Abstract
Background:
Procedural sedation required to improve the quality of Transthoracic Echocardiography (TTE) in infants and children. The ideal drug and route for sedation in children should have a rapid and reliable onset, atraumatic, palatable with minimal side effects, and rapid recovery. So, the aim of our study to evaluate and compare the efficacy and safety of intranasal midazolam and intranasal dexmedetomidine in pediatric patients for sedation during TTE.
Materials and Method:
Hundred children under three year of age, belonging to the American Society of Anaesthesiologists class-I and II, scheduled for TTE were divided into two groups by standard randomization technique. Patients in group-M received intranasal midazolam 0.2 mg/kg, whereas patients in group-D received intranasal dexmedetomidine 2 μg/kg prior to TTE under an adequately monitored anesthesia care. Onset and duration of sedation, heart rate, oxygen saturation, sonographer's, and parent's satisfaction scores were recorded.
Results:
All patients were successfully sedated for TTE. The average onset time, sedation time, awakening time and total time for Group-M were 7.3, 18.8, 29.51, 51 min and group-D were 10.1, 14.2, 24.9, 46.3 min, respectively and all were statistically significant (P < 0.001). TTE scan time of Group-M is 8.84 min and Group-D is 9.18 min and was statistically significant. Sonographer's and Parent's average satisfaction score for Group-M was 9.88, 10 and for Group-D was 7.64, 8.76, respectively, which were statistically significant (P < 0.001).
Conclusion:
Intranasal midazolam and dexmedetomidine are safe and effective for sedation in TTE. Intranasal midazolam was found to be comparatively more effective in view of onset of action, sonographers, and parental satisfaction score, while sedation time, awakening time and total duration was significantly higher as compared to intranasal dexmedetomidine.
Keywords: Intranasal dexmedetomidine, intranasal midazolam, transthoracic echocardiography
INTRODUCTION
Imaging studies are often needed to diagnose and treat medical conditions in young children. Sedation generally is required during these diagnostic tests because of lack of cooperation and difficulty in communication with pediatric patients. Particularly, in transthoracic echocardiography due to anxiety and movement of children interferes with diagnostic quality, thus appropriate medication is required to sedate these patients, who find it difficult to remain still.
The routine practice to sedate pediatric patient for echocardiography is to administer oral medications.[1] However, these patients often have cardiac malformations or cardiac dysfunction as well as hemodynamic disorders, respiratory diseases, or dysfunction of other vital organs, making the patients difficult to sedate, while increasing the risks associated with sedation. Transthoracic echocardiography (TTE) in pediatrics is frequently performed under moderate to deep sedation.[1]
To provide effective anxiolysis and conscious sedation and to facilitate a good echocardiography image quality were the objectives of our study. The ideal drug and route for sedation in children should have a rapid and reliable onset, should be atraumatic, palatable with minimal side effects, and rapid recovery.[2,3] Chloral hydrate has been commonly used to induce sedation in infants. However, it has well-known disadvantages, including a long half-life (trichloroethanol metabolite 12–24 h), delayed re-sedation, impaired respiration and upper airway mechanics, and limited availability in some countries.[4] Alternative sedatives to chloral hydrate are preferable now a day.
Thus, the intranasal route was selected, as all the criteria for an Ideal sedation are satisfied. Midazolam has already been used as sedation by various routes.[5] Oral and rectal roots for midazolam are well-known in pediatric patients.[6] The onset of action is slow via oral route (15–30 min), and its first pass metabolism results in lower and unpredictable systemic availability. Intranasal midazolam in preschool children was first described and advocated by Wilton and colleagues as premedication.[7] Dexmedetomidine is a newer alpha-2-agonist with a more selective action on the alpha2 adrenoreceptor and a shorter half-life. Its bioavailability is 81.8% (72.6-92.1%) when administrated via buccal mucosa.[8] Recent studies have found the intranasal route for dexmedetomidine to effectively produce preoperative and non-painful procedural sedation in children.[1] The aim of our study was to evaluate and compare the efficacy and safety of intranasal midazolam 0.2 mg/kg with intranasal dexmedetomidine 2 μg/kg in pediatric patients for sedation during echocardiography.
MATERIALS AND METHOD
After approval from hospital's scientific and ethical committees no. UNMICRC/C.ANESTHE/2018/13 and after obtaining written informed consent from the patient's parents, 100 Children in the age group 1 month to 3 years, belonging to American Society of Anesthesiologists (ASA) grade I or II scheduled for TTE were selected for the study. The present study was undertaken as a double blind prospective trial. Exclusion criteria consisted of lack of consent, abnormality of nasal structure, anticipated difficulty airway, patient with known allergy, organ dysfunction, cardiac dysrhythmia, mental retardation, and postoperative status.
Children were randomly allotted to either of the two groups (Group-M and Group-D) by computer generated randomization. Patients were fasted for 2 hours for food, formula, breast milk, or clear liquids. As a routine protocol, we secure peripheral intravenous line, and airway management tools kept ready for any probable complications if at all occurred, for all procedural sedation patients planned for echocardiography. Children in Group-M received intranasal midazolam 0.2 mg/kg while Group D children received intranasal dexmedetomidine 2 μg/kg via 1 ml syringe. Intranasal midazolam was prepared from the 5 mg/ml parenteral preparation in a 1-ml syringe, after appropriate dilution with 0.9% saline to make a final volume of 0.5 ml. Intranasal dexmedetomidine was prepared from the 100 μg/ml parenteral preparation diluted with 0.9% saline to make the final volume of 0.5 ml. All drugs were prepared by an independent investigator not involved in the study or conduct of anesthesia. Observers and attending anesthesiologist were blinded to the study drug given.
The drug was instilled into both nostrils using 1-ml syringe with the patient in recumbent position. Baseline heart rate (HR), Oxygen saturation (SpO2) was recorded, and observations of HR and SpO2 were made at 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 min after test drug administration. Sedation levels were measured using a modified Ramsay scale—responses to subxiphoid and suprasternal TTE probe placement were substituted for forehead tap[1,9] [Table 1]. TTE was performed once a Ramsay sedation level of 3 or greater was achieved. If a sufficient sedation level for successful TTE exam was not achieved within 10 min of the initial administration or maintained during the TTE, additional “rescue” dexmedetomidine 1 μg/kg or midazolam 0.1 mg/kg intranasally was administered to respective Group of patients. No mechanical restraints were utilized during the TTE scans. Heart rate and pulse oximetry were recorded as standard monitoring during echocardiography. A pediatric nurse and an anesthesiologist monitored the patient during the sedation. Heart rate, oxygen saturation, time to reach Ramsay sedation score 3 or greater (onset time), TTE scan time, Ramsay sedation score 3 or more to eye opening (sedation time), eye opening to discharge from the echocardiography suite (awakening time), and total time were recorded.
Table 1.
Modified Ramsay Sedation Scale
| Sedation Score | Response |
|---|---|
| 1 | Anxious and agitated or restless or both |
| 2 | Co-operative, oriented and tranquil |
| 3 | Responding to commands only |
| 4 | Brisk response to subxiphoid and suprasternal TTE probe placement |
| 5 | Sluggish response to subxiphoid and suprasternal TTE probe placement |
| 6 | No response to stimulus |
Sonographers scored the number of pauses >2 min due to inadequate sedation and overall quality of the sedation on a 10-point scale (1–10 lowest to highest satisfaction). Parental satisfaction with the sedation was elicited and graded on the same 10-point scale prior to discharge. Heart rate and oxygen saturation were compared to the respective patient's preprocedural measurements. Bradycardia was defined 20% decreases in heart rate below baseline value. Oxygen desaturation was defined as peripheral oxygen saturation (SpO2) below 92% for noncyanotic lesions, or 5% below baseline for cyanotic lesions.
After TTE scan completion, patients were moved to a post-anesthesia care unit (PACU) where they were allowed to wake up spontaneously. Patients were discharged after reaching a Ramsey score of 1 or 2, interacting with parents and nurse, demonstrating a heart rate, respiratory rate, and oxygen saturation within normal ranges for age or at baseline (modified Aldrete score 9/10).
Statistical analysis was performed using SPSS, Version 20.0 (Chicago, IL, USA). The independent sample t-test was used to compare continuous variables. The Chi-square test was used to compare the categorial variable. Data were presented as mean ± SD or proportion as appropriate. The “p” value less than 0.05 was considered to be significant.
RESULTS
Between September-2019 to November-2019 hundred children posted for echocardiography on OPD basis were enrolled for the study and evaluated for various parameters. All children accepted the intranasal drug instillation well without any vomiting. All children were studied in two groups, Group M (intranasal midazolam) and Group D (intranasal dexmedetomidine). Demographic characteristics were summarized in Table 2. Both the groups were comparable with respect to age, weight, height, gender, and number of cyanotic and acyanotic patients. No children complained of pain or discomfort with intranasal drug administration.
Table 2.
Demographic Parameters
| Demographic Parameters | Group M (n=50) | Group D (n=50) | P |
|---|---|---|---|
| Age (days; Mean±SD) | 423.9±193.92 | 416.2±180.7 | 0.8377 |
| Weight (kg; Mean±SD) | 8.18±2.07 | 8.14±2.11 | 0.9240 |
| Height (cm; Mean±SD) | 66.04±9.59 | 64.98±8.53 | 0.5606 |
| Sex (Male/Female) (n,%) | 29/21 (58%;42%) | 36/14 (72%/28%) | 0.1421 |
| Acyanotic (n,%) | 35 (70%) | 31 (62%) | 0.4008 |
| Cyanotic (n,%) | 15 (30%) | 19 (38%) | 0.4008 |
All patients were successfully sedated for TTE. Rescue sedation was required for three patients of Group D, while in Group M rescue sedation was not required in any patient, (difference was not statistically significant, P = 0.0802). The average onset time (sedation administration to Ramsay 3 or more) for Group M was 7.3 and Group D was 10.1, which was statistically significant (P < 0.001). The average sedation time (Ramsay 3 or more to eye opening) for Group M was 18.8 and Group D was 14.2, which was statistically significant (P < 0.001). The average awakening time (eye opening to discharge) for Group M was 29.51 and Group D was 24.9, the difference was statistically significant (P < 0.001). The average total time for Group-M 51 min and Group-D was 46.3 min respectively and was statistically significant (P < 0.001). TTE scan time was 8.84 min in Group M, while it was 9.18 min in Group D which was statistically significant (P = 0.0109) [Table 3].
Table 3.
Sedation Time and Satisfaction Score
| Group M (n=50) Mean±SD | Group D (n=50) Mean±SD | P | |
|---|---|---|---|
| Onset Time (Min) | 7.3±0.61 | 10.16±0.86 | <0.001 |
| Sedation Time (Min) | 18.18±1.18 | 14.2±1.52 | <0.001 |
| Awakening Time (Min) | 29.51±1.87 | 24.98±2.45 | <0.001 |
| Total Time (Min) | 51.04±7.43 | 46.34±3.13 | <0.001 |
| TTE Scan Time (Min) | 8.84±0.58 | 9.18±0.71 | 0.0109 |
| Sonographer Score | 9.88±0.38 | 7.64±0.82 | <0.001 |
| Parental Satisfaction score | 10±0.10 | 8.76±0.84 | <0.001 |
Sonographers graded their satisfaction with scanning conditions 10 (excellent) on a scale of 1–10, for 100 study patients. The average satisfaction score for Group M was 9.88 and for Group D was 7.64 and it was statistically significant (P < 0.001) [Table 3]. The number of sonographer pauses over 2 min was seen in one patient of Group D and was not statistically significant (P = 0.3173). All tests were successfully completed by sonographer report. Average parental satisfaction score of Group M was 10 and of Group D was 8.76 and it was found to be statistically significant (P < 0.001) [Table 3].
There were no statistically significant differences in Heart Rate and SpO2 in both the groups during sedation period [Figures 1 and 2]. None of the patients had a change in gross appearance of the nasal mucosa after intranasal dexmedetomidine or midazolam. There was no requirement of oxygen, any airway intervention, bradycardia (decrease of heart rate more than 20% from the baseline) in either of the groups.
Figure 1.

Comparison of Heart rate between the groups
Figure 2.
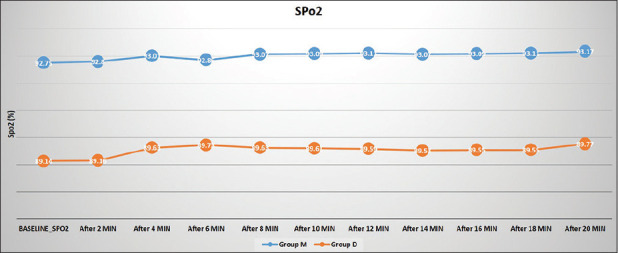
Comparison of saturation between the groups
DISCUSSION
Accurate transthoracic echocardiography is a mainstay for the diagnosis of congenital cardiac disease in pediatric patients. Most examinations are accomplished with patient cooperation; however, sedation is often required in infants and toddlers for getting clear image quality. Orally administered sedatives have been extensively utilized in this setting. The most commonly used oral sedative for pediatric sedation is Chloral hydrate.[10] However, the sedative effect of this drug is often associated with prolonged recovery and an undesirable recovery profile.
Ideal medication for sedation in pediatric patient for day care procedure should be easy to administer, with rapid onset and faster recovery.[11] Ketamin, midazolam, clonidine, dexmedetomidine, etc., possess ideal criteria for sedation such as rapid onset, good anxiolysis, sedation and rapid recovery. Previous studies regarding procedural sedation for MRI, CT Scan, dental procedures, etc., have shown that intranasal administration is an effective way for sedation and it provide rapid and reliable onset of action, predictable effect, good quality of sedation to children, it's relatively easy and non-invasive route with high bioavailability.[5,12,13]
Intranasal midazolam and intranasal dexmedetomidine are safely used in pediatric patient for sedation in non-painful procedures. In this prospective randomized double-blinded study, we compared intranasal dexmedetomidine with intranasal midazolam for sedation in 100 pediatric patients less than 3 years undergoing echocardiography.
Dexmedetomidine is an alpha-2 agonist, can be administrated intranasally or transbuccally, and has been recently introduced as a sedative in pediatric patients.[14] Primarily it has been used for pediatric sedation by intravenous route.[15] It is having minimal effects on the respiratory drive and upper airway dynamics.[16] It is odorless, intranasal administration is not irritating and well tolerated by children.[14] Dexmedetomidine has a half-life of 2 hours, which may lead to faster recovery. However, as an alpha-2 adrenergic receptor agonist, it can decreases heart rate and blood pressure.[17]
Midazolam is a benzodiazepine, which produce a calming effect on the central nervous system. It works by increasing the effect of Gamma Aminobutaric Acid in the brain. Although not analgesic, the advantageous properties of Midazolam include anxiolysis, sedative, amnesia, hypnotic, anticonvulsant, and muscle relaxant. Intranasal midazolam offers greater systemic bioavailability, as it avoids hepatic first pass metabolism in comparison to oral route. Intranasal route has faster and predictable onset than oral or rectal route.[18]
We observed that onset of sedation was 10 min in group D, which was comparable to the onset time of 13 min previously reported by Jeff Miller et al. with intranasal dexmedetomidine 2 and 3 mcg/kg dose.[1] In group M the average onset time was 7.3 min, this was comparable to onset time of 10 min in previously reported by P Bhakta et al. with intranasal midazolam 0.2 mg/kg dose and it was found to be statistically significant when compared to Group D (P < 0.001).[18] The average sedation time and awakening time in Group M were 18.8, 29.51 and in group D were 14.2, 24.9 respectively and the differences are statistically significant (P < 0.001). The awakening time in Group D was comparable to the awakening time of 30 min observed by Qing Yu et al. with the use of intranasal dexmedtomidine.[19] The average total time for Group M was 51 min, which coincide with the total time i.e sum of procedure time and recovery time of 51.7 min observed by F Peerbhay et al. The average total time for Group D was 46.34 min and was found to be statistically significant (P < 0.001) when compared with Group M.[20] The difference observed between both the groups may be due to a shorter half-life of dexmedetomidine in comparison to midazolam.
TTE scan time of Group M is 8.84 and Group D is 9.18 and it was statistically significant. The average sonographer's satisfaction score for group M was 9.88 and for group D was 7.64 and it was statistically significant (P < 0.001). Dexmedetomidine induces arousable sedation, thus patients can be awakened by background noise and the movement of the echocardiography probe on their body, thus the sonographer's satisfaction score was less and TTE scanned time was more in Group D. The number of sonographer pauses over 2 min was seen in one patient of Group D, but it was not statistically significant (P = 0.3173). Average parental satisfaction score of Group M was 10 and of Group D was 8.76, which was statistically significant (P < 0.001). This can be explained by intra-procedural calmness and smooth arousal of the child in Group M as compared to Group D.
Secondary endpoint like heart rate, oxygen saturation had no significant difference between two groups. No patient had a change in gross appearance of the nasal mucosa after intranasal dexmedetomidine or midazolam. There was no requirement of oxygen, any airway intervention, bradycardia in either of the groups and the results were consistent with the study done by J. Miller et al.[1]
We accept the fact that there are some limitations in our study. First of all, the sample size is small, we need to take a large sample size to have a significant power of analysis. Second, use of atomized intranasal delivery device that could have more effective drug delivery and intranasal absorption, but it is not available in India. Third, duration of TTE scan may vary between individual pediatric cardiologists.
CONCLUSION
Intranasal drug administrations for sedation in children posted for ecocardiography is simple, rapid and with predictable sedation. We have observed that this route is feasible for both the drugs. Intranasal midazolam was found to be comparatively more effective in view of onset of action, sonographers, and parental satisfaction score, while TTE Scan time, sedation time, awakening time and total duration was significantly higher as compared to intranasal dexmedetomidine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank pediatric cardiologists Dr Bhavik Champaneri and Dr Tarunkumar Parmar, for their contribution in providing satisfication scores.
REFERENCES
- 1.Miller J, Xue B, Hossain M, Zhang MZ, Loepke A, Kurth D. Comparison of dexmedetomidine and chloral hydrate sedation for transthoracic echocardiography in infants and toddlers: A randomized clinical trial. Pediatr Anesth. 2016;26:266–72. doi: 10.1111/pan.12819. [DOI] [PubMed] [Google Scholar]
- 2.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–9. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 3.Louon A, Reddy VG. Nasal midazolam and ketamine for paediatric sedation during computerised tomography. Acta Anaesthesiol Scand. 1994;38:259–61. doi: 10.1111/j.1399-6576.1994.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 4.Zilberman MV. How best to assure patient co-operation during a pediatric echocardiography examination? J Am Soc Echocardiogr. 2010;23:43–5. doi: 10.1016/j.echo.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Weber F, Wulf H, El Saeidi G. Premedication with nasal s-ketamine and midazolam provides good conditions for induction of anesthesia in preschool children. Can J Anaesth. 2003;50:470–5. doi: 10.1007/BF03021058. [DOI] [PubMed] [Google Scholar]
- 6.Lökken P, Bakstad OJ, Fonnelöp E, Skogedal N, Hellsten K, Bjerkelund CE, et al. Conscious sedation by rectal administration of midazolam or midazolam plus ketamine as alternatives to general anesthesia for dental treatment of uncooperative children. Scand J Dent Res. 1994;102:274–80. doi: 10.1111/j.1600-0722.1994.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilton NC, Leigh J, Rosen DR, Pandit UA. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–5. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Anttila M, Penttila J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56:691–3. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: A systematic review. Intensive Care Med. 2000;26:275–85. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- 10.Yuen VM, Li BL, Cheuk DK, Leung MK, Hui TW, Wong IC, et al. A randomized controlled trial of oral chloral hydrate vs intranasal dexmedtomidine plus buccal midazolam for auditory brainstem response testing in children. Pediatr Anesth. 2018;28:1022–8. doi: 10.1111/pan.13498. [DOI] [PubMed] [Google Scholar]
- 11.García-Velasco P, Román J, Beltrán de Heredia B, Metje T, Villalonga A, Vilaplana J. Nasal ketamine compared with nasal midazolam in premedication in pediatrics. Rev Esp Anestesiol Reanim. 1998;45:122–5. [PubMed] [Google Scholar]
- 12.Galinkin JL, Fazi LM, Cuy RM, Chiavacci RM, Kurth CD, Shah UK, et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93:1378–83. doi: 10.1097/00000542-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Steal-induction after clonidine premedication: A comparison of the oral and nasal route. Paediatr Anaesth. 2007;17:230–4. doi: 10.1111/j.1460-9592.2006.02080.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuen VM. Dexmedetomidine: Perioperative applications in children. Pediatr Anesth. 2010;20:256–64. doi: 10.1111/j.1460-9592.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 15.Mason KP, Lerman J. Dexmedetomidine in children: Current knowledge and future applications. Anesth Analg. 2011;113:1129–42. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoud M, Jung D, Salisbury S, McAuliffe J, Gunter J, Patio M, et al. Effect of increasing depth of dexmedetomidine and propofol anesthesia on upper airway morphology in children and adolescents with obstructive sleep apnea. J Clin Anesth. 2013;25:529–41. doi: 10.1016/j.jclinane.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Mason KP, Lonnqvist PA. Bradycardia in perspective-not all reductions in heart rate need immediate intervention. Pediatr Anesth. 2015;25:44–51. doi: 10.1111/pan.12584. [DOI] [PubMed] [Google Scholar]
- 18.Bhakta P, Ghosh BR, Roy M, Mukherjee G. Evaluation of intranasal Midazolam for preanasthtic sedation in paediatric patients. Indian J Anaesth. 2007;51:111–6. [Google Scholar]
- 19.Yu Q, Liu Y, Sun M, Zhang J, Zhao Y, Liu F, et al. Median effective dose of intranasal dexmedetomidine sedation for transthoracic echocardiography in pediatric patients with noncyonotic congenital heart disease: An up and down sequential allocation trial. Pediatr Anaesth. 2017;27:1108–14. doi: 10.1111/pan.13235. [DOI] [PubMed] [Google Scholar]
- 20.Peerbhay F, Elsheikhomer AM. Intranasal Midazolam sedation in a pediatric emergency dental clinic. Anesth Prog. 2016;63:122–30. doi: 10.2344/15-00016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


