Abstract
Toxoplasma gondii is widespread worldwide and can infect swine. This study evaluated the seroprevalence of T. gondii in swine from central China after an outbreak of African swine fever (ASF). A total of 2683 swine serum samples were collected from farms in four provinces. Of the serum samples, 1.42% (38/2683) (95% CI, 1.03–1.94) tested positive for T. gondii IgG antibody by a modified agglutination test (MAT) (cut-off: 1:25). Comparing with the results of previous studies, specifically our survey from before the outbreak, the seroprevalence of T. gondii in swine from central China was significantly decreased after the occurrence of ASF (OR = 7.679, 2015–2017 vs. 2019–2020). In general, the proportion of seropositive animals increased with the age of the swine, indicating post-natal transmission of T. gondii. Furthermore, there was a significant difference in seroprevalence between suckling pigs and weaned pigs (p < 0.05). This is the first large-scale investigation of T. gondii infection in swine after an ASF outbreak in China. The lower seroprevalence of T. gondii in swine after ASF may be due to stricter biosecurity measures on the farms, but results indicated swine exposure to zoonotic parasites despite these measures. This highlights that pigs must be considered a potential source of human T. gondii infections.
Keywords: Toxoplasma gondii, Seroepidemiology, Swine, African swine fever, China, Risk factors
Abstract
Toxoplasma gondii est répandu dans le monde entier et peut infecter les porcs. Cette étude a évalué la séroprévalence de T. gondii chez les porcs du centre de la Chine après une épidémie de peste porcine africaine (PPA). Au total, 2 683 échantillons de sérum de porc ont été prélevés dans des fermes de quatre provinces. Parmi les échantillons de sérum, 1,42 % (38/2683) (IC à 95 %, 1,03–1,94) étaient positifs pour les anticorps IgG de T. gondii par un test d’agglutination modifié (MAT) (seuil : 1:25). En comparaison avec les résultats d’études précédentes, en particulier notre enquête d’avant l’épidémie, la séroprévalence de T. gondii chez les porcs du centre de la Chine a été significativement diminuée après l’apparition de la PPA (OR = 7,679, 2015-2017 vs 2019-2020). En général, la proportion d’animaux séropositifs augmentait avec l’âge des porcs, indiquant une transmission postnatale de T. gondii. De plus, il y avait une différence significative entre la séroprévalence chez les cochons de lait et les cochons sevrés (p < 0,05). Il s’agit de la première enquête à grande échelle sur l’infection à T. gondii chez le porc après l’épidémie de PPA en Chine. La séroprévalence plus faible de T. gondii chez les porcs après la PPA peut être due à des mesures de biosécurité plus strictes dans les fermes, mais les résultats ont indiqué une exposition des porcs aux parasites zoonotiques malgré ces mesures. Cela souligne que les porcs doivent être constamment considérés comme une source potentielle d’infection humaine à T. gondii.
Introduction
Toxoplasma gondii is a widely distributed obligate intracellular parasitic protozoan that infects virtually all warm-blooded animals, including humans and swine [5]. About 1800 million people worldwide are chronically infected with T. gondii [14, 17]. Infection with T. gondii is usually asymptomatic in healthy people, but can be severe in people with immunodeficiency [5]. There are two main modes of transmission of T. gondii: horizontal transmission, mainly through oral infection, and vertical transmission through the placenta [11, 15].
Swine are susceptible to T. gondii, with seroprevalence ranging from 0% to 96.6% in different regions of the world [8]. China is the largest pork producer and consumer worldwide [18]. The positive serum rate of T. gondii in China was 32.9% from 2000 to 2017 [4], indicating that T. gondii infection is widespread on pig farms, which resulted in an adverse impact on the farmer’s income and human health. On August 3, 2018, there was an African swine fever (ASF) outbreak in China. Measures (early virus detection by clinical signs and laboratory diagnosis, and strict biosecurity) were implemented to control the spread of this disease [1–3]. This study investigated the seroprevalence of T. gondii infection in swine from Chinese farms and compared the seroprevalence before and after the ASF outbreak.
Materials and methods
Ethics approval and consent to participate
This study was conducted following the guideline recommendations for using samples from animals by the Beijing Association for Science and Technology (SYXK [Beijing] 2007-0023). The swine sera were collected with agreement from the farmers. The ethics committee of the Henan Agricultural University (China) further approved this study.
Investigation sites and serum samples
In this study, 2683 swine serum samples were collected from 16 farms in Henan (n = 5), Shaanxi (n = 9), Anhui (n = 1), and Shanxi (n = 1) provinces in 2019–2020 (Table 1 and Fig. 1). These blood samples were collected from live animals by veterinarians on the farms. The sera were used for porcine reproductive and respiratory syndrome and porcine circovirus antibodies screening, which also allowed us to survey T. gondii infection. These serum samples were transported to the Henan Agricultural University (Zhengzhou, Henan, China) in cooler boxes. Information including collection dates, age, sex, health (no abnormal clinical signs), unhealth (anorexia, fever, cough, miscarriage and diarrhea), whether pregnant, and farm locations (Henan, Shaanxi, Anhui and Shanxi) was also collected for the samples.
Table 1.
Seroepidemiology and background information on Toxoplasma gondii in 2683 swine.
| Province | No. of samples | MAT titers |
% (positive no.) | 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:25 | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1600 | 1:3200 | |||||
| Location | Henan | 703 | 7 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1.42% (10) | 0.74–2.64 |
| Shaanxi | 1867 | 10 | 13 | 3 | 1 | 1 | 0 | 0 | 0 | 1.50% (28) | 1.03–2.17 | |
| Anhui | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0–14.76 | |
| Shanxi | 86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0–5.13 | |
| Total | 2683 | 17 | 15 | 3 | 1 | 2 | 0 | 0 | 0 | 1.42% (38) | 1.03–1.94 | |
| Sampling time | 2019 | 265 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2.64% (7) | 1.18–5.46 |
| 2020 | 2418 | 12 | 13 | 3 | 1 | 2 | 0 | 0 | 0 | 1.28 (31) | 0.90–1.82 | |
| Total | 2683 | 17 | 15 | 3 | 1 | 2 | 0 | 0 | 0 | 1.42% (38) | 1.03–1.94 | |
| Growth stages | Suckling pig | 213 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 0–2.13 |
| Nursery pig | 452 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44% (2) | 0.01–1.71 | |
| Fattening pig | 595 | 10 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 2.18% (13) | 1.24–3.74 | |
| Adult pig | 1328 | 5 | 14 | 1 | 1 | 2 | 0 | 0 | 0 | 1.73% (23) | 1.14–2.60 | |
| Total | 2588 | 17 | 15 | 3 | 1 | 2 | 0 | 0 | 0 | 1.47% (38) | 1.07–2.01 | |
| Sex | Male | 104 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1.92% (2) | 0.10–7.17 |
| Female | 1276 | 7 | 11 | 0 | 1 | 1 | 0 | 0 | 0 | 1.57% (20) | 1.00–2.42 | |
| Total | 1380 | 7 | 12 | 1 | 1 | 1 | 0 | 0 | 0 | 1.59% (22) | 1.04–2.42 | |
| Pregnancy | Yes | 498 | 2 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 1.61% (8) | 0.76–3.19 |
| No | 292 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 3.42% (10) | 1.79–6.27 | |
| Total | 790 | 6 | 11 | 0 | 1 | 0 | 0 | 0 | 0 | 2.28% (18) | 1.42–3.60 | |
| Health condition | Health | 1821 | 13 | 15 | 3 | 1 | 1 | 0 | 0 | 0 | 1.81% (33) | 1.29–2.54 |
| Unhealthy | 130 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0% | 0–3.45 | |
| Total | 1951 | 13 | 15 | 3 | 1 | 1 | 0 | 0 | 0 | 1.69% (33) | 1.20–2.37 | |
| Parity | Multiparous | 576 | 3 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 1.74% (10) | 0.90–3.21 |
| Primiparous | 104 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.92% (2) | 0.10–7.17 | |
| Total | 680 | 5 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 1.76% (12) | 0.98–3.09 | |
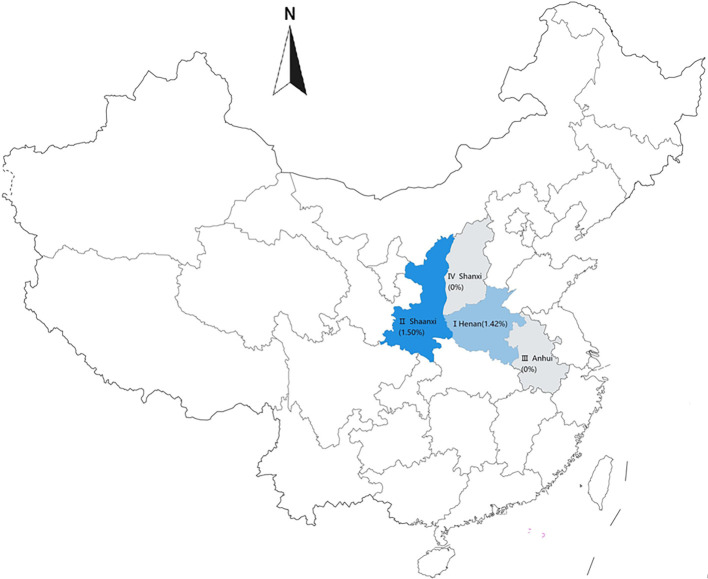
Figure 1.
Seroprevalence of T. gondii in swine from central China. I: Henan; II: Shaanxi; III: Anhui; IV: Shanxi. Map adapted from http://bzdt.ch.mnr.gov.cn/.
Serological testing
All the serum samples were tested for antibodies against T. gondii by a modified agglutination test (MAT) [6]. Sera with MAT titers of 1:25 or higher were considered positive for T. gondii [5]. Whole formalin-treated T. gondii tachyzoites were obtained from the University of Tennessee Research Foundation (Knoxville, TN, USA; https://utrf.tennessee.edu/). Toxoplasma gondii-positive mouse sera were provided by Dr. J. P. Dubey (ARS, USDA, Beltsville, MD, USA) as reference sera. All the serum samples were tested at 1:25, after which the dilution was doubled to 1:3200, and negative and positive controls were included in each plate.
Statistical analysis
The pig population was divided into four stages: suckling piglets (0–4 weeks), nursery pigs (>4 weeks to 10 weeks), fattening pigs (>10 weeks to 20 weeks), and adult pigs (>20 weeks). In addition, the swine farm locations, pregnancy, number of deliveries, and sampling time were also compared. The classification standards are summarized in Table 2.
Table 2.
Seroprevalence and risk factors for Toxoplasma gondii in swine tested by modified agglutination test.
| Factor | Classification standards | Classification standards | No. of samples | Seroprevalence (%) | Odds ratio (95% confidence interval) | p-value |
|---|---|---|---|---|---|---|
| Province | Shaanxi | – | 1867 | 1.50% | 1.005 (0.5098–2.184) | 0.8850 |
| Henan | – | 703 | 1.42% | 1 | ||
| Growth stages | Fattening pig | 11–20 week | 595 | 2.18% | 9.896 (0.5853–167.3) | 0.0296a,* |
| Adult pig | >21 week | 1328 | 1.73% | 5.026 (1.128–22.39) | 0.0188b,* | |
| Nursery pig | 5–10 week | 452 | 0.44% | 3.966 (0.9309–16.89) | 0.0442c,* | |
| Suckling pig | 0–4 week | 213 | 0% | 1 | ||
| Sex | Male | – | 104 | 1.92% | 1.231 (0.2837–5.344) | 0.7807 |
| Female | – | 1276 | 1.57% | 1 | ||
| Pregnancy | No | Replacement gilt, waiting for breeding | 292 | 3.42% | 2.172 (0.8473–5.568) | 0.0983 |
| Yes | – | 498 | 1.61% | 1 | ||
| Health condition | Health | – | 1821 | 1.81% | 4.889 (0.2977–80.29) | 0.1216 |
| Unhealthy | Illness, loss of appetite, miscarriage | 130 | 0% | 1 | ||
| Parity | Primiparous | – | 104 | 1.92% | 1.110 (0.2396–5.141) | 0.8940 |
| Multiparous | – | 576 | 1.74% | 1 | ||
| Sampling time | 2015–2017 | – | 2798d | 9.94% | 7.679 (5.447–10.83) | <0.0001* |
| 2019–2020 | This study | 2683 | 1.42% | 1 |
Note: *Statistically significant (p < 0.05).
Fattening pigs versus suckling pigs.
Fattening pigs versus nursery pigs.
Nursery pigs versus adult pigs.
Data were obtained from Su et al. [16].
These samples were collected from central China and detected by MAT.
Statistical analysis was performed using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). The results were analyzed by the Chi-square or Fisher’s exact test and the Monte Carlo test of simulated data to assess the risk factors associated with T. gondii infection. A p value of <0.05 was considered statistically significant.
Results
The total seroprevalence of T. gondii antibodies was 1.42% (38/2683, 95% CI, 1.03–1.94) in swine. Titers of 1:25 in 17, 1:50 in 15, 1:100 in three, 1:200 in one, and 1:400 in two were found (Table 1). The seroprevalence values for T. gondii in swine were 2.64% (7/265, 95% CI, 1.18–5.46) in 2019 and 1.28% (31/2418, 95% CI, 0.90–1.82) in 2020, but there was no significant difference between them (p = 0.0754). In Henan and Shaanxi, the seroprevalence values of T. gondii were 1.42% (10/703, 95% CI, 0.74–2.64) and 1.50% (28/1867, 95% CI, 1.03–2.17), respectively. However, the difference between the two provinces was not statistically significant (p = 0.8850). Also, no positive serum in swine was observed in Anhui (0/27) and Shanxi (0/86) provinces (Tables 1 and 2, Fig. 1).
The swine were divided into four groups according to their growth stages. No T. gondii antibodies were detected in suckling pigs (0/213). The seroprevalence of T. gondii was 0.44% (2/452, 95% CI, 0.01–1.71) for nursery pigs, 2.18% (13/595, 95% CI, 1.24–3.74) for fattening pigs, and 1.73% (23/1328, 95% CI, 1.14–2.60) for adult pigs. A comparison of different growth stages showed that the seroprevalence of T. gondii in pigs at the fattening stage was significantly higher than in suckling pigs (p = 0.0296) and nursery pigs (p = 0.0188). In addition, the seroprevalence of T. gondii in adult pigs was significantly higher than that in nursery pigs (p < 0.05), and there was no significant difference in other stages. In terms of males and females, pregnant sows and non-pregnant sows, primiparous sows and multiparous sows, there were no significant differences (p > 0.05) (Tables 1 and 2).
Discussion
Pork production is an important economic industry for many countries, including China. Toxoplasma gondii infection in swine threatens pork production and food security [8]. Therefore, it is necessary to understand the T. gondii infection status in swine from China. In this survey (2019–2020), the seroprevalence of T. gondii detected by MAT was 1.42% (38/2683) in swine from central China. According to the isolation results of T. gondii from 1000 swine hearts by bioassays in mice or cats, the percentage of isolation of viable T. gondii from cardiac tissue with MAT antibody titers in swine was: 37.1% (1:20), 38.1% (1:40), 60% (1:80), 75% (1:200) [7]. A titer of 1:25 in 17, 1:50 in 15, 1:100 in three, 1:200 in one, and 1:400 in two were found in this study (Table 1), which indicated that about 15 swines contained viable T. gondii parasites. The seroprevalence survey of T. gondii in this study (2019–2020) was lower than the results for swine sampled from 2015 to 2017 (9.94%, 278/2798) (p < 0.05); these samples were also collected from central China and tested using MAT [16]. The seroprevalence of T. gondii in swine from four provinces (Henan, Shaanxi, Anhui, and Shanxi) from 2003 to 2018 was summarized, and it was found to be higher than the seroprevalence obtained in this study (p < 0.05) (Fig. 2). Thus, the seroprevalence of T. gondii in swine significantly decreased after 2018. This difference may be related to the outbreak of ASF in China. The African swine fever virus (ASFV) first appeared in China in 2018 and spread rapidly to several provinces. ASFV was transmitted fast and showed a high mortality rate [3]. ASFV could be transmitted through various ways, including pigs and pork movement, the water and food supply chain, birds, arthropods, mammals, and mechanical vectors [3, 19]. At the beginning of the ASF outbreak, strict biosecurity measures were implemented to control this disease on all swine farms in China. Treatment measures included restricting human access to the farm, control of rats, mice, cats, birds, flies, and mosquitoes, frequent cleaning and disinfection, commercial feed, and using filtered water by carbon filter, and sealing feed and water. The main routes of swine infection by T. gondii include the consumption of meat containing tissue cysts (kitchen garbage), ingestion of oocyst-contaminated water or food, and contact mechanical carriers [5, 14]. These biosecurity practices against ASF also cut off the route of T. gondii transmission in swine, especially the oocyst route of transmission. This finding coincided with that of Gazzonis et al. [10]: they also found that the application stricter biosecurity procedures may decrease the risk of T. gondii infection on intensive swine farms.
Figure 2.
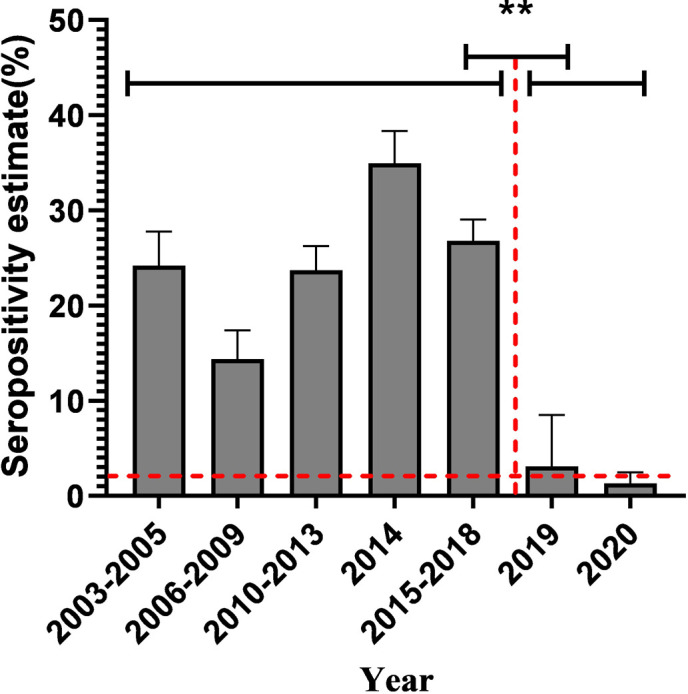
Comparison of seroprevalence of T. gondii in swine from four provinces before and after 2018. The four provinces were Henan, Shaanxi, Anhui and Shanxi. Data were obtained from Dong et al. [4] and Dubey et al. [8]. **p < 0.001.
There was no significant difference in the seroprevalence of T. gondii infection in pregnancy status, delivery number in sows, and sex. These findings are consistent with other studies [5, 8, 20]. In this experiment, the seropositivity increased with the age of the swine, and the seroprevalence of T. gondii was the highest in fattening pigs and the lowest in suckling piglets. Significant differences were found between older and younger swine (p < 0.05). This result confirmed that most T. gondii infection cases occur after birth, which is consistent with other reports [5, 8]. Here, the seroprevalence of T. gondii in adult swine is lower than that of fattening swine. Pregnant sows infected with T. gondii may miscarry or give birth to stillborn offspring, and severe toxoplasmosis can lead to death [8, 12]. Some sows may be culled due to decreased production efficiency after infection with T. gondii.
Anti-T. gondii maternal antibodies could be transmitted through milk to the piglets and begin to decline and disappear after weaning (3–4 months) [8, 9]. Low maternal antibodies in piglets indicated a low seroprevalence of T. gondii in sows. In this study, the seroprevalence rates of T. gondii in suckling piglets and nursery pigs were 0% and 0.44%, respectively. The low serum-positive rate of T. gondii in these piglets also indicated that the risk of T. gondii infection in the sows was decreased.
The global seroprevalence of T. gondii in swine was summarized by Dubey et al., it was 30.0% (21510/7182) in 2009–2017, and it was 11.2% (98/876) after 2018 [8]. This study showed that the seroprevalence of T. gondii antibodies in swine in central China was 1.42%, which was significantly lower than before the ASF outbreak. It indicates that strict biosecurity measures have greatly reduced the risk of T. gondii infection in swine from China. However, T. gondii still exists in swine and cannot be ignored. It is necessary to cook pork thoroughly, or freeze it completely, before consumption [13].
Declarations
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests. None of the authors of this report have financial or personal relationships with other people or organizations that could inappropriately influence its content.
Funding
This study was financed by the Higher Education Teaching Reform Research and Practice Project of Henan Province in 2019 (2019SJGLX006Y), China Agriculture Research System (CARS-35), the Key research projects of Henan higher education institutions (21A230009), and Natural Science Foundation of Henan Province (202300410214).
Authors’ contributions
WTX collected samples. SLX performed the data analysis and wrote the manuscript. NJ helped in collecting and testing samples. YRY designed the experiment and wrote the manuscript. GPZ, LXZ, and XRL helped in revision of the manuscript. All authors have read and approved the final version of the manuscript.
Cite this article as: Xie W, Xin S, Jiang N, Zhang G, Zhang L, Li X & Yang Y. 2021. Lower seroprevalence of Toxoplasma gondii in swine from central China after an outbreak of African swine fever. Parasite 28, 55.
Footnotes
Weitao Xie, and Shilin Xin contributed equally.
References
- 1.Alarcón LV, Alberto AA, Mateu E. 2021. Biosecurity in pig farms: a review. Porcine Health Management, 7(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng FW. 2020. Harm and control of African swine fever. Animal Husbandry and Veterinary Science, 13, 75–76. (in Chinese). [Google Scholar]
- 3.Dixon LK, Sun H, Roberts H. 2019. African swine fever. Antiviral Research, 165, 34–41. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Su R, Lu Y, Wang M, Liu J, Jian F, Yang Y. 2018. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000–2017) from China. Frontiers in Microbiology, 9, 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey JP. 2010. Toxoplasmosis of animals and humans. Boca Raton, Florida, USA: CRC Press, Taylor & Francis Group. p. 1–313. [Google Scholar]
- 6.Dubey JP, Desmonts G. 1987. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal, 19(4), 337–339. [DOI] [PubMed] [Google Scholar]
- 7.Dubey JP, Thulliez P, Powell EC. 1995. Toxoplasma gondii in Iowa sows: comparison of antibody titers to isolation of T. gondii by bioassays in mice and cats. Journal of Parasitology, 81, 48–53. [PubMed] [Google Scholar]
- 8.Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Hill D, Yang Y, Su C. 2020. All about Toxoplasma gondii infections in pigs: 2009–2020. Veterinary Parasitology, 288, 109185. [DOI] [PubMed] [Google Scholar]
- 9.García-Bocanegra I, Simon-Grifé M, Sibila M, Dubey JP, Cabezón O, Martín G, Almería S. 2010. Duration of maternally derived antibodies in Toxoplasma gondii naturally infected piglets. Veterinary Parasitology, 170(1–2), 134–136. [DOI] [PubMed] [Google Scholar]
- 10.Gazzonis AL, Marangi M, Villa L, Ragona ME, Olivieri E, Zanzani SA, Giangaspero A, Manfredi MT. 2018. Toxoplasma gondii infection and biosecurity levels in fattening pigs and sows: serological and molecular epidemiology in the intensive pig industry (Lombardy, Northern Italy). Parasitology Research, 117(2), 539–546. [DOI] [PubMed] [Google Scholar]
- 11.Hide G. 2016. Role of vertical transmission of Toxoplasma gondii in prevalence of infection. Expert Review of Anti-infective Therapy, 14, 335–344. [DOI] [PubMed] [Google Scholar]
- 12.Jiang HH, Wang SC, Huang SY, Zhao L, Wang ZD, Zhu XQ, Liu Q. 2016. Genetic characterization of Toxoplasma gondii isolates from pigs in Jilin Province, Northeastern China. Foodborne Pathogens & Disease, 13(2), 88–92. [DOI] [PubMed] [Google Scholar]
- 13.Kotula AW, Dubey JP, Sharar AK, Andrews CD, Shen SK, Lindsay DS. 1991. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. Journal of Food Protection, 54(9), 687–690. [DOI] [PubMed] [Google Scholar]
- 14.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet, 363(9425), 1965–1976. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Ferreira F, Caldart ET, Pasquali AKS, Mitsuka-Breganó R, Freire RL, Navarro IT. 2019. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerging Infectious Diseases, 25(12), 2177–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su R, Jiang N, Lu Y, Jian F, Wang H, Zhang G, Zhang L, Yang Y. 2020. Low prevalence of viable Toxoplasma gondii in swine from slaughter houses in the central of China. Parasitology International, 76, 102090. [DOI] [PubMed] [Google Scholar]
- 17.Waldman BS, Schwarz D, Wadsworth MH 2nd, Saeij JP, Shalek AK, Lourido S. 2020. Identification of a master regulator of differentiation in Toxoplasma. Cell, 180(2), 359–372.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang T, Luo Q, Huo X, Wang L, Liu T, Xu X, Wang Y, Lu F, Lun Z, Yu L, Shen J. 2012. Prevalence and genotypes of Toxoplasma gondii in pork from retail meat stores in Eastern China. International Journal of Food Microbiology, 157(3), 393–397. [DOI] [PubMed] [Google Scholar]
- 19.Wang BY, Liu YL, Ma J, Ma Y, Cao Y, Ni JQ, Wang CB, Liu Y. 2020. African swine fever: progress and analysis of its source and transmission route. Chinese Journal of Zoonotic Infectious Diseases, 28(5), 103–110. (in Chinese). [Google Scholar]
- 20.Wu F, Wang YL, Yang Z, Li XL, Li ZR, Lin Q. 2017. Seroprevalence and risk factors of Toxoplasma gondii in slaughter pigs in Shaanxi Province, Northwestern China. Vector Borne and Zoonotic Diseases, 17(7), 517–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.