ABSTRACT
Several studies reported a potential role of methane producing archaea in the pathophysiology of irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). We conducted a systematic review and meta-analysis to assess the prevalence of methane positive small intestinal bacterial overgrowth (SIBO) in IBS and IBD compared with controls. MEDLINE (PubMed) and Embase electronic databases were searched from inception until March 2021 for case-control and prevalence studies reporting SIBO in IBS and IBD. We extracted data from published studies and calculated pooled prevalence of SIBO in IBS or IBD, odds ratios (OR), and 95% CIs, utilizing a random effects model. The final dataset included 17 independent studies assessing the prevalence of methane positive SIBO in 1,653 IBS-patients and 713 controls, and 7 studies assessing the prevalence of methane positive SIBO in 626 IBD-patients and 497 controls, all utilizing breath test for SIBO diagnosis. Prevalence of methane positive SIBO in IBS and IBD was 25.0% (95% CI 18.8–32.4) and 5.6% (95% CI 2.6–11.8), respectively. Methane positive SIBO in IBS was not increased compared to controls (OR = 1.2, 95% CI 0.8–1.7, P = .37) but was significantly more prevalent in IBS-C as compared to IBS-D (OR = 3.1, 95% CI 1.7–5.6, P = .0001). The prevalence of methane-positive SIBO in patients with IBD was 3-fold lower at 7.4% (95% CI 5.4–9.8) compared to 23.5% (95% CI 19.8–27.5) in controls. The prevalence of methane positive SIBO was significantly lower in Crohn’s disease as compared to ulcerative colitis, (5.3%, 95% CI 3.0–8.5 vs. 20.2%, 95% CI 12.8–29.4). This systematic review and meta-analysis suggests methane positivity on breath testing is positively associated with IBS-C and inversely with IBD. However, the quality of evidence is low largely due to clinical heterogeneity of the studies. Thus, causality is uncertain and further studies are required.
KEYWORDS: Irritable bowel syndrome, inflammatory bowel disease, methane, small intestinal bacterial overgrowth, breath tests
Introduction
There is emerging evidence that microbial dysbiosis, defined as the alterations in the composition, density and function of the intestinal microbes plays an important role in a variety of gastrointestinal and extraintestinal conditions.1, 2 Small intestinal bacterial overgrowth (SIBO), a condition where the overall homeostasis of the small intestine becomes dysregulated through presence of altered number and type of microbes is an example of gut microbial dysbiosis.3 The current gold standard for diagnosing SIBO remains small bowel aspirate and culture, however in clinical practice breath testing has largely replaced culture methods given the simplicity and noninvasive nature of these tests.3 Breath tests are based on the principle that human cells do not produce hydrogen and/or methane gas4 and presence of these gases in the human breath indicates the metabolism of (non-digested) carbohydrates by gut microbes.5
Although certain Clostridium and Bacteroides spp. have been proposed to produce methane6 microbes from the third Domain of Life – the Archaea – are now widely believed to exclusively fill this metabolic niche. In humans, Methanobrevibacter smithii7 is the numerically predominant taxon, and principally rely on the production of methane from hydrogen (H2) and carbon dioxide (CO2) for their sole source of energy.8 Methane generally has not been found to have a physiologic role in humans,9 and is mainly excreted in flatus (80%), while a certain amount is excreted in breath (20%).8 Thus, methane can be detected during breath tests.
More than one third of healthy adult subjects are predominantly methane producers10 and there has been increasing interest in the association between methane and constipation11 and the potential effect on gastrointestinal intestinal transit.11,12 The role(s) of methane and methanogens in chronic diarrheal states such as inflammatory bowel disease (IBD) and pneumatosis cystoides,13 has been associated with significantly reduced concentrations of breath methane, as well as reduced methanogen positivity and counts in stool samples. Emphasizing the potential importance of methane production by archaea, the recent guideline of the American College of Gastroenterology on SIBO coined the term “intestinal methanogen overgrowth” (IMO), for methanogens rather than SIBO driven solely by bacteria.14 While previous systematic reviews and meta-analysis have assessed the link between SIBO and irritable bowel syndrome (IBS)15 or IBD,16 there are no systematic reviews, which have specifically explored the role of methane positive SIBO in relation to these conditions.
Hence, we decided to conduct a systematic review and meta-analysis to (1) assess and compare the prevalence of methane positive SIBO in patients with IBS and IBD (and their subtypes) and healthy controls; (2) explore the link between diagnostic modality (type of breath test) and variations in methane SIBO prevalence in patients with IBS and IBD; (3) assess the association between proton pump inhibitor (PPI) use and methane positivity on breath test; (4) assess the link between transit time and methane positivity on breath tests and; (5) assess the effect of antibiotic therapy on symptom improvement in patients with methane positive SIBO.
Materials and methods
Search strategy
A comprehensive literature search was performed using MEDLINE(PubMed) and Embase electronic databases from initiation (1966) up to March 2021 for all studies assessing the prevalence of SIBO in patients with IBD, IBS, and/or functional gastrointestinal disorders (FGIDs). The initial search was not limited to specific languages. A further advanced search was conducted. Grey literature was searched with Google and Google Scholar, and the ‘Snowball” method was also utilized which included pursuing through reference lists of articles as well as electronic citations, to identify all relevant articles. Search terms included “methane” OR “CH4” OR “breath test” OR “breath analysis” OR “methane breath test” OR “glucose breath test (GBT)” OR “glucose hydrogen breath test” OR “GBT” OR “lactulose breath test (LBT)” OR “lactulose hydrogen breath test (LHBT)” OR “LBT’ OR “LHBT” AND “constipation” OR “transit” OR “motility” OR “irritable bowel syndrome” OR “IBS” OR “irritable colon” OR “colonic inertia” OR “SIBO” OR “SBBO” OR “small bowel bacterial overgrowth (SBBO)” OR “small intestinal bacterial overgrowth” AND “Inflammatory bowel disease” OR “IBD”. Expert assistance was sought from the hospital librarian who helped conduct a detailed literature search strategy which is outlined in in the PRISMA flow diagram.
Selection of studies
An initial screen of abstracts and titles were conducted independently by two authors (A.G and A.S). Abstracts were eliminated in this initial screening if they were case series, case reports, animal studies; or if they did not investigate the association between methane positive SIBO and IBD or ulcerative colitis (UC) or Crohn’s disease (CD) or the association between methane positive SIBO and IBS. Full texts of the remaining articles were retrieved and reviewed. Studies recruiting unselected subjects meeting diagnostic criteria for IBS and IBD, that reported the prevalence of methane positive SIBO using clinically validated methods,17 and compared the prevalence of methane positive SIBO in IBS and IBD patients versus controls were eligible for inclusion. We also included studies that reported the efficacy data after antibiotic treatment of SIBO in IBS and IBD patients. The diagnosis of IBS and IBD (including CD and UC) was based upon clinical assessment, questionnaire data, or specific symptom-based criteria, including the Manning and Rome criteria. Studies not reporting original data, manuscripts not published as full papers or those that did not use clinically validated methods to diagnose SIBO17 were excluded. Individuals in the control group included healthy asymptomatic controls as well as ‘patient controls’ including patients undergoing evaluation for unexplained ‘gastrointestinal syndromes’ (e.g., anemia, dyspepsia, pyrexia of unknown origin). PPI and antibiotic data were extracted from the selected studies. Eligibility criteria for study inclusion are provided in Table 1. Disagreements between reviewers were resolved by mutual consensus after reference to the original published paper.
Table 1.
Eligibility criteria for the studies included in systematic review and meta-analysis
| Eligibility criteria |
|---|
|
|
|
|
| * Rome Criteria.18–21 ** Lactulose and/or Glucose breath test. |
IBS: irritable bowel syndrome; IBD: inflammatory bowel disease; SIBO: small intestinal bacterial overgrowth; CD: Crohn’s disease; UC: ulcerative colitis.
Data extraction and quality assessment
All data was extracted independently by two authors into a Microsoft Excel spreadsheet (2010 Professional edition; Microsoft Corp, Redmond, Washington, USA). During the data collection process, the following data was extracted from the studies; the author, the year of the study, country, source of controls, method of diagnosis of methane positive SIBO including test duration, quantity of substrate used and the cut off criteria for diagnosis of methane positive SIBO, gender, concurrent use of PPI and antibiotics, any significant co-morbidities including previous surgery for the patient and the control groups. In addition, for all patients with IBS and IBD, data regarding mode of diagnosis of IBS and IBD, sub-types, overlap with the other FGIDs, treatment of SIBO positive patients with antibiotics and objective and subjective response post treatment was recorded.
This systematic review and meta-analysis meets the preferred reporting items for systematic reviews and meta-analysis statement requirements (PRISMA).22 The quality of the included studies was assessed by using the Joanna Briggs Institute (JBI) critical appraisal tools for use in JBI systematic reviews for prevalence studies.23 The risk of bias was ranked as high when the study reached up to 49% of “yes” score, moderate when the study reached from 50 to 69% of “yes” score, and low when the study reached over 70% of “yes” score. In addition, the quality of the case-control included studies were assessed using the Newcastle-Ottawa scale (NOS) which judges the selection of the study groups, the comparability of the groups and the ascertainment of the exposure of interest, to assign a maximum score of 9 stars.24
Data analysis
The initial step involved determining the number of cases with IBS and controls (using various diagnostic modalities) in the respective cohorts. The same was done in patients with IBD and controls. This was followed by calculating the pooled estimates of prevalence and odds ratios (OR) and 95% confidence intervals (CI) for the prevalence of methane positive SIBO in IBS and IBD patients with their respective controls. Subgroup analysis stratified by diagnostic modalities, IBS and IBD subtypes, quality of the studies as assessed utilizing the quality assessment tools NOS/JBI critical appraisal tool were conducted. We also summarized PPIs/antibiotics data that were reported in included case-control studies. Finally, we did sensitivity analysis including only high-quality studies (assessed utilizing the NOS/JBI critical appraisal tool), reporting the prevalence of methane positive SIBO in IBS and IBD patients with their respective controls.
Analyses for the association between methane positive SIBO and patients with IBS or IBD and descriptive analyses were carried out utilizing the Statistical Package for Social Sciences (SPSS Version 23, Armonk NY: IBM Corporation) and comprehensive Meta-analysis (CMS) Version 3.3.070. The major statistical method for this review would be pooled proportion meta-analysis (uses logit transformation of proportions) for prevalence and odds ratio for comparisons between IBS/IBD and controls. Pooled estimates of disease prevalence were calculated using a random effects model to appropriately account for variability in the summary estimate. Between study variation was evaluated using Cochrane’s test25 and was quantified through the I2 index in which values close to 100 indicate substantial variation between studies while values close to zero indicate minimal between-study variation. Standard approaches (Egger Test26 and inspection of Funnel Plots), were applied to identify potential publication biases. If one or more cells had a value of 0, then the CMS software automatically adds a fixed value of 0.5 to the respective cell for computation of log odds ratio and variance. Further, either Chi2 test P < .10 or I2 > 50% were taken as indications of substantial heterogeneity.
Results
Selection outcome
The initial search strategy identified 1,179 publications, but only 52 appeared to be relevant to the study question and were retrieved for further evaluation. Of these, 30 were excluded for various reasons, leaving 15 eligible IBS studies12,27–40 and 5 IBD studies41–45 and two studies46,47 reporting on prevalence rates of methane positive SIBO in both IBS and IBD patients (PRISMA flow diagram and Table S9). The characteristics of all the studies in the current meta-analysis including the methodology pertaining to diagnosis of SIBO and patient characteristics, are outlined in Table 2, Table 3 and Tables S1, S2, and S3. Seven of the 22 studies were conducted in USA,27,28,33–35,39,40 four each in India12,29,36,44and Italy,32,37,41,42 three in Korea,31,43,48 and one each in Spain,38 Brazil,45 Australia,46 and Israel.47 The studies from Israel and Australia looked at both IBS and IBD patients.
Table 2.
Characteristics of studies showing mode of diagnosis and prevalence of methane positive SIBO in IBS patients
| No | Author | Study Year | Country | IBS, n |
IBS Subtype IBS-D IBS-C IBS-M |
Criteria for IBS diagnosis | Controls n | Type of control | Mode of diagnosis | Methane Positive SIBO in IBS, n (%) |
Methane positive SIBO in IBS subtypes, n (%) IBS-D IBS-C IBS-M |
Methane positive SIBO in controls, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shah A et al46 | 2020 | Australia | 62 | 18 | 8 | 38 | Rome IV | 63 | Healthy controls | GBT | 4 (25) | 1 (6.3) | 1 (6.3) | 2 (12.5) | 5 (31.3) |
| 2 | Ghoshal U et al12 | 2018 | India | 23 | NA | 23 | - | Rome III | 68 | Non-constipating IBS | LBT | 13 (56.5) | NA | 13 (56.5) | - | 25 (36.8) |
| 3 | Ghoshal U et al29 | 2016 | India | 25 | 13 | 12 | NA | Rome III | NA | NA | LBT | 8 (66.7) | 3 (23.1) | 8 (66.7) | NA | NA |
| 4 | Vega AB et al38 | 2015 | Spain | 48 | NA | 48 | NA | Rome III | 19 | Healthy controls | LBT | 29 (60.4) | NA | 29 (60.4) | NA | 10 (52.6) |
| 5 | Lee KN et al48 | 2013 | Korea | 68 | 35 | 23 | 10 | Rome III | 55 | Healthy controls | LBT | 10 (14.7) | 21 (60) | 14 (60.9) | 7 (70) | 5 (9.1) |
| 6 | Rana S et al36 | 2012 | India | 175 | 175 | NA | NA | Rome II | 150 | Healthy controls | GBT | 0 | 0 | NA | NA | 0 |
| 7 | Park JS et al31 | 2010 | Korea | 76 | 45 | 12 | 19 | NA | 40 | Healthy controls | LBT | 25 (32.9) | 14 (31.1) | 4 (33.3) | 7 (36.8) | 13 (32.5) |
| 8 | Hwang L et al39 | 2010 | USA | 56 | 23 | 24 | 9 | Rome I | NA | NA | LBT | 28 (50) | 6 (26.1) | 22 (91.7) | - | NA |
| 9 | Parodi A et al32 | 2009 | Italy | 130 | 51 | 31 | 48 | Rome III | 70 | Healthy controls | GBT | 35 (26.9) | 11 (21.6) | 10 (32.3) | 14 (29.2) | 17 (24.3) |
| 10 | Scarpellini E et al37^ | 2009 | Italy | 43 | 15 | 12 | 16 | Rome II | 56 | Healthy controls | LBT | 4 (9.3) | - | - | - | 0 |
| 11 | Bratten J et al27 | 2008 | USA | 224 | 114 | 92 | - | Rome II | 40 | Healthy controls | LBT | 44 (19.6) | 13 (11.4) | 25 (27.2) | - | 6 (15) |
| 12 | Majewski M et al40 | 2007 | USA | 204 | 149 | 30 | 25 | Rome II | NA | NA | GBT | 32 (15.7) | 20 (13.4) | 7 (23.3) | 4 (16) | NA |
| 13 | Chatterjee S et al28 | 2007 | USA | 87 | NA | NA | NA | Rome I | NA | NA | LBT | 20 (23) | NA | NA | NA | NA |
| 14 | Pimentel M et al33 | 2006 | USA | 39 | NA | 39 | NA | Rome I | NA | NA | LBT | 12 (30.8) | NA | 12 (30.8) | NA | NA |
| 15 | Pimentel M et al34 | 2003 | USA | 65 | 34 | 31 | NA | Rome I | NA | NA | LBT | 12 (24.0) | 0 | 12 (38.7) | NA | NA |
| 16 | Pimentel M et al35 | 2003 | USA | 296 | 111 | 120 | 65 | Rome I | NA | NA | LBT | 50 (16.9) | 6 (5.4) | 30 (25) | NA | NA |
| 17 | Peled Y et al47 | 1987 | Israel | 32 | 6 | 16 | - | Breath methane | 152 | Healthy controls | NA | 11 (34.4) | 1 (16.7) | 5 (31.3) | - | 76 (50) |
IBS: Irritable bowel syndrome; SIBO: small intestinal bacterial overgrowth; LBT: lactulose breath test; GBT: glucose breath test; IBS-D: IBS-diarrhea; IBS-C: IBS-constipation; IBS-M: IBS-mixed; NA: not applicable, ^ Study included pediatric patients with a diagnosis of IBS, *Information not available.
Table 3.
Characteristics of the IBD studies included in this systematic review and meta-analysis
| Study No | Author | Study Year | Study Type | Country | IBD, n | UC, n | CD, n | Diagnostic criteria for IBD | Controls n | Type of control | Mode of diagnosis of SIBO | Methane positive SIBO in IBD, n (%) | Methane positive SIBO in UC, n (%) | Methane positive SIBO in CD, n (%) | Methane positive SIBO in Controls (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shah A et al46 | 2020 | Case control | Australia | 81 | 48 | 33 | Known IBD | 63 | Healthy controls | GBT | 5 (31.3) | 4 (25) | 1 (6.3) | 5 (31.3) |
| 2 | Ricci J et al45 | 2017 | Case control | Brazil | 92 | NA | 92 | *IBD diagnosis | 97 | Nonspecific chronic GI symptoms | GBT | 9 (9.8) | NA | 9 (9.8) | 8 (8.2) |
| 3 | Greco A et al42 | 2015 | Prevalence | Italy | 68 | NA | 68 | Internationally accepted criteria | NA | NA | GBT | 1 (1.5) | NA | 1 (1.5) | NA |
| 4 | Lee JM et al43 | 2015 | Case control | Korea | 107 | 64 | 43 | Known IBD | 30 | Healthy controls | GBT | 3 (2.8) | - | - | 0 (0) |
| 5 | Rana S et al44 | 2013 | Case control | India | 137 | 95 | 42 | Confirmed by colonic biopsy | 115 | Healthy controls | GBT | 4 (2.9) | - | - | 28 (24.3) |
| 6 | Castiglione F et al41 | 2000 | Case control | Italy | 57 | NA | 57 | Known IBD | 40 | Healthy controls | LBT | 2 (3.5) | NA | 2 (3.5) | 0 (0) |
| 7 | Peled Y et al47 | 1987 | Case control | Israel | 84 | 51 | 33 | *IBD diagnosis | 152 | Healthy controls | Breath methane was measured | 18 (21.4) | 16 (31.4) | 2 (6.1) | 76 (50) |
IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease; SIBO: small intestinal bacterial overgrowth; LBT: lactulose breath test; GBT: glucose breath test; GI: gastrointestinal; NA: not applicable; *IBD diagnosis: clinical, radiological, colonoscopy and histological diagnosis of IBD.
IBS: Prevalence of methane positive SIBO
All studies: Overall, the 17 studies (10 case control studies12,27,31,32,36–38,46–48 and 7 prevalence studies28,29,33–35,39,40) assessing the prevalence of methane positive SIBO included 1,653 patients with IBS. The prevalence of methane positive SIBO in IBS patients was 25.0% (95% CI 18.8–32.4), Figure S1), with significantly high heterogeneity in the studies included in this analysis (I2=86.28, p = .0001). Visual inspection of the funnel plot revealed overall asymmetry, suggesting the potential for publication bias (Figure S2), consistent with the results of Egger’s test.
Case control studies: Ten12,27,31,32,36–38,46–48 of the 17 studies were case control studies and included 881 patients with IBS and 713 controls. The prevalence of methane positive SIBO in IBS patients (175/881, 19.95% [95% CI 17.3–22.7]) was not different from controls (157/713, 22.0% [95% CI 19.0–25.3]). Overall, the odds of methane positive SIBO was not statistically different in IBS patients as compared to controls (OR = 1.2 (95% CI 0.8–1.7), P = .373, Figure 1). There was no significant heterogeneity in the studies included in the analysis (I2=13.70, P = .320) and visual inspection of the funnel plot showed overall symmetry, suggesting minimal potential for publication bias, Figure 2.
Figure 1.

Forest plot of case-control studies showing methane positive SIBO in patients with IBS and controls, utilizing breath tests (OR = 1.2 (95% CI 0.8–1.7), P = .373), (I2=13.70, P = .320)
Figure 2.
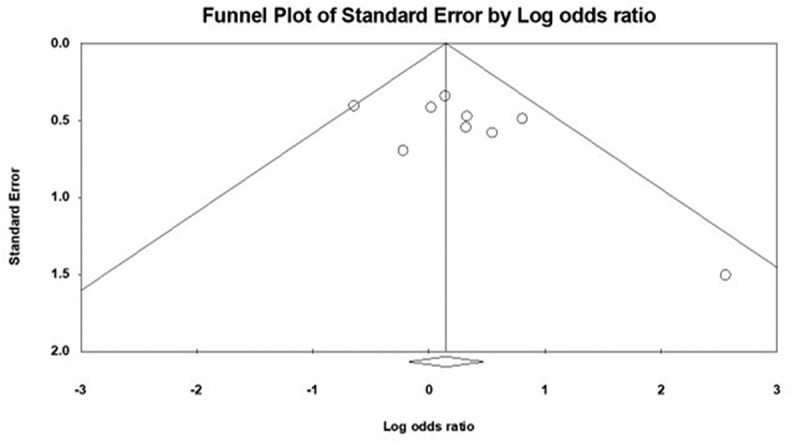
Funnel plot of methane positive SIBO in patients with IBS and controls
Influence of selection criteria for controls, and risk of bias on the prevalence of methane positive SIBO in patients with IBS and controls
The quality of the included studies (all prevalence studies and the case group (only IBS patients) of the case control studies) as assessed by the JBI critical appraisal tool is shown in Table S6. Out of the ten case-control studies, eight studies27,31,32,36–38,46,48 presented a low risk of bias/high methodological quality, one12 (utilizing LBT for SIBO diagnosis) presented a moderate risk of bias/moderate methodological quality, and one47 (measuring breath methane) high risks of bias/low methodological quality. All seven prevalence studies28,29,33–35,39,40 presented a moderate risk of bias/moderate methodological quality. Furthermore, the majority (8/10, 80%) of the case-control studies were of high-quality, defined as a score of ≥6 using the Newcastle-Ottawa assessment scale (NOS), Table S5.
High-quality studies with low risk of bias: Including only studies with low risk of bias based upon the JBI critical appraisal tool and the NOS (supplementary material Tables S5 and S6), yielded a OR of 1.2 (95% CI 0.9–1.8, P = .246, Figure S3) for methane positive SIBO in IBS patients as compared to healthy asymptomatic controls. Furthermore, including only high-quality studies resulted in reducing heterogeneity to 0 (I2=0%, P = .744). Three32,36,46 out of the eight studies utilized GBT and the remaining five27,31,37,38,48 utilized LBT for diagnosis of methane positive SIBO in IBS patients and controls.
Healthy controls: If only studies27,31,32,36–38,46–48 where healthy asymptomatic controls were included, the OR for methane positive SIBO in IBS-patients was 1.1 (95% CI 0.8–1.5), P = .704, Figure S4). There was no significant heterogeneity (I2=3.303, P = .404) in the studies included in this analysis. This analysis excluded only one study,12 with non-constipating IBS as controls.
Comparison of methane positive SIBO in IBS according to the type of breath test utilized for SIBO diagnosis.
Twelve studies12,27–29,31,33–35,37–39,48 utilized LBT and four studies32,36,40,46 utilized GBT for SIBO diagnosis. One study47 measured breath methane and considered a subject methane producer if the breath methane concentration was at least 1 parts per million (ppm)above ambient air. The overall prevalence of methane positive SIBO in IBS was almost 3-fold higher for studies utilizing LBT (29.0% (95% CI 20.9–38.6), Figure S5), as compared to studies utilizing GBT (11.5% (95% CI 5.0–24.3), fFigure S7). There was significantly high heterogeneity in both these analysis (I2=87.10, P = .0001 for LBT and 86.32, P = .0001 for GBT). Visual inspection of the funnel plot revealed asymmetry, suggesting publication bias, Figure S6.
The OR for methane positive SIBO in IBS of 1.5 (95% CI 1.0–2.3), P = .06, Figure S8) for studies using LBT was higher as compared to 1.1 (95% CI 0.6–2.0), P = .824, Figure S9) for studies utilizing GBT. Moreover, there was no significant heterogeneity in the studies utilizing LBT (I2=0, P = .590) or GBT for diagnosing methane positive SIBO in IBS (I2=0, P = .641).
IBS subtypes and prevalence of methane positive SIBO
Eleven27,31,32,34,35,39,40,46–49 out of 17 studies reported on methane positive SIBO in IBS-subtypes (Table 2). Of these, only five studies31,32,40,46,48 reported prevalence rates of methane positive SIBO in all distinct IBS-subtypes. The prevalence of SIBO was higher in patients with IBS-C at 37.7% (95% CI 33.5–42.1) as compared to 24.3% (95% CI 17.4–32.3) in IBS-M and almost 3-fold higher as compared to patients with IBS-D 12.4% (95% CI 10.2–14.9). The odds of methane positive SIBO in IBS-C was significantly higher as compared to IBS-D, (OR = 3.1 (95%CI 1.7–5.6), P = .0001, Figure S10) with substantial heterogeneity in the included studies (I2=52.23, p = .02). However, including only high-quality studies the prevalence of methane positivity on breath test remained significantly higher in IBS-C as compared to IBS-D (OR = 2.0, (95% CI 1.3–3.2),P = .002), and there was no significant heterogeneity in this analysis (I2=0, P = .540). There was no significant difference in the odds of methane positive SIBO in IBS-C as compared to IBS-M or IBS-D as compared to IBS-M (data not shown).
Association between methane positive SIBO in patients with IBS and oro-cecal transit time
Only two12,38 out of 17 studies included in this systematic review and meta-analysis reported on the intestinal transit time in methane producers and compared it to that in non-methane producers. Vega et al,38 reported a significantly longer oro-cecal transit time (OCTT) in both healthy controls and constipated, methane-producing subjects as compared to non-methane producers. Similarly, Ghoshal et al,12 reported longer OCTT in methane producers as compared to non-methane producers, however it was not significant (P = .06).
Effect of proton pump inhibitors on the prevalence of methane positive SIBO in IBS
Overall, only one29 out of 17 studies included IBS patients who were not on PPI therapy. 15 studies12,27,28,31–40,47,48 were excluded because data about effect of PPI on methane positivity on breath test in IBS patients could not be extracted, Table S1. Only one case control study46 assessed the effect of PPI therapy on methane positivity in IBS patients. The prevalence of methane positive SIBO was higher in 3/26 (11.5%, 95% CI 2.4–30.1) IBS patients on PPI as compared to those 2/36 (5.5%, 95% CI 0.6–18.6) not on a PPI, a non-significant difference.
Effect of antibiotic therapy on the prevalence of methane positive SIBO in IBS
Only three12,33,38 out of 17 studies included in this systematic review and meta-analysis, assessed the effect of treatment (one study of rifaximin12 and one study of neomycin,33 and one study with fiber38) on symptom improvement in methane positive IBS-C patients, Table S4. While studies could not be combined for meta-analysis due to markedly different treatment modalities (type of antibiotic, fiber), all three studies demonstrated that the treatment of methane positive IBS-C patients with antibiotics/fiber (as compared to placebo) resulted in symptom improvement and improvement or normalization of follow-up breath tests in a large proportion of treated patients.
IBD: Prevalence of methane positive SIBO
All studies: In total, seven studies assessed the prevalence of methane positive SIBO in 626 adult patients with IBD. The prevalence of methane positive SIBO in patients in IBD patients was 5.6% (95% CI 2.6–11.8, Figure S11). Substantial heterogeneity was noted in the overall analysis, (I2=80.4, p = .0001). Five studies utilized GBT42–46 and only one study utilized LBT.41 One study47 measured breath methane and considered a subject a methane producer if the breath methane concentration was at least 1 ppm above ambient air. Hence, we did not do a subgroup analysis according to the type of breath test utilized to diagnose SIBO in patients with IBD.
Case control studies: Six41,43–47 of the of the seven studies were case control studies and included 558 adult patients with IBD and 497 controls. The prevalence of methane positive SIBO in patients with IBD was approximately 3-fold lower at 7.4% (95% CI 5.4–9.8) compared to 23.5% (95% CI 19.8–27.5) in controls. Overall analysis comprising all patients with IBD (including patients with CD and UC) found no difference in the odds of methane positive SIBO in IBD (OR = 0.5 (95% CI 0.2–1.3, P = .172 Figure 3), with substantial heterogeneity detected between the studies included in this analysis (I2=70.8, P = .004).
Figure 3.
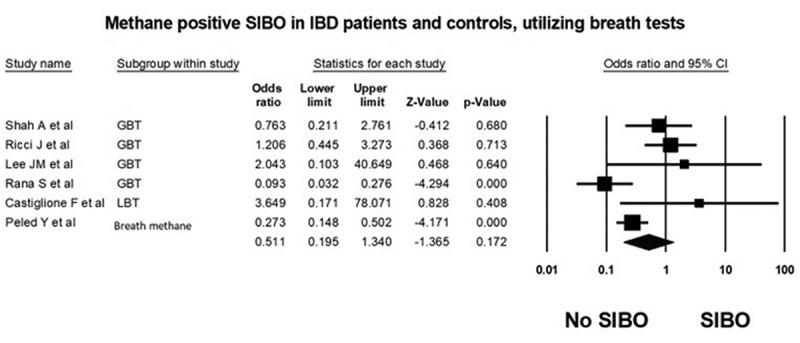
Forest plot of case control studies showing prevalence of methane positive SIBO in patients with IBD and controls, utilizing breath tests (OR = 0.5 [95% CI 0.2–1.3], P = .17), (I2=70.8, P = .004)
Influence of selection criteria for controls, and risk of bias, type of breath test on the prevalence of methane positive SIBO in patients with IBD and controls
The quality of the included studies based on the JBI critical appraisal tool and the NOS is shown in Tables S7 and S8. Out of the seven studies, five studies presented a low risk of bias/high methodological quality and two studies (one utilizing GBT45 and one measured breath methane47) presented high risk of bias/low methodological quality.
High-quality studies with low risk of bias: Including only five studies41–44,46 with low risk of bias based upon the quality assessment tools (supplementary material Tables S7 and S8), 5 high-quality studies, yielded a significantly low prevalence for methane positive SIBO at 3.6% (95% CI 2.2–6.0, Figure S12) in patients with IBD. There was no heterogeneity in this subgroup analysis (I2=0, P = .595). Conducting a sensitivity analysis with studies utilizing GBT, the results remained unchanged (data not shown).
Methane positive SIBO in IBD subtypes
In regard to IBD subtypes, three41,42,45 out of seven studies included patients with CD, while four included both UC and CD patients43,44,46,47 (all case control studies). Data from two out the four studies43,44 could not be extracted due to insufficient information on methane positive SIBO in IBD subtypes. Methane positive SIBO was approximately 4-fold higher in UC patients at 20.2% (95% CI 12.8–29.4) as compared to 5.3% (95% CI 3.0–8.5) in CD patients, Table 3.
Assessment of risk factors for methane positive SIBO in IBD
None of the studies reported on the influence of risk factors such as disease duration, prior surgery, fibro-stenosing disease, location of IBD, and the influence of immunotherapy on methane positive SIBO in IBD patients, hence we were unable to conduct a subgroup analyses to assess the impact of risk factors of methane positivity on breath test in IBD patients. With regards to transit time, Rana et al,50 did not find any significant difference in the oro-cecal transit time measured utilizing LBT between methane positive IBD patients (145.2 ± 28.7) as compared to methane negative IBD patients (117.7 ± 33.45). This association between transit time and methane was not reported in the remaining studies included in this analysis.
Effect of antibiotic therapy and proton pump inhibitors on the prevalence of methane positive SIBO in IBD
We found no studies that directly evaluated the effect antibiotic treatment has on the prevalence of methane positive SIBO in IBD. Four41,43–45 out of seven studies, included IBD patients that were not on a PPI and in two42,47 out of seven studies information about PPI use was not available, (Table S2). Only one case control study46 assessed the effect of PPI therapy on methane positive SIBO in IBD patients. The prevalence of methane positive SIBO was higher in IBD patients on PPI (15.4%, 95% CI 1.9–45.4) as compared to patients not on PPI (4.4%, 95% CI 9.2–12.3).
Discussion
This is the first systematic review and meta-analysis of methane positive SIBO in patients with IBS and IBD and is based on 22 peer-reviewed case control and prevalence studies from eight different countries that included 1,653 patients with IBS, 626 patients with IBD, and 1,210 controls. We found patients with IBS-C have a significantly higher prevalence of methane positivity on breath tests as compared to those with IBS-D (OR = 3.1, 95% CI 1.7–5.6). In contrast, if IBS patients are not differentiated according to their subtype, the prevalence of methane positive SIBO is not increased in IBS as compared to controls (OR = 1.2, 95% CI 0.8–1.7). Methane positive SIBO was not associated with IBD (OR 0.5, 95% CI 0.2–1.3), although patients with UC have a significantly greater prevalence of breath methane positivity as compared to those with CD. The primary analysis (including both prevalence and case-control studies) reporting methane positive SIBO in IBS and IBD patients revealed substantial heterogeneity. This could at least partially be explained by the inherent limitations of the studies and the limitations of the available tests for SIBO diagnosis. Hence, we conducted subgroup analysis, according to the study type, type of controls, the quality of the studies as assessed utilizing the quality assessment tools (NOS and JBI appraisal tool), and the type of breath test used for SIBO diagnosis.
In these analyses we found very little or even no heterogeneity and the prevalence rates for methane positive SIBO was not higher in IBS patients as compared to controls. Importantly, the majority (8/10) of the case control studies scored high on the quality assessment tools, while all prevalence studies had a moderate risk of bias. Finally, conducting subgroup analysis according to the type of breath test, we found a 3-fold (significantly) increased prevalence of methane positive SIBO in IBS patients in studies utilizing LBT as compared to those utilizing GBT, with significant heterogeneity in both analyses. On the other hand, although the OR for methane positive SIBO in IBS patients was significantly higher in case-control studies utilizing LBT as compared to those utilizing GBT, there was no heterogeneity in these analyses. This suggests that the type of study (prevalence vs. case-control) rather than the type of breath test used for SIBO diagnosis, contributed to the high heterogeneity in the primary analyses.
Next, we examined studies reporting methane positive SIBO in IBD patients. Due to the limited number of studies, we were unable to make comparisons on methane prevalence rates in IBD patients according to the type of study or type of breath test. However, including only high-quality studies (5/7), we found significantly lower prevalence rates for methane positive SIBO in IBD patients and there was no heterogeneity in studies included in this analysis.
One of the important findings of this systematic review and meta-analysis is the significant association between methane on breath tests and IBS- and IBD subtypes. We found a 3-fold higher prevalence of methane positive SIBO in patients with IBS-C as compared to those with IBS-D, but there was substantial heterogeneity in the analysis. However, conducting subgroup analysis, including only high-quality studies, the odds of methane positive SIBO were still significantly higher in IBS-C as compared to IBS-D (OR 2.0, 95% CI 1.3–3.2) and importantly the heterogeneity in this analysis was reduced to zero. Our findings were in keeping with those by Kunkel et al,11 who reported a significantly increased prevalence of methane positive SIBO in patients with IBS-C and functional constipation, but also noted very high heterogeneity in the studies included in the analysis. Only two studies included in this systematic review and meta-analysis reported on oro-cecal transit time and methane. Both found longer oro-cecal transit time in methane producers as compared to non-methane producers. There is experimental51 and clinical evidence that methane is likely capable of slowing intestinal transit,11,52 implicating either a direct or indirect action of methane inducing constipation. Analyzing stool microbiome utilzing qPCR, Kim et al53 and more recently Ghoshal et al,29 demonstrated that Methanobrevibacter smithii is more abundant in methane producing IBS-C subjects as compared to non-methane producing IBS-C patients. Interestingly, they also demonstrated a correlation between the concentration of M. smithii and the functional consequence of their metabolic activity, i.e., quantity of methane in exhaled air. However, other human studies have shown that slow intestinal trasit may facilitate the growth of methanogenic bacteria.47,54 Thus, the question does methane slow the transit time or does a delayed transit time promotes the growth of methanogens (hence the production of methane) remains to be elucidated.
Although only limited data are available, we found a significantly (almost 4-fold) lower prevalence of methane positive SIBO in patients with CD (5.3%) as compared to those with UC (20.2%). More importantly, only one50 out the seven studies included in the meta-analysis assessed the transit time in IBD patients. This study did not find any significant association between oro-cecal transit time and methane status in IBD patients. These findings are intriguing, as in our recent systematic review and meta-analysis,16 we found a significantly a higher prevalence of non-methanogenic SIBO utilizing breath testing in IBD patients (and higher in patients with CD as compared to UC) versus non-IBD controls (OR 9.5, 95% CI 3.39–26.68). Moreover, the oro-cecal transit time was prolonged in IBD patients compared to healthy controls, and the oro-cecal transit time was significantly increased in SIBO positive compared to SIBO negative IBD patients. Collectively, these findings suggest that breath methane, methanogen positivity as measured by PCR/culture and OCTT are not inextricably linked, and that methanogen persistence is differentially affected by other alterations to the nutritional and/or environmental landscape of the gut milieu in CD and UC.
One of the key limitations of this systematic review is the small number of studies measuring both, methane and hydrogen measurements during breath tests. We did not include conference abstracts, since the brevity of abstracts frequently fails to provide adequate information to appropriately appraise the design, methods, risk of bias, and outcomes of the studies included in the abstracts. Balancing the risk of publication bias against potentially misleading data, we opted to include only fully peer-reviewed published manuscripts. Indeed, a recent study comparing abstracts with full-length journal articles concluded that the information presented in abstracts was not dependable55 Attempts were made to contact authors of the studies included in this systematic review and meta-analysis to get additional information and/or clarification whenever this deemed necessary. Two research groups responded, and the additional information was incorporated in the analyses. Even though this systematic review and meta-analysis has not been prospectively registered, we acknowledge that prospective registration of systematic reviews is now recommended since it promotes transparency, reduces potential for bias and avoids duplication of reviews.56
Although there more than 60 peer-reviewed published studies (both case-control and prevalence studies) assessing the link between SIBO and IBS and IBD, less than half have measured both, methane and hydrogen on breath testing. It is now well acknowledged that lack of measurement of methane on routine breath testing could underestimate SIBO prevalence in various gastrointestinal conditions.14 Another important limitation is that all studies included in this systematic review and meta-analysis have only utilized breath tests (indirect testing), which are surrogate markers for diagnosing bacterial overgrowth. Methanogens are strict anaerobes57 and are exceedingly difficult to culture, requiring very specific conditions and culture media.58 Moreover, currently there is no consensus regarding the gold standard for the diagnosis of SIBO. While direct (small bowel aspirate and culture) tests are invasive, time consuming and require an endoscopy, they have been largely replaced in clinical practice by (indirect) breath tests. However, compared with culture-based methods, the GBT has a sensitivity of 62.5% and a specificity of 81.7% and the LBT has a sensitivity of 52.4% and a specificity of 85.7%.59Molecular techniques (PCR-based tests), utilizing specific primers to quantify small intestinal bacterial colonization are emerging as alternative diagnostic approaches.46
The assessment of the link between methanogens and IBS or IBD is further limited by the paucity of studies assessing the effect of antibiotic and PPI use specifically on methane positive SIBO in these patients. It is now well established that PPI is a risk factor for SIBO60 in various gastrointestinal disorders. Proton pump inhibitors cause chronic acid suppression and the resultant hypochlorhydria alters the intraluminal environment to promote growth of the microbes in the small intestine. Only one study46 specifically assessed the effect of PPI on methane positive SIBO in IBS and IBD and found a higher prevalence rate of methane positive SIBO in both IBS and IBD patients on a PPI, as compared to those not on a PPI.
Due to the limited number of studies, it was not possible to conduct subgroup analysis to assess the effect of antibiotic therapy on symptom improvement, effect on transit time and normalization of follow-up breath tests in patients with IBS and IBD. Ghoshal et al,12 in their recent randomized controlled trial showed in thirteen methane producers, treatment with rifaximin as compared to placebo resulted in significant improvement in weekly stool frequency (P = .05), stool form (P = .08) and area under the curve (AUC) for methane on post treatment breath test (P = .005). Similarly, Pimentel et al33 showed that in twelve methane positive IBS-C patients, treatment with neomycin as compared to placebo was associated with significant improvement in constipation and normalization of methane on follow-up breath test in those treated with neomycin. In an elegant study by Vega et al,38 treatment of constipated patients (IBS-C and functional constipation) with fiber was associated with a significant improvement in the colonic transit time, symptoms and decreased methane production. This may support the concept that slow transit causes methane positivity but is not definitive.
In summary, this is the first systematic review and meta-analysis specifically examining the prevalence of methane positive “SIBO” or “IMO” in patients with IBS and IBD (Table 4). Based on the available data the prevalence of methane positive SIBO is increased in patients with IBS-C and decreased in patients with IBD, importantly in those with CD. Although PPI appeared to be a risk factor for methane positive SIBO in IBS and IBD patients, and antibiotic therapy was associated with improvement in symptoms and transit time, these data is extremely limited and need validation in larger studies. All this emphasizes the importance of measuring methane in addition to hydrogen on routine breath tests and the need for more robust tests (including isolation and culturing the fastidious methanogens) or utilizing molecular techniques (PCR-based techniques) to diagnose small intestinal methanogen overgrowth. While this systematic review and meta-analysis suggests a link between methane positive SIBO and gastrointestinal disorders, the quality of evidence is low, and this can be attributed mainly to substantial clinical heterogeneity seen in the prevalence studies. Thus, more appropriately designed studies are required that not only assess the prevalence of SIBO but also characterize intestinal dysbiosis in various gastrointestinal disorders.
Table 4.
Summary of findings of the outcomes reported in this systematic review and meta-analysis
| Mode of diagnosis of SIBO in IBS/IBD | No of studies | IBS/IBD n | Controls n | SIBO in IBS /IBD patients n | SIBO in Controls n | Prevalence rates of SIBO in IBS/IBD patients, % (95% CI) | Prevalence rates of SIBO in controls, % (95% CI) | Prevalence of SIBO, OR (95% CI) | Assessment of heterogeneity between studies |
|---|---|---|---|---|---|---|---|---|---|
| Irritable Bowel Syndrome | |||||||||
| All studies | 17 | 1653 | - | 340 | - | 25.0 (18.8–32.4)* | - | P = .825 | I2=86.28, p = .0001 |
| Only high-quality studies (All were case control studies) | 8 | 826 | 493 | 151 | 56 | 18.3 (15.7–21.1) | 11.4 (8.7–14.5) | 1.2 (0.9–1.8), p = .246 | I2=0, p = .744 |
| Case control studies ONLY | 10 | 881 | 713 | 175 | 157 | 19.9 (17.3–22.7) | 22.0 (19.0–25.3) | 1.2 (0.8–1.7), p = .373 P = .124 |
I2=13.7, p = .320 |
| Case control studies including only healthy controls | 9 | 858 | 645 | 162 | 132 | 18.9 (16.2–21.7) | 20.5 (17.4–23.8) | 1.1 (0.8–1.5), p = .704 | I2=3.30, p = .404 |
| Case control studies utilizing LBT | 6 | 482 | 278 | 125 | 59 | 25.9 (22.7–30.1) | 21.2 (16.6–26.5) | 1.5 (1.0–2.3), p = .06 | I2=0, p = .59 |
| Case control studies utilizing GBT | 3 | 367 | 283 | 39 | 22 | 10.6 (7.7–14.2) | 7.8 (4.9–11.5) | 1.1 (0.6–2.0), p = .824 | I2=0, p = .641 |
| IBS subtypes (IBS-C versus IBS-D) | 11 | 509 (IBS-C) |
774 (IBS-D) | 192 (IBS-C) | 96 (IBS-D) | 37.7 (33.5–42.1) | 12.4 (10.2–14.9) | 3.1 (1.7–5.6), p = .0001 P = .314 |
I2=52.23, p = .02 |
| Inflammatory Bowel Disease | |||||||||
| All studies | 7 | 626 | - | 42 | - | 5.6 (2.6–11.8)* | - | - | I2=80.4, p = .0001 |
| Case control studies ONLY | 6 | 558 | 497 | 41 | 117 | 7.4 (5.4–9.8) | 23.5 (19.8–27.5) | 0.5 (0.2–1.3), p = .172- | I2=70.8, p = .004 |
| CD | 2 | 283 | - | 15 | - | 5.3 (3.0–8.5) | - | - | |
| UC | 2 | 99 | - | 20 | - | 20.2 (12.8–29.4) | - | - | - |
| Only high-quality studies (4 case control & 1 cohort study) | 5 | 450 | - | 15 | - | 3.6 (2.2–6.0)* | - | - | I2=0, p = .595 |
IBS: Irritable bowel syndrome; IBS-D: IBS-diarrhea; IBS-C: IBS-constipation; IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease; SIBO: small intestinal bacterial overgrowth; LBT: lactulose breath test; GBT: glucose breath test; OR: odds ratio; *pooled prevalence rate of SIBO in respective GI conditions calculated utilizing the CMA software, P indicates values from Eggers’s test.
Supplementary Material
Acknowledgments
The authors would like to acknowledge our Librarian, Ms Gina Velli who has assisted with the literature search.
Funding Statement
This work was supported by the National Health and Medical Research Council and the Princess Alexandra Hospital Research Foundation
Author contributions
Arjun Gandhi, Ayesha Shah, and Gerald Holtmann - study idea, concept and design, data extraction and interpretation of data, drafting of the manuscript.
Mark Morrison, Natasha Koloski, and Nicholas J Talley - interpretation of data, drafting of the manuscript, review of final manuscript.
Mike Jones - data analysis, review of final manuscript.
Declaration of Interests:
GH report to be on the advisory boards Australian Biotherapeutics, Glutagen, Bayer and received research support from Bayer, Abbott, Pfizer, Janssen, Takeda, Allergan. He serves on the Board of West Moreton Hospital and Health Service, Queensland, UQ Healthcare, Brisbane and the Gastro-Liga, Germany. He has a patent for the Brisbane aseptic biopsy device. Editor of the Gastro-Liga Newsletter.
NJT reports personal fees from Allakos, from Aviro Health, from Antara Life Sciences, from Arlyx from Bayer, from Danone, from Planet Innovation, from Takeda, from Viscera Labs, from twoXAR, from Viscera Labs, from Dr Falk Pharma, from Censa, from Cadila Pharmaceuticals, from Progenity Inc, from Sanofi-aventis, from Glutagen, from ARENA Pharmaceuticals, from IsoThrive, from BluMaiden, from HVN National Science Challenge, non-financial support from HVN National Science Challenge NZ, outside the submitted work; In addition, NJT has a patent Biomarkers of IBS licensed (#12735358.9 −1405/2710383 and (#12735358.9 −1405/2710384), a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent NTU Ref: TD/129/17 “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued and copyright Nepean Dyspepsia Index (NDI) 1998 and Editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press). NJT participates Committees: Australian Medical Council (AMC) Council Member (2016–2019), MBS Review Taskforce (2016-2020), NHMRC Principal Committee, Research Committee (2016–2021), Asia Pacific Association of Medical Journal Editors (APAME) (current), GESA Board Member (2017–2019). NJT Misc: Avant Foundation (judging of research grants) (2019). NJT community and patient advocacy groups: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). NJT acknowledges funding from the National Health and Medical Research Council (NHMRC) for the Centre for Research Excellence in Digestive Health. NJT holds an NHMRC Investigator grant.
Supplementary material
supplemental data for this article can be accessed on the publisher’s website
References
- 1.Lynch SV, Pedersen O.. The human intestinal microbiome in health and disease. New England Journal of Medicine. 2016;375(24):2369–16. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 2.Shah A, Holtmann G. Clinical conditions associated with small intestinal bacterial overgrowth. gastrointestinal diseases and their associated infections. in: Gastrointestinal Diseases and their associated Infections. Edited by Guy D. Eslick. St. Louis, MO, United States: 67-83 Elsevier Inc. 2019;67–83. [Google Scholar]
- 3.Shah A, Morrison M, Holtmann GJ. Gastroduodenal “Dysbiosis”: a new clinical entity. Curr Treat Options Gastroenterol. 2018;16(4):591–604. doi: 10.1007/s11938-018-0207-x. [DOI] [PubMed] [Google Scholar]
- 4.Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281(3):122–127. doi: 10.1056/NEJM196907172810303. [DOI] [PubMed] [Google Scholar]
- 5.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55(3):297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay LF, Holbrook WP, Eastwood MA. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand B. 1982;90(3):257–260. doi: 10.1111/j.1699-0463.1982.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 7.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. 2010;55(8):2135–2143. doi: 10.1007/s10620-009-1012-0. [DOI] [PubMed] [Google Scholar]
- 9.Bond JH Jr., Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971;133(3):572–588. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt MD, Furne JK, Kuskowski M, et al. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol. 2006;4(2):123–129. doi: 10.1016/j.cgh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel D, Basseri RJ, Makhani MD, et al. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011;56(6):1612–1618. doi: 10.1007/s10620-011-1590-5. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal UC, Srivastava D, Misra A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: a pilot study. Indian J Gastroenterol. 2018;37(5):416–423. doi: 10.1007/s12664-018-0901-6. [DOI] [PubMed] [Google Scholar]
- 13.McKay LF, Eastwood MA, Brydon WG. Methane excretion in man–a study of breath, flatus, and faeces. Gut. 1985;26(1):69–74. doi: 10.1136/gut.26.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pimentel M, Saad RJ, Long MD, et al. ACG clinical guideline: small intestinal bacterial overgrowth. Am J Gastroenterol. 2020;115(2):165–178. doi: 10.14309/ajg.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Talley NJ, Jones M, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. Am J Gastroenterol. 2020;115(2):190–201. doi: 10.14309/ajg.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 16.Shah A, Morrison M, Burger D, et al. Systematic review with meta-analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics. 2019;49(6):624–635. doi: 10.1111/apt.15133. [DOI] [PubMed] [Google Scholar]
- 17.Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north american consensus. Am J Gastroenterol. 2017;112(5):775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drossman D, Thompson WG, Talley N, et al. Identification of sub-groups of functional gastrointestinal disorders. Gastroenterology International. 1990;3:159–172. [Google Scholar]
- 19.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 21.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;19:S0016-5085(16)00223-7. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 24.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):1–173. :iii-x. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103(4):958–963. doi: 10.1111/j.1572-0241.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, Park S, Low K, et al. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102(4):837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghoshal U, Shukla R, Srivastava D, et al. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in methanobrevibacter smithii which is associated with higher methane production. Gut Liver. 2016;10(6):932–938. doi: 10.5009/gnl15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KM, Paik CN, Chung WC, et al. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol. 2013;25(6):726–732. doi: 10.1097/MEG.0b013e32835eb916. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Yu JH, Lim HC, et al. [Usefulness of lactulose breath test for the prediction of small intestinal bacterial overgrowth in irritable bowel syndrome]. Korean J Gastroenterol. 2010;56(4):242–248. doi: 10.4166/kjg.2010.56.4.242. [DOI] [PubMed] [Google Scholar]
- 32.Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, Isola L, De Conca S, Marabotto E, Savarino V, et al. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43(10):962–966. doi: 10.1097/MCG.0b013e3181a099a5. [DOI] [PubMed] [Google Scholar]
- 33.Pimentel M, Chatterjee S, Chow EJ, et al. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51(8):1297–1301. doi: 10.1007/s10620-006-9104-6. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98(2):412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 35.Pimentel M, Mayer AG, Park S, et al. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48(1):86–92. doi: 10.1023/A:1021738515885. [DOI] [PubMed] [Google Scholar]
- 36.Rana SV, Sharma S, Kaur J, et al. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion. 2012;85(3):243–247. doi: 10.1159/000336174. [DOI] [PubMed] [Google Scholar]
- 37.Scarpellini E, Giorgio V, Gabrielli M, et al. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155(3):416–420. doi: 10.1016/j.jpeds.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Vega AB, Perelló A, Martos L, et al. Breath methane in functional constipation: response to treatment with Ispaghula husk. Neurogastroenterol Motil. 2015;27(7):945–953. doi: 10.1111/nmo.12568. [DOI] [PubMed] [Google Scholar]
- 39.Hwang L, Low K, Khoshini R, et al. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010;55(2):398–403. doi: 10.1007/s10620-009-0778-4. [DOI] [PubMed] [Google Scholar]
- 40.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–142. [PubMed] [Google Scholar]
- 41.Castiglione F, Del Vecchio Blanco G, Rispo A, et al. Orocecal transit time and bacterial overgrowth in patients with Crohn’s disease. J Clin Gastroenterol. 2000;31(1):63–66. doi: 10.1097/00004836-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Greco A, Caviglia GP, Brignolo P, Ribaldone DG, Reggiani S, Sguazzini C, Smedile A, Pellicano R, Resegotti A, Astegiano M, et al. Glucose breath test and Crohn’s disease: diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol. 2015;50(11):1376–1381. doi: 10.3109/00365521.2015.1050691. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Lee KM, Chung YY, et al. Clinical significance of the glucose breath test in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2015;30(6):990–994. doi: 10.1111/jgh.12908. [DOI] [PubMed] [Google Scholar]
- 44.Rana SV, Sharma S, Malik A, et al. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig Dis Sci. 2013;58(9):2594–2598. doi: 10.1007/s10620-013-2694-x. [DOI] [PubMed] [Google Scholar]
- 45.Ricci JERJ, Chebli LA, Ribeiro T, Castro ACS, Gaburri PD, Pace FHDL, Barbosa KVBD, Ferreira LEVVDC, Passos MDCF, Malaguti C, et al. Small-intestinal bacterial overgrowth is associated with concurrent intestinal inflammation but not with systemic inflammation in Crohn’s disease patients. J Clin Gastroenterol. 2018;52(6):530–536. doi: 10.1097/MCG.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 46.Shah A, Talley NJ, Koloski N, Macdonald GA, Kendall BJ, Shanahan ER, Walker MM, Keely S, Jones MP, Morrison M, et al. Duodenal bacterial load as determined by quantitative polymerase chain reaction in asymptomatic controls, functional gastrointestinal disorders and inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(1):155–167. doi: 10.1111/apt.15786. [DOI] [PubMed] [Google Scholar]
- 47.Peled Y, Weinberg D, Hallak A, et al. Factors affecting methane production in humans. Gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci. 1987;32(3):267–271. doi: 10.1007/BF01297052. [DOI] [PubMed] [Google Scholar]
- 48.Lee KN, Lee OY, Koh DH, Sohn W, Lee SP, Jun DW, Lee HL, Yoon BC, Choi HS, Hahm JS, et al. Association between symptoms of irritable bowel syndrome and methane and hydrogen on lactulose breath test. J Korean Med Sci. 2013;28(6):901–907. doi: 10.3346/jkms.2013.28.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghoshal UC, Srivastava D, Misra A, et al. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28(3):281–289. doi: 10.1097/MEG.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 50.Rana SV, Sharma S, Kaur J, et al. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. Journal of Crohn’s and Colitis. 2014;8(8):859–865. doi: 10.1016/j.crohns.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2006;290(6):G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 52.Attaluri A, Jackson M, Paulson J, et al. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105(6):1407–1411. doi: 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim G, Deepinder F, Morales W, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci. 2012;57(12):3213–3218. doi: 10.1007/s10620-012-2197-1. [DOI] [PubMed] [Google Scholar]
- 54.Fiedorek SC, Pumphrey CL, Casteel HB. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 1990;10(4):473–477. doi: 10.1097/00005176-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Mayo-Wilson E, Li T, Fusco N, Bertizzolo L, Canner JK, Cowley T, Doshi P, Ehmsen J, Gresham G, Guo N, et al. Cherry-picking by trialists and meta-analysts can drive conclusions about intervention efficacy. J Clin Epidemiol. 2017;91:95–110. doi: 10.1016/j.jclinepi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Tawfik GM, Giang HTN, Ghozy S, Altibi AM, Kandil H, Le -H-H, Eid PS, Radwan I, Makram OM, Hien TTT, et al. Protocol registration issues of systematic review and meta-analysis studies: a survey of global researchers. BMC Med Res Methodol. 2020;20(1):213. doi: 10.1186/s12874-020-01094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forterre P, Brochier C, Philippe H. Evolution of the Archaea. Theor Popul Biol. 2002;61(4):409–422. doi: 10.1006/tpbi.2002.1592. [DOI] [PubMed] [Google Scholar]
- 58.Khelaifia S, Raoult D, Drancourt M, Versatile A, Driessen A. A versatile medium for cultivating methanogenic archaea. PLOS ONE. 2013;8(4):e61563. doi: 10.1371/journal.pone.0061563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the rome consensus conference. Aliment Pharmacol Ther. 2009;29(Suppl 1):1–49. [DOI] [PubMed] [Google Scholar]
- 60.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–490. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


