Abstract
Serum procollagen type I N-propeptide (PINP) is designated the reference marker of bone formation in osteoporosis; the reference marker for resorption is C-terminal telopeptide of type I collagen (CTX). PINP has very low circadian and biological variation, is not affected by food intake, and is very stable in serum after venepuncture. The two automated commercial assays for PINP provide similar results in subjects with normal renal function, allowing reference intervals to be used interchangeably. Bone turnover markers (BTM) are currently not recommended for fracture risk assessment and therefore not included in fracture risk calculators. In the management of osteoporosis, the main utility of BTM including PINP is for monitoring therapy, both antiresorptive as well as anabolic agents; monitoring is thought to help improve adherence. PINP as well as CTX may also be used in assessing offset of drug action following a pause in bisphosphonate therapy, to help decide when to re-instate therapy, or following cessation of denosumab therapy to assess efficacy of follow-on bisphosphonate therapy. PINP may also be used in the diagnosis of Paget’s disease of bone as well as in monitoring response to therapy and for recurrence. Although BTM other than bone alkaline phosphatase are currently not recommended for use in metabolic bone disease of chronic kidney disease, PINP measured by assays specific to the intact molecule has potential in this condition. Further studies are needed to examine this area, as well as in malignant bone disease.
Introduction
The bone formation marker procollagen type I N-propeptide (PINP) is a trimeric peptide with a molecular mass of about 35,000 kDa, consisting of two type 1 procollagen-α1 chains and a procollagen-α2 chain which are bonded non-covalently (Figure 1).1 Procollagen I molecule is synthesised by osteoblasts, and the pro-peptide extensions at the amino- and carboxy-terminals (PINP and PICP respectively) of the procollagen molecule are cleaved off and released into circulation when collagen molecule is laid down to form the osteoid matrix during bone formation. Since the PINP and PICP molecules are produced in equimolar amounts with collagen-1 molecule, their concentrations in the circulation might be expected to reflect bone formation rate. In practice, serum PINP (sPINP) has been found to reflect histomorphometric measures of bone formation,2 and has been identified as the most promising marker of bone formation and designated the reference marker of bone formation in osteoporosis by the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC),3 and endorsed by the National Bone Health Alliance (NBHA) in the US.4 PINP in the circulation is metabolised in the liver.5 In addition to the intact (trimeric) PINP molecule described above, monomers of the molecule, which are degradation products of its metabolism mainly in the liver, are also found in blood and are recognised by some immunoassays for sPINP.1,6 The monomeric fragments are likely excreted by the kidney and accumulate disproportionately when renal function is impaired. Hence, high concentrations are reported in dialysis patients when PINP is measured by ‘total PINP’ assays that recognise the monomer in addition to the intact PINP molecule.7
Figure 1.
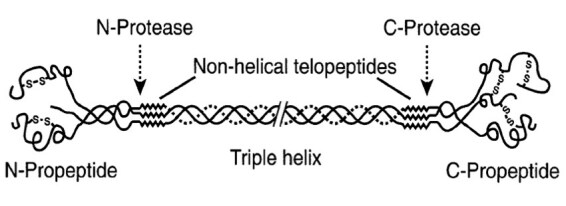
The structure of the collagen type I molecule. (Figure courtesy of Simon Robins, Aberdeen, UK.)
Pre-Analytics
There is a perception that biological variation is unusually high for bone turnover markers (BTM).8 This perception is true for the early markers which were measured in urine. Analytes in urine generally suffer from large biological variation as exhibited by the commonly used test of urine albumin in diabetes; three repeat measurements are recommended to confirm albuminuria to address this problem.9,10 Creatinine is also added for spot urine measurements to correct for the effect of dilution but this adds to the biological and analytical variation. Creatinine correction does not correct for inter-individual variation, but in fact can accentuate it due to inter-individual variation in muscle mass.11 At extremes of muscle mass, creatinine correction of urine analytes may lead to misleading results.12 On the other hand, measurements in blood are less prone to the effects of biological variation; sPINP has a within-subject biological variation of 7–9%.13,14 In fact, PINP in blood is minimally affected by circadian variation and feeding. PINP decreases 3.8% (+/− 0.9%) following intake of food.15 The coefficient of cyclic variation of PINP is in the range of 3–5% which is very low.16 Hence blood sampling can be performed at any time of the day regardless of fasting status, which is a major advantage of using sPINP as a bone marker.17 Either serum or EDTA plasma sample may be used for PINP measurement and the results treated interchangeably as they are equivalent. In addition, PINP is stable in serum (or plasma) after venesection. PINP in both EDTA plasma and serum is stable for at least 24 h at room temperature and for 5 days at 4 °C.17
Analytics
Three commercial immunoassays are currently available for measuring sPINP, including two chemiluminescence immunoassays on automated platforms: Immunodiagnostic Systems PLC on the iSYS automated analyser (IDS, Boldon, UK); and Roche Diagnostics (Mannheim, Germany) instruments. A manual radioimmunoassay (RIA) produced by Orion Diagnostica (UniQ PINP RIA, Orion Diagnostica, Espoo, Finland) is also available. Whilst the Orion Diagnostica RIA assay is not used widely in Australia unlike the assays on the automated platforms, it is popular in the US since it is approved by the FDA for clinical use.
The IDS iSYS chemiluminescence immunoassay and the Orion Diagnostica RIA for sPINP have the advantage of being more specific to the trimeric (intact PINP) molecule and do not recognise the monomer or fragments of the PINP molecule; the calibrator for these two assays is purified trimeric PINP.1 The calibrator for the Roche Diagnostics assays is a synthetic amino procollagen peptide made from pre-procollagen-α1.1 The Roche assay for ‘total’ PINP has the limitation of measuring both the intact PINP molecule and the PINP monomer or fragments of the PINP molecule which accumulate in the circulation in late stage chronic kidney disease (i.e. when the glomerular filtration rate is <30 mL/min/1.73m2) providing a spuriously elevated, potentially misleading result.
Even though the two automated assays for PINP (Roche and IDS) measure total and intact PINP respectively, the results produced by the two assays are similar when renal function is normal. A multi-centre study of 796 patients (with eGFR >30 mL/min/1.73m2) who presented to osteoporosis clinics in four centres in Europe measured PINP in serum and EDTA plasma by the three methods described above.18 The results confirmed that serum and EDTA plasma gave equivalent results. The results by the two automated assays (Roche Cobas and IDS iSYS) were similar (Passing-Bablok regression: Cobas = 0.91 x iSYS + 2.6.). However, the Orion Diagnostica RIA showed a proportional bias (non-agreement) with the two automated assays but with good correlation (Passing-Bablok regressions: Cobas = 1.22 x Orion + 0, iSYS = 1.35 x Orion RIA – 3.2). These results indicate that the Roche and IDS iSYS assays for sPINP and reference intervals may be used interchangeably in osteoporosis patients with eGFR >30 mL/min/1.73m2.19 The harmonised Australian reference intervals for sPINP in adults published previously for the Roche assay can now also be used for the IDS iSYS assay.20
Post-Analytics
Osteoporosis
Fracture Risk Assessment
Osteoporosis is diagnosed on the basis of bone mineral density (BMD) measurement, with a T score of −2.5 or lower below healthy young adult norms (WHO diagnostic criterion). Fracture risk assessment is key to treatment decisions for individual patients. FRAX® is the fracture risk calculator used worldwide,21 whilst the Garvan risk calculator is also popular in Australia and in older frailer populations due to the inclusion of an older cohort (up to 96 y) and falls risk.22 In univariate analyses, BTM have been shown in some studies to be associated with fracture risk, especially at the spine.3 A recent meta-analysis demonstrated a modest but significant association between sPINP and the risk of fracture. The hazard ratio per SD increase in sPINP (gradient of risk) was 1.23 (95 % CI 1.09–1.39) for men and women combined unadjusted for BMD, similar to sCTX.21 The study was not able to determine the extent to which fracture risk prediction was independent of BMD. BTM would be useful for inclusion in fracture risk calculations only if their association with fracture risk were independent of BMD. The literature in this matter is inconsistent and there is a lack of adequate data due to variable assays and inconsistencies in design between studies.23 At present, BTM are currently not included in fracture risk calculations.24 However, the role of PINP in lower BTM states where antiresorptive treatments are less effective, and in older populations, may be important in identifying the potential role for anabolic instead of antiresorptive therapy.25
Monitoring of Osteoporosis Treatment
The aim of treatment for osteoporosis is to prevent or reduce the risk of fracture. Therefore, the outcome measure of success of treatment is fracture incidence. However, one does not want to wait until fractures occur in a patient to find out that treatment has been unsuccessful in that patient. Hence, the use of surrogate markers to evaluate treatment efficacy. Traditionally BMD and BTM have been used as surrogate markers for this purpose.3 The increase in BMD following treatment has been shown to be associated with fracture risk reduction in some studies.26 Similarly, the magnitude of decrease in BTM following antiresorptive therapy has been shown to be proportional to fracture risk reduction.27 The change in BMD is modest and slow, reaching significance (> least significant change (LSC)) with bisphosphonate therapy in 18–24 months, a long time to wait to find out if treatment has been effective.28 On the other hand, BTM changes are rapid and large, reaching significance within weeks or months of initiating therapy.3 For these reasons, BTM are used initially to assess efficacy of treatment including adherence and persistence with treatment. The direction of BTM change is generally the same for bone formation markers and bone resorption markers due to ‘coupling’; for antiresorptive therapies (bisphosphonates, selective oestrogen receptor modulators, denosumab), there is a reduction in bone resorption markers followed by a later reduction in formation markers.29 Similarly, following anabolic therapies (teriparatide, abaloparatide), there is an increase in formation markers followed by a smaller increase in resorption markers (Figure 2).29 Hence PINP, a bone formation marker, may in fact be used for monitoring antiresorptive therapy as well as anabolic therapy, and is used in practice for monitoring both modalities of treatment. Even though the use of PINP for monitoring antiresorptive therapy may seem counterintuitive, it has been shown to perform well for this purpose.30 Dual action therapies such as romosozumab, which was recently licenced for use in Australia, and strontium ranelate which is no longer in wide use, cause an increase in formation markers and a reduction in resorption markers (uncoupling).31 The use of BTM in monitoring romosozumab therapy remains to be ascertained. It is likely that the transient increase in sPINP and the more sustained decrease in sCTX may be useful markers of effective uncoupling and efficacy.
Figure 2.
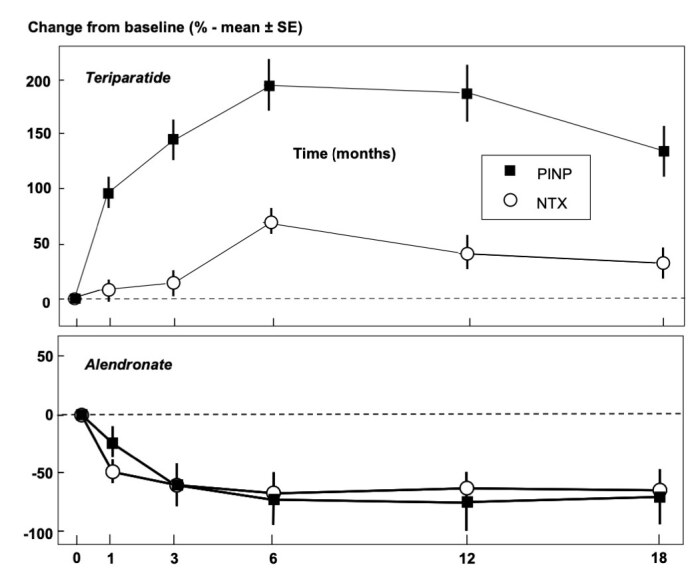
Changes (% ± SEM) in sPINP and uNTX following treatment with an anti-resorptive therapy (alendronate) and an anabolic therapy (teriparatide). (Ref. 3; reprinted with permission.)
sPINP, serum procollagen type I N-propeptide; uNTX, urine N-terminal telopeptide.
The TRIO study was designed to examine the responses of several BTM (both formation markers and resorption markers) to various bisphosphonates.32 At 12 weeks of therapy with alendronate, 98% of patients had a significant change in sCTX compared to 82% of patients based on sPINP. By 48 weeks, the change in sPINP was significant in 94% of subjects. At 12 weeks, sCTX had decreased to below the treatment target (premenopausal median) in 96% of patients, and sPINP in 82% of patients increasing to 94% by 48 weeks.32 The premenopausal median for PINP is 35 μg/L for both Roche and IDS assays; for sCTX the median is assay-dependent but approximately 250 ng/L.29 These differences reflect the earlier reduction in bone resorption markers followed by a later reduction in formation markers with antiresorptive therapies. However, by 6 months of oral therapies, BTM would generally have reached a plateau at their reduced concentrations. A two-tailed analysis was performed in calculating the LSC in the TRIO study.32 In fact, since the direction of change is known, one-tailed analysis may be more appropriate, the use of which would lead to close to 100% of patients achieving a reduction in both sCTX and sPINP with alendronate therapy.33 Studies that have specifically examined the utility of PINP and CTX in monitoring bisphosphonate therapy, comparing it to BMD measurement, reiterate the utility of sPINP for monitoring bisphosphonate therapy.30 The attractions of sPINP include its lower circadian variation and lack of impact of food intake as indicated above, meaning testing can be performed any time during the day without the need for fasting, as well as the stability of PINP in the blood sample after venesection.
One of the major problems with long-term oral therapy for any chronic condition such as osteoporosis is lack of adherence, with evidence suggesting that the majority of patients do not continue to take medications long term, especially since the benefit of therapy is not apparent to the patient. An important tool used by clinicians to improve adherence and persistence with therapy is to share with the patient the results of BTM that confirm response to therapy, and to encourage persistence with the therapy.34
The changes in sPINP and sCTX after denosumab therapy have been well studied.35 The decreases in these markers are even greater than those seen with bisphosphonate therapy.36 Median decrease in CTX at three months in the denosumab-treated group was 89%, significantly greater than the 66% change seen in the alendronate-treated group. The median change in sPINP at three months was 76% with denosumab compared to 56% with alendronate. Most patients on denosumab therapy would therefore show significant and major reductions in both sPINP and sCTX, with PINP concentrations usually decreasing to well below 20 μg/L. sCTX also decreases to below 200 ng/L. Whilst both markers are sensitive enough to demonstrate the reduction in bone resorption following denosumab therapy, their utility in monitoring denosumab therapy has become more topical due to their observed rebound increase following cessation of therapy which is associated with an increased risk of vertebral fractures. The recommendation is to treat patients who cease denosumab therapy with a potent bisphosphonate such as zoledronic acid infusion or oral alendronate.37,38 The effectiveness of such therapy to counteract the rebound increase in bone turnover following cessation of denosumab therapy may be monitored using sPINP or sCTX. The aim is to maintain the BTM below the premenopausal median.
The offset of bisphosphonate action after a pause in therapy (‘drug holiday’) following long-term treatment, such as five years of oral alendronate or three infusions of zoledronic acid needs to be managed to mitigate detrimental effects during the drug holiday. When treatment is paused, BTM may be monitored during the drug holiday period for this purpose in order to decide when and if to re-institute treatment. Whilst evidence on how this should be managed optimally is still awaited,39 it has been suggested that an increase in sPINP or sCTX greater than the LSC and/or to above the median of the premenopausal reference interval could be considered an indication for re-instituting therapy.40
Paget’s Disease of Bone
Paget’s disease of bone is relatively rare and is mainly seen in people of northern European descent. It presents with bone pain and sometimes deformity or fracture. Biochemistry is integral to the diagnosis and management of Paget’s disease, together with imaging studies. Total alkaline phosphatase (ALP) is the best characterised blood test for diagnosis and for monitoring.41 Although ALP in blood is of bone and liver origin in equal proportions (and other sources such as placenta and intestine less often), its increase in Paget’s disease is usually high enough for it to be of adequate sensitivity for diagnosis and for monitoring recurrence after treatment. In the rare form of monostotic Paget’s where ALP may not be increased above the reference interval, bone-specific ALP (BALP) or PINP may be used as they are more sensitive and also specific for bone.42 The resorption marker in urine (N-terminal telopeptide, NTX) is an alternative or addition to the formation markers. The aim of treatment is to normalise BTM to the lower part of the reference interval. Patients in remission are monitored with six-monthly BTM measurements; relapse is heralded by pain and an increase in BTM >LSC, with a gradual increase to above the reference interval. A recent meta-analysis found that Paget’s disease activity is best monitored using sP1NP.43
Mineral Bone Disease of Chronic Kidney Disease
The Kidney Disease Improving Global Outcomes (KDIGO) guideline recommends measurement of ALP as part of biochemistry to examine for mineral bone disease of chronic kidney disease (CKD-MBD).44 This may be of limited benefit in the setting of concurrent liver disease when BALP would be more informative. Monitoring of CKD-MBD with biochemistry includes measurement of parathyroid hormone or BALP. Other BTM are not recommended as they are mostly affected by renal failure itself. However, since intact PINP measurement is not affected by renal failure, studies of intact PINP in CKD MBD may be warranted to examine its utility in management.
Conclusion
PINP is a useful marker in the diagnosis and/or management of metabolic bone diseases (Table). PINP may be used for the monitoring of osteoporosis therapy with both antiresorptive and anabolic agents. It may be useful to identify who may more likely benefit from antiresorptive therapy for osteoporosis or be considered for anabolic therapy instead. PINP may be used for diagnosis and monitoring of Paget’s disease of bone. Its use in other conditions such as CKD-MBD as well as metastatic bone disease warrants further study.45,46
Table.
Roles of PINP in the management of metabolic bone disorders.
| 1. Assessment of bone formation |
| Mainly in the osteoporosis research setting, including drug trials.2–4 |
| 2. Assessment of bone turnover |
| PINP reflects bone turnover overall due to coupling of bone resorption and bone formation. Mainly used in the research setting currently.2–4 |
| 3. Assessment of osteoporosis risk i.e. stratification |
| Whilst a raised PINP is an independent risk factor for fracture risk, there are not enough data to confirm its role as an independent factor in fracture risk calculations when all other risk factors are included.23,24 |
| 4. Assessment of response to osteoporosis treatments – anabolic and antiresorptives |
| The main role is assessment and monitoring of BTM in osteoporosis treatment. A significant decrease in PINP following antiresorptive therapy and a significant increase following anabolic therapy confirms response to therapy.29,30 |
| 5. Monitoring of response to treatment |
| Useful for confirming adherence i.e. compliance and persistence with treatment.34 |
| 6. Monitoring for offset of treatment on cessation |
| On treatment cessation, an increase in PINP to baseline or greater than the premenopausal median may indicate offset of treatment effect i.e. increased bone turnover and increase in risk of fracture which may inform the need to restart bisphosphonate therapy. With denosumab therapy, a rapid rebound increases the risk of fracture (return to baseline risk) and may require restarting denosumab, or instituting bisphosphonate therapy to blunt the rapid offset before cessation.37,40 |
| 7. Monitoring of safety e.g. bone suppression and adynamic bone disease in CKD |
| Currently there are no data for the use of PINP to diagnose over-suppression of turnover and risk of osteonecrosis of the jaw or atypical fracture in osteoporosis therapy. |
| There is potential for the use of intact PINP assays to monitor bone turnover in CKD; however, data from clinical studies are lacking for this purpose. |
| 8. Diagnosing Paget’s disease of bone |
| Whilst total ALP or bone ALP (if liver disease is present) measurement, together with imaging studies, is adequate for the diagnosis of Paget’s disease of bone, the increased sensitivity and specificity of PINP may be useful in diagnosing monostotic Paget’s disease.41,42 |
| 9. Monitoring of disease activity in Paget’s disease |
| As above, total ALP is adequate in most cases of Paget’s disease of bone to monitor therapy and detect recurrence; PINP is more sensitive and may be used as back-up or where ALP is elevated due to other causes.41–43 |
| 10. Metastatic bone disease |
| There is potential for the use of PINP both in detecting metastatic bone disease as well as in monitoring of treatment, especially for osteoblastic secondaries such as prostate cancer, breast cancer etc. This area requires further study.46 |
BTM, bone turnover markers; CKD, chronic kidney disease; ALP, alkaline phosphatase.
Footnotes
Competing Interests: None declared.
References
- 1.Koivula MK, Risteli L, Risteli J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin Biochem. 2012;45:920–7. doi: 10.1016/j.clinbiochem.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Chavassieux P, Portero-Muzy N, Roux JP, Garnero P, Chapurlat R. Are biochemical markers of bone turnover representative of bone histomorphometry in 370 postmenopausal women? J Clin Endocrinol Metab. 2015;100:4662–8. doi: 10.1210/jc.2015-2957. [DOI] [PubMed] [Google Scholar]
- 3.Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 4.Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, et al. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012;23:2425–33. doi: 10.1007/s00198-012-2049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melkko J, Hellevik T, Risteli L, Risteli J, Smedsrod B. Clearance of NH2-terminal propeptides of types I and III procollagen is a physiological function of the scavenger receptor in liver endothelial cells. J Exp Med. 1994;179:405–12. doi: 10.1084/jem.179.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melkko J, Kauppila S, Niemi S, Risteli L, Haukipuro K, Jukkola A, et al. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clin Chem. 1996;42:947–54. [PubMed] [Google Scholar]
- 7.Cavalier E, Lukas P, Carlisi A, Gadisseur R, Delanaye P. Aminoterminal propeptide of type I procollagen (PINP) in chronic kidney disease patients: the assay matters. Clin Chim Acta. 2013;25:117–8. doi: 10.1016/j.cca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;11(Suppl 6):S2–17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:S1–150. [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 11.Houlihan CA, Tsalamandris C, Akdeniz A, Jerums G. Albumin to creatinine ratio: a screening test with limitations. Am J Kidney Dis. 2002;39:1183–9. doi: 10.1053/ajkd.2002.33388. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo M, Laurenzi M, Mancini M, Zanchetti A, De Santo NG. Low muscular mass and overestimation of microalbuminuria by urinary albumin/creatinine ratio. Hypertension. 2006;47:56–61. doi: 10.1161/01.HYP.0000197953.91461.95. [DOI] [PubMed] [Google Scholar]
- 13.Cavalier E, Lukas P, Bottani M, Aarsand AK, Ceriotti F, Coşkun A, et al. European Biological Variation Study (EuBIVAS): within- and between-subject biological variation estimates of β-isomerized C-terminal telopeptide of type I collagen (β-CTX), N-terminal propeptide of type I collagen (PINP), osteocalcin, intact fibroblast growth factor 23 and uncarboxylated-unphosphorylated matrix-Gla protein – a cooperation between the EFLM Working Group on Biological Variation and the International Osteoporosis Foundation-International Federation of Clinical Chemistry Committee on Bone Metabolism. Osteoporos Int. 2020;31:1461–70. doi: 10.1007/s00198-020-05362-8. [DOI] [PubMed] [Google Scholar]
- 14.Westgard QC. Desirable specifications for Total Error, Imprecision, and Bias, derived from intra- and inter-individual biologic variation. [Accessed 17 March 2021]. https://www.westgard.com/biodatabase1.htm.
- 15.Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30:886–90. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- 16.Redmond J, Fulford AJ, Jarjou L, Zhou B, Prentice A, Schoenmakers I. Diurnal rhythms of bone turnover markers in three ethnic groups. J Clin Endocrinol Metab. 2016;101:3222–30. doi: 10.1210/jc.2016-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–56. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- 18.Cavalier E, Eastell R, Rye Jørgensen NR, Makris K, Tournis S, Vasikaran S, et al. IFCC-IOF Joint Committee for Bone Metabolism (C-BM) A multicenter study to evaluate harmonization of assays for N-terminal propeptide of type I procollagen (PINP): a report from the IFCC-IOF Joint Committee for Bone Metabolism. Clin Chem Lab Med. 2019;57:1546–55. doi: 10.1515/cclm-2019-0174. [DOI] [PubMed] [Google Scholar]
- 19.Vasikaran SD, Bhattoa HP, Eastell R, Heijboer AC, Jørgensen NR, Makris K, et al. Harmonization of commercial assays for PINP; the way forward. Osteoporos Int. 2020;31:409–12. doi: 10.1007/s00198-020-05310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasikaran SD, Chubb SP, Ebeling PR, Jenkins N, Jones GR, Kotowicz MA, et al. Harmonised Australian reference intervals for serum PINP and CTX in adults. Clin Biochem Rev. 2014;35:237–42. [PMC free article] [PubMed] [Google Scholar]
- 21.FRAX® Fracture Risk Assessment Tool. [Accessed 17 March 2021]. https://www.sheffield.ac.uk/FRAX/tool.aspx?country=31.
- 22.Garvin Institute of Medical Research. Bone Fracture Risk Calculator. [Accessed 17 March 2021]. https://www.garvan.org.au/bone-fracture-risk.
- 23.Johansson H, Odén A, Kanis JA, McCloskey EV, Morris HA, Cooper C, et al. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int. 2014;94:560–7. doi: 10.1007/s00223-014-9842-y. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey EV, Vasikaran S, Cooper C. Official Positions for FRAX® clinical regarding biochemical markers from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom. 2011;14:220–2. doi: 10.1016/j.jocd.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Inderjeeth C, Nair PA, Chan K, Raymond W, Lim EM. Bone turnover markers in old vs early postmenopausal women. MOJ Gerontol Ger. 2019;4:22–6. [Google Scholar]
- 26.Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632–42. doi: 10.1002/jbmr.3641. [DOI] [PubMed] [Google Scholar]
- 27.Bauer DC, Black DM, Bouxsein ML, Lui LY, Cauley JA, de Papp AE, et al. Foundation for the National Institutes of Health (FNIH) Bone Quality Project. Treatment-related changes in bone turnover and fracture risk reduction in clinical trials of anti-resorptive drugs: a meta-regression. J Bone Miner Res. 2018;33:634–42. doi: 10.1002/jbmr.3355. [DOI] [PubMed] [Google Scholar]
- 28.Inderjeeth CA, Chan K, Kwan K, Lai M. Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab. 2012;30:493–503. doi: 10.1007/s00774-012-0349-1. [DOI] [PubMed] [Google Scholar]
- 29.Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. Diagnosis of Endocrine Disease: Bone turnover markers: are they clinically useful? Eur J Endocrinol. 2018;178:R19–31. doi: 10.1530/EJE-17-0585. [DOI] [PubMed] [Google Scholar]
- 30.Bell KJ, Hayen A, Glasziou P, Irwig L, Eastell R, Harrison SL, et al. Potential usefulness of BMD and bone turnover monitoring of zoledronic acid therapy among women with osteoporosis: secondary analysis of randomized controlled trial data. J Bone Miner Res. 2016;31:1767–73. doi: 10.1002/jbmr.2847. [DOI] [PubMed] [Google Scholar]
- 31.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 32.Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, et al. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int. 2016;27:21–31. doi: 10.1007/s00198-015-3145-7. [DOI] [PubMed] [Google Scholar]
- 33.Tan RZ, Loh TP, Vasikaran S. Bone turnover marker monitoring in osteoporosis treatment response. Eur J Endocrinol. 2020;183:C5–7. doi: 10.1530/EJE-19-0970. [DOI] [PubMed] [Google Scholar]
- 34.Diez-Perez A, Naylor KE, Abrahamsen B, Agnusdei D, Brandi ML, Cooper C, et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int. 2017;28:767–74. doi: 10.1007/s00198-017-3906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 36.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–61. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 37.Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2021;106:264–81. doi: 10.1210/clinem/dgaa756. [DOI] [PubMed] [Google Scholar]
- 38.Kendler D, Chines A, Clark P, Ebeling PE, McClung M, Rhee Y, et al. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020;105:e255–64. doi: 10.1210/clinem/dgz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 40.Naylor KE, McCloskey EV, Jacques RM, Peel NFA, Paggiosi MA, Gossiel F, et al. Clinical utility of bone turnover markers in monitoring the withdrawal of treatment with oral bisphosphonates in postmenopausal osteoporosis. Osteoporos Int. 2019;30:917–22. doi: 10.1007/s00198-018-04823-5. [DOI] [PubMed] [Google Scholar]
- 41.Ralston SH, Corral-Gudino L, Cooper C, Francis RM, Fraser WD, Gennari L, et al. Diagnosis and management of Paget’s disease of bone in adults: a clinical guideline. J Bone Miner Res. 2019;34:579–604. doi: 10.1002/jbmr.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer FR, Bone HG, Hosking DJ, Lyles KW, Murad MH, Reid IR, et al. Endocrine Society. Paget’s disease of bone: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:4408–22. doi: 10.1210/jc.2014-2910. [DOI] [PubMed] [Google Scholar]
- 43.Al Nofal AA, Altayar O, BenKhadra K, Qasim Agha OQ, Asi N, Nabhan M, et al. Bone turnover markers in Paget’s disease of the bone: a systematic review and meta-analysis. Osteoporos Int. 2015;26:1875–91. doi: 10.1007/s00198-015-3095-0. [DOI] [PubMed] [Google Scholar]
- 44.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salam S, Gallagher O, Gossiel F, Paggiosi M, Khwaja A, Eastell R. Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol. 2018;29:1557–65. doi: 10.1681/ASN.2017050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Oronzo S, Brown J, Coleman R. The value of biomarkers in bone metastasis. Eur J Cancer Care (Engl) 2017;26:1–10. doi: 10.1111/ecc.12725. [DOI] [PubMed] [Google Scholar]


