Abstract
Context:
Atorvastatin is considered as lipid reductive drugs with anti-inflammatory and pleotherapic effects in coronary artery bypass graph (CABG).
Aim:
This study is conducted to evaluate the effects of atorvastatin in CABG.
Setting and Design:
Patients with a coronary bypass graph procedure in Nemazee hospital in Shiraz were divided into two 50-groups receiving high-dose (80 mg) and low-dose (20 mg) atorvastatin.
Materials and Methods:
Troponin I, creatinine kinase-MB (CK-MB), atrial fibrillation (AF) after CABG, duration of mechanical ventilation, inotrope duration of consumption, blood sugar profile, liver and renal function, death during 30 days of CABG, MACE (major advance cardiac events) during admission in ICU, and 1 month follow up were surveyed.
Statistical Analysis:
Collected data were analyzed by independent and paired t-test and Chi square.
Results:
AST was increased, ALT, ALK-P after CABG were decreased, and urine volume in the second day of admission in ICU was increased in the high-dose group. There was an increase and following decrease in blood sugar of patients in the high-dose after CABG. An inflammatory marker after CABG was raised in both groups, ck-mb had an increase, and then followed by a reduction. Troporin had no significant differences between groups. Patients with high-dose atorvastatin had better glomerular filtration rate and renal performance. Along with decreasing AF in the case group, hemodynamics' disorder reduced and there was less bleeding.
Conclusion:
According to the above, it seems that a short-time prescription of high dose of atorvastatin in CABG can lead to better renal function, decreasing of arrhythmia and AF.
Keywords: Atorvastatin, coronary artery bypass graph, preconditioning
INTRODUCTION
Coronary artery bypass graft (CABG) is a familiar surgery that is associated with major mortality and morbidity from peri-procedural myocardial injury that leads to short- and long-term clinical complications.[1,2]
Ischemic preconditioning alludes to the transient periods of ischemia, which increases the resistance of myocardium to consequent reperfusion events and ischemia injury.[3] Nevertheless, intracellular mechanisms' discernment of ischemic preconditioning reveals new possibilities for introduction of pharmacological preconditioning in human models.[4]
Recently, some clinical trials suggested that the beneficial effect of statins is due to their lipid lowering properties and some other pleiotropic function like anti-inflammatory effect on endothelial cells, regulatory immune mechanism, antioxidant effect, and antithrombotic action of these drugs.[5,6]
A randomized trial of atorvastatin for reduction of myocardial damage during angiography showed that preoperative adjuvant pharmacological therapy with atorvastatin for 7 days resulted in 81% reduction in the risk of heart failure in patients with stable angina undergoing the percutaneous revascularization.[7] In another study conducted in Iran, it was concluded that high doses of atorvastatin for 6 months prior to open heart surgery may reduce the plasma serum high sensitivity c-reactive protein (hs-CRP).[8]
Considering the existence of diverse studies and lack of a comprehensive study showing the preconditioning effect of atorvastatin, we decided to investigate the short-term pretreatment influence of high and low doses of atorvastatin in reducing mortality and morbidity, myocardial ischemia, arrhythmias, and the pattern of inflammatory response characterized by inflammatory markers associated with surgery, after CABG in patients that chronically use low dose of atorvastatin. The results of this study can be an important step in the treatment of CABG complications and reduces the cost of health care in cardiovascular status.
MATERIALS AND METHODS
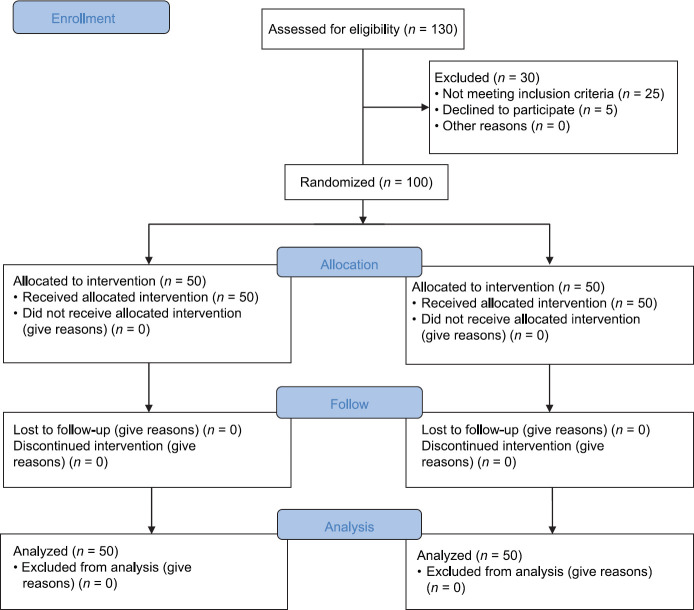
After receiving approval from ethics committee of Shiraz University of Medical Sciences and completing the informed consent by patients, 100 patients were enrolled in this triple-blind, randomized, clinical trial. The sample size was calculated based on the obtained results of similar studies with the p1 = 0.16, p2 = 0.7, α = 5%, and the power of 85%. This prospective triple blind randomized clinical trial (IRCT No.: 2017080619470N61) was performed in a sample population of patients who were undergoing on-pump CABG at Nemazee hospital in Shiraz, southern Iran cardiology center and were selected by Randomized block design and divided into two groups of A and B. Fifty patients were assigned in the intervention group and 50 patients in the control group. The study was conducted according to the CONSORT criteria [Figure 1].
Figure 1.
CONSORT flow chart
The inclusion criterion was patients who had been taking (atorvastatin 20 mg daily) at least one year prior to the cardiac surgery. Although the exclusion criteria included unstable angina, all of the patients with the history of MI or hospital admission or any evidence related to MI for the 6 previous months before the study, history of cardiac surgery, congenital heart disease, history of cardiac arrhythmias (arterial fibrillation) before surgery, receiving antiarrhythmic drugs (except Beta blockers), having pacemaker, left ventricle ejection fraction less than 30%, uncontrolled hypertension, arterial or ventricular arrhythmia, pregnancy, patient who are undergoing diabetes treatment, increased level of liver enzymes, renal failure with creatinine more than 2 mg/dl, active inflammation or immune deficiency, positive history of muscle disease, or reaction to the stations. Confidential patients data were gathered by a code (ID).
Patients in the study group received 80 mg atorvastatin daily, for 3 days before surgery and low dose of 20 mg atorvastatin daily during the ICU stay, after surgery, and after discharge, the treatment continued at home with a single dose of 20 mg atorvastatin daily. On the other side, patients in the control group received 20 mg atorvastatin up to surgery and during the one month follow-up period they continued their medication with 20 mg atorvastatin daily, while a single dose of atorvastatin was given to the patients every night, an hour after the meal.
The atorvastatin pills were prepared in separated packages (each package contains 3 pills of 80 mg or 3 pills of 20 mg atorvastatin). The subject, tester, and monitoring committee were unaware of which package is 80 mg or 20 mg atorvastatin, so the experiment was carried in a triple blinded way through groups A and B. Moreover, to avoid the effects of anesthetic drugs on the patient's outcome, the method of anesthesia in all patients was the same.
Before induction of anesthesia, arterial line was obtained from the left radial artery to hemodynamic and heart monitoring. Premedication in all patients of both groups was with midazolam, fentanyl, morphine, and induction was administered by thiopental and Pancuronium, while 1 MAC of isoflurane was administered during surgery and also central line and folly catheter were inserted after induction. Surgical procedures in all patients were similar. After the surgery, patients were transferred to the (cardiac surgery) ICU and the pills were given via the nasogastric tube if the patient was under mechanical ventilation, postoperatively.
The primary outcomes of this study were troponin I, creatinine kinase-MB (CK-MB) and hs-CRP levels, the incidence of postoperative arrhythmia and ventricular fibrillation, mechanical ventilation duration in the ICU. Secondary outcomes included DC shock frequency, ICU and hospital stay length, ejection fraction level, ICU blood intake, need for inotrope at the pomp off time or in the ICU, blood glucose profile, liver status glomerular filtration rate (GFR), and urine output. Postoperative complications such as delirium rate, postsurgery bleeding, low cardiac output, hemodynamic instability (any perturbation in heart rate, blood pressure, or central vein pressure), major advance cardiac events including death, nonfatal myocardial infarction, repeated revascularization incidence due to stroke, and cardiac arrest either during ICU stay or one month of follow up were also recorded.
Troponin I measured using electrochemiluminescence immunoassay method (Cobas, E411, Japan), CK-MB assessed by enzymatic method of clut sampling (autoanalyser dirui, Cs-800, China), and hs-CRP appraised with turbidimetry method (Biosystems SA, Barcelona, Spain) before, 24 and 48 hours after operation. Any type of arrhythmia that needs intervention or last over 20 minutes was studied at different time intervals containing aorta declamping term, off pump time, and during ICU stay. Peri- and postoperative Inotrope consumption, the incidence of arrhythmic events in ICU, the type of inotrope, and duration of its administration were also monitored.
Kidney function tests (e.g., GFR, creatinine, and bilirubin) were checked before surgery and at the first 3 days of ICU stay. Urine output volume was monitored at the beginning and during the operation; also, at off pump time and every day during the ICU stay. Liver function alkaline phosphatase (ALK-P), alanine aminotransferase (ALT), aspartate aminotransferase (AST), direct, and total bilirubin were evaluated preoperatively and in the second day after surgery to follow the effects of stations on the liver. Patients' blood sugar was checked preoperative, during surgery and after the off pump and then every 6 hours a day in ICU for 48 hours, and then it was daily monitored by glucometer kit during the hospitalization. In addition to hemodynamic status (blood pressure, heart rate and central Venus pressure) monitoring, delirium was also followed daily in ICU by CAM ICU (confusion assessment method for the ICU).
Moreover, one month after surgery, all of the patients were followed by the surgeon considering their cardiac function using echocardiography (ejection fraction and cardiac output measures), probability of cardiac risk occurrence, cardiac arrest, and revascularization necessitation.
Data analysis and statistical description was performed by SPSS 16 software (SPSS Inc., Chicago, IL) enrolling statistical tests including repeated measurement test, Man–Whitney U-test, Chi-square test, t-test, paired t-test, Kolmogorov–Smirnov test, and fisher exact test. P value ≤0.05 was considered as significant.
RESULTS
Out of 130 patients who were screened for enrollment, 30 patients were excluded. In total, 5 patients declined to participate and 25 patients did not fulfill the inclusion criteria. Therefor, 100 patients participated in the study, 50 patients were in the treatment group and 50 patients in the control group [Figure 1].
Demographic criteria including age, gender, BMI, probability of any pre-existence disease (like hypertension, diabetic mellitus, chronic obstructive pulmonary disease, and hyperlipidemia), smoking condition and rate, the ejection fraction value, cardio pulmonary support, aortic cross clamp, total time of CABG, intraaortic balloon pomp, perioperative inotrope, and perioperative arrhythmias were not significantly different in control and case groups.
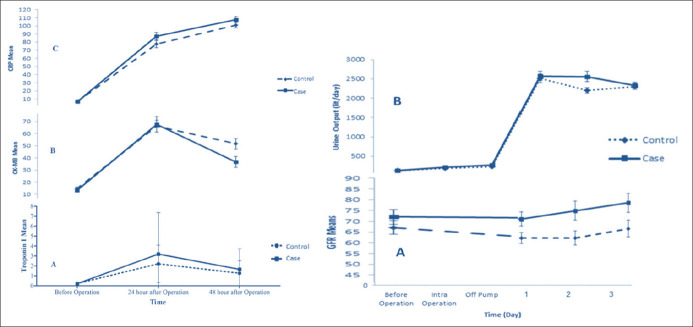
Heart markers consisting of troponin I and CK-MB increased postoperatively in both groups and subsequently decreased 24 hours after surgery; troponin I variation was not different between two groups (P-value >0.05), but CK-MB was significantly lower in case group (P-value = 0.018) [Figure 2: right a and b]. Moreover, inflammatory marker (hs-CRP) was raised in both groups without any significant difference between groups (P-value >0.05) [Figure 2: right c].
Figure 2.
Left: (A) Troponin I changes in different time intervals in high- and low-dose atorvastatin pretreated groups. (B) CK-MB changes in different time intervals in high- and low-dose atorvastatin pretreated groups. (C) hs-CRP changes in different time intervals in high- and low-dose atorvastatin pretreated groups. Right: (A) GFR changes in different time intervals in high- and low-dose atorvastatin pretreated groups. (B) Urine output changes in different time intervals in high- and low-dose atorvastatin pretreated groups
The number of arrhythmia related to off pump time and during ICU stay days, inotrope utilized cases and its dosage during ICU stay and postoperatively, ejection fraction values and length of ICU or hospital stay of patients in two groups did not show statistical differences; however, the incidence of arrhythmia during declamping, the AF cases count per day, mechanical ventilation, blood bag consumption in ICU, and the number of employed DC shock were significantly lower in high dose atorvastatin group. Moreover, postoperative complication assessment showed no differences in delirium event, low cardiac output existence, mortality rate, myocardial infarction incidence, and revascularization happening during ICU stay or 1 month follow-up. Nevertheless, hemodynamic instability degree and bleeding prevalence after surgery were significantly lower in the patients of high dose atorvastatin group [Table 1].
Table 1.
Different arrhythmias occurrences and postoperative factors comparison in patients undergoing coronary artery bypass graft surgery in two groups, variables are presented as mean±SD or number (percentage)
| Outcomes | Case | Control | P |
|---|---|---|---|
| Declamping arrhythmia | 15 (30.00%) | 26 (52.00%) | 0.025 |
| Off pomp arrhythmia | 1 (2.00%) | 3 (6.00) | 0.617 |
| Atrial fibrillation/day | 2.20±0.83 | 5.00±2.6 | 0.017 |
| ICU arrhythmia/day | 0.42±0.81 | 0.20±0.53 | 0.173 |
| Mechanical ventilation duration (hour) | 11.81±3.30 | 14.48±4.51 | 0.001 |
| DC shock | 0.44±0.99 | 0.86±1.27 | 0.046 |
| Postoperative inotrope | 15 (30.00%) | 23 (46.00%) | 0.099 |
| Postoperative inotrope dosage (μg/min) | 0.045±0.027 | 0.049±0.026 | 0.52 |
| ICU inotrope consumption | 23 (46.00%) | 25 (50.00%) | 0.689 |
| Inotrope dosage (μg/kg/min) | 0.10±0.39 | 0.10±0.42 | 0.936 |
| Inotrope consumption duration (day) | 5.82±9.09 | 9.12±12.69 | 0.138 |
| ICU stay length (hour) | 52.12±20.94 | 58.30±19.80 | 0.133 |
| Hospital stay length (hour) | 99.82±21.71 | 106.42±19.76 | 0.115 |
| Ejection fraction | 54.60±5.3 | 52.7±7.64 | 0.146 |
| ICU Blood consumption (bag) | 1.73±0.86 | 2.30±1.60 | 0.029 |
| Postoperative complication | |||
| ICU Myocardial infarction | 0 (0.00%) | 0 (0.00%) | 1 |
| ICU Death | 1 (2.00%) | 0 (0.00%) | 1 |
| Low cardiac output | 7 (14.00) | 7 (14.00%) | 1 |
| Postsurgery bleeding | 0 (0%) | 4 (8%) | 0.041 |
| Hemodynamic instability | 2 (4.00%) | 9 (18.00%) | 0.025 |
| Delirium | 3.00 (6.00%) | 1.00 (2.00%) | 0.617 |
| 1 month Follow up | |||
| Myocardial infarction | 1 (2.00%) | 3 (6.00%0) | 0.617 |
| Revascularization | 0 (0.00%) | 1 (2.00%) | 1 |
The blood glucose profile of included patients were increased in both groups during the follow-up period up to 12 hours in control group and 6 hours after surgery in intervention group and had a consequent decrease soon after without significant differences in two groups, except the blood sugar sample of intervention group at hour 6, which was significantly upper than the control group (P-value = 0.005) [Figure 3].
Figure 3.
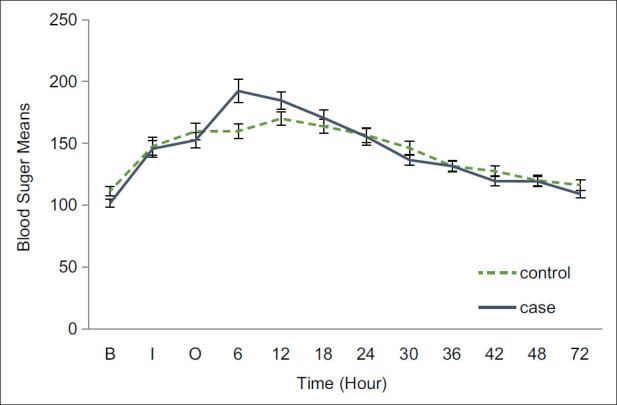
Glucose profile in patients undergoing coronary artery bypass graft surgery pretreated with high and low doses of atorvastatin
AST alteration of high-dose atorvastatin group was significantly more than low-dose atorvastatin group; nevertheless, in the case of ALT, ALK-P, and bilirubin variation, there were no differences in two groups [Table 2].
Table 2.
Liver function status in patients undergoing coronary artery bypass surgery in two groups, their preoperative, postoperative, and subtracted values considered as time 0 and 1 and Diff, respectively. Variables are presented as mean±SD
| Liver marker | Case | Control | P |
|---|---|---|---|
| AST0 (mg/dl) | 23.10±9.06 | 29.64±21.09 | 0.406 |
| AST1 (mg/dl) | 52.34±28.95 | 43.10±18.69 | 0.139 |
| Diff AST1-0 | 29.24±31.53 | 13.46±23.39 | 0.043 |
| ALT0 (mg/dl) | 26.96±20.21 | 29.76±24.15 | 0.600 |
| ALT1 (mg/dl) | 26.56±13.23 | 27.76±14.75 | 0.885 |
| Diff ALT1-0 | -4.00±19.58 | -2.00±19.58 | 0.738 |
| Alk-p0 (U/L) | 194.12±77.19 | 205.96±78.19 | 0.448 |
| ALK-P1 (U/L) | 142.24±51.32 | 155.20±58.51 | 0.242 |
| Diff ALK-P1-0 | -51.88±55.27 | -50.76±100.67 | 0.945 |
| Bilirubin Total0 (mg/dl) | 0.69±0.27 | 0.63±0.29 | 0.299 |
| Bilirubin Total1 (mg/dl) | 1.25±0.67 | 1.28±0.71 | 0.830 |
| Diff bilirubin total1-0 | 0.56±0.64 | 0.65±0.71 | 0.846 |
| Bilirubin direct0 (mg/dl) | 0.21±0.27 | 0.23±0.23 | 0.721 |
| Bilirubin direct1 (mg/dl) | 0.36±0.24 | 0.40±0.26 | 0.388 |
| Diff bilirubin direct1-0 | 0.14±0.34 | 0.17±0.35 | 0.404 |
Furthermore, at the second day of ICU stay, kidney function markers including GFR and urine output volume in the treatment group were higher than the control group (P-value <0.05 and P value = 0.23, respectively), but the statistical analysis of other evaluated days showed no difference between two groups [Figure 2: right].
DISCUSSION
Our data showed that pharmacological adjuvant therapy with high-dose atorvastatin did not influence the values of troponin I changes after surgery in consistent with the results of Ludman et al., which reports administration of high-dose atorvastatin does not further reduce the troponin I values after coronary artery bypass surgery.[9] So, it may assume that it does not lessen the perioperative myocardial injury. But CK-MB value, as a cardiac marker, shows an increase and soon after a reduction in both group a significant decrease in the case group. Accordingly, it is identified that the mean CK-MB is significantly increased in the patients who medicated with high-dose statin comparing to low dose after off-pump coronary artery bypass.[10] This could be considered as a profitable effect of high-dose pretreatment with atorvastatin in patients who undergo surgery, although more studies are needed to provide better insight into the impact of this cardiac protection effect.
Although it is shown in other studies that high-dose statin medication may lead to further hs-CRP value reduction and head to lower cardiovascular events and better results in the patients' quality of life,[8] we did not recognize any difference between postoperative hs-CRP changes in two groups. In spite of our study, administration of different doses of atorvastatin reduces the hs-CRP amount in most patients; although this diminution is not significant at all doses and in all patients, in high-dose patients, it occurred faster and greater than other groups.[8] Thus, it can be concluded that high-dose administration of atorvastatin with long-time hs-CRP follow-up is needed to conduct more accurate results according to the beneficial effects of statins medication.
Here, on the contrary of other articles that only have examined the AF incidence, all arrhythmias peri- and postoperatively and during the ICU stay were assessed. Moreover, type and length of arrhythmia and treatment were completely considered which as it is clear, in the high dose atorvastatin group; number of utilized DC shock in operating room, incidence of arrhythmia during declamping, and AF in the ICU was lower in comparison with the low-dose group. Distinctly, atrial fibrillation is the most common arrhythmia following open heart surgery associated with the events such as postoperative thromboembolism, impaired hemodynamic, ventricular dysrhythmias, and higher mortality rate.[11] Reduction in AF significantly has diminished the hemodynamic instability but did not moderate mortality. Several factors are associated with postoperative AF, including inotropes, prolonged ventilation, acute kidney injury, intra-aortic balloon pump, and central venous pressure elevation,[12] as was seen in our study; the length of ventilation was shorter and renal function was improved in the case group in favor of AF reduction. Conversely, a study carried out by Kourliouros et al., the beneficial effect of pretreatment by high-dose atorvastatin 80 mg compared to 10 mg on postoperative AF has not improved.[13]
These results demonstrate that, in the case of the ICU mortality rate, ICU and hospital stay length, delirium and low cardiac output incidence, MI occurrence either in the ICU or during one month follow-up, and also revascularization probability, no differences were found between the two groups. But the duration of mechanical ventilation in the group of high-dose atorvastatin was significantly less than the low-dose group and the patients get off from the ventilator in a shorter time which may lead to minor side effects due to long-term mechanical ventilation (e.g., Ventilator-Associated Pneumonia). Furthermore, the intervention group had more stable hemodynamic status and, interestingly, bleeding and blood transfusion requirement was significantly lower comparing to the control group.
Our findings are in consistent with the result of Youn et al., which shows no significant difference in major cardiovascular events observed over the 30 days after pretreatment with high dose of rosuvastatin.[10] On the contrary, in LaRos et al. study, high dose of atorvastatin adjuvant therapy showed significant reduction in the rate of major cardiovascular events which is probably due to the difference in duration of the follow-up period or the lack of any surgical procedures and even can be as a result of prolonged treatment with high dose of atorvastatin.[14]
We have found a peak rise in blood glucose profile 6 hours after operation in high-dose atorvastatin group which returned to normal range without any intervention. Thus, it can be assumed that short-term medication of high-dose atorvastatin has no noticeable diabetogenic effect on post CABG cases. Similarly, Masana[6] has not detected any alteration in insulin sensitivity in patients who received rosuvastatin 20 mg/day. Moreover, some other studies clarified the LDL lowering effect of atorvastatin in patients with type 2 diabetes without exacerbating the insulin resistance.[15,16] In spite of our study, a number of investigations highlighted conceivable relation among statin therapy and elevated risk of diabetic mellitus. For instance, Preiss et al. meta-analysis[17] and Ray[18] study revealed a significant association between statin therapy and increase in type 2 diabetes.
Recent written reports clearly support the liver toxicity effect of atorvastatin as the most frequently used statin.[19,20] Further investigation in the hepatotoxicity effect of satins shows higher incidence of this side effect with high-dose atorvastatin comparing to the other type of statins.[21] Accordingly, we have found a significant increase in the postoperative AST level with no alteration in the amount of other liver markers. However, the hepatotoxic possibility of statins remains as a controversial issue, as in one study in India none of the patients had liver enzymes elevation or hepatotoxicity, which may be due to genetic differences or duration of atorvastatin administration.[22]
In the current study, GFR and urine output was improved in the patients receiving high dose of atorvastatin that may enable us to progress the renal function by high-dose atorvastatin administration without adverse effects in short term. Our finding is in consistence with other reports that indicated GFR in the statin treated group was higher than placebo group in patient without any chronic renal disease, while in the group of chronic renal disease, such difference was not reported that may be as a result of patients characteristics variation rather than statin effects.[23] Furthermore, it is demonstrated that the creatinine level and its clearance reduced in atorvastatin pretreatment patients with acute coronary syndrome undergoing percutaneous coronary procedure, which may be due to protective effect of statins on renal function.[24] Although atorvastatin may protect patients receiving radio contrast materials from nephropathy, usefulness of pretreatment with high dose of atorvastatin in contrast-induced nephropathy declining in patients with preexisting chronic renal disease is not established.[25,26] Thus, we can conclude that high dose of atorvastatin may not be equally effective in all situations, or in patients with chronic kidney disease may have different effects.
Two major limitations are detectable in our study. First of all it is clarified that atorvastatin administration leads to lower cholesterol value and arthrosclerosis risk, which similarly reduces the MI occurrences in long term.[27] So, it seems necessary to follow the patients in longer time duration than 48 hours for MI probability and compare the effects of acute high-dose effects of atorvastatin in a somewhat chronic way. Second, here we did not study the mechanistic effect of acute high-dose atorvastatin on different factors containing the cardiac, kidney, liver markers, and especially its influence on glucose levels either through insulin resistance or insulin secretion alteration. Moreover, we did not perform a dose and response atorvastatin analysis, which should be considered in future investigation.
CONCLUSION
In summary, we found that high-dose atorvastatin pretreatment in adult patients who already receive an atorvastatin medication regimen would improve their preconditioning after CABG surgery. However, the encountered myocardial injury during operation did not show further reduction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Watabe H, Sato A, Akiyama D, Kakefuda Y, Adachi T, Ojima E, et al. Impact of coronary plaque composition on cardiac troponin elevation after percutaneous coronary intervention in stable angina pectoris: A computed tomography analysis. J Am Coll Cardiol. 2012;59:1881–8. doi: 10.1016/j.jacc.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A, Gersh BJ, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, et al. Prognostic significance of periprocedural versus spontaneously occurring myocardial infarction after percutaneous coronary intervention in patients with acute coronary syndromes: An analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54:477–86. doi: 10.1016/j.jacc.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Slagsvold KH, Moreira JB, Rognmo O, Høydal M, Bye A, Wisløff U, et al. Remote ischemic preconditioning preserves mitochondrial function and activates pro-survival protein kinase Akt in the left ventricle during cardiac surgery: A randomized trial. Int J Cardiol. 2014;177:409–17. doi: 10.1016/j.ijcard.2014.09.206. [DOI] [PubMed] [Google Scholar]
- 5.Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost. 2009;7:514–20. doi: 10.1111/j.1538-7836.2008.03235.x. [DOI] [PubMed] [Google Scholar]
- 6.Masana L. Pitavastatin in cardiometabolic disease: Therapeutic profile. Cardiovasc Diabetol. 2013;12(Suppl 1):S2. doi: 10.1186/1475-2840-12-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G ARMYDA Investigators. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: Results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–8. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 8.Nesar Hossein V, Yosef Nejad K, Abdollahian F. Short-term therapy with high dose atorvastatin in patients with coronary artery disease can reduce inflammatory process. Acta Med Iran. 2010;48:218–21. [PubMed] [Google Scholar]
- 9.Ludman AJ, Hausenloy DJ, Babu G, Hasleton J, Venugopal V, Boston-Griffiths E, et al. Failure to recapture cardioprotection with high-dose atorvastatin in coronary artery bypass surgery: A randomised controlled trial. Basic Res Cardiol. 2011;106:1387–95. doi: 10.1007/s00395-011-0209-5. [DOI] [PubMed] [Google Scholar]
- 10.Youn YN, Park SY, Hwang Y, Joo HC, Yoo KJ. Impact of high-dose statin pretreatment in patients with stable angina during off-pump coronary artery bypass. Korean J Thorac Cardiovasc Surg. 2011;44:208–14. doi: 10.5090/kjtcs.2011.44.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samadikhah J, Golzari SE, Sabermarouf B, Karimzadeh I, Tizro P, Mohammad Khanli H, et al. Efficacy of combination therapy of statin and vitamin C in comparison with statin in the prevention of post-CABG atrial fibrillation. Adv Pharm Bull. 2014;4:97–100. doi: 10.5681/apb.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariscalco G, Musumeci F, Banach M. Factors influencing post-coronary artery bypass grafting atrial fibrillation episodes. Kardiol Pol. 2013;71:1115–20. doi: 10.5603/KP.2013.0291. [DOI] [PubMed] [Google Scholar]
- 13.Kourliouros A, Valencia O, Hosseini MT, Mayr M, Sarsam M, Camm J, et al. Preoperative high-dose atorvastatin for prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2011;141:244–8. doi: 10.1016/j.jtcvs.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.LaRosa JC, Deedwania PC, Shepherd J, Wenger NK, Greten H, DeMicco DA, et al. Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the Treating to New Targets [TNT] trial) Am J Cardiol. 2010;105:283–7. doi: 10.1016/j.amjcard.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Yu D, Wang Y, Chi J, Sun M, Guo L, Jiang L, et al. [Impacts of atorvastatin on blood lipids and arterial media thickness in new-onset type 2 diabetes patients] Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35:733–6. [PubMed] [Google Scholar]
- 16.Canas JA, Ross JL, Taboada MV, Sikes KM, Damaso LC, Hossain J, et al. A randomized, double blind, placebo-controlled pilot trial of the safety and efficacy of atorvastatin in children with elevated low-density lipoprotein cholesterol (LDL-C) and type 1 diabetes. Pediatr Diabetes. 2015;16:79–89. doi: 10.1111/pedi.12245. [DOI] [PubMed] [Google Scholar]
- 17.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 18.Ray K. Statin diabetogenicity: Guidance for clinicians. Cardiovasc Diabetol. 2013;12(Suppl 1):S3. doi: 10.1186/1475-2840-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrascosa MF, Salcines-Caviedes JR, Lucena MI, Andrade RJ. Acute liver failure following atorvastatin dose escalation: Is there a threshold dose for idiosyncratic hepatotoxicity? J Hepatol. 2015;62:751–2. doi: 10.1016/j.jhep.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, et al. Hepatotoxicity associated with statin use: Analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig. 2014;106:246–54. [PubMed] [Google Scholar]
- 21.Chang CH, Chang YC, Lee YC, Liu YC, Chuang LM, Lin JW. Severe hepatic injury associated with different statins in patients with chronic liver disease: A nationwide population-based cohort study. J Gastroenterol Hepatol. 2015;30:155–62. doi: 10.1111/jgh.12657. [DOI] [PubMed] [Google Scholar]
- 22.Kaul U, Varma J, Kahali D, Hiremath MS, Dani S, Dalal J, et al. Post-marketing study of clinical experience of atorvastatin 80 mg vs 40 mg in Indian patients with acute coronary syndrome- a randomized, multi-centre study (CURE-ACS) J Assoc Physicians India. 2013;61:97–101. [PubMed] [Google Scholar]
- 23.Natsuaki M, Furukawa Y, Morimoto T, Sakata R, Kimura T. CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Renal function and effect of statin therapy on cardiovascular outcomes in patients undergoing coronary revascularization (from the CREDO-Kyoto PCI/CABG Registry Cohort-2) Am J Cardiol. 2012;110:1568–77. doi: 10.1016/j.amjcard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Patti G, Ricottini E, Nusca A, Colonna G, Pasceri V, D'Ambrosio A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty--contrast-induced nephropathy] trial. Am J Cardiol. 2011;108:1–7. doi: 10.1016/j.amjcard.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105:288–92. doi: 10.1016/j.amjcard.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, et al. Efficacy of short-term high-dose atorvastatin for prevention of contrast-induced nephropathy in patients undergoing coronary angiography. Angiology. 2010;61:711–4. doi: 10.1177/0003319710364216. [DOI] [PubMed] [Google Scholar]
- 27.Campeau L. Lipid lowering and coronary bypass graft surgery. Current Opinion in Cardiology. 2000;15:395–9. doi: 10.1097/00001573-200011000-00004. [DOI] [PubMed] [Google Scholar]