Targeting the B-lineage surface antigen CD19 in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is one of the most successful examples of T-cell-based immunotherapies.1,2 The CD3/CD19 bispecific T-cell engager, blinatumomab, produces excellent responses in adult and pediatric patients with relapsed/refractory BCP-ALL.1-3 However, a significant proportion of patients do not respond to therapy or relapse.1,3 Acting as a strong selective factor, CD19-directed immunotherapy can drive the specific, but still not completely understood immune escape mechanism by the loss of CD19 expression on leukemic blasts, thereby leading to CD19-negative relapses.3,4 This loss of CD19 creates significant challenges to the application of multicolor flow cytometry (MFC) for monitoring minimal residual disease (MRD). Moreover, the expression of other markers can also change, and the frequency of these changes is still unclear. As accurate detection of residual tumor cells has emerged as a key tool in evaluating efficacy and predicting failures after CD19-directed therapies,5 these obstacles to flow cytometry are highly significant. The current report briefly summarizes our data on MFC-MRD and relapse detection in patients treated with blinatumomab with emphasis on changes in the expression of markers that are relevant for MFC-MRD investigations.
We carried out a retrospective review of 90 pediatric patients with relapsed/refractory ВCP-ALL who received blinatumomab between December 2015 and August 2020. The characteristics of the patients, including their cytogenetic data, are presented in Online Supplementary Table S1. All patients, except for two in whom treatment was interrupted because of disease progression, received at least one 28-day course of blinatumomab (median number of courses 1; range, 1-4). Blinatumomab was kindly provided by Amgen as part of a named-patient extended-access program. Blinatumomab treatment was administered to 67 of the 90 studied patients as a “bridge therapy” to allogeneic stem cell transplantation (Online Supplementary Table S1). Morphological examination and MFC-MRD detection in bone marrow aspirates were performed before and after each cycle of blinatumomab, while, for patients who underwent hematopoietic stem cell transplantation, MFC-MRD evaluation was carried out on days +30, +90, +120, +180, +360 after transplantation or in cases of suspected relapse.
All patients underwent routine diagnostic immunophenotyping and MRD detection by eight- or ten-color flow cytometry according to the standard protocols of the Moscow-Berlin group.6,7 During the study period MFC was performed on FACS Canto II, FACS Celesta (both from Becton Dickinson, BD; USA), CytoFlex and Navios (both from Beckman Coulter, BC; USA) flow cytometers. EuroFlow guidelines for machine performance monitoring were used.8 The immunophenotype of the tumor cells was analyzed with focus on markers applicable for MFCMRD investigation.4,9 Online Supplementary Table S2 provides a list of monoclonal antibodies used for MFC-MRD monitoring. CD22 and CD24 were additionally studied, mainly after the blinatumomab courses.10 Expression of surface antigens was deemed positive if the antigen was expressed on more than 20% of tumor cells.6 An increase or decrease of expression of each single antigen was defined as a change of the percentage of positive cells by more than 25%. Proportions of cases with stable and changed expression of each single antigen between CD19-negative and CD19-positive relapses were compared using the Fisher exact test.
Figure 1.
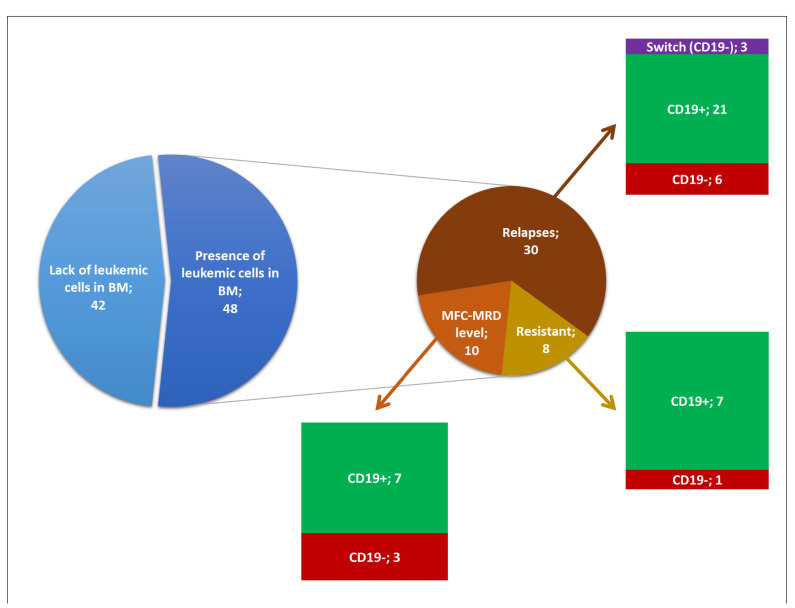
Outcome after blinatumomab treatment in the studied patients (n=90) with emphasis on the expression of CD19. Patients who achieved complete multicolor flow cytometry (MFC)-minimal residual disease (MRD)-negative remission and those with bone marrow (BM) MFC-MRD-negativity, but with progression of extramedullary disease are grouped together and named “Lack of leukemic cells in BM”. Remaining cases (n=48) include resistant ones (n=8), relapses (21 CD19-positive, 6 CD19-negative and 3 switches to acute myeloid leukemia) and patients with blasts detected by MFC at MRD-level in bone marrow at least once (n=10).
The treatment outcomes of the studied patients are summarized in Figure 1. Thirty-nine patients achieved complete MFC-MRD-negative remission and three achieved bone marrow MFC-MRD-negativity, but with progression of extramedullary disease. These patients never had detectable leukemia in the bone marrow during follow-up, so they were excluded from analysis of immunophenotypic changes. Overall, modulation of antigen expression was studied in 48 patients with tumor blasts detectable in bone marrow at least once after a course of treatment with blinatumomab.
We focused separately on the status of CD19 expression on leukemic cells (Figure 1), since this is the sole possible immunophenotypic change directly linked to the administration of blinatumomab. Thirty patients experienced relapse (>5% of blasts cells by MFC). In 21 cases, leukemic cells at relapse were CD19-positive and in six cases they were CD19-negative. Three children (2 with KMT2A gene rearrangements and 1 with germline KMT2A) developed relapses through “lineage switch” to CD19-negative acute myeloid leukemia (n=1), mixedphenotype acute leukemia (n=1) and acute unclassifiable leukemia (n=1). Eight patients were resistant to blinatumomab therapy, and the expression of CD19 on blast cells was retained in the bone marrow of seven of them while one patient had CD19-negative leukemic blasts after immunotherapy. Blast cells of patients who had leukemic cells in bone marrow only at the level of MFCMRD (<5% by MFC) (n=10), were either CD19-positive (n=7) or CD19-negative (n=3).
Figure 2.
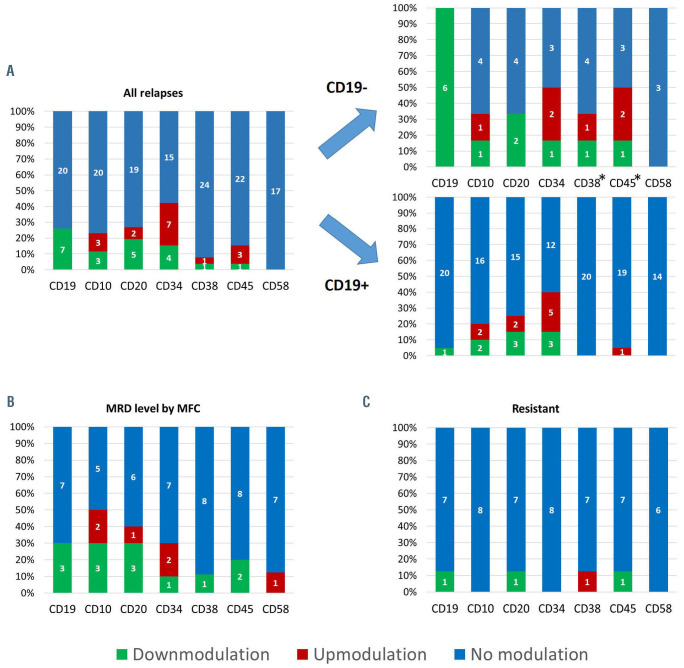
Frequency of changes in immunophenotype of leukemic blasts. (A-C) Frequency of changes in immunophenotype of leukemic blasts in relapsed cases (A), cases with detectable blasts in bone marrow only at a minimal residual disease level (B), and in resistant cases (C). Cases of “lineage switch” are not shown. *Differences in frequencies of antigen expression changes are statistically significant (P<0.05). MRD: minimal residual disease; MFC: multicolor flow cytometry.
The immunophenotypic changes of leukemic cells in 27 relapsed patients (3 cases of lineage-switch excluded) are presented in Figure 2A. For all antigens applicable for MFC-MRD assessment, except CD58, changes in expression, either up- or down-modulation, were demonstrated in substantial proportions of cases. We found different frequencies of changes in the expression of two markers among CD19-negative and CD19-positive relapses: CD45 and CD38 were less stable on CD19-negative blasts (Figure 2A). Expression of CD22 and CD24, which are suggested as candidates to replace CD19,10 was studied at relapse in 24 and 19 patients, respectively. Total positivity (≥90%) for these antigens was found in 20 and 17 cases, respectively. We analyzed ten patients, who did not relapse, but had leukemic cells at a MRD level by MFC in bone marrow at least once during the follow-up period (Online Supplementary Table S2). Besides the understandable downmodulation of CD19 in three out of ten patients, expression of CD10, CD20 and CD34 had changed in five, four and three cases, respectively (Figure 2B). Leukemic cells in eight resistant patients had rather stable immunophenotypic profiles with only very rare changes in antigen expression (Figure 2C).
Since cytometric residual leukemia detection is based on investigation of the B-cell compartment, CD19 is a vital antigen for conventional MFC-MRD monitoring.9 Possible loss of this marker during CD19-negative relapse could break the well-established algorithm of MFC-MRD gating.10 If modulation in the expression of other antigens also occurs, cytometric MRD studies could become very tricky. The implication is that assessment of immunophenotypic changes is crucial for improving antibody panels and gating algorithms in patients who undergo CD19- directed treatment.
Overall, leukemic blasts were CD19-negative in 27.1% of patients with relapses, progression or MFC-MRD-positivity after blinatumomab. It was shown previously that CD19 is completely lost in 10-25% of relapses.3,4 In our series lack of the targeted antigen was noted in nine out of a total of 30 relapses (30.0%) including three “switches” to acute myeloid leukemia. Contrary to data published by Jabbour et al.,11 not all patients resistant to blinatumomab preserved high CD19 expression: in one of eight cases (12.5%) leukemic cells became completely CD19- negative. Although CD19-positive relapses were mainly preceded by CD19-positive MFC-MRD and CD19-negative relapses were mainly preceded by CD19-negative MFC-MRD, we observed some exceptions, demonstrating that this is not a strict pattern (Online Supplementary Figure S1).
Studying the expression of other antigens commonly used for MFC-MRD evaluation (CD10, CD20, CD34, CD45, CD58, CD38), we also observed frequent changes both in the percentage of positive cells and the distribution of the level of positivity. Phenotypic shifts between diagnostic and relapse samples have been reported with a frequency of up to 70% in patients with BCP-ALL.12 Moreover, corticosteroid-mediated changes of antigen expression profile have been observed during remission induction.13 In our series, CD58 demonstrated outstanding stability: no cases of reduced expression of this antigen were noted. All remaining markers, usually useful for MFC-MRD detection, underwent either increased or decreased expression in substantial proportions of relapses and MRD-positive patients. Nevertheless, we were not able to point to any trend in immunological changes.
Figure 3.
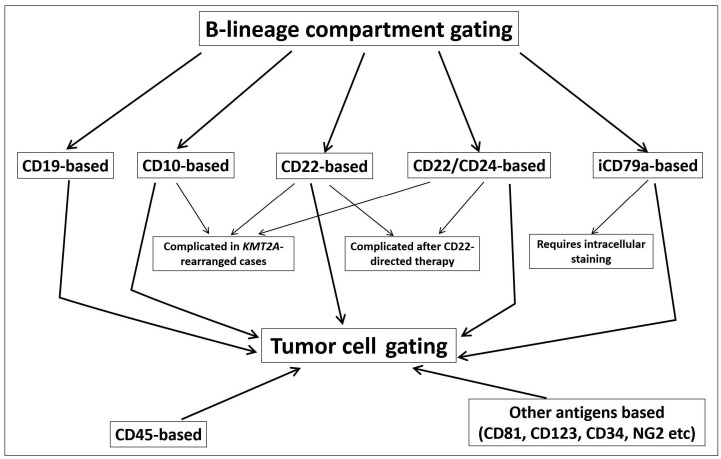
Possible algorithm for searching for residual leukemic cells in patients with B-cell precursor acute lymphoblastic leukemia after CD19-targeted therapy. Various ways of B-lineage gating are shown with their limitations indicated. Consecutive investigation of all these B-cell compartments together with the CD45-defined blast region, precursor regions and aberrantly expressed antigens can help to overcome changes in expression of CD19 and other antigens, applicable for minimal residual disease monitoring.
Frequent loss of CD19 expression under selective pressure of CD19-targeted therapy leads to weakness of the application of CD19 as the main gating antigen in searches for neoplastic B-cell precursors. As suggested by Cherian et al., CD22 and CD24 could be added to aid in monitoring BCP-ALL if CD19-negativity develops.10 However, both of these markers could be negative on leukemic cells particularly when KMT2A gene rearrangement occurs (12% of cases in current study).14 We have found total positivity for these antigens in the majority but not in all patients who developed relapse after blinatumomab treatment. Other antigens could also be used (Figure 3) for primary gating,4 although their application might be based on initially detected expression.
Our data show that not only CD19 could be downmodulated under the pressure of blinatumomab. Expression of almost all other markers that are useful for MFC-MRD monitoring in BCP-ALL could be changed between ALL diagnosis, MRD and relapse. This suggests that MFCMRD monitoring after CD19 targeting should be based on a sophisticated approach with combinations of multiple markers and flexible gating strategies (Figure 3) in order to minimize the possibility of false negative results. In fact, more than a half of patients with disease progression or reappearance preserved CD19 expression, thus it has no sense to exclude this conventional antigen from tumor-cell gating. However, if residual leukemia is not found among CD19-positive cells, other B-cell compartments should be studied with consideration of the blast immunophenotype detected before CD19 targeting (Figure 3).4,10,15 Moreover, taking into account possible myeloid switching under the selective pressure of blinatumomab therapy, the distribution of cells according to CD45 expression and light scatter should also be investigated.
Thus, large and relatively individualized panels of antibodies with additional B-lineage and aberrant markers (myeloid antigens, NG2, etc.) should be applied to increase the effectiveness of MFC-MRD detection in BCP-ALL patients after CD19-directed treatment.
Supplementary Material
Funding Statement
Funding: the KMT2A rearrangement assessment study was supported by RFBR grant n. 17-29-06052 and Presidential grant n. MK-1645.2020.7 (n. 075-15-2020-338)
References
- 1.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389. [DOI] [PubMed] [Google Scholar]
- 3.Topp MS, Gokbuget N, Zugmaier G, et al. Phase II trial of the anti- CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory Bprecursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134-4140. [DOI] [PubMed] [Google Scholar]
- 4.Mejstrikova E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour E, Short NJ, Jorgensen JL, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. 2017;123(2):294-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novikova I, Verzhbitskaya T, Movchan L, et al. Russian-Belarusian multicenter group standard guidelines for childhood acute lymphoblastic leukemia flow cytometric diagnostics. Oncohematology. 2018;13(1):73-82. [Google Scholar]
- 7.Popov A, Belevtsev M, Boyakova E, et al. Standardization of flow cytometric minimal residual disease monitoring in children with Bcell precursor acute lymphoblastic leukemia. Russia–Belarus multicenter group experience. Oncohematology. 2016;11(4):64-73. [Google Scholar]
- 8.Kalina T, Flores-Montero J, Lecrevisse Q, et al. Quality assessment program for EuroFlow protocols: summary results of four-year (2010-2013) quality assurance rounds. Cytometry A. 2015;87(2):145-156. [DOI] [PubMed] [Google Scholar]
- 9.Karawajew L, Dworzak M, Ratei R, et al. Minimal residual disease analysis by eight-color flow cytometry in relapsed childhood acute lymphoblastic leukemia. Haematologica. 2015;100(7):935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherian S, Miller V, McCullouch V, et al. A novel flow cytometric assay for detection of residual disease in patients with B-lymphoblastic leukemia/lymphoma post anti-CD19 therapy. Cytometry B Clin Cytom. 2018;94(1):112-120. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour E, Dull J, Yilmaz M, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93(3):371-374. [DOI] [PubMed] [Google Scholar]
- 12.Borowitz MJ, Pullen DJ, Winick N, et al. Comparison of diagnostic and relapse flow cytometry phenotypes in childhood acute lymphoblastic leukemia: implications for residual disease detection: a report from the children's oncology group. Cytometry B Clin Cytom. 2005;68(1):18-24. [DOI] [PubMed] [Google Scholar]
- 13.Dworzak MN, Gaipa G, Schumich A, et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: evidence for a drug-induced regulatory phenomenon. Results of the AIEOP-BFM-ALL-FLOW-MRDStudy Group. Cytometry B Clin Cytom. 2010;78(3):147-153. [DOI] [PubMed] [Google Scholar]
- 14.De Zen L, Bicciato S, te Kronnie G, Basso G. Computational analysis of flow-cytometry antigen expression profiles in childhood acute lymphoblastic leukemia: an MLL/AF4 identification. Leukemia. 2003;17(8):1557-1565. [DOI] [PubMed] [Google Scholar]
- 15.Cherian S, Stetler-Stevenson M. Flow cytometric monitoring for residual disease in B lymphoblastic leukemia post T cell engaging targeted therapies. Curr Protoc Cytom. 2018;86(1):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.