Abstract
Risk of outcome variability challenges therapeutic innovation. Selection of the most suitable candidates is predicated on reliable response indicators. Especially for emergent regenerative biotherapies, determinants separating success from failure in achieving disease rescue remain largely unknown. Accordingly, (pre)clinical development programs have placed increased emphasis on the multi-dimensional decoding of repair capacity and disease resolution, attributes defining responsiveness. To attain regenerative goals for each individual, phenotype-based patient selection is poised for an upgrade guided by new insights into disease biology, translated into refined surveillance of response regulators and deep learning-amplified clinical decision support.
Keywords: : biomarkers, deep learning, heart failure, heterogeneity, imaging, prediction, regenerative medicine, stem cells, stratification
It is more important to know what sort of person has a disease than to know what sort of disease a person has. Hippocrates (Greek physician, c. 460–370 BC)
Therapeutic response disparity
From refractory patients to super responders, individual response to treatment is commonly unpredictable across disease conditions and management strategies. Predicting therapeutic effectiveness remains limited in the setting of all major causes of morbidity and mortality [1–6]. The clinical reality of mixed benefits mandates a better understanding of individual variation with the goal of achieving tailored care [7].
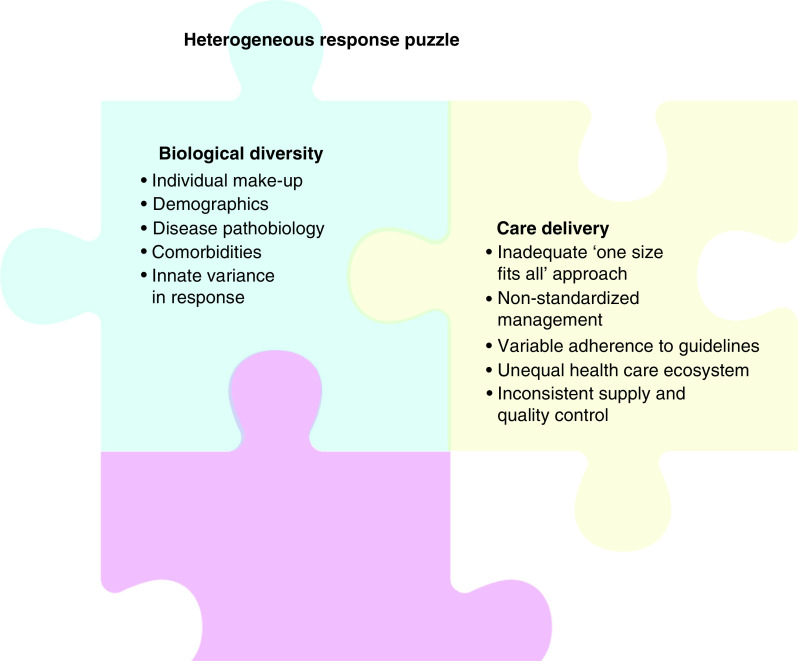
Clinical development programs focus initially on feasibility, safety and signs of efficacy, with stratification of responders and nonresponders typically considered during post hoc surveillance. Accordingly, contributors delineating best responders remain partially understood (Figure 1). Emphasis is placed on deciphering the impact of the individual’s genetic make-up, primary disease severity, comorbidities, vulnerability to adverse effects, adherence to treatment regimen and/or social cofounding factors [8–10]. This recognized complexity highlights the necessity for multi-parametric assessment to guide clinical decision making in candidate characterization, therapeutic delivery and outcome analysis.
Figure 1. . Heterogeneous response puzzle.
Unpredictable refractoriness to therapeutic interventions is a major challenge in healthcare. Mixed results have not fully characterized multi-factorial causes. Biological diversity among individuals and nonuniform care delivery refute a ‘one size fits for all’ paradigm. Personalized approaches are required to overcome heterogeneous outcomes in practice.
The experience of regenerative science-driven practice advancement exemplifies the need to enhance responder screening in the context of deploying new therapies [11]. Propelled by the promise of curative technologies, uptake of regenerative medicine in cardiology is however constrained by heterogeneity in patient outcome [12]. The clinical readiness of the regenerative toolkit is indeed lagging, requiring informed means to ensure targeted benefit [13,14]. The present Special Report offers a perspective toward achieving theragnostics strategies, namely diagnostic-guided treatment improvements [15], in the setting of regenerative therapy for heart failure.
Multi-level pathophysiology
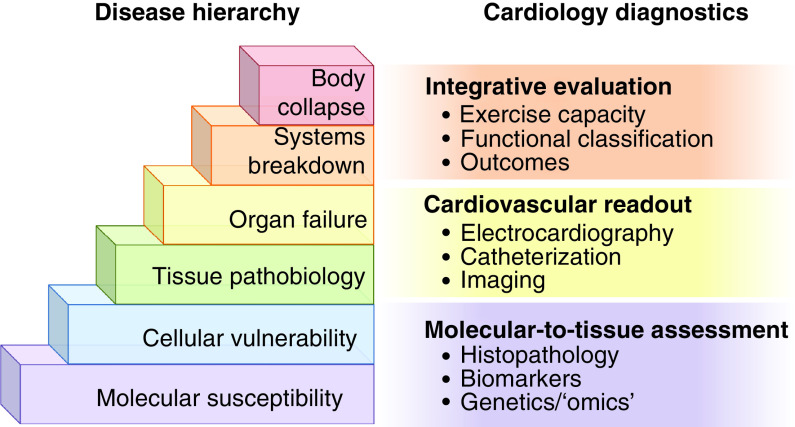
Heart failure reflects a compromise in force generation [16,17]. A normal heart cycle orchestrates billions of cardiomyocytes, turning ∼μm of contraction/cell into a ∼5 l/min organ output to fulfill body needs. Susceptible to diverse pathologies, aberrancy in this force-generating system evolves from latent molecular defects to advanced symptomatic disability (Figure 2). Congenital cardiomyopathy, due to a monogenic defect, exemplifies progression from a primal sarcomeric deficit into overt organ failure [18]. Distinctively, myocardial infarction, caused by coronary blood flow blockade, results in metabolic, electrical, mechanical and/or structural damage ultimately leading to ischemic cardiomyopathy [19]. Isolated cardiac dyssynchrony, with disparity in wall motion and conduction, precipitates suboptimal pumping function [20]. Regardless of the initial insult, an assault on the cardiac force-generating hierarchy provokes a common downward end-stage spiral (Figure 2 left). While treatments in later stages of disease are often limited to symptom mitigation and palliation, pinpointing the disease substrate at an earlier, presymptomatic phase of pathogenesis would offer proactive management options [21,22]. Adequate detection of silent risk and prognostication of outcome are thus required for actionable therapeutic targeting.
Figure 2. . Disease complexity and diagnostic toolkit.
Left: body health is supported by molecular, cellular, organ and systems well-being. Multi-level compensatory mechanisms engage to maintain integrative homeostasis. Harm exceeding intrinsic safeguard triggers progressive disease manifestation, and ultimately refractory state leading in extremis to cardiovascular collapse. Right: vulnerability, stress load and response readouts are useful in assessing health versus disease. In clinical cardiology, diagnostic armamentarium has expanded from blood extracted biomarkers to imaging and signal detection modalities, covering all levels of the disease hierarchy.
Therapy puzzle
Heart failure management incorporates lifestyle modifications, pharmacotherapy, interventional and surgical procedures, device implantation and heart transplant. Reperfusion therapy for myocardial infarction has reduced acute mortality; yet survivors suffer from a high incidence of heart failure [23,24]. Bi-ventricular pacing for cardiac resynchronization therapy (CRT), despite evidence for benefit in heart failure, does not eliminate nonresponders, accounting for a third of all recipients [25]. CRT indications are based on the New York Heart Association functional classification, left ventricular (LV) ejection fraction and QRS duration with bundle branch block [26], criteria nonspecific for the dyssynchronous myocardium (Figure 2 right). Scar size has been identified as a culprit impeding effective CRT pacing [27]. Thus, for advanced heart failure with extensive tissue damage, reparative approaches may be valuable [28]. Among regenerative strategies aimed at cardiac repair, the use of adult stem cells is the most advanced in clinical translation [29,30].
Responsiveness outlook
Grading therapeutic responsiveness according to clinical outcome stratifies super-responders, hypo-responders (with partial success) and nonresponders (refractory/failing therapy). Selectively targeting super-responders offers a practical approach to maximize current modalities [31]. Rescue of nonresponders and refinement for hypo-responders require an overhaul of treatment/delivery options and/or deeper understanding of efficacy determinants. To optimize and standardize effectiveness, specific and selective biomarkers are needed, fortified by deep learning algorithms using real-world experience [32]. Next-generation biomarkers would contribute to: early diagnosis through subclinical detection of molecular defects, enabling proactive intervention; preemptive control of risk factors in identified vulnerable individuals or at risk cohorts; and informed discovery of druggable pathways underpinning disease pathogenesis with development of corresponding therapeutic solutions.
Pretherapy stratification
Selection of optimal candidate biotherapeutics and/or recipients prior to therapy is ongoing. Limited uniformity in stem cell procurement necessitates standardized sourcing, manufacturing and quality assessment before patient delivery [33]. Uncertainty in clinical grade product repair proficiency has propelled development of potency assays [34], and cardio-reparative guidance protocols [35]. In fact, recent clinical trials incorporate prespecified cell potency criteria as exemplified by the CardiAMP Heart Failure trial using bone marrow mononuclear cell therapy for ischemic heart failure [36]. In parallel, release criteria for successful regenerative capacity enhancement of mesenchymal stem cells have been integrated into the C-CURE [37] and CHART-1 [38,39] heart failure clinical trials to ensure procurement of cardio-reparative cardiopoietic cells [40,41]. The DREAM-HF clinical trial employs mesenchymal precursor cells in subgroups of patients with advanced heart failure to address the biological plausibility of regenerative immunotherapy [42]. Beyond optimization of the biotherapeutics, the potential value of candidate recipient selection has been considered using distinct strategies. In this regard, genetics impact regenerative efficacy. In contrast to noncarriers, carriers with confirmed pathogenic or likely pathogenic cardiomyopathic variants exhibit non/hypo-response following autologous or allogeneic bone marrow-derived mesenchymal stem cell treatment [43]. Notwithstanding, genetic variants have been proven conclusive in a relatively modest portion of refractory patient cohorts warranting exploration of nonpolymorphic determinants of therapy evasion [44]. Alternatively, plasma profiling of circulating biomarkers in the setting of bone marrow mononuclear cell therapy for acute myocardial infarction offers a tool to probe for likelihood of response [45]. Furthermore, phenotype-based stratification prior to intervention could facilitate screening of putative super-responders. Conventionally, selection of candidates for cardiac stem cell therapy relies on a cut-off value, such as LV ejection fraction <40%. Recent clinical study sub-analyses, however, document signs of cardiopoietic stem cell efficacy associated with a tight phenotypic profile, namely a range of LV dilatation, a marker of organ remodeling [46,47]. Candidate titration was validated in a model of staged infarction size. While intramyocardial stem cell delivery was beneficial, outcomes diverged based on pretherapy LV end-diastolic volume (LVEDV). Super responders, defined as achieving functional and structural restitution, presented with an LVEDV equivalent to 200–370 ml in human heart [48]. In end-stage disease (pretherapy LVEDV equivalent to >370 ml in human heart), cell therapy hindered further deterioration into terminal heart failure syndrome, compared with the untreated cohort, but did not improve function or structure compared with pretherapy [31]. LV enlargement dependent response implicates a ‘Goldilocks principle’ for success with cardiac regenerative interventions [48]. Disease severity should be considered for: exclusion of nonresponders; and adjustment of therapeutic goals matched to the individual patient. In advancing toward adoptable regenerative care, narrowing the pool of patient responders would thus benefit from companion diagnostic criteria delineating pathogenesis.
Restoring disease substrate
Clinical decision making is assisted by identifying in real-time the origin and extent of disease, and responsiveness to therapy. Among cardiology modalities (Figure 2), analytical extension of echocardiography and magnetic resonance by tissue deformation imaging (speckle tracking) has enabled detection of early myocardial dysfunction and prediction of cardiac events [49]. Applied in regenerative medicine protocols, speckle tracking has proven useful in guiding stem cell delivery targeted to an early stage of mechanical dyssynchrony [50]. This diagnostic methodology enables proactive intervention for reparative prophylaxis and tissue repair (Figure 3). Regional motion abnormality is thus considered a theragnostic target reflecting organ symptomatology and underlying pathobiology [51]. Indeed, cell therapy has the potential to correct the molecular disease substrate, transitioning infarcted hearts from a cardiomyopathic trajectory back to predisease state [52].
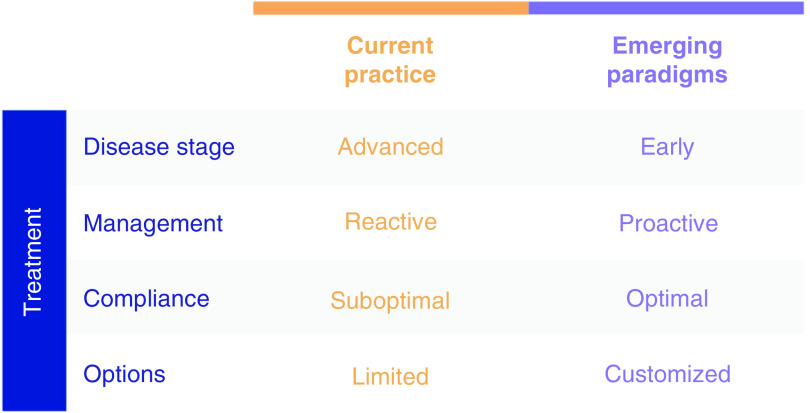
Figure 3. . Disease management evolution: from reactive to proactive.
Current practice targeting advanced disease is suboptimal offering limited options. Emerging paradigms are poised to ensure optimized and customized treatment solutions.
Knowledge overflow
The growth of medical knowledge has shortened its doubling time, from 50 years in 1950 to a mere 3.5 years in 2010 and down to 0.2 years in 2020 [53]. New knowledge influx exceeds the decision making capacity of a singular healthcare provider. Steadily, for example, numerous biomarkers, at each level of the disease hierarchy (Figure 2), have been introduced to facilitate disease management. In particular, cardiac biomarkers are a major focus of interest, exemplified by a number of families ranging from natriuretic peptides; neuro-hormones reflecting the renin-angiotensin and sympathetic nervous system; extracellular matrix proteins and metalloproteases associates with organ remodeling; inflammation and oxidative stress biomarkers; myocardial enzymes and proteins leaked from damaged tissues; and cardiovascular disease risk factors [54]. Beyond blood extracted markers, diverse bio-sampling approaches, as well as imaging modalities and bio-signal detection platforms, have extended the intricacy in interpreting information from biomarker-based datasets (Figure 2 right). Data processing relying on human manpower is limited in the context of stem cell therapy, where the complex identities of the biotherapeutics and recipients exaggerate the multifaceted nature inherent to biomarker readouts. Public repositories, such as Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) and ProteomeXchange Consortium (http://www.proteomexchange.org/), list an increasing number of decoded altered transcripts (∼700 out of >20,000) and proteins (450 out of 4000) in failing hearts [52,55]. Concomitantly, in specialty clinics, an expert can extract, in a single echocardiography session, over 100 parameters with 2D, M-mode, Doppler and speckle-tracking analysis. Thus, a pressing challenge is to translate the ever-growing, versatile knowledge base into a user-friendly algorithm amenable for daily practice (Figure 4).
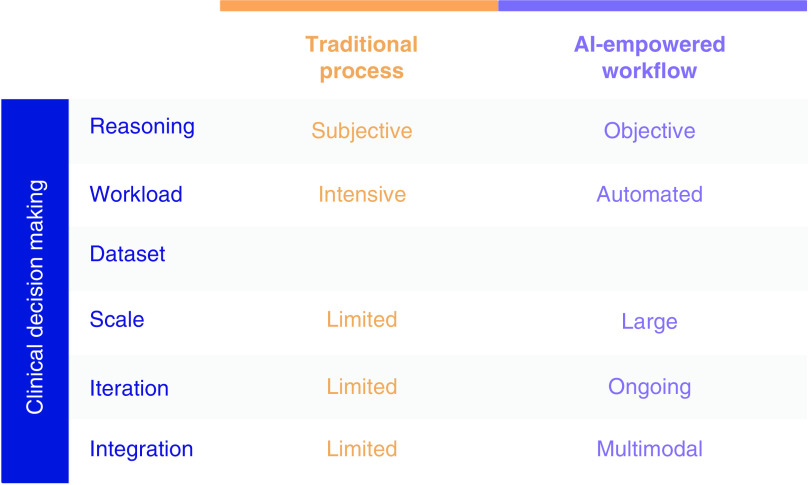
Figure 4. . Artificial intelligence supported clinical practice.
With increasing overflow of new knowledge, clinical decision making benefits from AI-empowered algorithms.
AI: Artificial intelligence.
Artificial intelligence decision support
Handling large datasets is critical for the enhanced management of lifelong diseases, as it is for responding to urgent needs of acute outbreaks. Incorporation of artificial intelligence (AI) is a transformative approach ushering the future of medicine [56]. Applicable to cardiac practice, disease diagnosis has been refined by AI in the setting of electrocardiography and cardiac imaging. The classic electrocardiogram, invented by Einthoven 125 years ago, has evolved into a microcomputer-based system with automatic analysis in the last 40 years, and recently through AI-powered applications has further adopted a predictive capacity to screen individuals with asymptomatic LV dysfunction or high-risk atrial fibrillation during normal sinus rhythm [57,58]. Indeed, AI-assisted preselection of patients at risk would enable proactive treatment planning [59], including potential use of regenerative solutions. A 3D print enhanced by AI processing is also poised to contribute to preinterventional planning in complex cases, and in training of new regenerative procedures. At present, AI diagnostics iteratively assimilate information from multiple modalities within the same level, or between neighboring levels, of the disease hierarchy (Figure 2). Deep leaning, which learns from unlabeled data and without human supervision [60], is expected to achieve comprehensive diagnosis through cross-sectional integration across the disease pyramid (Figure 4). AI has been deployed to accelerate research cycles that have required 10–15 years of investment, up to US$2.8 billion in expenses per drug through advanced clinical testing, with a 10% success rate to reach US FDA approval [61]. As conventional empirical approaches are considered an Achilles heel in clinical development, applications of AI to design clinical trials would assist in improved selection of patients, recruitment and monitoring of large cohorts. In fact, deep learning has been introduced to ensure quality control of clinical grade regenerative products [62]. It is anticipated that emerging cardiac regenerative therapy will increasingly rely on AI-based guidance for optimal use of cutting-edge technologies, and to maximize the precision of applied reparative options (Figure 5). In this regard, a call for an AI-augmented multinational biorepository of pertinent datasets is timely to enable image-based pilots, followed by phenomenological predictions in larger cohorts [63–65].
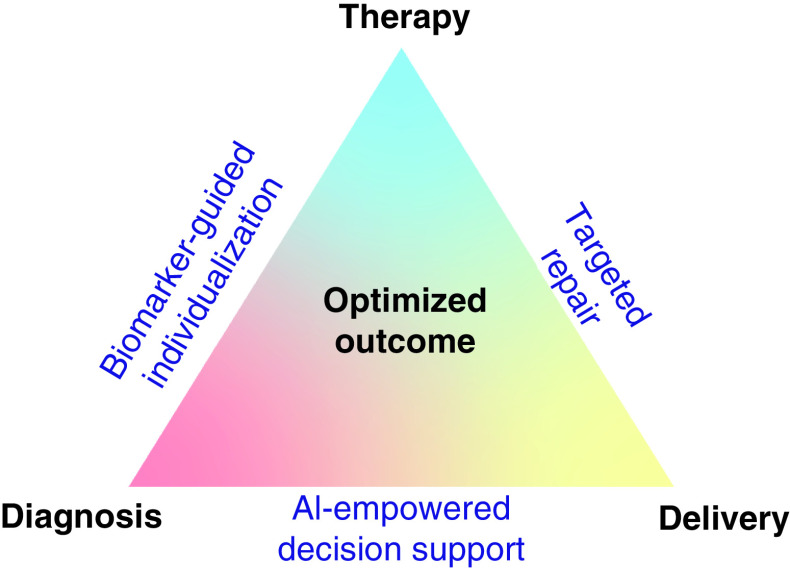
Figure 5. . Iterative optimization of individual outcome.
The diagnosis–therapy–delivery triad is increasingly transformed by reliance on biomarker-guided personalized care, targeted cures and AI-empowered clinical decision making.
AI: Artificial intelligence.
Summary
Regenerative medicine principles extend the reach of traditional therapeutic goals by offering the opportunity to achieve targeted cures [66–68]. However, recognized outcome heterogeneity has hampered adoption of the regenerative toolkit [69,70]. Biological diversity among recipients, unpredictable biotherapeutic efficacy and inconsistent care delivery, all underscore refractoriness to therapy. Supported by randomized controlled studies, and enriched by a mechanistic-driven reasoning, the diagnosis–therapy–delivery paradigm has advanced from empirical to evidence-based to pathobiology-informed decision making (Figure 5). Indeed, ongoing efforts to standardize effectiveness have encompassed a (patho)phenotype-based optimization in patient selection, a (bio)marker-enforced proactive intervention aiming on the disease substrate and more recently an AI-powered approach to support quality control of regenerative products, clinical trial design and practice delivery (Figure 5).
Future perspective
Regenerative medicine ushers an era of curative aspiration. To overcome heterogeneity in the therapeutic response and maximize the reach of regenerative therapy, achievable upcoming milestones include: in discovery science, advancement of knowledge that decodes disease pathobiology and biological diversity; in clinical development, automation and scale up manufacturing of standardized patient-ready regenerative products; and in care delivery, roll-out of individual-centered practice guidelines optimizing indications, procedures and outcome readouts. Ongoing digital transformation amplifies population-validated datasets in an increasingly maturing regenerative medicine ecosystem.
Executive summary.
Clinical unmet needs
Variance in individual therapy response is commonly encountered in practice.
New therapies are challenging due to limited clinical experience and absence of long-term pharmacovigilance.
Heterogeneous outcomes associated with regenerative therapies compromise standardized adoption.
Advanced heart failure relies on palliative strategies with phenotype-based readouts nonspecific to underlying disease.
Proactive regenerative interventions may refine heart failure management.
Individualized theragnostic algorithms would empower regenerative care.
Decision support for patient selection
Genetic make-up determines regenerative response. Carriers of cardiomyopathic variants appear to benefit less from stem cell therapy, encouraging preinterventional screening to identify best responders.
Structural parameters of heart remodeling, namely left ventricular size, appear to predict stem cell effectiveness. Disease severity stratification may help streamline patient selection.
Transforming future practice
Evolving biomarkers-guided individualized diagnosis, targeted repair and artificial intelligence-empowered decision making have the potential to transform clinical practice and optimize outcomes.
Footnotes
Author contributions
S Yamada contributed toward conception and design, data acquisition, analysis and interpretation, drafting the work, final approval of the manuscript and agreement to be accountable for all aspects of the work; R Jeon, A Garmany and A Behfar contributed toward data acquisition, final approval of the manuscript and agreement to be accountable for aspects of the work; A Terzic contributed toward conception and design, data acquisition, analysis and interpretation, drafting the work, final approval of the manuscript, financial and administrative support and agreement to be accountable for all aspects of the work.
Financial & competing interests disclosure
The authors are supported by the National Institutes of Health (R01 HL134664), Regenerative Medicine Minnesota, Marriott Family Foundation, Van Cleve Cardiac Regenerative Medicine Program, Michael S and Mary Sue Shannon Family, Center for Regenerative Medicine, Center for Biomedical Discovery and Medical Scientist Training Program at Mayo Clinic. A Terzic holds the Marriott Family Professorship in Cardiovascular Diseases Research, and is Michael S and Mary Sue Shannon Director of the Mayo Clinic Center for Regenerative Medicine. S Yamada, A Behfar and A Terzic are inventors on regenerative sciences related intellectual property disclosed to Mayo Clinic. Previously, Mayo Clinic has administered research grants from Celyad. Mayo Clinic, A Behfar and A Terzic have interests in Rion LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This Special Report refers to human and animal experimental investigations conducted under respective regulatory and ethical approvals.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sulaica EM, Wollen JT, Kotter J, Macaulay TE. A review of hypertension management in black male patients. Mayo Clin. Proc. 95(9), 1955–1963 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 575(7782), 299–309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicini FA, Cecchini RS, White JR et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, Phase III, equivalence trial. Lancet 394(10215), 2155–2164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis JM. Precision medicine in Type 2 diabetes: using individualized prediction models to optimize selection of treatment. Diabetes 69(10), 2075–2085 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey ES, Langton D, Katelaris C et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur. Respir. J. 55(5), 1902420 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Bondar J, Caye A, Chekroud AM, Kieling C. Symptom clusters in adolescent depression and differential response to treatment: a secondary analysis of the treatment for adolescents with depression study randomised trial. Lancet Psych. 7(4), 337–343 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp. Clin. Trials Commun. 11, 156–164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazio M, Ablain J, Chuan Y, Langenau DM, Zon LI. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat. Rev. Cancer 20(5), 263–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauffenburger JC, Choudhry NK. Call for a systems-thinking approach to medication adherence: stop blaming the patient. JAMA Intern. Med. 178(7), 950–951 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Kent DM, Paulus JK, van Klaveren D et al. The predictive approaches to treatment effect heterogeneity (PATH) statement: explanation and elaboration. Ann. Intern. Med. 172(1), W1–W25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leask F, Terzic A. Regenerative outlook: offering global solutions for equity of care. Regen. Med. 15(11), 2249–2252 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Terzic A, Behfar A. Regenerative medicine in the practice of cardiology. Eur. Heart J. 37(14), 1089–1090 (2016). [PubMed] [Google Scholar]
- 13.Banerjee MN, Bolli R, Hare JM. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ. Res. 123(2), 266–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston PV, Duckers HJ, Raval AN, Cook TD, Pepine CJ. Not all stem cells are created equal: the case for prospective assessment of stem cell potency in the CardiAMP heart failure trial. Circ. Res. 123(8), 944–946 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Frangos S, Buscombe JR. Why should we be concerned about a “g”? Eur. J. Nucl. Med. Mol. Imaging 46, 519 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E. Heart failure. JACC Heart Fail. 1(1), 1–20 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Shah SJ, Borlaug BA, Kitzman DW et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 141(12), 1001–1026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill TJ, Ashrafian H, Watkins H. Genetic cardiomyopathies causing heart failure. Circ. Res. 113(6), 660–675 (2013). [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJ. Clinical practice. Systolic heart failure. N. Engl. J. Med. 362(3), 228–238 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ. Res. 113(6), 765–776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Normand C, Kaye DM, Povsic TJ, Dickstein K. Beyond pharmacological treatment: an insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 393(10175), 1045–1055 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Yamada S, Terzic A. Path toward proactive therapy for patent ductus arteriosus. Clin. Pharmacol. Ther. 106(6), 1187–1190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terzic A, Behfar A. Stem cell therapy for heart failure: ensuring regenerative proficiency. Trends Cardiovasc. Med. 26(5), 395–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honig P, Terzic A. Affairs of the heart: innovation in cardiovascular research and development. Clin. Pharmacol. Ther. 102(2), 162–168 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136(6), e137–e161 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Salden OAE, Vernooy K, van Stipdonk AMW, Cramer MJ, Prinzen FW, Meine M. Strategies to improve selection of patients without typical left bundle branch block for cardiac resynchronization therapy. JACC Clin. Electrophysiol. 6(2), 129–142 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Heggermont W, Auricchio A, Vanderheyden M. Biomarkers to predict the response to cardiac resynchronization therapy. Europace 21(11), 1609–1620 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Yamada S, Nelson TJ, Kane GC et al. Induced pluripotent stem cell intervention rescues ventricular wall motion disparity, achieving biological cardiac resynchronization post-infarction. J. Physiol. 591(17), 4335–4349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzic A, Behfar A. Regenerative heart failure therapy headed for optimization. Eur. Heart J. 35(19), 1231–1234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 385(9970), 812–824 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Behfar A, Terzic A. Regenerative medicine clinical readiness. Regen. Med. 16(3), 309–322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho D. Artificial intelligence in cancer therapy. Science 367(6481), 982–983 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Terzic A, Behfar A, Filippatos G. Clinical development plan for regenerative therapy in heart failure. Eur. J. Heart Fail. 18(2), 142–144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raval AN, Cook TD, Duckers HJ et al. The CardiAMP Heart Failure trial: a randomized controlled pivotal trial of high-dose autologous bone marrow mononuclear cells using the CardiAMP cell therapy system in patients with post-myocardial infarction heart failure: trial rationale and study design. Am. Heart J. 201, 141–148 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Bartunek J, Terzic A, Behfar A, Wijns W. Clinical experience with regenerative therapy in heart failure: advancing care with cardiopoietic stem cell interventions. Circ. Res. 122(10), 1344–1346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raval AN, Johnston PV, Duckers HJ et al. Point of care, bone marrow mononuclear cell therapy in ischemic heart failure patients personalized for cell potency: 12-month feasibility results from CardiAMP heart failure roll-in cohort. Int. J. Cardiol. 326, 131–138 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Bartunek J, Behfar A, Dolatabadi D et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 61(23), 2329–2338 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Bartunek J, Terzic A, Program CHART et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 38(9), 648–660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teerlink JR, Metra M, Investigators CHART et al. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the congestive heart failure cardiopoietic regenerative therapy (CHART-1) study. Eur. J. Heart Fail. 19(11), 1520–1529 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Behfar A, Yamada S, Crespo-Diaz R et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J. Am. Coll. Cardiol. 56(9), 721–734 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behfar A, Terzic A. Stem cell in the rough: repair quotient mined out of a bone marrow niche. Circ. Res. 115(10), 814–816 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase III DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ. Res. 125(3), 265–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieger AC, Myerburg RJ, Florea V et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine 48, 377–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This hypothesis generating study highlights the impact of genetic profiles on stem cell therapy responsiveness in the setting of nonischemic heart failure.
- 44.Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19(1), 39–56 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Jokerst JV, Cauwenberghs N, Kuznetsova T et al. Circulating biomarkers to identify responders in cardiac cell therapy. Sci. Rep. 7(1), 4419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartunek J, Terzic A, Davison BA et al. Cardiopoietic stem cell therapy in ischaemic heart failure: long-term clinical outcomes. ESC Heart Failure 7(6), 3345–3354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This long-term follow-up of patients in the largest to-date stem cell clinical trial for ischemic heart failure suggests in patients with significant left ventricular dilatation, receiving adequate dosing, beneficial outcomes with reduced death or hospitalization.
- 47.Frljak S, Poglajen G, Zemljic G et al. Larger end-diastolic volume associates with response to cell therapy in patients with non-ischemic dilated cardiomyopathy. Mayo Clin. Proc. 95(10), 2125–2133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada S, Arrell DK, Rosenow CS, Bartunek J, Behfar A, Terzic A. Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy. Stem Cells Transl. Med. 9(1), 74–79 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bax JJ, Di Carli M, Narula J, Delgado V. Multimodality imaging in ischaemic heart failure. Lancet 393(10175), 1056–1070 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Yamada S, Arrell DK, Kane GC et al. Mechanical dyssynchrony precedes QRS widening in ATP-sensitive K+ channel-deficient dilated cardiomyopathy. J. Am. Heart Assoc. 2(6), e000410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada S, Arrell DK, Martinez-Fernandez A et al. Regenerative therapy prevents heart failure progression in dyssynchronous nonischemic narrow QRS cardiomyopathy. J. Am. Heart Assoc. 4(5), e001614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arrell DK, Rosenow CS, Yamada S, Behfar A, Terzic A. Cardiopoietic stem cell therapy restores infarction-altered cardiac proteome. NPJ Regen. Med. 5, 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Densen P. Challenges and opportunities facing medical education. Trans. Am. Clin. Climatol. Assoc. 122, 48–58 (2011). [PMC free article] [PubMed] [Google Scholar]
- 54.Chow SL, Maisel AS, Anand I et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 135(22), e1054–e1091 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Barth AS, Kumordzie A, Frangakis C et al. Reciprocal transcriptional regulation of metabolic and signaling pathways correlates with disease severity in heart failure. Circ. Cardiovasc. Genet. 4(5), 475–483 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N. Engl. J. Med. 380(14), 1347–1358 (2019). [DOI] [PubMed] [Google Scholar]; • This report describes core structural changes in health care systems necessary to enact the promise of machine learning in medicine.
- 57.Attia ZI, Kapa S, Lopez-Jimenez F et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 25(1), 70–74 (2019). [DOI] [PubMed] [Google Scholar]; •• This artificial intelligence aided detection of asymptomatic left ventricular dysfunction from routine electrocardiograms offers high-accuracy, low-cost screening of cohorts at risk.
- 58.Attia ZI, Noseworthy PA, Lopez-Jimenez F et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394(10201), 861–867 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Kather JN, Calderaro J. Development of AI-based pathology biomarkers in gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 17(10), 591–592 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Dey D, Slomka PJ, Leeson P et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73(11), 1317–1335 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 323(9), 844–853 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaub NJ, Hotaling NA, Manescu P et al. Deep learning predicts function of live retinal pigment epithelium from quantitative microscopy. J. Clin. Invest. 130(2), 1010–1023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This deep-learning refined clinically compatible platform for prediction of cell functionality and outlier identity optimized for release of cell therapy products prior to delivery.
- 63.Lee Y, Ragguett RM, Mansur RB et al. Applications of machine learning algorithms to predict therapeutic outcomes in depression: a meta-analysis and systematic review. J. Affect. Disord. 241, 519–532 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Hilton CB, Milinovich A, Felix C et al. Personalized predictions of patient outcomes during and after hospitalization using artificial intelligence. NPJ Digit. Med. 3(3), 51 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Sperrin M, Ashcroft DM, van Staa TP. Consistency of variety of machine learning and statistical models in predicting clinical risks of individual patients: longitudinal cohort study using cardiovascular disease as exemplar. BMJ 371, m3919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernández-Avilés F, Sanz-Ruiz R, The TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes) writing group et al. Global position paper on cardiovascular regenerative medicine. Eur. Heart J. 38(33), 2532–2546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marbán E. A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat. Biomed. Eng. 2(6), 353–361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blau HM, Daley GQ. Stem cells in the treatment of disease. N. Engl. J. Med. 380(18), 1748–1760 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Menasche P. Cardiac cell therapy: current status, challenges and perspectives. Arch. Cardiovasc. Dis. 113(4), 285–292 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Cossu G, Fears R, Griffin G, Meulen V. Regenerative medicine: challenges and opportunities. Lancet 395(10239), 1746–1747 (2020). [DOI] [PubMed] [Google Scholar]