Abstract
Background
B cells play an important role in allergies through secretion of IgE. IL-4 receptor α (IL-4Rα) is key in allergic asthma and regulates type 2 cytokine production, IgE secretion, and airway hyperresponsiveness. IL-4 activation of B cells is essential for class switching and contributes to the induction of B effector 2 (Be2) cells. The role of Be2 cells and signaling via IL-4Rα in B cells is not clearly defined.
Objective
We sought to find out whether IL-4Rα–responsive B cells or Be2 function was essential in experimental allergic asthma.
Methods
Mice lacking IL-4Rα on B cells (mb1creIL-4Rα−/lox) or littermate controls (IL-4Rα−/lox) and mice lacking IL-4 or IL-4/IL-13 on B cells were sensitized and challenged with high-dose house dust mite (>10 μg) or with low-dose house dust mite (<3 μg). We also adoptively transferred naive IL-4Rα−/lox or IL-4Rα−/− B cells into μMT−/− mice a day before sensitization or a day before challenge. We analyzed lung inflammation, cellular infiltrate, and airway hyperresponsiveness.
Results
We found that IL-4Rα signaling on B cells was important for optimal TH2 allergic immune responses mainly when the load of antigen is limited. IL-4Rα signaling on B cells was essential for germinal centers and in the effector phase of allergic responses. Be2 cells were essential in airway hyperresponsiveness, but not in other parameters.
Conclusions
IL-4Rα signaling on B cells is deleterious in allergic asthma because it is required for optimal TH2 responses, Be2 function, germinal center formation, and T follicular helper cells, especially when the load of the antigen is limiting.
Key words: IL-4Rα, TH2 cells, B cells, germinal center, T follicular helper cell, B effector 2 cells
Abbreviations used: AHR, Airway hyperresponsiveness; APC, Allophycocyanin; Be2, B effector 2; GC, Germinal center; HDM, House dust mite; IL-4Rα, IL-4 receptor alpha; mLN, Mediastinal lymph node; TFH, T follicular helper
Graphical abstract
Asthma is a chronic debilitating disease affecting more than 300 million people worldwide, with at least 250,000 people dying from complications associated with the disease.1 The immune response to the disease is characterized by TH2 immune cells such as eosinophils and type 2 cytokines IL-4, IL-5, and IL-13 and B cells secreting IgE.2,3 Secreted IgE binds to high-affinity receptors FcεR on the surfaces of mast cells and basophils, resulting in activation and degranulation of these cells and release of histamines, proteases, and membrane phospholipids such as leukotrienes and prostaglandins.4,5 Priming of long-lived type 2 memory T cells is attributed to dendritic cells,6,7 with earlier studies demonstrating a minimal contribution from B cells, despite their ability to present antigens to T cells.8,9
The role of B cells in experimental allergic asthma is contradictory; earlier studies using ovalbumin as a model antigen showed a redundant role for B cells in allergic asthma.10, 11, 12 Mice deficient of B cells (μMT−/−) developed similar airway hyperresponsiveness (AHR), eosinophilia, and TH2 airway responses when sensitized and challenged with ovalbumin.10, 11, 12 More recent evidence using a clinically relevant allergen, house dust mite (HDM), suggests an essential function of B cells in allergic asthma.13, 14, 15 However, despite empirical evidence demonstrating a key role played by B cells in allergic asthma, caveats and contradictions in literature still exist and require further clarification. Some studies have suggested that B cells are either not important at all9 or are essential only in priming of CD4 T cells and induction of T follicular helper (TFH) cells during the sensitization stage, but play no part during challenge stages of HDM-induced asthma.13 Other studies have shown that B cells are essential in both priming of CD4 T cells15 and effector stages of HDM-induced allergic asthma.14 Furthermore, the load of antigen seems to be critical in the involvement of B cells in HDM-induced allergic asthma.14 At high doses of HDM antigen, B cells play minimal role in antigen uptake, processing, and presentation to CD4 T cells, whereas at low doses of HDM antigen, B cells have more access to antigen and can uptake, process, and present antigen to T cells, playing an essential part in the development of TFH cells.14 Interestingly, the inability of B cells to present antigen at the sensitization stage leads to TH1 and TH17 airway responses and not TH2 responses.15
IL-4 receptor alpha (IL-4Rα) is central in TH2 allergic airway asthma16, 17, 18 and other type 2 diseases.19,20 In allergic asthma, we and others have shown temporal21 and cell-specific requirement of IL-4Rα in dendritic cells,22 T cells,23 and epithelial cells24 and also the redundant role of this IL-4/IL-13 signaling receptor in macrophages25 and airway smooth muscle cells.26,27
We have recently shown that early IL-4 production by B cells influences type 2 CD4 T-cell differentiation in lymph nodes, which leads to protective type 2 responses against certain parasitic infections.28, 29, 30 Given the complexity around the importance of B cells in allergic asthma, we set to investigate the role of IL-4Rα signaling, specifically in B cells, during HDM-induced allergic asthma.
We challenged mice with high-dose or low-dose HDM to assess whether antigen load matters in the requirement of IL-4Rα–responsive B cells in allergic asthma. We found that although mice lacking IL-4Rα on B cells (mb1creIL-4Rα−/lox) had reduced AHR at high-dose HDM, in other parameters, they were comparable to littermate control mice. This was contrary to what we observed at low-dose HDM, where mb1creIL-4Rα−/lox mice had reduced AHR, type 2 responses, eosinophilia, TFH cells, and ability to produce TH2 cytokines. By adoptively transferring naive IL-4Rα–deficient B cells into μMT−/− mice sensitized to low-dose HDM, we demonstrated the importance of IL-4Rα signaling on B cells at both sensitization and effector stages. Interestingly, lack of IL-4 or IL-4/IL-13 production by B cells resulted in reduced AHR, which suggested a key contribution in this parameter, but less so in airway inflammation or antibody production.
Here, we show an essential role for IL-4Rα–responsive B cells in optimal type 2 allergic airway inflammation, especially when the load of HDM is limited.
Methods
Mice
To generate mice deficient of IL-4Rα only on B cells (mb1creIL-4Rα−/lox), we intercrossed homozygous mb1cre mice31 with IL-4Rα−/−32 on Balb/c background. We then further mated mb1cre IL-4Rα−/− mice with homozygous IL-4Rαlox/lox mice19 to generate hemizygous mb1creIL-4Rα−/lox33 mice, which were backcrossed up to 10 generations in Balb/c background. Hemizygous littermates (IL-4Rα−/lox) expressing single functional IL-4Rα allele was used as a wild-type control in all experiments. Mice were housed in independently ventilated cages under specific pathogen-free conditions at the University of Cape Town Animal Facility. All mice were used at age 8 to 10 weeks, and animal procedures were performed according to strict recommendation by the South African Veterinary Council and were approved by the University of Cape Town Animal Ethics Committee (reference no. 018/013).
HDM-induced allergic airway disease
A high-dose and a low-dose treatment schedule was used to induce symptoms of allergic asthma in mice.14 Mice were anesthetized with ketamine 80 mg/kg (Anaket-V; Centaur Labs, Johannesburg, South Africa) and xylazine 16 mg/kg (Rompun; Bayer, Isando, South Africa). For the high-dose schedule, mice were sensitized intratracheally on day 0 with 100 μg of HDM (Stellergens Greer Laboratories, Lenoir, NC) and intranasally challenged with 10 μg HDM on days 8, 9, 10, 11, and 12. For low-dose treatment, mice were sensitized with 1 μg and challenged with 3 μg of HDM. AHR was measured on day 15. After the procedure, mice were euthanized and tissue samples were collected for analysis.
Adoptive transfer
Spleens were collected from naive IL-4Rα−/lox, mb1creIL-4Rα−/lox, IL-4−/−, or IL-4/IL-13−/− mice and passed through a 40-μm strainer to obtain single-cell suspensions. Cells were stained with Flourescein isothiocyanate (FITC)-B220 and allophycocyanin (APC)-CD19 for 30 minutes at 4oC. A dead cell exclusion dye (7AAD) was added before sorting on BD FACS Aria I to at least 96% purity. A total of 2 × 106 to 5 × 106 cells were adoptively transferred intravenously into μMT−/− recipient mice a day before HDM sensitization. In other experiments, sorted B cells were adoptively transferred intravenously into low-dose HDM-sensitized mice a day before challenge with low-dose HDM.
Airway hyperresponsiveness
Airway resistance and elastance of the whole respiratory system (airways, lung chest wall) after intranasal challenge were determined by forced oscillation measurements as described previously25 with the Flexivent system (SCIREQ, Montreal, Canada) by using the single-compartment (‘‘snapshot’’) perturbation. Measurements were carried out on mice with increasing doses (0, 5, 10, 20, and 40 mg/mL) of acetyl-β-methylcholine (methacholine, Sigma-Aldrich, Aston Manor, South Africa) treatment. Differences in the dose-response curves were analyzed by repeated-measures 2-way ANOVA with the Bonferroni posttest. Only mice with acceptable measurements for all doses (coefficient of determination >0.90) were included in the analysis.
Flow cytometry
Bronchoalveolar lavage fluid cells were obtained as previously described.26 Single-cell suspensions were prepared from lymph nodes in RPMI media (Gibco, Paisley, United Kingdom) by passing them through a 100-μm strainer. To obtain single-cell suspensions from lung tissues, a left lobe was digested for 1 hour at 37oC in RPMI media containing 13 mg/mL DNase I (Roche, Randburg, South Africa) and 50 U/mL collagenase IV (Gibco, Waltham, Mass) and passed through a 70-μm strainer. Antibodies used in these experiments included the following: phycoerythrobilin-conjugated anti–Siglec-F (clone E50-2440), anti-CD124 (IL-4Rα, clone M-1), anti–IL-5 (clone TRFK5), anti-CD44 (clone KM114), FITC-conjugated anti–Gr-1 (clone RB6-8C5), CD45 (clone 30-F11), IL-4 (clone 11B11), PerCP Cy5.5–conjugated anti-Ly6C (clone AL-21), anti–IL-17 (clone TC11-18H10), APC-conjugated anti-CD11c (clone HL3), anti-FoxP3 (clone MF23), V450-conjugated anti-CD11b (clone M1/70), anti-CD62L (clone MEL-14), anti-IgG1 (clone A110-1), AlexaFlour 700–conjugated anti-CD3ε (clone 145-2C11), anti–IFN-γ, V500–anti-CD4 (clone RM4-5) and anti-B220 (clone RA3-6B2), APC-Cy7–conjugated anti-CD19 (clone 1D3) and anti-CD8 (clone 53-6.7), BV786-conjugated anti-IgE (clone R35-72) and anti–IL-33R (ST2) (clone U29-93), and biotin-CD25 (clone, 7D4), which were purchased from BD Pharmingen (San Diego, Calif), phycoerythrobilin-cynanine7 anti-F4/80 (clone BM8), anti–IL-13 (clone eBio13A), AlexaFlouro 700–conjugated anti–MHC II (clone M5/114), APC-conjugated anti–IL-21 (clone FFA21), and live/dead Fixable Yellow stain (Qdot605 dead cell exclusion dye), which were purchased from eBiosciences. Biotin-labeled antibodies were detected by Texas Red–conjugated phycoerythrobilin (BD Biosciences, San Diego, Calif). For staining, cells (1 × 106) were stained and washed in PBS, 3% FCS FACS buffer. For intracellular cytokine staining, cells were restimulated with phorbal 12-myristate 13-acetate (Sigma-Aldrich) (50 ng/mL), ionomycin (Sigma-Aldrich) (250 ng/mL), and monensin (Sigma-Aldrich) (200 mM in Iscove's Modified Dulbecco's Medium (IMDM)/10% FCS) for 5 hours at 37˚C and then fixed in 2% PFA and permeabilized with Foxp3 transcriptional factor staining buffer kit (eBioscience) before intracellular staining with appropriate cytokine antibodies and acquisition through LSR Fortessa machine (BD Immunocytometry System, San Jose, Calif) and data were analyzed using FlowJo software (Treestar, Ashland, Ore).
Histology
Left upper-lung lobes was fixed in 4% formaldehyde/PBS and embedded in paraffin. Tissue sections were stained with periodic acid-Schiff for mucus secretion and hematoxylin and eosin stain for inflammation. Slides were scanned at 20× magnification on the virtual slide VS120 microscope (Olympus, Hamburg, Germany). Downstream processing of images was done through Image J (FIJI) for image extraction at series 15, and Ilastik software was used for mucus area quantification on whole-lung sections. Data shown are from 1 experiment from at least 3 independent experiments (n = 5-7 mice per experiment).
Antibody and cytokine ELISAs
Antibody ELISAs were carried out as previously described26 using 5 μg/mL HDM to coat for specific IgGs. Total IgE in serum was measured using antimouse IgE (BD Biosciences, 553413) to coat, mouse IgE (κ, anti-TNP, BD Biosciences, 557079) as standard, and biotin antimouse IgE (BD Biosciences, 553419) as secondary antibody.
For in vitro cytokine production analysis, single-cell suspensions were prepared from mediastinal lymph nodes (mLNs) of HDM-treated and littermate control mice. Cells (2 × 105 cells, in 200 μL) were incubated for 5 days in IMDM/10% FCS (Delta Bioproducts, Kempton Park, South Africa) in 96-well plates. Cells were stimulated with either HDM (30 μg/mL) or anti-CD3 (10 μg/mL) and supernatants were collected after a 5-day incubation period. Concentrations of IL-4, IL-5 (BD Biosciences), and IL-13 (R&D Systems, Minneapolis, Minn) were measured using ELISAs according to the manufacturer’s protocol.
Statistical analysis
P values were calculated in GraphPad Prism 6 (GraphPad Software, Inc, San Diego, Calif) by using nonparametric Mann-Whitney Student t test or 2-way ANOVA with Bonferroni posttest for multiple comparisons, and results are presented as SEM or mean of SD. Differences were considered significant if P was less than .05.
Results
IL-4Rα–responsive B cells are not essential in high-dose HDM-induced allergic asthma
The role of B cells in asthma is controversial,9,14 and recent evidence suggested that the load of antigen is crucial in influencing the role of B cells.14 We used a standard high dose of 100 μg HDM to sensitize mice at day 0 and challenged with a reduced dose of 10 μg on days 8 to 1234,35 (Fig 1, A). First, we showed that at both steady state and during HDM challenge, there was reduced IL-4Rα expression in both lung and mLNs in mice lacking IL-4Rα on B cells (mb1creIL-4Rα−/lox) when compared with littermate (IL-4Rα−/lox) control mice or IL-4Rα–deficient mice (see Fig E1, A and B, in this article’s Online Repository at www.jacionline.org). We found that mice lacking IL-4Rα on B cells had a moderately reduced airway resistance and elastance when compared with littermate mice sensitized and challenged with high-dose HDM (Fig 1, B).
Fig 1.
IL-4Rα–responsive B cells regulate AHR and IgE production during high-dose HDM exposure, but have little impact on airway inflammation and TH2 responses. A, Schematic diagram showing sensitization and challenge protocol where mice (mb1creIL-4Rα−/lox) and wild-type littermate control (IL-4Rα−/lox) were sensitized with HDM 100 μg intratracheally on day 0 and challenged with HDM 10 μg on days 8 to 12. Analysis was done on day 15. B, Airway resistance and elastance were measured with increasing doses of acetyl methacholine (0-40 mg/mL). C, Total lung cell numbers, eosinophil numbers, neutrophil numbers, and B-cell numbers were stained and analyzed by flow cytometry and enumerated from % of live cells. D, Number of lung CD4 T cells producing IL-4, IL-5, and IL-13 after 5-hour stimulation with PMA/ionomycin in the presence of monensin. E, Total serum IgE and HDM-specific IgG1 titers measured by ELISA. F, Histology analyses of lung sections (magnification ×20), stained with periodic acid-Schiff. A.U., Arbitrary units; i.n., intranasal; i.t., intratrachael; OD, optical density; PMA, phorbal 12-myristate 13-acetate. Shown are means ± SDs from 1 representative experiment of 2 (n = 4-6). Significant differences between groups were analyzed by Student t test (Mann-Whitney) (Fig 1, C, D, and F) or by 2-way ANOVA with Benforroni posttest (Fig 1, B and E) and are described as ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Fig E1.
Characterization of IL-4Rα expression on B cells. A, Representative histogram plots of IL-4Rα expression on B cells in mLNs. B, Quantification of IL-4Rα expression on B cells in mLNs and lungs represented as MFI. C, Frequencies of eosinophils (live+CD11clow CD11bhigh Ly6Glow SiglecFhi) and neutrophils (live+CD11clowCD11bhighLy6Ghigh) analyzed by flow cytometry (part of Fig 1). D, Total number of lung CD4 T cells and CD4 T cells producing IFN-γ after 5-hour stimulation with PMA/ionomycin in the presence of monensin in mb1creIL-4Rα−/lox and littermate control IL-4Rα−/lox mice. Shown are means ± SDs from 1 representative experiment of 3 or more (n = 6-7). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗∗P < .01. MFI, Median fluorescent intensity; PMA, phorbal 12-myristate 13-acetate; PMN, polymorphonuclear.
We then measured cellular infiltrates within the lung tissue after HDM challenge and observed a comparable increase in total cellular infiltration, which was mainly represented by eosinophils in both mb1creIL-4Rα−/lox and littermate mice challenged with HDM (Fig 1, C, and Fig E1, C). We then measured type 2 cytokines produced by CD4 T cells in the lung after stimulation with phorbal 12-myristate 13-acetate/ionomycin for 5 hours. We observed increased but comparable levels of CD4 T cells producing IL-4, IL-5, and IL-13 in both mb1creIL-4Rα−/lox and littermate mice challenged with high-dose HDM (Fig 1, D). Levels of CD4 T cells producing IL-4, IL-5, and IL-13 were low in mb1creIL-4Rα−/lox and littermate control IL-4Rα−/lox mice challenged with PBS when compared with high-dose HDM-challenged mice of the same genotype (Fig 1, D). We also observed no differences in CD4 T-cell numbers or IFN-γ–producing CD4 T cells in all mutants challenged with high-dose HDM (Fig E1, D). We observed significantly higher total IgE production and HDM-specific IgG1 titers in IL-4Rα−/lox mice when compared with mb1creIL-4Rα−/lox mice challenged with high-dose HDM (Fig 1, E). We observed no differences in mucus production and inflammation between mb1creIL-4Rα−/lox and IL-4Rα−/lox mice (Fig 1, F). Overall, these results demonstrated that IL-4Rα on B cells plays a minimal role in the development of allergic asthma after a challenge with high-dose HDM.
IL-4Rα–responsive B cells play an essential role in low-dose HDM-induced allergic asthma
B-cell–deficient mice (μMT−/−) showed increased eosinophilic airway inflammation when challenged with high- dose HDM, comparable to that observed in wild-type mice, even under chronic challenges.14,36 Titration of HDM below 3 μg reduced the influx of eosinophils, proliferation of Derp-1–specific T cells, and type 2 cytokine production when compared with wild-type mice.14 We then used this low-dose HDM sensitization and challenge protocol to assess whether type 2 airway inflammation depended on the dose of inhaled HDM (Fig 2, A). We found robust differences in airway resistance and elastance in mb1creIL-4Rα−/lox mice sensitized and challenged with low-dose HDM when compared with littermate IL-4Rα−/lox mice (Fig 2, B). Mb1creIL-4Rα−/lox and IL-4Rα−/lox mice challenged with saline had similarly low levels of resistance and elastance compared with HDM-exposed mice (Fig 2, B). We then analyzed total lung infiltrate and found a significant increase in total cells and eosinophils in IL-4Rα−/lox littermates compared with mb1creIL-4Rα−/lox or global IL-4Rα–deficient mice challenged with low-dose HDM (Fig 2, C). We did not observe any changes in neutrophil numbers when comparing low-dose HDM-challenged mice and control mice that were challenged with saline (Fig 2, C). We then analyzed type 2 cytokine production by CD4 T cells in the lung and found a significant increase in percentages and number of CD4 T cells producing IL-4, IL-5, and IL-13 in IL-4Rα−/lox littermate mice when compared with mb1creIL-4Rα−/lox and IL-4Rα−/− mice sensitized and challenged with low-dose HDM (Fig 2, D; see Fig E2, A and B, in this article’s Online Repository at www.jacionline.org). We also found similar trends of reduced TH2 cytokine levels in IL-4Rα B-cell–deficient mLNs stimulated for 5 days with anti-CD3 (see Fig E3 in this article’s Online Repository at www.jacionline.org). There were low number of cytokine-producing CD4 T cells in both mb1creIL-4Rα−/lox and littermate control mice sensitized and challenged with saline (Fig 2, D). We analyzed lung tissue for signs of inflammation and stained for mucus-producing cells (Fig 2, E). We found similar levels of mucus area in both mb1creIL-4Rα−/lox and IL-4Rα−/lox littermate control mice, and there were no detectable mucus-producing cells in control mice challenged with saline (Fig 2, E). Overall, these results demonstrate that at low-dose HDM exposure, IL-4Rα on B cells contributes significantly to the development of allergic asthma and TH2-type lung inflammation.
Fig 2.
IL-4Rα–responsive B cells are essential in optimal TH2 immune responses during low-dose HDM exposure. A, Schematic diagram showing sensitization and challenge protocol where mice (mb1creIL-4Rα−/lox) and wild-type littermate control (IL-4Rα−/lox) were sensitized with HDM 1 μg intratracheally on day 0 and challenged with HDM 3 μg on days 8 to 12. Analysis was done on day 15. B, Airway resistance and elastance were measured with increasing doses of acetyl methacholine (0-40 mg/mL). C, Total lung cell numbers, eosinophil numbers, and neutrophil numbers were stained and analyzed by flow cytometry and enumerated from % of live cells. D, Number of lung CD4 T cells producing IL-4, IL-5, and IL-13 after 5-hour stimulation with PMA/ionomycin in the presence of monensin. Representative FACS plots are shown in Fig E2. E, Histology analyses of lung sections (magnification ×20), stained with periodic acid-Schiff. A.U., Arbitrary units; i.n., intranasal; i.t., intratrachael; PMA, phorbal 12-myristate 13-acetate. Shown are means ± SDs from 1 representative experiment of 3 (n = 6-7). Significant differences between groups were analyzed by Student t test (Mann-Whitney) (Fig 2, C, D, and E) or by 2-way ANOVA with Benforroni posttest (Fig 2, B) and are described as ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Fig E2.
IL-4Rα–responsive B cells are essential in TH2 and IL-21 production. Representative flow cytometry plots showing lung CD4 T cells producing IL-4, IL-5, IL-13, and IL-21. Frequencies of CD4+cytokine+ cells are quantified on the right. This is part of Figs 2 and 3, D. Shown are means ± SDs from 1 representative experiment of 3 (n = 4-9). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗P < .05, ∗∗P < .01.
Fig E3.
IL-4Rα–responsive B cells are essential in TH2 cytokine release after anti-CD3 stimulation. mLNs were stimulated with anti-CD3 (10 μg/mL) for 5 days and supernatants were used to measure levels of IL-4, IL-5, and IL-13. Cytokines were not detected in unstimulated or HDM-stimulated mLNs (30 μg). Shown are means ± SDs from 1 experiment (n = 6-7). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗P < .01.
IL-4Rα–responsive B cells are important for accumulation of germinal center B cells and TFH cells in secondary lymphoid tissue at low antigen load
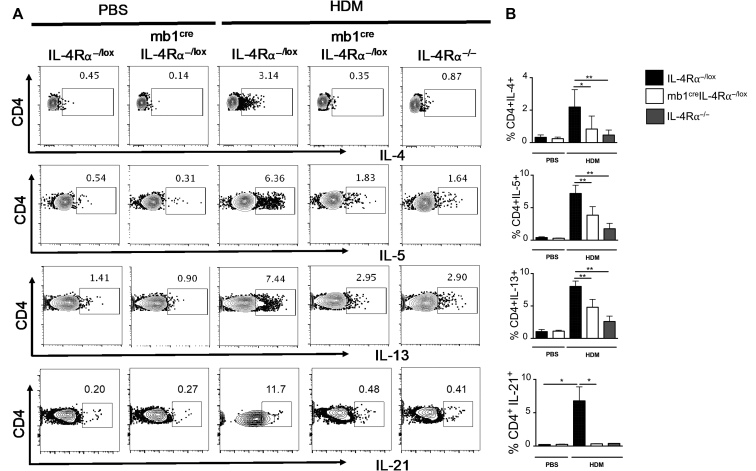
B cells have been shown to be important in the development of TFH cells, and these TFH cells acted as precursors for IL-4/IL-13–committed CD4 T cells that migrated to the lung to recruit eosinophils and caused disease.13 First, we measured percentages and number of B cells in the mLNs and found frequencies to be intact (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). However, total numbers were significantly reduced when comparing mb1creIL-4Rα−/lox to IL-4Rα−/lox littermate mice and global IL-4Rα–deficient mice (Fig 3, A). We then compared germinal center (GC) B cells in mLNs and found comparably low levels between mb1creIL-4Rα−/lox and IL-4Rα−/lox littermate control mice (Fig 3, B). We observed significantly higher percentages (represented by high expression of GL7 and FAS) and number of GC B cells in IL-4Rα−/lox littermate mice when compared with mb1creIL-4Rα−/lox mice challenged with low-dose HDM (Fig 3, B). We did not observe major changes in frequencies of follicular B cells (Fig E4, B), but observed significantly increased frequencies of marginal-zone B cells (Fig E4, B) in mb1creIL-4Rα−/lox mice when compared with IL-4Rα−/lox littermate mice challenged with low-dose HDM, probably as a compensatory mechanism to increase non-GC antibody production. We then looked for TFH cells of which we know B cells play a crucial role in their development particularly at low-dose HDM. We found significantly reduced frequencies and numbers of TFH cells (represented by high expression of PD-1 and CXCR5) in mLNs of mb1creIL-4Rα−/lox and IL-4Rα−/− when compared with IL-4Rα−/lox littermate controls (Fig 3, C). To understand whether these TFHs could be contributing to effector TH2 cells in the lung, we analyzed IL-21 intracellular levels produced by CD4 T cells in the lung.37 We found significantly reduced frequencies and numbers of IL-21–producing T cells in mb1creIL-4Rα−/lox and IL-4Rα−/− when compared with IL-4Rα−/lox littermate controls (Fig 3, D, and Fig E2, A and B). Our data suggested that IL-4Rα–responsive B cells in secondary lymphoid tissues are important for the accumulation of GC B cells and development of TFH cells, which might be contributing to overall TH2 cells.
Fig E4.
B cells produce other TH2-type cytokines. A, Frequencies of B cells (live+B220+CD19+MHCII+) in mLNs of mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice. B, Frequencies of follicular (FO) B cells (live+B220+CD19+MHCII+CD23+CD21/CD35low) and marginal-zone (MZ) B cells (live+B220+CD19+MHCII+CD23lowCD21/CD35+) in mLNs of mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice. C, Frequencies of IL-5–producing B cells (live+B220+CD19+MHCII+IL-5+) in the lungs of mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice. D, Total number of IL-13–producing B cells (live+B220+CD19+MHCII+IL-13+) in the lungs of mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice. Shown are means ± SDs from 1 representative experiment of 3 (n = 6-7). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗∗P < .01.
Fig 3.
IL-4Rα signaling on B cells is essential for GC formation and TFH cells during low-dose HDM exposure. A, Total number of B cells in the mLNs in mb1creIL-4Rα−/lox, littermate control (IL-4Rα−/lox), and IL-4Rα−/− mice sensitized and challenged as in Fig 2. B, Representative flow cytometry plots of GCs and number of GCs (live+B220+CD19+MHCII+GL7+FAS+) in the mLNs in mb1creIL-4Rα−/lox and littermate control IL-4Rα−/lox mice. C, Representative flow cytometry plots of TFH cells (live+CD3+CD4+CD44+PD-1+CXCR5+) and number of TFH cells in the mLNs. D, Number of lung CD4 T cells producing IL-21 after 5-hour stimulation with PMA/ionomycin in the presence of monensin. Representative FACS plots are shown in Fig E2. FAS, FS-7-associated surface; PMA, phorbal 12-myristate 13-acetate. Shown are means ± SDs from 1 representative experiment of 3 (n = 4-6). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗P < .05.
IL-4Rα–responsive B cells are required for optimal TH2 airway responses and antibody production
Effector B cells producing type 2 cytokines (B effector 2 [Be2]) have been shown to be important during parasitic infections, and early expression of IL-4 by these B cells promotes differentiation of type 2 CD4 T cells.28,30,38 We measured cytokine production by effector B cells and found increased frequencies and numbers of mLN B cells producing IL-5 in IL-4Rα−/lox littermate mice when compared with mb1creIL-4Rα−/lox and IL-4Rα−/− mice challenged with low-dose HDM (Fig 4, A and B, and Fig E4, C). We also observed a similar reduction in the number of IL-13–producing B cells in mb1creIL-4Rα−/lox and IL-4Rα−/− when compared with IL-4Rα−/lox littermate mice challenged with low-dose HDM (Fig E4, D). We then measured total serum IgE and HDM-specific IgG1 by ELISA and found significantly reduced titers in mb1creIL-4Rα−/lox and IL-4Rα−/− when compared with IL-4Rα−/lox littermate mice challenged with low-dose HDM (Fig 4, C). We analyzed IgE and IgG1 surface expression by B cells using flow cytometry. We found significantly reduced levels of IgE and IgG1 expression in B cells when comparing mb1creIL-4Rα−/lox to IL-4Rα−/lox littermate mice (Fig 4, D). B- and T-cell engagement through CD86 and CD28 in T-cell zones is essential for TFH-cellgeneration and class switching to IgE.39 We measured CD86 and other costimulatory molecules on the surface of B cells and found reduced expression of CD86, but not CD80 and MHCII (Fig 4, E; see Fig E5, A and B, in this article’s Online Repository at www.jacionline.org), which may suggest an incomplete T-cell engagement via CD28 and explain lack of class switching. Thus far, our data suggested that IL-4Rα signaling on B cells is essential for Be2-cell function and class switching to IgE and this contributes to overall TH2 responses.
Fig 4.
IL-4Rα signaling on B cells is essential for B effector 2 function and class switching. A, Representative flow cytometry plots of IL-5–producing B cells (live+B220+CD19+MHCII+IL-5+) in the lung of mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice. B, Quantification of total number of IL-5–producing B cells in the lung. C, Total serum IgE and HDM-specific IgG1 titers in mb1creIL-4Rα−/lox, littermate control IL-4Rα−/lox, and IL-4Rα−/− mice measured by ELISA. D, Surface expression of IgE (live+B220+CD19+MHCII+IgE+) and IgG1 (live+B220+CD19+MHCII+IgG1+) on mLN B cells, represented as MFI. E, CD86 surface expression on mLN B cells (Live+B220+CD19+MHCII+CD86+), represented as MFI. MFI, Median fluorescent intensity. Shown are means ± SDs from 1 representative experiment of 3 (n = 4-6). Significant differences between groups were analyzed by Student t test (Mann-Whitney) (Fig 4, B, D, and E) or by 2-way ANOVA with Benforroni posttest (Fig 4, E) and are described as ∗∗∗P < .001, ∗∗∗∗P < .0001.
Fig E5.
Antigen uptake and processing is intact in IL-4Rα–deficient B cells. A, Quantification of CD80 expression on B cells (live+B220+CD19+) in mLNs represented as MFI. B, Quantification of MHCII expression on B cells (live+B220+CD19+) in mLNs and lungs represented as MFI. MFI, Median fluorescent intensity. Shown are means ± SDs from 1 representative experiment of 3 (n = 6-7). Significant differences between groups were analyzed by Student t test (Mann-Whitney) and are described as ∗∗P < .01.
IL-4Rα–responsive B cells are essential in type 2 airway inflammation during the effector phase
Previous studies had suggested that B cells were important in TFH-cell development at the sensitization stage, but not at the effector stage, and played minimal role in disease if adoptively transferred after sensitization.13 We asked whether IL-4Rα–responsive B cells were important only at the sensitization stage. We sensitized B-cell–deficient μMT−/− mice with low-dose HDM and a day before challenge, we transferred naive B cells having either sufficient or lacking IL-4Rα (Fig 5, A). We found that μMT−/− with B cells from mb1creIL-4Rα−/lox mice had significantly reduced resistance and elastance when compared with μMT−/− receiving B cells from IL-4Rα−/lox littermate mice (Fig 5, B). This reduced AHR in mb1creIL-4Rα−/lox mice was accompanied by reduced eosinophil recruitment in the lung, but not total cell or B-cell numbers (Fig 5, C). We then analyzed CD4 T-cell numbers that were producing type 2 cytokines and found significantly reduced IL-5– producing CD4 T cells in the lung of μMT−/− mice that received mb1creIL-4Rα−/lox B cells when compared with μMT−/− mice receiving IL-4Rα−/lox B cells (Fig 5, D). We then measured total IgE by ELISA and found significantly increased IgE in μMT−/− mice that received IL-4Rα−/lox B cells compared with μMT−/− mice that received mb1creIL-4Rα−/lox B cells (Fig 5, E). We observed comparable mucus area between μMT−/− mice receiving IL-4Rα−/lox B cells or mb1creIL-4Rα−/lox B cells (Fig 5, F). No mucus-producing cells were detected in control mice challenged with saline (Fig 5, F). Our findings suggested that IL-4Rα signaling on B cells was also essential at the effector phase for optimal TH2 airway responses.
Fig 5.
IL-4Rα signaling on B cells is essential at the effector phase of allergic asthma through regulation of AHR and TH2 airway responses. A, Schematic diagram showing sensitization and challenge protocol where μMT−/− mice were sensitized with HDM 1 μg intratracheally on day 0 and naive B cells (live+B220+CD19+) from mb1creIL-4Rα−/lox or IL-4Rα−/lox mice (2-5 × 106 cells) were adoptively transferred intravenously a day before challenge with HDM 3 μg on days 8 to 12. Analysis was done on day 15. B, Airway resistance and elastance were measured with increasing doses of acetyl methacholine (0-20 mg/mL). C, Total lung cell numbers, eosinophil numbers, and B-cell numbers were stained and analyzed by flow cytometry and enumerated from % of live cells. D, Number of lung CD4 T cells producing IL-5 after 5-hour stimulation with PMA/ionomycin in the presence of monensin. E, Total serum IgE production from 2 independent experiments pooled together. F, Histology analyses of lung sections (magnification ×20), stained with periodic acid-Schiff. A.U., Arbitrary units; i.n., intranasal; i.t., intratrachael; i.v., intravenous; PMA, phorbal 12-myristate 13-acetate. Shown are means ± SEM from 2 independent experiments pooled (n = 10-14). Significant differences between groups were analyzed by Student t test (Mann-Whitney) (Fig 5, C-F) by 2-way ANOVA with Benforroni posttest (Fig 5, B) and are described as ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001.
IL-4/IL-13–producing B cells contribute to AHR but not inflammation
We then investigated whether the production of type 2 cytokines by these B cells is essential for allergic airway inflammation. We adoptively transferred naive B cells either sufficient or deficient of IL-4 or double deficient of IL-4/IL-13 into μMT−/− mice, sensitized and challenged with low-dose HDM (Fig 6, A). We measured lung function and found both resistance and elastance to be significantly reduced in μMT−/− mice that received IL-4–deficient or IL-4/IL-13–double-deficient B cells when compared with μMT−/− mice that received wild-type B cells (Fig 6, B). We then measured cellular infiltrate in the lung and found no major changes in total lung infiltrate, eosinophils, and CD4 T cells in all recipient mice (Fig 6, C). The number of B cells was lower in μMT−/− mice that were adoptively transferred with B cells from various strains compared with control mice; however, no differences were observed between mice that were challenged with a low dose of HDM (Fig 6, C). Total IgE measured by ELISA was not changed between groups of μMT−/− mice adoptively transferred with B cells and sensitized and challenged with low-dose HDM (Fig 6, D). We also did not observe any changes in type 2 cytokines produced by CD4 T cells in the absence of type 2 cytokines produced by B cells when comparing μMT−/− mice that received wild-type B cells to those that received IL-4–deficient or IL-4/IL-13–double-deficient B cells (Fig 6, E). Taken together, these results suggested that although B cells producing type 2 cytokines are essential in AHR, they play minimal role in airway inflammation.
Fig 6.
TH2 cytokine production by B cells is important only in regulation of AHR but not eosinophilia or TH2 airway responses. A, Schematic diagram showing sensitization and challenge protocol where naive B cells (live+B220+CD19+) from IL-4−/− or IL-4−/−IL-13−/− mice (2-5 × 106 cells) were adoptively transferred intravenously into μMT−/− mice a day before sensitization with HDM 1 μg intratracheally on day 0 and challenged with HDM 3 μg on days 8 to 12. Analysis was done on day 15. B, Airway resistance and elastance were measured with increasing doses of acetyl methacholine (0-20 mg/mL). C, Total lung cell numbers, eosinophil numbers, and CD4 T-cell numbers were stained and analyzed by flow cytometry and enumerated from % of live cells. D, Total IgE production from 2 independent experiments pooled together. E, Number of lung CD4 T cells producing IL-5 after 5-hour stimulation with PMA/ionomycin in the presence of monensin. i.n., Intranasal; i.t., intratrachael; i.v., intravenous; PMA, phorbal 12-myristate 13-acetate.Shown are means ± SEM from 2 independent experiments pooled (n = 10-14). Significant differences between groups were analyzed by Student t test (Mann-Whitney) (Fig 6, C-F) by 2-way ANOVA with Benforroni posttest (Fig 6, B) and are described as ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Discussion
B cells secrete IgE and are important in activating mast cells and basophils degranulation, which initiates a cascade of inflammatory signals. However, contradictory findings on the requirement of B cells exist in studies using mice lacking B cells. More recent evidence suggested that antigen load determines the importance of B cells, particularly in interactions with TH cells and generation of TFH cells. IL-4 is critical in class switching of B cells to generate IgE40; however, whether signaling through the IL-4Rα on B cells is required for the generation of TFH cells and IgE has not been investigated in the context of allergic asthma. Here, we showed that IL-4Rα–responsive B cells are important mainly when the load of HDM antigen is limiting. We further showed that IL-4Rα–responsive B cells regulate AHR, IgE secretion, and TFH-cell generation, and that B-cell–derived type 2 cytokines are required for optimal TH2 responses.
We first challenged mice with high-dose HDM and found IL-4Rα–responsive B cells to be important in AHR and IgE production, but not for eosinophil recruitment or type 2 cytokine production. This is consistent with previous studies where B-cell–deficient mice showed similar levels of eosinophilia and type 2 cytokines when sensitized and challenged with high-dose HDM.14 B-cell–deficient mice have significantly increased AHR at high-dose HDM, a phenotype different to what we observe in IL-4Rα–deficient B cells, which suggested that IL-4Rα–responsive B cells may be involved in driving AHR during allergic asthma. Differences in susceptibility to disease is also observed between μMT−/− mice and mb1creIL-4Rα−/lox mice during chronic Schistosoma mansoni infection, which is attributed to differences in immunomodulatory IL-10 production seen in μMT−/− mice, but not in mb1creIL-4Rα−/lox mice.28,29
Similar to what was reported by Dullaers et al,14 mb1creIL-4Rα−/lox mice that were sensitized with 1 μg HDM and challenged with 3 μg HDM displayed a great reduction in AHR, eosinophil recruitment, and type 2 cytokine production compared with littermate IL-4Rα−/lox mice. We also observed significant reduction in GC B cells and TFH cells, but no changes in follicular and slight increase in marginal-zone B cells, probably as a compensatory mechanism to increase non-GC antibody production. The ability of B cells to produce type 2 cytokines was also significantly reduced, suggesting that IL-4Rα B cells help contribute to the overall type 2 immune response output. This is consistent with previous studies, where IL-4Rα responsiveness by B cells is crucial in early IL-4 production in mLNs, defining a dichotomy in subsequent CD4 TH-cell differentiation.28, 29, 30,38 B cells receive the IL-4 signal from CD4 T cells in GCs to initiate class switching to produce IgE, which is likely to be dependent on IL-4Rα signaling on B cells.33,41 Although IgE can directly be generated from IgM, particularly with low antigen load,42 we think that in our model, lack of IL-4Rα signaling on B cells led to reduced sequential class switching from IgM to IgG1 and to IgE. Sequential class switching occurs in the GCs and results in high-affinity plasma cell IgE.41,43,44 These high-affinity IgE plasma cells are direct precursors of IgG1 plasma cells because they share similar CDR3 repertoire in the context of helminth infections, skin cancers, and alum/ovalbumin-induced asthma.44,45 In human B cells from the tonsil, IL-4Rα signaling is required for GC maintenance and generation of high-affinity IgE BCRs that select for plasma cell compartments.46,47
There has been contrasting evidence regarding whether B cells are important during the sensitization phase, the effector phase, or in both phases of allergic response.13,15 B cells were found to be critical in shaping IL-4–committed TFH cells during HDM sensitization stage, which contributed to the effector TH2 pool during challenge stages. However, blocking of TFH cells with BCL6 inhibitor after the sensitization stage did not reduce TH2 allergic airway inflammation, which was attributed to the redundant role of B cells at this stage. This is in contrast with recent findings where blocking B cells with anti-CD20 before HDM challenge significantly reduced TH2 airway responses.15 To consolidate these findings, we transferred IL-4Rα–deficient B cells after HDM sensitization and before challenge. Our data showed that IL-4Rα–responsive B cells are required at the challenge stage, because we observed reduced AHR, TH2 cytokines, and total IgE when we transferred B cells lacking IL-4Rα into recipient mice. Both previous studies had used a similar dose of 20 μg of HDM to sensitize and challenge and we used 3 μg of HDM to challenge. It is likely that the choice of method used to target B cells or their function might be a major contributing factor between the 2 studies and not necessarily the load of HDM antigen. In our studies, we transferred naive B cells a day before challenge, whereas Ballesteros-Tato et al13 had blocked TFH cells using BCL6 inhibitor and Wypych et al15 targeted B cells using anti-CD20 mAb. All in all, our data demonstrated that IL-4Rα on B cells is important in TH2 allergic asthma at both sensitization and challenge stages and contributes to overall TH2 responses when the antigen load is limited.
Previous studies have shown a controversial role of IL-21 in TFH cells that eventually developed into committed TH2 cells. Ballesteros-Tato et al13 suggested that lung TH2 cells were direct descendants of IL-21+Bcl6+ TFH cells and developed 6 days after multiple sensitization with 25 μg of HDM exposure. In contrast, Coquet et al37 found that IL-21+ TFH cells did not differentiate efficiently into ST2+ TH2 cells and migrated into the lung without all key features of TFH cells such as CXCR5 expression. This idea was recently supported by Tibbitt et al,48 where a trajectory single-cell analysis of differentiating TH2 cells up until day 10 suggested that naive CD4 T cells acquired many features of TFH cells but did not express Bcl6 or CXCR5, which suggested that TH2 cells did not descend directly from GC TFH-cell precursors. In our study, the absence of IL-4Rα signaling on B cells resulted in reduced IL-21 production in the lung, which might explain reduced GCs and TH2 cells. Appropriate experiments to answer this complex function of IL-21 in TFH cells that commit to TH2 cells are needed and should use a double (Bcl6 and IL-21) or triple (Bcl6, IL-21 and IL-4) reporter transgenic mouse or a fate reporter transgenic mouse that can trace naive CD4 T cells as they differentiate into intermediate and committed TH2 cells in multiple tissues.
Be2 cells producing IL-4 or IL-13 have been shown to be important in worm expulsion or in Leshmania major disease susceptibility.28, 29, 30,38,49 These Be2 cells are dependent on IL-4 and IL-4Rα and require the presence of intact TH2 cells.28,38,49 Because we had observed that IL-4Rα signaling on B cells was essential for optimal TH2 allergic airway immune responses, we then investigated whether production of cytokines by these Be2 cells was essential for optimal TH2 immune responses. We transferred B cells from IL-4 or IL-4/IL-13–deficient mice into μMT−/− before sensitization. Interestingly, Be2 cells were essential for AHR, but played no role in lung eosinophil recruitment, total IgE production, or type 2 cytokine production by CD4 T cells. This suggested that although the presence of IL-4/IL-13 cytokine production by Be2 cells was required for AHR, it was redundant in other parameters. It is likely that TH2 cells can compensate for the lack of IL-4/IL-13 production by B cells; however, how TH2 cells fail to compensate for AHR is currently unclear and requires further investigation. B cells in lymph nodes secrete early IL-4 production, which may be important for CD4 T-cell differentiation.28,29 It is likely that B cells produce early IL-4 in mLNs, which act in an autocrine fashion to upregulate IL-4Rα, but whether this IL-4 plays a major role in CD4 TH2 differentiation, we can only speculate.
Blocking B cells with anti-CD20 before sensitization did not affect TH2 cytokine production ex vivo, but resulted in reduction in eosinophils and IFN-γ secretion.15 Unfortunately, AHR was not investigated in this setting, making it difficult to draw parallel conclusions regarding the function of B cells in AHR. We can speculate that other intrinsic Be2-cell mechanisms are at play in regulation AHR. B cells are known to take up HDM and present it to naive T cells, priming them to become TH2 cells both in vitro and in vivo, and lack of MHCII in B cells results in reduced TH2 priming.14,15 IL-4Rα–deficient B cells have been shown to have reduced MHC II expression and antigen uptake, which contributed in reduced TH2 priming on secondary exposure to Nippostrongylus brasiliensis, leading to increased worm burdens.30 We did not observe any changes in CD80 or MHCII expression on IL-4Rα signaling–deficient B cells when compared with IL-4Rα–sufficient mice (Fig E5, A and B). However, we did observe a reduction in CD86 costimulatory molecule (Fig 4, E), which may suggest an incomplete T-cell engagement via CD28 and reduced IgE potentiation.39 Our findings do not suggest antigen uptake and processing as a potential mechanism for reduced TH2 priming, but a lack of complete costimulatory engagement in the absence of IL-4Rα signaling on B cells.
Conclusions
We showed that IL-4Rα–responsive B cells play a nonredundant role in allergic asthma in an antigen load–dependent manner. We further showed that IL-4Rα signaling on B cells is crucial at both sensitization and challenge stages and produces cytokines that help in optimal TH2 allergic airway responses. We further showed that Be2-cell function is important only for AHR but redundant in eosinophilia. Our study highlighted a previously unappreciated function of IL-4Rα signaling on B cells and brings evidence for targeting of this signaling axis in allergic asthma.
Key messages.
-
•
IL-4Rα–responsive B cells play a critical role in HDM-induced allergic asthma when the load of HDM is limited.
-
•
IL-4Rα signaling on B cells is required at both sensitization and effector stages of allergic disease.
-
•
IL-4Rα–responsive B cells are required for Be2 function of B cells and help maintain optimal TH2 during allergic asthma.
Acknowledgments
We thank the UCT Research Animal Facility for maintaining mice and Munadia Ansari for genotyping mice. We thank Dr Faruk Ramadan (Kings College London) for helpful discussions on the manuscript. We are grateful to Lizette Fick for excellent histology services. We are also grateful to Ronnie Dreyer for cell sorting expertise. We thank FlowJo for proving free service to Africa.
Footnotes
This work was supported by ICGEB, Cape Town Component, Medical Research Council (MRC) South Africa as well as by the South African National Research Foundation (NRF) Research Chair initiative and Wellcome Trust CIDRI-Africa (grant no. 203135Z/16/Z to F.B.). S.H. is supported by NRF Thuthuka Grant (grant no. 117721), South African MRC under a Self-Initiated Research grant, and Robert Bosch Stiftung Fellowship (grant no.12.5.F081.0008.0). F.K. is funded by NRF Competitive Support for Unrated Researchers (CSUR) (grant no. 111946).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interests.
Contributor Information
Sabelo Hadebe, Email: sabelo.hadebe@uct.ac.za.
Frank Brombacher, Email: brombacherfrank@gmail.com.
Appendix
References
- 1.Lambrecht B.N., Hammad H. The immunology of asthma. Nat Immunol. 2014;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd C.M., Hessel E.M. Functions of T cells in asthma: more than just TH2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geha R.S., Jabara H.H., Brodeur S.R. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 5.Gould H.J., Ramadani F. IgE responses in mouse and man and the persistence of IgE memory. Trends Immunol. 2015;36:40–48. doi: 10.1016/j.it.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Plantinga M., Guilliams M., Vanheerswynghels M., Deswarte K., Branco-Madeira F., Toussaint W. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Hammad H., Plantinga M., Deswarte K., Pouliot P., Willart M.A.M., Kool M. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archambault A.S., Carrero J.A., Barnett L.G., McGee N.G., Sim J., Wright J.O. Cutting edge: conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J Immunol. 2013;191 doi: 10.4049/jimmunol.1201598. 545LP-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKnight C.G., Jude J.A., Zhu Z., Panettieri R.A., Finkelman F.D. House dust mite–induced allergic airway disease is independent of IgE and FcεRIα. Am J Respir Cell Mol Biol. 2017;57:674–682. doi: 10.1165/rcmb.2016-0356OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsgren M., ält J.S.E., Korsgren O., Sundler F., Persson C.G.A. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell–deficient mice. J Exp Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean J.A., Sauty A., Luster A.D., Drazen J.M., De Sanctis G.T. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–387. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]
- 12.Hamelmann E., Takeda K., Schwarze J., Vella A.T., Irvin C.G., Gelfand E.W. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999;21:480–489. doi: 10.1165/ajrcmb.21.4.3659. [DOI] [PubMed] [Google Scholar]
- 13.Ballesteros-Tato A., Randall T.D., Lund F.E., Spolski R., Leonard W.J., León B. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dullaers M., Schuijs M.J., Willart M., Fierens K., Van Moorleghem J., Hammad H. House dust mite–driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol. 2017;140:76–88.e7. doi: 10.1016/j.jaci.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Wypych T.P., Marzi R., Wu G.F., Lanzavecchia A., Sallusto F. Role of B cells in TH cell responses in a mouse model of asthma. J Allergy Clin Immunol. 2018;141:1395–1410. doi: 10.1016/j.jaci.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomkinson A., Duez C., Cieslewicz G., Pratt J.C., Joetham A., Shanafelt M.-C. Inhibition of the IL-4/IL-13 receptor system prevents allergic sensitization without affecting established allergy in a mouse model for allergic asthma. J Allergy Clin Immunol. 2001;166:1361–1369. doi: 10.1067/mai.2003.1527. [DOI] [PubMed] [Google Scholar]
- 17.Grünig G., Warnock M., Wakil A.E., Venkayya R., Brombacher F., Rennick D.M. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 19.Herbert D.R., Hölscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Horsnell W.G.C., Vira A., Kirstein F., Mearns H., Hoving J.C., Cutler A.J. IL-4Rα-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol. 2011;4:83–92. doi: 10.1038/mi.2010.46. [DOI] [PubMed] [Google Scholar]
- 21.Khumalo J., Kirstein F., Scibiorek M., Hadebe S., Brombacher F. Therapeutic and prophylactic deletion of IL-4Rα signaling ameliorates established ovalbumin-induced allergic asthma. Allergy. 2020;75:1347–1360. doi: 10.1111/all.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwenhuizen N.E., Kirstein F., Hoving J.C., Brombacher F. House dust mite induced allergic airway disease is attenuated in CD11ccreIL-4Rα−/l°x mice. Sci Rep. 2018;8:885. doi: 10.1038/s41598-017-19060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirstein F., Nieuwenhuizen N.E., Jayakumar J., Horsnell W.G.C., Brombacher F. Role of IL-4 receptor α–positive CD4+ T cells in chronic airway hyperresponsiveness. J Allergy Clin Immunol. 2016;137:1852–1862.e9. doi: 10.1016/j.jaci.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Kuperman D.A., Huang X., Nguyenvu L., Holscher C., Brombacher F., Erle D.J. IL-4 Receptor signaling in clara cells is required for allergen-induced mucus production. J Immunol. 2005;175:3746–3752. doi: 10.4049/jimmunol.175.6.3746. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuizen N.E., Kirstein F., Jayakumar J., Emedi B., Hurdayal R., Horsnell W.G.C. Allergic airway disease is unaffected by the absence of IL-4Rα-dependent alternatively activated macrophages. J Allergy Clin Immunol. 2012;130:743–750.e8. doi: 10.1016/j.jaci.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Kirstein F., Horsnell W.G.C., Kuperman D.A., Huang X., Erle D.J., Lopata A.L. Expression of IL-4 receptor alpha on smooth muscle cells is not necessary for development of experimental allergic asthma. J Allergy Clin Immunol. 2010;126:347–354. doi: 10.1016/j.jaci.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins C., Yanase N., Smulian G., Gildea L., Orekov T., Potter C. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med. 2011;208:853–867. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurdayal R., Ndlovu H.H., Revaz-Breton M., Parihar S.P., Nono J.K., Govender M. IL-4–producing B cells regulate T helper cell dichotomy in type 1- and type 2-controlled diseases. Proc Natl Acad Sci U S A. 2017;114:E8430–E8439. doi: 10.1073/pnas.1708125114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndlovu H., Nono J.K., Abdel Aziz N., Nieuwenhuizen N.E., Brombacher F. Interleukin-4 receptor alpha expressing B cells are essential to down-modulate host granulomatous inflammation during schistosomasis. Front Immunol. 2018;9:2928. doi: 10.3389/fimmu.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsnell W.G.C., Darby M.G., Hoving J.C., Nieuwenhuizen N., McSorley H.J., Ndlovu H. IL-4Rα-associated antigen processing by B cells promotes immunity in Nippostrongylus brasiliensis infection. PLoS Pathog. 2013;9:1–12. doi: 10.1371/journal.ppat.1003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobeika E., Thiemann S., Storch B., Jumaa H., Nielsen P.J., Pelanda R. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci. 2006;103 doi: 10.1073/pnas.0605944103. 13789LP-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohrs M., Ledermann B., Köhler G., Dorfmüller A., Gessner A., Brombacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 33.Hoving J.C., Kirstein F., Nieuwenhuizen N.E., Fick L.C.E., Hobeika E., Reth M. B cells that produce immunoglobulin E mediate colitis in BALB/c mice. Gastroenterology. 2012;142:96–108. doi: 10.1053/j.gastro.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debeuf N., Haspeslagh E., van Helden M., Hammad H., Lambrecht B.N. Current protocols in mouse biology. John Wiley & Sons, Inc; Hoboken, NJ: 2016. Mouse models of asthma; pp. 169–184. [DOI] [PubMed] [Google Scholar]
- 36.Vroman H., Bergen I.M., Li B.W.S., van Hulst J.A.C., Lukkes M., van Uden D. Development of eosinophilic inflammation is independent of B–T cell interaction in a chronic house dust mite-driven asthma model. Clin Exp Allergy. 2017;47:551–564. doi: 10.1111/cea.12834. [DOI] [PubMed] [Google Scholar]
- 37.Coquet J.M., Schuijs M.J., Smyth M.J., Deswarte K., Beyaert R., Braun H. Interleukin-21-producing CD4+ T cells promote type 2 immunity to house dust mites. Immunity. 2015;43:318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Wojciechowski W., Harris D.P., Sprague F., Mousseau B., Makris M., Kusser K. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeannin P., Delneste Y., Lecoanet-Henchoz S., Gauchat J.-F., Ellis J., Bonnefoy J.-Y. CD86 (B7-2) on human B cells: a functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J Biol Chem. 1997;272:15613–15619. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 40.Shimoda K., van Deursent J., Sangster M.Y., Sarawar S.R., Carson R.T., Tripp R.A. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted State6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 41.Mandler R., Finkelman F.D., Levine A.D., Snapper C.M. IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J Immunol. 1993;150 407LP-418. [PubMed] [Google Scholar]
- 42.Sudowe S., Rademaekers A., Kölsch E. Antigen dose-dependent predominance of either direct or sequential switch in IgE antibody responses. Immunology. 1997;91:464–472. doi: 10.1046/j.1365-2567.1997.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y.L., Stubbington M.J.T., Daly M., Teichmann S.A., Rada C. Intrinsic transcriptional heterogeneity in B cells controls early class switching to IgE. J Exp Med. 2016;214:183–196. doi: 10.1084/jem.20161056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turqueti-Neves A., Otte M., Schwartz C., Schmitt M.E.R., Lindner C., Pabst O. The extracellular domains of IgG1 and T cell-derived IL-4/IL-13 are critical for the polyclonal memory IgE response in vivo. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford G., Hayes M.D., Seoane R.C., Ward S., Dalessandri T., Lai C. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat Immunol. 2018;19:859–870. doi: 10.1038/s41590-018-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramadani F., Bowen H., Upton N., Hobson P.S., Chan Y.-C., Chen J.-B. Ontogeny of human IgE-expressing B cells and plasma cells. Allergy. 2017;72:66–76. doi: 10.1111/all.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanagihara Y., Ikizawa K., Kajiwara K., Koshio T., Basaki Y., Akiyama K. Functional significance of IL-4 receptor on B cells in IL-4-induced human IgE production. J Allergy Clin Immunol. 1995;96:1145–1151. doi: 10.1016/s0091-6749(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 48.Tibbitt C.A., Stark J.M., Martens L., Ma J., Mold J.E., Deswarte K. Single-cell RNA sequencing of the T helper cell response to house dust mites defines a distinct gene expression signature in airway Th2 cells. Immunity. 2019;51:169–184.e5. doi: 10.1016/j.immuni.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Harris D.P., Goodrich S., Mohrs K., Mohrs M., Lund F.E. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor α, and Th2 cells. J Immunol. 2005;175 doi: 10.4049/jimmunol.175.11.7103. 7103LP-7. [DOI] [PubMed] [Google Scholar]