CD38, a transmembrane glycoprotein, is widely expressed on multiple immune cell populations.1,2 High expression of CD38 on myeloma cells makes it a target of choice for therapeutic antibodies targeting cell surface molecules in multiple myeloma (MM).2 CD38 functions as a receptor for CD31 and as an ectoenzyme catalyzing the reaction between NAD+ and NADP+ to generate cyclic ADP ribose (ADPR), NAADP, and ADPR.3
Daratumumab, a human IgG1κ monoclonal antibody (mAb) targeting CD38, eliminates MM cells through several direct mechanisms: antibody-dependent cellular phagocytosis (ADCP), complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and apoptosis,4-7 as well as immunomodulatory mechanisms.2,8 Other CD38-targeting mAb in clinical development, isatuximab (ISA) and TAK-079, are reported to act similar to daratumumab.9,10 It remains unclear how the pleiotropic mechanisms of CD38-targeting mAb collectively impact tumor cytolysis and exhibit anti-tumor effects in a comprehensive ex vivo immune milieu. Here, we report results of mechanistic comparison studies of three CD38-targeting mAb: daratumumab and analogs of ISA and TAK-079 (generated based on the published antigen-binding fragment sequences for ISA11 and TAK-079,12 respectively).
In order to assess antibody binding, CD38-expressing Daudi and LP-1 tumor cells were coated with daratumumab, ISA analog, or TAK-079 analog antibodies at varying concentrations. Cells were washed and stained with Live/Dead® (Invitrogen, Carlsbad, CA, USA) and Alexa Fluor 647–conjugated goat anti-human Fc (Jackson ImmunoResearch, West Grove, PA, USA), and binding was analyzed by flow cytometry on a fluorescence-activated cell sorting (FACS) Celesta instrument (BD Biosciences, San Diego, CA, USA). CD38 expression was measured using CD38 (clone HIIT2) PerCp-Cy5.5 (BioLegend, San Diego, CA, USA). All three CD38 mAb (daratumumab, ISA analog, and TAK-079 analog) demonstrated similar relative binding to the target cells, which is in line with earlier findings of the binding properties of the three mAb.5
CDC activity of the three CD38 mAb was tested on multiple cell lines with a range of CD38 surface expression and CDC sensitivity levels (Online Supplementary Figure S1A).13 In Daudi, LP-1, and MOLP-8 cells, daratumumab resulted in higher levels of CDC activity compared with the other CD38 mAb, with a more pronounced difference seen in Daudi cells (Online Supplementary Figure S1B). In contrast, LP-1 and MOLP- 8 cells were susceptible to CDC activity with all three CD38 mAb. However, in LP-1 cells, daratumumab exhibited higher maximal cytotoxicity versus ISA and TAK-079 analogs and lower half maximal effective concentration (EC50) versus TAK-079 (Online Supplementary Figure S1C). Similarly, in MOLP-8 cells, daratumumab exhibited higher maximal cytotoxicity versus ISA analog and lower EC50 versus TAK-079 analog.
In ADCC assays (E:T ratio, 50:1) using peripheral blood mononuclear cells as effectors, all three CD38 mAb induced similar levels of target cell death (Online Supplementary Figure S2A). Compared to Daudi cells, MOLP-8 and LP-1 cells were less susceptible to ADCC activity. In ADCP assays (E:T ratio 4:1) using monocytederived M2c macrophages as effectors, all three CD38 mAb induced similar levels of target cell phagocytosis as detected by pHrodo labeling of target cells after 4 hours (Online Supplementary Figure S2B). Daudi cells and MOLP-8 cells were phagocytosed by M2c macrophages by all three CD38 antibodies. However, LP-1 cells were relatively the most resistant to phagocytosis.
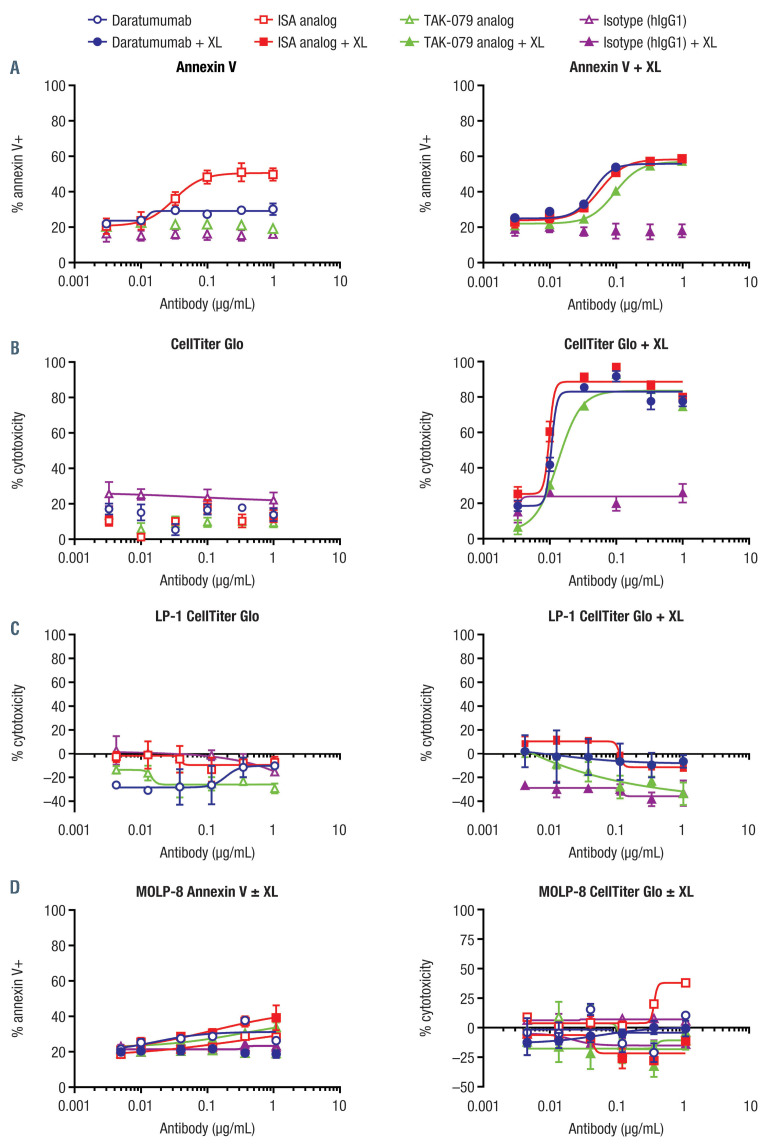
Apoptosis was assessed in the presence and absence of Fc receptor (FcR) crosslinking. Phosphatidylserine translocation to the cell surface was induced by the ISA analog in the absence of FcR crosslinking as measured by annexin V staining, similar to previously published reports (Figure 1A).9 Neither daratumumab nor the TAK- 079 analog could elicit annexin V staining in the absence of crosslinking. However, in the presence of crosslinking, all three CD38 mAb could induce annexin V staining in Daudi cells. In order to probe the level of cell death over time, we used a 5-day cytotoxicity assay to detect metabolically active cells (Figure 1B and C). All three CD38 mAb elicited comparably high levels of cell death in the presence of the FcR crosslinker and low levels in its absence. Minimal activation-induced cell death (AICD) was observed with LP-1 (Figure 1D) or MOLP-8 cells (Figure 1D).
Daratumumab has been shown to reduce CD38 expression levels partly by trogocytosis.14 In order to assess trogocytosis, we utilized Daudi cells as targets and THP-1 cells as effectors. Time-dependent loss of CD38 mAb staining on Daudi cells was correlated with a gain of signal on THP-1 cells (Online Supplementary Figure S3A). Daratumumab with a silent Fc did not mediate loss of CD38 on cell surfaces, indicating the effect is Fc dependent. Membrane dye was transferred in addition to CD38 (Online Supplementary Figure S3B), and imaging showed comparable efficiency of target transfer from Daudi to THP-1 cells among all three CD38 mAb (Online Supplementary Figure S3C). In the absence of THP-1, there was a negligible loss of signal for all three mAb observed on Daudi cells. The lysosome-associated membrane protein 1 (CD107a), co-localized with CD38 in effector cells, suggesting that CD38 is degraded after trogocytosis (Online Supplementary Figure S3D). All three CD38 mAb showed comparable results, suggesting that all can mediate trogocytosis.
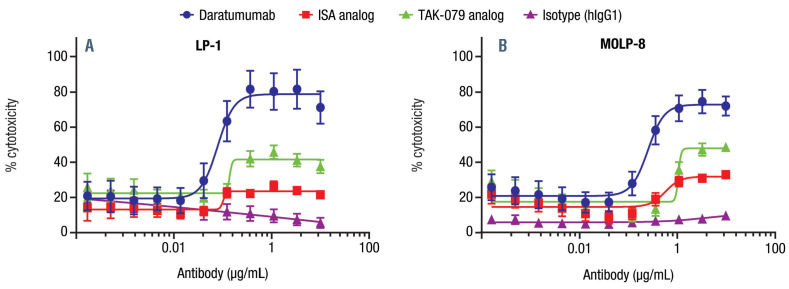
We aimed to compare cytotoxicity of the CD38 mAb ex vivo utilizing all mechanisms of action (MOA). The cumulative effect of the CD38 mAb was compared using europium-labeled LP-1 and MOLP-8 cells in the presence of whole blood from healthy donors containing both effector cells and complement. Within the assay, daratumumab demonstrated a significantly higher maximal cytotoxicity than comparator mAb in LP-1 cells (P<0.0001 for both comparisons) and MOLP-8 cells (P=0.0016 for both comparisons; Figure 2). Moreover, the EC50 was significantly lower for daratumumab versus the TAK-079 analog in both cell lines (P<0.0001) and was lower than the ISA analog in MOLP-8 cells (P=0.0008). Similar trends were seen with a 24-hour assay using flow cytometry as a read-out.
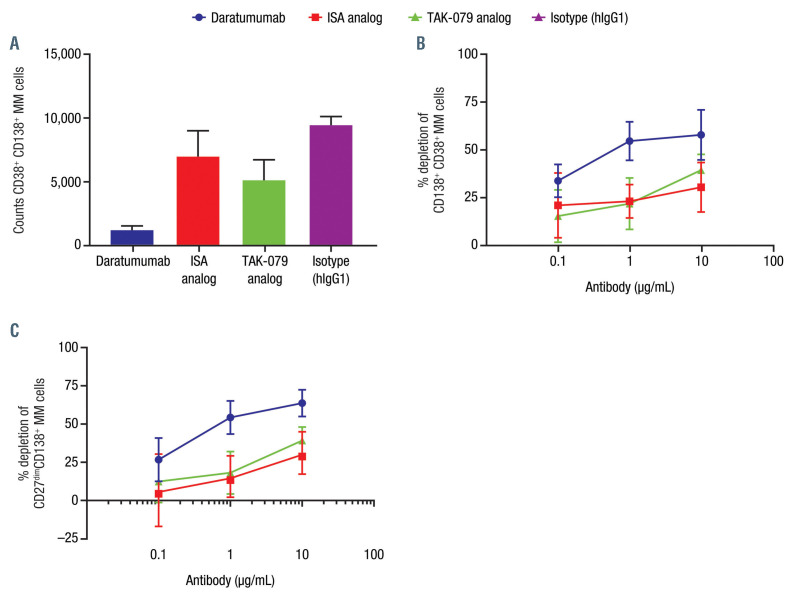
Bone marrow samples from untreated newly diagnosed patients, containing tumor cells and autologous immune effector cells, were obtained commercially to compare the cumulative impact of the mode of action (MOA) of CD38 mAb ex vivo. Depletion of the CD19–CD20–CD38+ CD138+ MM cells was measured by flow cytometry after 3 days in the presence of CD38 mAb and human complement (Figure 3A). Daratumumab elicited higher percent cytotoxicity of the CD38+CD138+ MM cells compared with ISA and TAK- 079 analogs (Figure 3B). CD38 was detected using HuMab, which was developed to not compete with daratumumab. It also did not compete with the ISA analog or TAK-079 analog. Data were similar with gating on CD19–CD20–CD138+CD27dim cells (Figure 3C)
Figure 1.
Activation-induced cell death activity of CD38 monclonal antibodies, in the absence and presence of crosslinker. Analyzed at 24 hoursa in Daudi cells by (A) annexin V staining and (B) at 5 daysb by CellTiter Glo Assay; no activity was seen in (C) LP-1 cells at 5 daysb by CellTiter Glo Assay and (D) MOLP-8 cells at 48 hoursa by annexin V staining and at 5 daysb by CellTiter Glo Assay. Daudi cells were plated with or without crosslinker (AffiniPure F(ab')₂ fragment goat antihuman IgG, Fcγ fragment specific, in which endotoxin was removed; Jackson ImmunoResearch, West Grove, PA, USA). Antibody dilutions were added and incubated for 24 hours (annexin V staining) or 5 days (CellTiter Glo Luminescent Cell Viability Assay). After 24 hours, cells were washed and stained with Live/Dead (Invitrogen) followed by annexin V (BioLegend, San Diego, CA, USA) and analyzed by flow cytometry (FACSCanto). After 5 days, CellTiter Glo reagent was prepared according to the manufacturer’s recommendations and added to a second plate to assess viability. Luminescence was measured and normalized to “plate max” and “plate min”. This analysis was also conducted using MOLP-8 cells, with 48-hour (annexin V staining) or 5-day (CellTiter Glo) incubation. aData are a summary of three independent experiments performed in duplicate; bdata are a summary of three independent experiments performed in triplicate. ISA: isatuximab; XL: crosslinking.
Figure 2.
Daratumumab demonstrated higher cytotoxicity than comparator CD38 monoclonal antibodies in whole blood assays with (A) LP-1 and (B) MOLP- 8 cells. Multiple myeloma cell lines LP-1 or MOLP-8 were labeled with DELFIAR europium solution (PerkinElmer, Pittsburgh, PA, USA) according to the manufacturer’s protocol. Healthy donor blood samples were added with a titration of daratumumab, isatuximab (ISA) analog, TAK-079 analog, or isotype control. Europium release was measured after 3-hour incubation. Percent cytotoxicity = [(experimental lysis – min lysis)/(max lysis – min lysis)] x 100. Daudi cells did not effectively label with europium in this assay, which is a phenomenon that has been previously described. Data are shown as representative experiments (LP-1, n=6 donors; MOLP-8, n=5 donors).
The results from this study add to the literature on the MOA of CD38 mAb and can provide insight into potential clinical differences that may be seen among the agents. We confirmed that all three mAb bind to CD38 at a similar level. Additionally, all three mAb demonstrated CDC, ADCC, ADCP, AICD, and trogocytosis MOA. Although most mechanisms were similar among the three mAb, daratumumab demonstrated higher CDC activity and, in the presence of human serum (which allows all possible MOA for antibody activity), showed stronger depletion of MM cells.
The cell lines tested varied in their sensitivity to different effector functions, partly due to differing expression levels of CD38 and complement inhibitory proteins. Regardless, daratumumab had greater CDC activity across cell lines compared to the ISA analog and TAK-079 analog. In contrast to a report published by Jiang et al.,9 we did not observe AICD in MOLP-8 cells. Although it is unclear why this was observed, one possibility is that the MOLP-8 cells used in our study had lower CD38 levels or were more resistant to AICD. The difference between the results from the 24-hour annexin V staining and the 5-day CellTiter Glo Assay in the absence of crosslinking suggests that the impact of AICD over time without crosslinking is minimal; cells may be able to recover and continue to proliferate. However, in the presence of crosslinking, AICD resulted in more effective tumor killing by all three CD38 mAb in Daudi cells. The structural differences between daratumumab and ISA have previously been hypothesized to account for the differing interactions with FcR crosslinking.15 Neither MOLP-8 nor LP-1 cells were susceptible to AICD in this study.
The ex vivo assay using healthy donor blood and MM cell lines was performed within 3 hours. At this timepoint, ADCC, CDC, and ADCP were the major mechanisms responsible for MM cell ablation. Whole blood contains endogenous complement, natural killer (NK) cells, and monocytes, which function as effector cells. This assay was repeated at 24 hours with similar results using absolute cell counts by flow cytometry of the labeled MM cell lines. In the MM patient samples, which contain endogenous NK cells and monocytes, the superiority of daratumumab killing was maintained even after 3 days. It is likely that daratumumab had the highest maximal cytotoxicity because of its superior CDC activity.
Our study has several limitations. First, daratumumab was compared with analogs of comparators, ISA and TAK-709. Second, not all anti-tumor mechanisms of CD38 mAb, including direct inhibition of enzymatic activity, were tested in this study.5 Last, observed differences in CDC were tested in blood, and findings may vary in the setting of a bone marrow compartment in MM patients.
In conclusion, daratumumab and surrogate analogs of ISA and TAK-079 have generally similar MOA. It remains to be determined in clinical trials if these in vitro differences lead to differences in clinical benefit.
Figure 3.
Daratumumab depletes multiple myeloma cells in patient samples as depicted by (A) counts of CD38+CD138+, (B) percent depletions of CD138+CD38+, and (C) percent depletion of CD27dimCD138+ multiple myeloma cells.a Peripheral blood mononuclear cells or bone marrow mononuclear cells from multiple myeloma (MM) patients were obtained from MM patients according to the guidelines of the Ethics Committee of the Discovery Life Sciences (Huntsville, AL, USA) and in compliance with Declaration of Helsinki protocols. Cells were thawed and measured for viability/density, and 200,000 live cells were seeded to assay plates. MM patient cells were treated with daratumumab, isatuximab (ISA) analog, or TAK-079 analog at specified concentrations in the presence of 10% human complement. After 3 days, MM cell numbers were measured using Precision Count BeadsTM (BioLegend) and by gating on live CD19-CD20–CD138+CD38+ (HuMab; does not compete with tested CD38 monoclonal antibodies). The percent cytotoxicity was determined relative to the corresponding IgG1 control. Complement was present in each experiment. Data are shown as representative experiment at 10 mg/mL treatment. aPeripheral blood mononuclear cells or bone marrow–derived macrophages from MM patients (n=5 donors).
Supplementary Material
Acknowledgmemts
The authors would like to thank Amy Wong, Amy Axel, and Mi Ta of Oncology at Janssen Research & Development, LLC, for providing additional experimental support.
Funding Statement
Funding: the analysis was funded by Janssen Global Services, LLC; editorial and medical writing support were provided by Tara Abraham, PhD, and Grace Wang, PharmD, of MedErgy, and were funded by Janssen Global Services, LLC.
References
- 1.van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13-29. [DOI] [PubMed] [Google Scholar]
- 2.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konen JM, Fradette JJ, Gibbons DL. The good, the bad and the unknown of CD38 in the metabolic microenvironment and immune cell functionality of solid tumors. Cells. 2020;9(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840-1848. [DOI] [PubMed] [Google Scholar]
- 5.Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014; 124(21):3474. [Google Scholar]
- 6.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overdijk MB, Jansen JH, Nederend M, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor-mediated cross-linking. J Immunol. 2016; 197(3):807-813. [DOI] [PubMed] [Google Scholar]
- 8.Adams HC, 3rd, Stevenaert F, Krejcik J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A. 2019;95(3):279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399-408. [DOI] [PubMed] [Google Scholar]
- 10.Moreno L, Perez C, Zabaleta A, et al. The mechanism of action of the anti-CD38 monoclonal antibody isatuximab in multiple myeloma. Clin Cancer Res. 2019;25(10):3176-3187. [DOI] [PubMed] [Google Scholar]
- 11.Rojkjaer L, Boxhammer R, Endell J, Winderlich M, Samuelsson C, MORPHOSYS AG. (2011) WO2012/041800. Anti-CD38 antibody and lenalidomide or bortezomib for the treatment of multiple myeloma and NHL. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012041800&recNum=5&office=&queryString=WO2012%2F041800+%28MOR03087%29&prevFilter=&sortOption=Pub+Date+Desc&maxRec=5. Accessed in June, 2020. [Google Scholar]
- 12.Elias KA, Landes G, Singh S, et al. (2012). WO2012/092612. Anti- CD38 antibodies. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012092612&recNum=113&docAn=US2011068235&queryString=%20&. Accessed in June, 2020. [Google Scholar]
- 13.Nijhof IS, Casneuf T, van Velzen JF, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959-970. [DOI] [PubMed] [Google Scholar]
- 14.Krejcik J, Frerichs KA, Nijhof IS, et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res. 2017;23(24):7498-7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malavasi F, Faini AC. Mechanism of action of a new anti-CD38 antibody: enhancing myeloma immunotherapy. Clin Cancer Res. 2019; 25(10):2946-2948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.