Abstract
Osteoarthritis is a debilitating disease that results in pain and joint stiffness. Currently, steroidal and nonsteroidal anti-inflammatory drugs and supplements aimed at restoring lubrication to the affected joint are the most successful with respect to improving patient comfort. Due to the success in lubricating therapies, there exists a keen interest to develop better therapies that mimic how lubrication occurs naturally in the joint. Here we describe the results obtained using a chondroitin sulfate chain to which is conjugated peptides that bind to either hyaluronic acid (found in high concentrations in the synovial fluid) or collagen type II (present on the cartilage surface). Our study investigates the effect of binding to the cartilage surface and interacting with hyaluronic acid on lubrication at the cartilage surface. The results described here suggest that binding to the cartilage surface is critical to supporting lubrication and did not require the addition of hyaluronic acid to reduce friction.
Keywords: Lubrication, Cartilage, Osteoarthritis, Friction, Lubricin, Hyaluronic Acid
Graphical Abstract
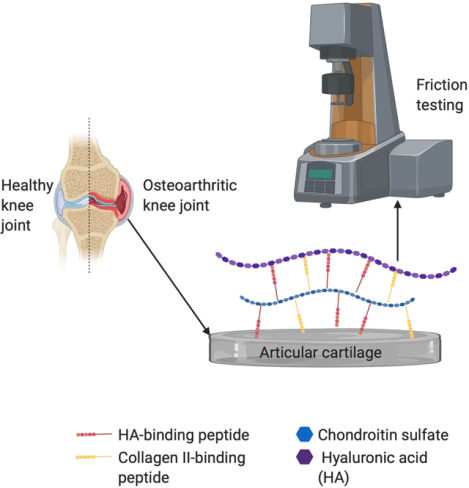
1.0. Introduction
Osteoarthritis (OA) is a painful and debilitating disease, with world-wide estimates suggesting that as many as 250 million people world-wide are afflicted with the disease1. In addition to symptoms of pain, stiffness, and reduced mobility, it is often characterized by the degradation of articular cartilage2. The loss of lubrication in the synovial joints is a primary reason for the decrement of articular cartilage. Therefore, it is believed that further damage to articular cartilage can be avoided if proper lubrication is restored through lubricant supplement treatments. Various hyaluronic acid (HA)-based injectable treatments, termed viscosupplements, are in use today, but there is little clinical evidence to support that these products are effective in treating the long-term effects of osteoarthritis3.
Lubricin, a lubricating proteoglycan found in the human synovial fluid, has become a potential alternative to HA injections. Lubricin concentration in the synovial fluid is often diminished in people with osteoarthritis4, making replenishment of lubricin an attractive target. Importantly, native lubricin interacts with cartilage oligomeric matrix protein (COMP), fibronectin and collagen type II on the cartilage surface, but COMP is susceptible to matrix metalloprotease cleavage, which in turn liberates lubricin form the surface5. Current research has focused on making synthetic, injectable lubricants, which mimic the lubricating function of native lubricating polymers but differ in structure and enzymatic susceptibility6–10. As yet, the synthetic lubricin molecules have not been shown to be as effective at reducing friction at the articular surface as native molecules; which suggests that targeting lubricin binding to the surface molecules other than COMP may be beneficial. Native lubricin interacts with both the cartilage surface and macromolecules within the synovial fluid, namely HA, and these interactions are thought to play a prominent role in friction reduction11. With the goal of defining the degree to which surface interaction and interaction with HA play a role in reducing friction at the articular cartilage surface, we have investigated the in vitro friction reduction properties of a molecule containing a chondroitin sulfate (CS) backbone to which is conjugated varying numbers of HA- and Col II-binding peptides (Fig. 1A). Herein we describe the synthesis and the function at articular surfaces and demonstrate that surface binding of CS is critical to function, whereas binding to HA appears to be less important and potentially unnecessary.
Figure 1.
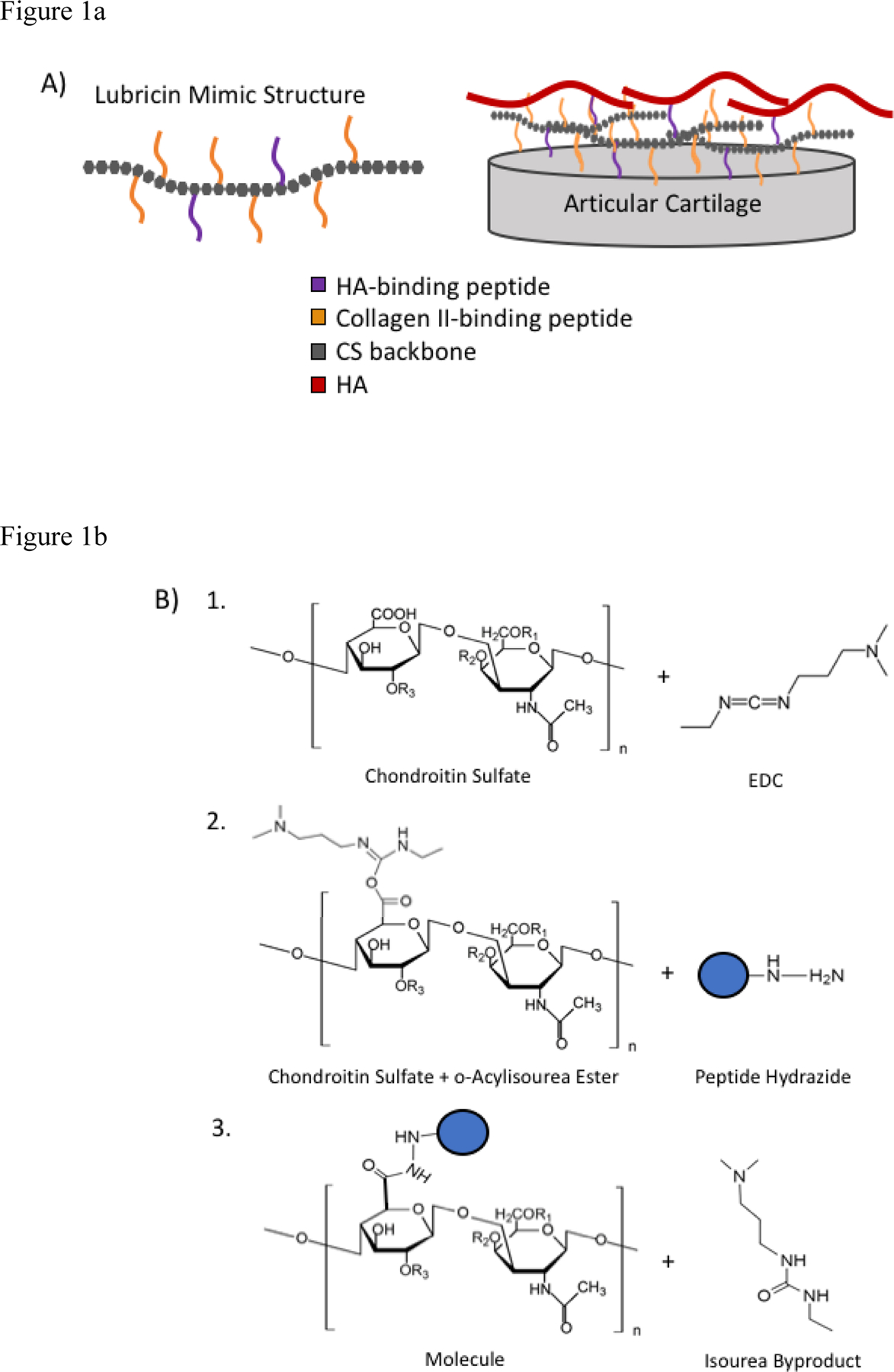
A) Design of biomimetic lubricin. B) Schematic of lubricin mimic synthesis using EDC chemistry and peptide hydrazides. R1=H, R2=SO4, and R3=H for chondroitin 6-sulfate.
2.0. Materials and Methods
2.1. Peptide Synthesis
The collagen type II-binding peptide WYRGRLGSG (WYR)12 and HA-binding peptide GAHWQFNALTVRGGGSG (GAH)13 were synthesized using a using standard Fmoc solid phase peptide synthesis8. Both GAH and WYR were synthesized on a Cl-(Trt)-Cl resin each with a hydrazide group coupled first to the CL-(Trt)-CL resin followed by a GSG spacer followed by the peptide sequence. Biotinylated peptides had an extra biotin group added at the N-terminus before peptide cleavage. To purify the peptides, a Vydac C18 column (Grace Davison Discovery Sciences, Deerfield, IL) was used on an ÄKTA Explorer 100 FPLC (GE Healthcare, Piscataway, NJ) with a 0.1% TFA and acetonitrile gradient. Matrix-assisted laser desorption ionization time-of-flight (MALDI TOF) mass spectrometry on a Voyager DE PRO analyzer (Applied Biosystems, Foster City, CA) was used to verify the molecular weight of the peptides.
2.2. Synthesis of Bioconjugates
Collagen type II- and HA-binding molecules were synthesized using EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) chemistry to conjugate peptide hydrazides to the carboxylic acid groups on chondroitin sulfate (CS). Using an 8 M urea solution and 0.05 mM EDC, the carboxyl groups on the CS backbone were activated at a pH of 4.5 for no more than 5 minutes at room temperature. All of the bioconjugates included the addition of one biotinylated peptide per CS backbone to support biomolecule imaging at the cartilage surface using confocal microscopy. The CS backbone was then functionalized with either WYR or GAH peptides to yield WYR_CS or GAH_CS. To stop the reaction, the pH was adjusted to 8 and allowed to sit at room temperature for 30 minutes. The molecules were purified using size exclusion chromatography on an ÄKTA Purifier FPLC (GE Healthcare, Piscataway, NJ) with Bio-Scale Mini Bio-Gel columns packed with polyacrylamide beads (Bio-Rad Laboratories, Hercules, CA) and then lyophilized. A Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA) was used to quantify the conjugated peptides on the CS backbone. The peptide coupling efficiencies were determined by dividing the amount of bound peptide, as determined by absorbance measurements, by the amount of peptide added to the reaction mix. Hydrazide dye (Alexa Fluor® 488 hydrazide) was conjugated to CS using EDC chemistry for later detection using confocal microscopy.
2.3. Tissue Harvest and Treatment
Osteochondral explants were harvested from 18–36 month old bovine knee joints obtained 24 hours after slaughter (Lampire Biological Laboratories, Inc., Pipersville, PA). The explants were taken from the load bearing regions of the femoral condyle using a 6-mm-diameter coring reamer (Arthrex, Inc., Naples FL) and trimmed to a thickness of approximately 3–4 mm. In order to remove residual synovial fluid, samples were shaken vigorously overnight at 4°C in PBS containing a SigmaFAST protease inhibitor cocktail (PIC) tablet (Sigma-Aldrich, St. Louis, MO) and 0.5% penicillin/streptomycin. Cartilage samples were randomly divided and submerged in a variety of treatment groups and stored at 4°C until mechanical testing took place. Samples were treated for at least 2 hours (or longer depending on in what order they were tested), and testing order was randomized with respect to treatment. Samples were tested within one week of harvest. All of the treatments were dissolved in PBS/PIC solution except for the bovine synovial fluid (Animal Technologies, Tyler, TX). The chondroitin sulfate and bioconjugate treatments were all dissolved at a concentration of ~8.4 μmol, which corresponded to ranges of 0.3 to 0.5 mg/ml of bioconjugate, which correlate to normal concentration of the native molecule in healthy joints9.
2.4. Measurement of Coefficient of Friction
Where Reff represents the radius of the cartilage, in this case 3.0 mm. Static COF was calculated by taking the maximum calculated coefficient of friction during the first 10 degrees (~2 sec). The kinetic COF was calculated by averaging the COF calculated from the second rotation. In all, at least eight samples (n=8) were tested per treatment group. All of the osteochondral plugs were tested within one week following harvest.
2.5. Immunostaining of Peptidoglycans at Cartilage Surface
Following tribological testing, the cartilage surface (~1 mm thick) was sliced off the osteochondral plugs, rinsed three times with PBS, fixed with 4% paraformaldehyde overnight at 4ᵒC, and rinsed three times with PBS. A midsagittal cut was made through the cartilage samples; the two halves were embedded in O.C.T compound (Tissue Tek) and frozen at −80ᵒC. The frozen samples were sectioned at 7 μm thickness, air dried, and stored at −20ᵒC for immunostaining. For detection of biotin-labeled bioconjugates at the cartilage surface, the sections were rinsed with PBS to remove residual O.C.T. compound and blocked with 1% BSA (lyophilized powder, ≥96% (agarose gel electrophoresis), Sigma-Aldrich) for 30 minutes at room temperature. The sections were immunostained for 15 minutes at room temperature with Alexa Fluor® Streptavidin 633 (Thermo Scientific) diluted 1:200 in 1% BSA and counterstained with DAPI diluted 1:500 in the same solution. The sections were rinsed and mounted with ProLong Gold antifade mounting medium (Thermo Scientific) before they were imaged (CS control at 488 and all other surfaces at 633) under a confocal microscope (Leica Microsystems, Buffalo Grove, IL). Chondroitin sulfate-Alexa Fluor® 488 (Thermo Scientific) conjugates with no biotinylated peptides were analyzed as a control.
2.6. Statistical Analysis
Single factor equal variance ANOVA and Tukey’s post hoc tests were performed using GraphPad Prism for analysis of static and kinetic friction coefficients. A probability value of 95% (P < 0.05) was used to determine statistical significance.
3.0. Results and Discussion
Previously, a lubricin mimic was developed in our lab that was termed mLUB due to its ability to mimic some lubricin function15. After comparing different ratios, the most effective formulation of mLUB had 1 mol of a CS backbone covalently bound to ~15 mol collagen type II-binding peptides (named WYR), which bound the cartilage surface and thus created a protective boundary layer similar to that of native lubricin, and ~5 mol HA-binding peptides (named GAH), which allowed the lubricin mimic to also bind to HA either within the cartilage or in the synovial fluid at the cartilage surface and thus imitate lubricin-HA interactions. The peptides were covalently attached using an oxidation process that included a BMPH (N-β-maleimidopropionic acid hydrazide) crosslinker. This synthesis method resulted in opening many of the rings on the CS backbone thereby producing a random coil polymer as opposed to the more rigid structure of unmodified CS. Furthermore, the stability of the molecule was compromised due to the oxidation step, which reduced shelf-life of the molecules. To remedy these issues, EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) chemistry was used here to conjugate peptide hydrazides to the carboxylic acid groups on the CS backbone and form amide bonds between the peptide and the CS (Fig. 1B).
In addition to modifying the chemistry, we hypothesized that the interaction of the CS-peptide bioconjugate with HA at the articular cartilage surface was necessary for proper lubrication due to previous studies showing lubricin/HA interactions16. In contrast to our prior results with mLUB15, preliminary tests surprisingly showed that the addition of the bioconjugates reduced friction at the cartilage-glass interface without the addition of HA. In fact, as shown in supplemental data Figure S1, neither the addition of high molecular weight HA nor the addition of low molecular weight HA to the testing medium further reduced friction beyond that seen with the 2GAH_13WYR_CS. This finding is consistent with studies demonstrating that HA is perhaps more important to prevent wear rather than reduce friction17. To further understand our initial results, a series of friction tests were completed to determine which components of the synthesized lubricin mimetic (GAH or WYR) were crucial for reducing friction at the cartilage surface. Further, by varying the molar ratios of HA-binding or collagen II-binding peptide attached to the CS backbone, we investigated the number of each peptide needed to reduce friction at the cartilage surface. Ultimately, this study provided additional insights into the molecular interactions contributing to reduced friction at the cartilage surface.
Bioconjugates with a variety of peptide-CS conjugation ratios were synthesized (Table 1) for evaluation of their ability to reduce friction at the surface. In particular, 2GAH_13WYR_CS, which contained one molecule of CS conjugated to 2 mol of HA-binding peptide (GAH) and 13 mol of collagen II-binding peptide (WYR), was designed to contain ~15 WYR peptides as did the successful mLub formulation15. As noted in Table 1, CS was conjugated to varying ratios of either the GAH or WYR peptides to investigate the contributions of GAH and WYR to limiting friction at the surface.
Table 1.
Biotinylated bioconjugate synthesis and peptide quantification
| WYR Addition | ||||||
|---|---|---|---|---|---|---|
| Molecule | WYR added (mols per CS) | Absorbance@280 nm | Peptide Bound (mols per CS) | Coupling Efficiency | CoF (kinetic) | CoF (static) |
| 2GAH_13WYR_CS | 16.5 | 2.1 | 13.2 | 80% | 0.19±0.04 | 0.22±0.02 |
| 5WYR_CS | 5.5 | 1.1 | 5.5 | 100% | 0.30±0.01 | 0.26±0.03 |
| 9WYR_CS | 11 | 1.6 | 9.0 | 82% | 0..26±0.05 | 0.23±0.03 |
| 15WYR_CS | 16.5 | 2.2 | 14.6 | 89% | 0.19±0.04 | 0.22±0.03 |
| GAH Addition | ||||||
| Molecule | GAH added (mols per CS) | Absorbance@280 nm | Peptide Bound (mols per CS) | Coupling Efficiency | CoF (kinetic) | CoF (static) |
| 2GAH_13WYR_CS | 2.4 | 0.4 | 2.0 | 84% | 0.19±0.04 | 0.22±0.02 |
| 2GAH_CS | 2.4 | 0.4 | 2.0 | 84% | 0.31±0.04 | 0.27±0.03 |
| 4GAH_CS | 5.5 | 0.7 | 4.0 | 73% | 0.31±0.02 | 0.26±0.02 |
| 9GAH_CS | 11 | 1.3 | 9.0 | 82% | 0.27±0.02 | 0.23±0.02 |
| 12GAH_CS | 16.5 | 1.7 | 12.3 | 75% | 0.28±0.03 | 0.26±0.02 |
The static and kinetic coefficient of friction (COF) for each treatment was measured with a rheometer using a cartilage-on-glass setup (Fig. 2). Synovial fluid was used as a positive control to mimic the COF of articular cartilage in the synovial joint. The results show that none of the treatments were able to match the static COF of synovial fluid (Fig. 2A). However, 15WYR_CS reduced the static friction more than phosphate buffered saline (PBS), CS, and 2GAH. The 2GAH_13WYR_CS molecule also had a lower static COF than PBS and CS. All the other treatments, excluding synovial fluid, were not statistically different from the CS or PBS samples. These findings indicate that the presence of a high amount of WYR can reduce the static COF more effectively than the other synthesized molecules, including those with a high amount of GAH attached to the CS backbone.
Figure 2.
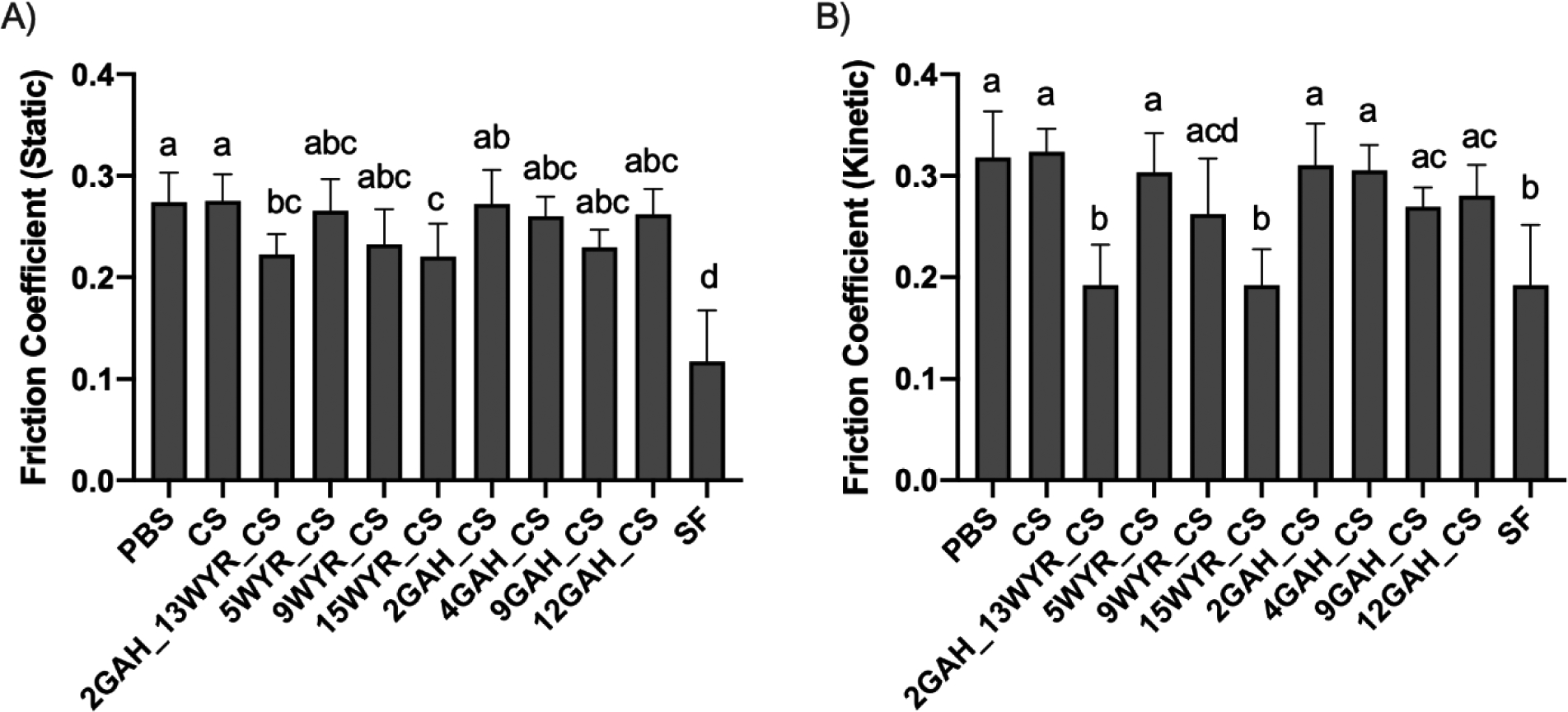
Coefficient of friction (COF) values measured at the cartilage surface. A) Static and B) kinetic COF were measured with PBS; 2GAH_13WYR_CS; 5, 9, or 15WYR; 2, 4, 9, or 12GAH; and synovial fluid (SF) treatments. Groups (n=8) with the same letter are statistically similar (p > 0.05).
The kinetic COF for both 15WYR_CS and 2GAH_13WYR CS were statistically the same as the SF treatment (Fig. 2B). None of the other treatments were able to match the kinetic COF of the synovial fluid sample. The kinetic COF of 12GAH_CS was statistically the same as the PBS control, and this result indicates that, in the range tested, increasing the amount of GAH bound to the CS backbone did not improve the ability of GAH_CS to lubricate cartilage. The addition of WYR to the lubricin mimic was found to be crucial for lowering the kinetic COF. We did observe that 9WYR_CS did not significantly reduce the kinetic COF. A higher collagen II-binding peptide concentration of ~14.6 mols of WYR per mol of CS (15WYR_CS) was required to sufficiently reduce the kinetic COF. Increasing the amount of WYR most likely increased the binding efficiency of the lubricin mimic to the osteochondral plug surface and allowed it to stay in place during the repetitive motion experienced during friction testing. Treatments with fewer peptides could have been worn away due to the high shear forces present during rheology testing of the osteochondral plugs. To test this hypothesis, we imaged the molecules on the cartilage plugs after rheological testing.
To determine whether 15WYR_CS and 2GAH_13WYR_CS created a protective boundary layer at the articular cartilage surface similar to that provided by lubricin in native cartilage, we imaged the various molecules bound to the cartilage surface following friction testing. From the confocal images, we observed that some bioconjugates remained bound to the cartilage surface better than others (Fig. 3). As expected, the images showed no fluorescence at the cartilage surface for the cartilage plugs treated with PBS or fluorescently labeled CS. The WYR_CS treatments appeared to be bound at the plug surface in higher concentrations compared to the GAH_CS formulations. This finding supports the claim that WYR facilitated bioconjugate adsorption to the cartilage surface. Although 9WYR_CS showed a significant amount of molecule present (Fig. 3), friction testing showed that it did not significantly reduce the friction at the articular cartilage surface (Fig. 2). On closer examination, there appeared to be discontinuations of the 9WYR_CS coating on the cartilage surface (Fig. 3). This result suggests that a complete protective boundary layer is required for effective lowering of the COF. This claim is supported by evidence suggesting that both 15WYR_CS and 2GAH_13WYR_CS effectively coated the entire surface (Fig. 3) and significantly reduced the static and kinetic COF (Fig. 2). We observed the presence of the GAH_CS molecules bound to the cartilage surface (Fig. 3) despite the fact that GAH was not included for its ability to bind cartilage. Although collagen II is the most prominent component of articular cartilage, there was enough HA present within the tissue to allow GAH_CS to bind but at lower levels to those seen when using the WYR peptide. It is possible that if the GAH_CS molecules could be redesigned to increase binding to the cartilage surface that the GAH_CS molecules would also be able to form a protective boundary layer that reduces friction. Ultimately, only the 15WYR_CS and 2GAH_13WYR_CS were seen to create a full protective boundary layer at the articular cartilage surface and reduce the COF. Importantly, the reduction of the kinetic COF observed when testing 15WYR_CS matched that seen with synovial fluid.
Figure 3.
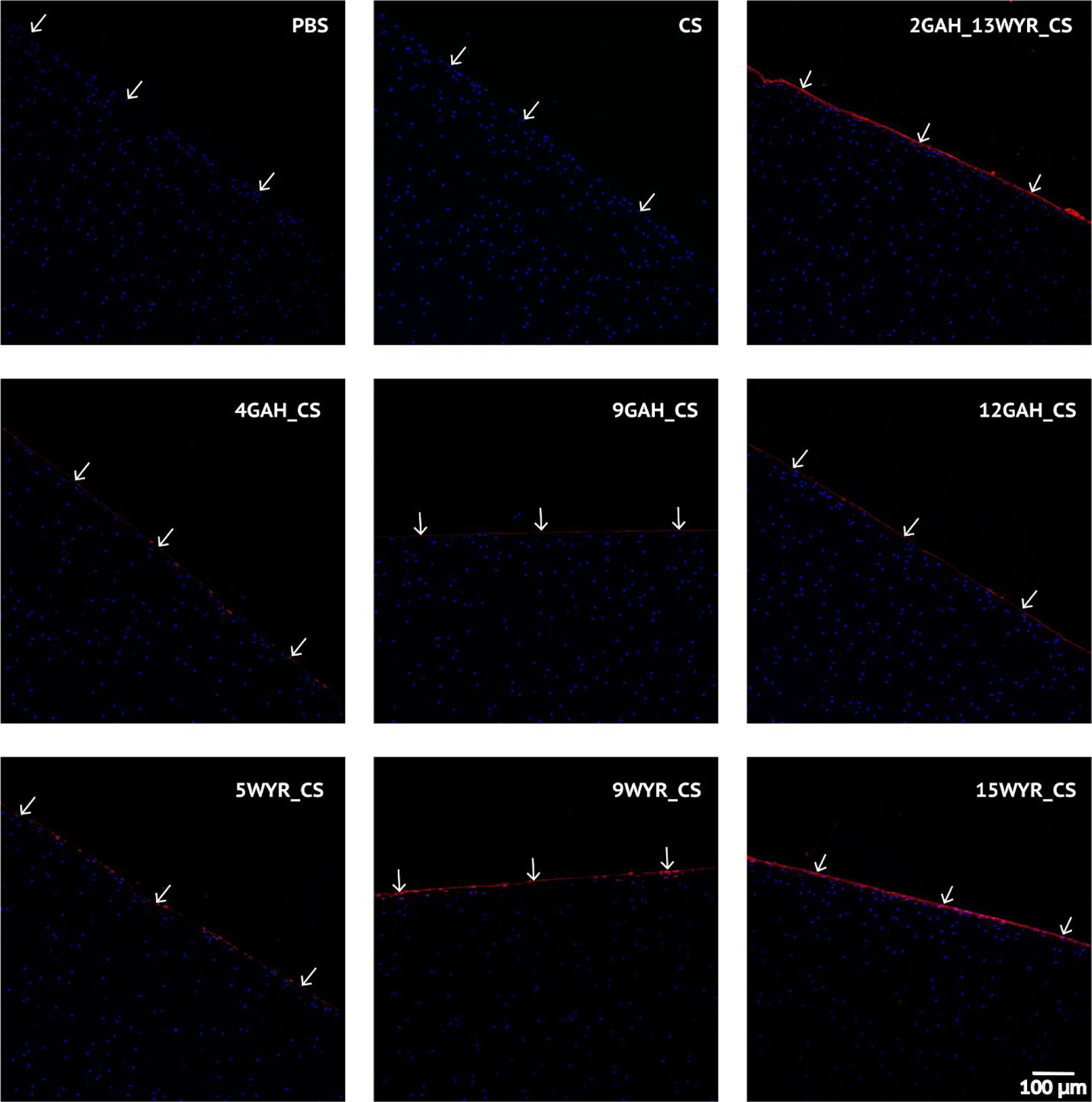
Confocal imaging of post-rheology cartilage samples. The nuclei of chondrocytes were stained with DAPI (blue). The biotinylated peptidoglycan treatments at the cartilage surface were stained with Alexa Fluor® Streptavidin 633 (red). White arrows indicate cartilage surface.
4.0. Conclusions
The 15WYR_CS bioconjugate significantly reduced the coefficient of friction at the articular cartilage surface. The addition of collagen II-binding peptides to the synthesized biomolecule was shown to be crucial to the attachment and development of complete coverage of the molecule to the articular cartilage surface. Like lubricin, the key player in cartilage lubrication, the bioconjugate was able to successfully create a protective boundary layer at the articular cartilage surface. Unlike previous designs, the 15WYR_CS bioconjugate synthesized with only collagen II-binding peptides did not require the addition of HA to successfully reduce friction at the articular cartilage surface. The benefits to this molecule formulation are not only ease of synthesis but also an increased potential for the molecule to reduce friction in the presence of degradation enzymes such as hyaluronidase that target HA, especially in an osteoarthritic condition. Furthermore, concentrations of HA are significantly reduced in joints affected by osteoarthritis. Thus, it appears that the osteoarthritic environment would not hinder the effectiveness of 15WYR_CS as much as formulations that incorporate HA since 15WYR_CS did not require HA to successfully reduce friction at the articular cartilage surface. With these considerations, 15WYR_CS showed potential as a long-term supplemental lubrication treatment for OA patients; however, further in vitro and in vivo analysis is necessary to fully substantiate this claim.
Supplementary Material
Highlights.
Glycosaminoglycan binding to the cartilage surface is critical to reducing friction
Hyaluronic acid may not be required to reduce friction at the cartilage surface
Peptide-glycosaminoglycan conjugates can reduce cartilage friction
Acknowledgements
Support for this work was provided via funding from The National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, R01065398
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Hu X, Cheng J, et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat Commun 2019;10:1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: From standard procedures to the synthesis of difficult sequences. Nature Protocols 2007;2:3247–56. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis Rheum 2012;64:3963–71. [DOI] [PubMed] [Google Scholar]
- 5.Thorson C, Galicia K, Burleson A, et al. Matrix Metalloproteinases and Their Inhibitors and Proteoglycan 4 in Patients Undergoing Total Joint Arthroplasty. Clin Appl Thromb Hemost 2019;25:1076029619828113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wathier M, Lakin BA, Bansal PN, Stoddart SS, Snyder BD, Grinstaff MW. A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage. Journal of the American Chemical Society 2013;135:4930–3. [DOI] [PubMed] [Google Scholar]
- 7.Cooper BG, Lawson TB, Snyder BD, Grinstaff MW. Reinforcement of articular cartilage with a tissue-interpenetrating polymer network reduces friction and modulates interstitial fluid load support. Osteoarthritis Cartilage 2017;25:1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgese G, Cavalli E, Müller M, Zenobi-Wong M, Benetti EM. Nanoassemblies of Tissue-Reactive, Polyoxazoline Graft-Copolymers Restore the Lubrication Properties of Degraded Cartilage. ACS Nano 2017;11:2794–804. [DOI] [PubMed] [Google Scholar]
- 9.Samaroo KJ, Tan M, Putnam D, Bonassar LJ. Binding and lubrication of biomimetic boundary lubricants on articular cartilage. Journal of Orthopaedic Research 2017;35:548–57. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Feeney E, Guan Y, et al. Boundary mode lubrication of articular cartilage with a biomimetic diblock copolymer. Proc Natl Acad Sci U S A 2019;116:12437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang DP, Abu-Lail NI, Coles JM, Guilak F, Jay GD, Zauscher S. Friction Force Microscopy of Lubricin and Hyaluronic Acid between Hydrophobic and Hydrophilic Surfaces. Soft Matter 2009;5:3438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater 2008;7:248–54. [DOI] [PubMed] [Google Scholar]
- 13.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med 2000;192:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2007;15:35–47. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence A, Xu X, Bible MD, Calve S, Neu CP, Panitch A. Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface. Biomaterials 2015;73:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jay GD, Lane BP, Sokoloff L. Characterization of a bovine synovial fluid lubricating factor. III. The interaction with hyaluronic acid. Connective tissue research 1992;28:245–55. [DOI] [PubMed] [Google Scholar]
- 17.Lee DW, Banquy X, Das S, Cadirov N, Jay G, Israelachvili J. Effects of molecular weight of grafted hyaluronic acid on wear initiation. Acta Biomater 2014;10:1817–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


