased platelet aggregation in PC4d positive patients suggests a mechanistic link between PC4d, platelet activity and thrombotic outcome.
In summary, PC4d levels were increased in patients with a prior history of venous and arterial vascular events. A PC4d cutoff at 10 net MFI had the optimal sensitivity and specificity to identify patients with past events, independent of aPL antibodies. Platelets isolated from patients with PC4d≥10 net MFI were fewer in count, larger in size, and hyperactive, suggesting that PC4d may be a biomarker for thrombotic risk. Future prospective studies are needed to validate PC4d as a predictor of vascular events in SLE patients.
HIGHLIGHTS:
In this study of 150 SLE patients, arterial events were associated with higher PC4d levels; this association remained significant after adjusting for potential confounders.
PC4d≥10 net MFI had the optimal sensitivity/specificity for the association with vascular events. This association was strongest in patients with both aPL and PC4d ≥10.
Higher PC4d levels correlate with lower platelet count and larger platelet volume. The platelet function evaluated using light transmission aggregometry showed that platelets isolated from patients with PC4d≥10 aggregate more readily as compared to platelets isolated from patients with PC4d<10, supporting a mechanistic link between PC4d formation, platelet activation and thrombosis risk.
PC4d may be a useful clinical biomarker for increased platelet aggregation and thrombosis risk. Prospective studies are needed to evaluate the role of PC4d on vascular risk stratification in SLE.
Funding:
YG – National Center for Advancing Translational Sciences at the National Institutes of Health [TL1 TR001875]; American Philosophical Society Daland Fellowship
Footnotes
Conflicts of interest/Competing interests:
RVA – employee at Exagen Inc; JC – employee at Exagen Inc
Ethics approval:
The study was approved by the Columbia University Institutional Review Board.
Consent to participate:
Written informed consent was obtained from all patients.
Consent for publication:
Not applicable
Availability of data and material:
De-identified data is available upon reasonable request made to the corresponding author.
Code availability:
Not applicable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Yen EY, Singh RR. Brief Report: Lupus-An Unrecognized Leading Cause of Death in Young Females: A Population-Based Study Using Nationwide Death Certificates, 2000–2015. Arthritis Rheumatol. 2018;70(8):1251–5. Epub 2018/04/20. doi: 10.1002/art.40512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis care & research. 2014;66(4):608–16. Epub 2013/10/10. doi: 10.1002/acr.22173. [DOI] [PubMed] [Google Scholar]
- 3.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176(8):708–19. Epub 2012/10/02. doi: 10.1093/aje/kws130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurbel PA, Jeong YH, Navarese EP, Tantry US. Platelet-Mediated Thrombosis: From Bench to Bedside. Circ Res. 2016;118(9):1380–91. Epub 2016/04/30. doi: 10.1161/CIRCRESAHA.115.307016. [DOI] [PubMed] [Google Scholar]
- 5.Nhek S, Clancy R, Lee KA, Allen NM, Barrett TJ, Marcantoni E, et al. Activated Platelets Induce Endothelial Cell Activation via an Interleukin-1beta Pathway in Systemic Lupus Erythematosus. Arterioscler Thromb Vasc Biol. 2017;37(4):707–16. Epub 2017/02/06. doi: 10.1161/ATVBAHA.116.308126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2(47):47ra63. Epub 2010/09/03. doi: 10.1126/scitranslmed.3001001. [DOI] [PubMed] [Google Scholar]
- 7.Berlacher MD, Vieth JA, Heflin BC, Gay SR, Antczak AJ, Tasma BE, et al. FcgammaRIIa ligation induces platelet hypersensitivity to thrombotic stimuli. Am J Pathol. 2013;182(1):244–54. Epub 2012/11/13. doi: 10.1016/j.ajpath.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Habets KL, Trouw LA, Levarht EW, Korporaal SJ, Habets PA, de Groot P, et al. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis. Arthritis Res Ther. 2015;17:209. Epub 2015/08/14. doi: 10.1186/s13075-015-0665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey-Goldman R, Li J, Dervieux T, Alexander RV. Cell-bound complement activation products in SLE. Lupus Sci Med. 2017;4(1):e000236. Epub 2017/12/08. doi: 10.1136/lupus-2017-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putterman C, Furie R, Ramsey-Goldman R, Askanase A, Buyon J, Kalunian K, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med. 2014;1(1):e000056. Epub 2014/11/15. doi: 10.1136/lupus-2014-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merrill JT, Petri MA, Buyon J, Ramsey-Goldman R, Kalunian K, Putterman C, et al. Erythrocyte-bound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Sci Med. 2018;5(1):e000263. Epub 2018/06/06. doi: 10.1136/lupus-2018-000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navratil JS, Manzi S, Kao AH, Krishnaswami S, Liu CC, Ruffing MJ, et al. Platelet C4d is highly specific for systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):670–4. Epub 2006/02/01. doi: 10.1002/art.21627. [DOI] [PubMed] [Google Scholar]
- 13.Petri MA, Conklin J, O’Malley T, Dervieux T. Platelet-bound C4d, low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci Med. 2019;6(1):e000318. Epub 2019/06/07. doi: 10.1136/lupus-2019-000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao AH, Navratil JS, Ruffing MJ, Liu CC, Hawkins D, McKinnon KM, et al. Erythrocyte C3d and C4d for monitoring disease activity in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):837–44. Epub 2010/02/27. doi: 10.1002/art.27267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao AH, McBurney CA, Sattar A, Lertratanakul A, Wilson NL, Rutman S, et al. Relation of platelet C4d with all-cause mortality and ischemic stroke in patients with systemic lupus erythematosus. Transl Stroke Res. 2014;5(4):510–8. Epub 2013/12/11. doi: 10.1007/s12975-013-0295-9. [DOI] [PubMed] [Google Scholar]
- 16.Lood C, Tyden H, Gullstrand B, Sturfelt G, Jonsen A, Truedsson L, et al. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS One. 2014;9(6):e99386. Epub 2014/06/13. doi: 10.1371/journal.pone.0099386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CC, Schofield T, Tang A, Manzi S, Ahearn JM. Potential Roles of Antiphospholipid Antibodies in Generating Platelet-C4d in Systemic Lupus Erythematosus. Antibodies (Basel). 2017;6(3). Epub 2017/07/02. doi: 10.3390/antib6030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lood C, Eriksson S, Gullstrand B, Jonsen A, Sturfelt G, Truedsson L, et al. Increased C1q, C4 and C3 deposition on platelets in patients with systemic lupus erythematosus--a possible link to venous thrombosis? Lupus. 2012;21(13):1423–32. Epub 2012/08/11. doi: 10.1177/0961203312457210. [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86. Epub 2012/05/04. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenungsson E, Gustafsson JT, Grosso G, Rossides M, Gunnarsson I, Jensen-Urstad K, et al. Complement deposition, C4d, on platelets is associated with vascular events in systemic lupus erythematosus. Rheumatology (Oxford). 2020. Epub 2020/04/08. doi: 10.1093/rheumatology/keaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. Epub 1997/10/27. doi: . [DOI] [PubMed] [Google Scholar]
- 22.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91. Epub 2002/02/13. [PubMed] [Google Scholar]
- 23.Hui-Yuen JS, Gartshteyn Y, Ma M, O’Malley T, Conklin J, Eichenfield AH, et al. Cell-bound complement activation products (CB-CAPs) have high sensitivity and specificity in pediatric-onset systemic lupus erythematosus and correlate with disease activity. Lupus. 2018:961203318809181. Epub 2018/11/01. doi: 10.1177/0961203318809181. [DOI] [PubMed] [Google Scholar]
- 24.Kalunian KC, Chatham WW, Massarotti EM, Reyes-Thomas J, Harris C, Furie RA, et al. Measurement of cell-bound complement activation products enhances diagnostic performance in systemic lupus erythematosus. Arthritis Rheum. 2012;64(12):4040–7. Epub 2012/08/31. doi: 10.1002/art.34669. [DOI] [PubMed] [Google Scholar]
- 25.Shimbo D, Child J, Davidson K, Geer E, Osende JI, Reddy S, et al. Exaggerated serotonin-mediated platelet reactivity as a possible link in depression and acute coronary syndromes. Am J Cardiol. 2002;89(3):331–3. Epub 2002/01/26. [DOI] [PubMed] [Google Scholar]
- 26.Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638–45. Epub 2009/11/21. doi: 10.1111/j.1365-2141.2009.07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103(3 Suppl):20A–6A. Epub 2009/02/14. doi: 10.1016/j.amjcard.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Pizzulli L, Yang A, Martin JF, Luderitz B. Changes in platelet size and count in unstable angina compared to stable angina or non-cardiac chest pain. Eur Heart J. 1998;19(1):80–4. Epub 1998/03/21. doi: 10.1053/euhj.1997.0747. [DOI] [PubMed] [Google Scholar]
- 29.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338(8780):1409–11. Epub 1991/12/07. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim H, Kleiman NS. Platelet pathophysiology, pharmacology, and function in coronary artery disease. Coron Artery Dis. 2017;28(7):614–23. Epub 2017/06/24. doi: 10.1097/MCA.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 31.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322(22):1549–54. Epub 1990/05/31. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 32.Aurigemma C, Scalone G, Tomai F, Altamura L, De Persio G, Stazi A, et al. Persistent enhanced platelet activation in patients with acute myocardial infarction and coronary microvascular obstruction: clinical implications. Thromb Haemost. 2014;111(1):122–30. Epub 2013/10/03. doi: 10.1160/TH13-02-0166. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson O, Mohlin C, Nilsson B, Ekdahl KN. The Human Platelet as an Innate Immune Cell: Interactions Between Activated Platelets and the Complement System. Front Immunol. 2019;10:1590. Epub 2019/07/30. doi: 10.3389/fimmu.2019.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonati PA, Scavone M, Gerosa M, Borghi MO, Pregnolato F, Curreli D, et al. Blood Cell-Bound C4d as a Marker of Complement Activation in Patients With the Antiphospholipid Syndrome. Front Immunol. 2019;10:773. Epub 2019/04/30. doi: 10.3389/fimmu.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peerschke EI, Yin W, Alpert DR, Roubey RA, Salmon JE, Ghebrehiwet B. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus. 2009;18(6):530–8. Epub 2009/04/28. doi: 10.1177/0961203308099974. [DOI] [PMC free article] [PubMed] [Google Scholar]