ABSTRACT
To identify the specific region of eCG involved in FSH-like activity, the following mutant expression vectors were constructed targeting the amino acid residues 102–104 of the eCG β-subunit: single mutants, eCGβV102G/α, eCGβF103P/α, and eCGβR104K/α; double mutants, eCGβV102G;F103P/α, eCGβV102G;R104K/α, and eCGβF103P;R104K/α; triple mutant, eCGβV102G;F103P;R104K/α. The LH-like and FSH-like activities of eCG mutants were examined in CHO-K1 cells expressing rat LH/CG receptor and rat FSH receptor. The levels of eCGβV102G/α, eCGβR104K/α, and eCGβV102G;R104K/α in the culture supernatant were markedly lower than those of eCGβ/α-wt. The other mutants and rec-eCGβ/α-wt were efficiently secreted into the culture supernatant. The LH-like activities of eCGV104G/α, eCGβV102G;R104K/α, and eCGβF103P;R104K/α were approximately 61%, 52%, and 54%, respectively, of those of eCG-wt. The Rmax values of the mutants were 58.9%–78.8% those of eCG-wt with eCGβR104K/α exhibiting the lowest value. The FSH-like activities of single mutants were only 16%–20% of those of eCG-wt. Additionally, the FSH-like activity of double mutants was less than 10% of that of eCG-wt. In particular, the FSH-like activities of βV102G;R104K/α and βF103P;R104K/α were 2.5–2.9% of that of eCG-wt. These results suggest that the amino acid residues 102–104 of the eCG β-subunit are dispensable and that the residue 104 of the eCG β-subunit plays a pivotal role in signal transduction through the rat FSH receptor. Thus, these mutants may aid future studies on eCG interactions with mammalian FSH receptors in vitro and in vivo.
KEYWORDS: eCG, CHO-S cells, rLH/CGR, rFSHR, cAMP assay
Introduction
Equine chorionic gonadotropin (eCG) is a unique member of the glycoprotein hormone family as it exhibits both luteinizing hormone (LH)-like and follicle-stimulating hormone (FSH)-like activities in non-equid species (Stewart and Allen 1981; Apparailly and Combarnous 1994; Chopineau et al. 2001; Galet et al. 2009; Min et al. 2004, 2019). The administration of eCG increases the ovulation rates (Pacala et al. 2010; Garcia-Ispierto et al. 2012; Sim and Min 2014; Sim et al. 2017). Mice were superovulated by injection of eCG and then human CG (hCG) after 48 h, and prostaglandin E2 is a key paracrine mediator of ovulation in granulosa cell proliferation (Lundberg et al. 2020). However, eCG exhibits only LH-like activity in equidaes (Galet et al. 2009). eCG is produced by the endometrial cups of placenta after the first trimester (Murphy and Martinuk 1991) and hCG is expressed from placenta at high levels soon after fertilization (Jameson and Hollenberg 1993; Kim et al. 2020).
eCG comprises a common α-subunit and a hormone-specific β-subunit (Pierce and Parsons 1981). The β-subunits of eCG and eLH, which are translated from the same gene, have identical primary structures (Sherman et al. 1992). However, eCG and eLH exhibit differential glycosylation patterns with eCG containing sialylated oligosaccharides and eLH containing sulfated oligosaccharides (Smith et al. 1993; Matsui et al. 1994). The eCG α-subunit contains two N-linked glycosylation sites at the amino acid residues 56 and 82, while the eCG β-subunit contains only one N-linked glycosylation site at the amino acid residue 13 (Sugino et al. 1987; Min et al. 2004). Additionally, the eCG β-subunit contains approximately 12 O-linked glycosylation sites in the carboxyl-terminal peptide (CTP) region (Bousfield et al. 1985). Among all known glycoprotein hormones, eCG has the highest carbohydrate content (more than 40%) (Aggarwal and Papkoff 1981). Thus, eCG has unique biological functions and carbohydrate profiles.
Previous studies examining the LH-like activity of glycoprotein hormones have revealed that the Asn56 and Asn52 glycosylation sites of the eCG α-subunit (Min et al. 1996, 2004, 2019) and hCG α-subunit (Bielinska et al. 1989; Matzuk et al. 1989), respectively, play an essential role in signal transduction. Similarly, the Asn56 and Asn52 glycosylation sites of eFSH α-subunit (Saneyoshi et al. 2001) and hFSH α-subunit (Bishop et al. 1994; Flack et al. 1994; Valove et al. 1994) are critical for signal transduction. These findings indicated that the Asn52 and Asn56 glycosylation sites of α-subunit mediated the LH-like and FSH-like activities of eCG, eFSH, hCG, and hFSH.
Previously, we had reported that the FSH-like activity of dimeric recombinant eCGαΔ56/β (rec-eCGαΔ56/β) was similar to that of wild-type eCG (Min et al. 1996; Saneyoshi et al. 2001). Additionally, the single-chain rec-eCGβ/αΔ56 mutant exhibited slightly decreased FSH-like activity in the primary cultures of rat granulosa cells (Min et al. 2004). However, the FSH-like activities of various mutants were similar in vivo. The proportion of nonfunctional oocytes in the rec-eCG-treated groups (2%) was significantly lower than that in the native eCG-treated group (Min et al. 2019). Recently, microarray analysis revealed the differentially expression of ovulation-related genes between the rec-eCG-treated and native-eCG -treated groups (Min et al. 2020). Thus, the FSH-like activity of eCG, which varies in vitro and in vivo, is dependent on the glycosylation sites.
The FSH-like activity of the eCG β-subunit mutant in which the residues 94–96 were substituted with alanine was completely inhibited (Park et al. 2010). The residues 102–104 of the β-subunit mediated the binding of eCG to the FSH receptor (FSHR) (Chopineau et al. 2001). A recent study reported that the superovulation rate and embryo development were not markedly different between the rec-eCG-treated and natural-eCG-treated groups irrespective of the mouse strain (Crispo et al. 2021). The dose of hFSH 37LVY39→37AAA39 β-subunit mutant required to elicit cyclic adenine monophosphate (cAMP) response was higher than that of wild-type hFSH. This is indicated that the receptor ability of the mutant was lower that of wild-type hFSH. Additionally, the half-maximal effective dose (ED50) of the Q48A mutant was higher than that of wild-type hFSH, which was consistent with the decreased receptor binding activity of the Q48A mutant (Roth and Dias 1996). A synthetic peptide corresponding to residues 34–37 (TRDL) of the hFSH β-subunit promotes the onset of puberty in immature male and female mice (Grasso et al. 1997). Crystal structure analysis revealed that the disulfide bond formation between Cys 26 and Cys 110 of the hCG β-subunit is completed after the interaction between α and β subunits (Huth et al. 1992; Lapthorn et al. 1994). Additionally, intercysteine loop sequence 93–100 of β-subunit is critical for hCG activity (Keutmann et al. 1989).
In this study, the homology of subunit sequence from CG and FSH was comparatively analyzed to determine the specific sites mediating the FSH-like activity other than the glycosylation sites. The amino acid residues 102–104 of the hCG β-subunit, hFSH β-subunit, and eFSH β-subunit were comparatively analyzed. The single-chain mutants of eCG were constructed by substituting the amino acid residues V102, F103, and R104 of the eCG β-subunit with G102, P103, and K104, respectively. The following seven mutants were constructed: single mutants, eCGβV102G, eCGβF103P, and eCGβR104K; double mutants, eCGβV102G;F103P, eCGβV102G;R104K, and eCGβF103P;R104K; triple mutant, eCGβV102G;F103P;R104K. The mutants were transfected into CHO-S cells for the production of rec-eCG mutants. Finally, the activities of rec-eCG mutants were examined in cells expressing rat LH receptor (rLHR) and rat FSHR (rFSHR) in vitro. The findings of this study indicated that the amino acid residues 102–104 of the eCG β-subunit were more important for mediating the FSH-like activity than mediating the LH-like activity.
Materials and methods
Materials
Polymerase chain reaction (PCR) reagents were purchased from Takara Inc. (Osaka, Japan). The primers were synthesized by GenoTech (Daejeon, Korea). The pcDNA3 mammalian expression vector, Chinese hamster ovary (CHO)-suspension (CHO-S) cells, FreeStyle CHO medium, FreeStyleTM MAX transfection reagent, antibiotics (penicillin and streptomycin), LipofectamineTM-3000 reagents, and anti-myc antibody were purchased from Invitrogen (Carlsbad, CA, USA). The CHO-K1 cells were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). pGEMTeasy, Ham’s F-12 medium, Opti-MEM 1, and CHO-S-serum-free medium (SFM) II were obtained from Gibco BRL (Grand Island, NY, USA). The pCORON1000 SP VSV-G tag and enhanced chemiluminescence (ECLTM) detection system for western blot analysis were purchased from GE Healthcare (USA). The homogeneous time-resolved fluorescence (HTRF) cAMP assay kit was purchased from Cisbio (Codolet, France). The pregnant mare serum gonadotropin (PMSG) enzyme-linked immunosorbent assay (ELISA) kit was obtained from DRG International Inc. (Mountainside, NJ, USA). The QIAprep-Spin plasmid kits were purchased from Qiagen Inc. (Hilden, Germany). Disposable spinner flasks were purchased from Corning Inc. (Corning, NY, USA). Centrifugal filter devices were purchased from Amicon Bio (Billerica, MA, USA). eCG α-subunit and β-subunit cDNAs were prepared as previously reported (Min et al. 2019). All other reagents used in this experiment were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Wako Pure Chemicals.
Construction of single-chain eCGβ/α mutants
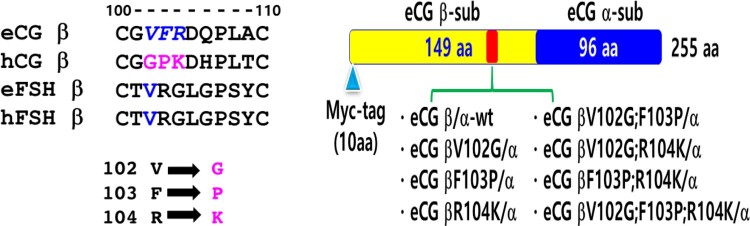
An overlap extension PCR with eCG α-subunit and β-subunit cDNAs was performed to generate single-chain mutants as described previously (Min et al. 2019). Two different sets of PCR primers were used to amplify each mutant fragment. In the first PCR, the forward primer of the β-subunit and the reverse primer of each mutant point were used to amplify the first fragment. The second fragment was amplified with the forward primer of each mutant point and the reverse primer of the α-subunit carboxyl-terminal region, including the stop codon. In the second PCR, the amplified fragments from the first PCR were used as a template to amplify the single-chain (tethered) eCG as shown in Figure 1. Next, myc-tag single-chain eCGβ/α was also constructed using the same method. A 10-amino acid myc-tag was added between the first and second amino acids of the eCG mature protein.
Figure 1.
Schematic diagram of single chain equine chorionic gonadotropin (eCGβ/α) proteins with mutant residues 102–104 of the β-subunit. (A) Comparative analysis revealed that the amino acid residues 102–104 of the β-subunit were markedly different between eCG and follicle-stimulating hormone. Thus, the valine102, phenylalanine103, and arginine104 of the eCG β-subunit were substituted with glycine, proline, and lysine, respectively. (B) Identification of single-chain eCGβ/α-wt. Myc-tag was inserted between the first amino acid and second amino acid of the β-subunit of the mature protein. The single-chain eCGβ/α-wt protein with myc-tag comprised 255 amino acids. The following eight expression vectors were constructed: designated as pcDNA3-eCGβ/α-wt, pcDNA3-eCGβV102G/α, pcDNA3-eCGβF103P/α, pcDNA3-eCGβR104K/α, pcDNA3-eCGβV102G;F103P/α, pcDNA3-eCGβV102G;R104K/α, pcDNA3-eCGβF103P;R104K/α, and pcDNA3-eCGβV102G;F103P;R104K/α.
The common regions among the eCG β-subunit, eFSH β-subunit, and hFSH β-subunit and hCG β-subunit were comparatively analyzed (Figure 1). To construct the mutants (targeting the amino acid residues 102, 103, and 104) of the eCG β-subunit, single-chain wild-type eCG (eCG β/α-wt) cDNA was used as a template. The amplicons obtained from second PCR were cloned into the pGEMTeasy vector. The plasmids were extracted and sequenced to confirm the Kozak site, myc-tag, mutation, and PCR errors. Next, the plasmids cloned into pGEMTeasy were digested with EcoRI and SalI and subcloned into the EcoRI and XhoI sites of the eukaryotic expression vector pcDNA3. The following eight expressing vectors were constructed: pcDNA3-eCG β/α-wt, pcDNA3-eCGβV102G/α, pcDNA3-eCGβF103P/α, pcDNA3-eCGβR104K/α, pcDNA3-eCGβV102G;F103P/α, pcDNA3-eCGβV102G;R104K/α, pcDNA3-eCGβF103P;R104K/α, and pcDNA3-eCGβV102G;F103P;R104K/α. The schematic diagrams of single-chain eCG mutants are shown in Figure 1.
Expression of rec-eCGβ/α mutants in CHO-Scells
The mammalian vectors expressing eCGβ/α mutants were transfected into CHO-S cells using the FreeStyle MAX reagent, following the manufacturer’s instructions. Briefly, the CHO-S cells (1 × 107 cells/30 mL) were cultured in FreeStyle CHO expression medium for 3 days. One day before transfection, the cells (5–6 × 105 cells/mL) were passaged with CHO expression medium (125 mL) in a disposable spinner flask. On the day of transfection, the cell density was approximately 1.2–1.5 × 106 cells/mL. Next, DNA (160 μg) was mixed with 1.2 mL of OptiPRO SFM, while FreeStyle MAX reagent (160 μL) for transfection was mixed with 1.2 mL of the OptiPRO SFM. The two media were mixed and incubated for 5 min at room temperature. The cells were incubated with the complex (2.4 mL) in a cell suspension flask. To analyze the levels of rec-eCG proteins, the culture medium was collected at 72 h post-transfection. The culture medium was centrifuged at 15,000 rpm at 4°C for 10 min to remove cell debris and the supernatant was stored at −80°C until analysis. The sample was aliquoted (1 mL) for ELISA analysis, while the remaining samples was concentrated approximately 10 times using freezer-drying. The concentrated samples were subjected to ELISA, western blotting, and biological activity assay analyses.
Quantitation of rec-eCGβ/α proteins
The rec-eCG proteins were quantified using PMSG ELISA with anti-PMSG monoclonal antibody, horseradish peroxidase-conjugated secondary antibody, and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate, following the manufacturer’s instructions. The culture medium (100 μL) was dispensed into the wells of a 96-well microplate coated with a monoclonal antibody against a unique antigenic site on the eCG molecule. The reaction mixture was incubated for 60 min at room temperature and washed thrice with distilled water, followed by incubation with 100 μL of horseradish peroxidase-conjugated secondary antibody for 60 min at room temperature. The samples were washed five times with 300 µL of distilled water and incubated with TMB (100 μL) for 30 min at room temperature. Further, the sample was incubated with 50 μL of stop solution and the absorbance of the reaction mixture at 450 nm was measured using a plate reader within 30 min.
Western blotting analysis
The concentrated samples were subjected to sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) using a 12.5% gel, following the protocols of Laemmli (1970). The resolved proteins were electroblotted to a polyvinylidene difluoride membrane (0.2 µm) for 2 h using the Bio-Rad Mini Trans-Blot Electroblotter apparatus. The membrane was blocked with a 1% blocking reagent for 1 h and incubated with monoclonal mouse anti-myc-tag antibody (1:5000) for 1–2 h. Next, the membrane was washed to remove the unbound antibody and incubated with horseradish peroxidase-conjugated anti-mouse IgG secondary antibodies for 30 min. The membrane was then washed, incubated for 1–5 min with 2 mL ECLTM western blotting detection reagents, and exposed to an X-ray film for 1–10 min.
Construction of rat luteinizing hormone/chorionic gonadotropin receptor (rLH/CGR) and rat FSHR (rFSHR) expression vector
The rLH/CGR and rFSHR encoding sequences were cloned into the pcDNA3 expression vector and the recombinant plasmids were transfected into CHO-K1 cells as previously reported (Park et al. 2009). The amplified rLH/CGR and rFSHR sequences were cloned into the pcDNA3 mammalian expression vector at the EcoRI and XhoI sites (designated as pcDNA3-rLH/CGR and pcDNA3-rFSHR, respectively). Additionally, the rLH/CGR and rFSHR cDNAs were subcloned into the eukaryotic expression vector pCORON 1000SP VSV-G tag for transfection (designated as pVSVG-rLH/CGR and pVSVG-rFSHR, respectively) (Park et al. 2017).
Transient transfection into the CHO-K1 cells
The CHO-K1 cells were transfected using the previously reported liposome transfection method (Byambaragchaa et al. 2020). The CHO cells were cultured in growth medium [Ham’s F-12 media supplemented with penicillin (50 U/mL), streptomycin (50 µg/mL), glutamine (2 mM), and 10% fetal bovine serum] to 80%–90% confluency in six-well plates. The cells were transfected with diluted plasmid DNA-Lipofectamine 3000 complex for 5 h. Next, the cells were cultured in CHO growth medium supplemented with 20% fetal bovine serum. The CHO growth medium was replaced at 24 h post-transfection. The transfected cells were seeded into a 384-well plate (104 cells per well) and subjected to cAMP analysis at 48–72 h post-transfection.
cAMP analysis using HTRF assays
The levels of cAMP in CHO-K1 cells were measured using the cAMP Dynamics 2 competitive immunoassay kits. Briefly, the CHO-K1 cells transfected with wild-type rLHR and rFSHR were seeded (10,000 cells per well) into a 384-well plate at 48 h post-transfection). In this assay, a cryptate-conjugated anti-cAMP monoclonal antibody and d2-labeled cAMP were used. To prevent cAMP degradation, MIX was added to the cell dilution buffer. The concentration range of the standard samples was 0.17–712 nM. The cells were stimulated with the agonist for 30 min at room temperature. Next, the cells were incubated with cAMP-d2 (5 µL) and anti-cAMP-cryptate antibodies (5 µL) at room temperature for 1 h after sealing the wells. cAMP was detected by measuring the decrease in HTRF energy transfer (665 nm/620 nm) using an Artemis K-101 HTRF microplate reader (Kyoritu Radio, Tokyo, Japan). The delta F% (energy transfer) value is inversely proportional to the concentration of cAMP in the standard or sample. The results were expressed as delta F% (cAMP inhibition) as follows:
The cAMP concentrations were calculated using Prism software (GraphPad, Inc., La Jolla, CA, USA).
Data analysis
The sequences were compared using the Multalin interface-multiple sequence alignment tool. The dose–response curves were analyzed from experiments performed in duplicates. The cAMP levels in the transfected cells were subtracted from those in the mock-transfected cells. GraphPad Prism 6.0 was used to analyze cAMP production, EC50 values, and stimulation curves. The curves fitted in a single experiment were normalized to the background signal measured in the mock-transfected cells (Figures 4 and 5). The results are expressed as mean ± standard error from three independent experiments. The data were analyzed using one-way analysis of variance, followed by Tukey’s comparison tests with GraphPad Prism 6.0. The differences were considered significant at p < 0.05.
Figure 4.
Effect of recombinant equine chorionic gonadotropin (rec-eCG) β-subunit mutants on cyclic adenine monophosphate (cAMP) production in cells expressing rat luteinizing hormone/chorionic gonadotropin receptor (rLH/CGR). Cells transiently transfected with rLH/CGR were seeded in 383-well plates (10,000 cells per well) on day 1 post-transfection and incubated with rec-eCG proteins for 30 min at room temperature. cAMP production was detected using a homogeneous time-resolved fluorescence assay. cAMP accumulation is represented as delta F%. cAMP concentrations were calculated using GraphPad Prism software. The results of the mock-transfected cells were subtracted from each dataset (see Methods). Each data point represents mean ± standard error of mean from triplicate experiments. The mean data were fitted to the equation for generating a one-phase exponential decay curve. The blank circles show the same curves as the wild-type receptor. The open circle was shown the eCG β/α-wt and black circles were indicated each eCG mutants in the 102–104 amino residue of eCG β-subunit. (A) eCGβV102G/α; (B) eCGβF103P/α; (C) eCGβR104K/α; (D) eCGβV102G;F103P/α; (E) eCGβV102G;R104K/α; (F) eCGβF103P;R104K/α; (G) eCGβV102G;F103P;R104K/α.
Figure 5.
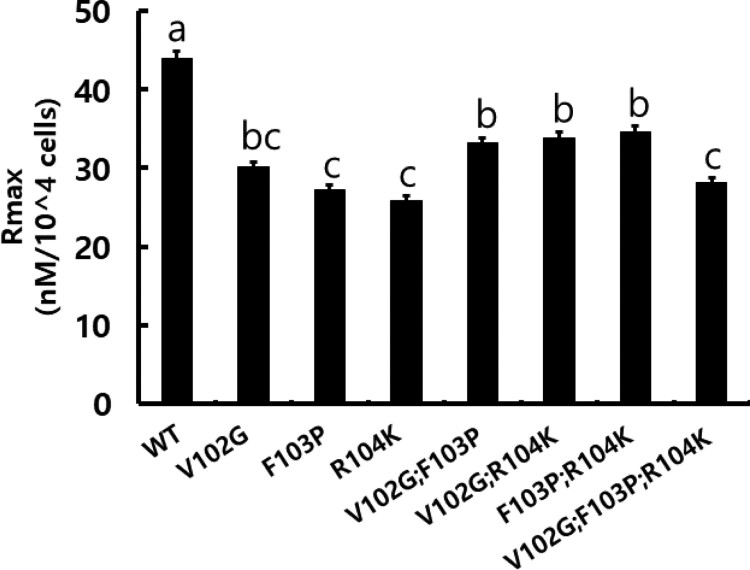
The Rmax values of wild-type and mutant recombinant equine chorionic gonadotropin (rec-eCGβ/α) in cells expressing rLH/CGR. Data are represented as mean ± standard deviation from triplicate experiments. Values with different superscripts were significantly different (P < 0.05).
Results
Secretion quantities of rec-eCG mutants from CHO-S cells
The culture supernatant of CHO-S cells transiently transfected with the expression vectors of rec-eCG mutants was collected at 72 h post-transfection to quantify the levels of rec-eCG mutants using ELISA (Figure 2). The levels of rec-eCGβ/α-wt (589 ± 50 mIU/mL) and rec-eCGβF103P/α mutant (554 ± 43 mIU/mL) were similar in the culture supernatant. However, the levels of rec-eCGβV102G/α (263 ± 32 mIU/mL) and rec-eCGβR104 K/α mutants (421 ± 45 mIU/mL) in the culture supernatant were markedly lower than those of rec-eCGβ/α-wt. This indicated that the amino acid residues 102 and 104 of the eCG β-subunit were critical for the secretion of eCG into the culture medium. Furthermore, the levels of rec-eCGβV102G;F103P/α, rec-eCGβV102G;R104 K/α, and rec-eCGβF103P;R104 K/α in the culture supernatant were 528 ± 63, 342 ± 51, and 432 ± 65 mIU/mL, respectively. Compared with that of rec-eCGβ/α-wt, the level of rec-eCGβF103P;R104K/α was slightly lower but the level of rec-eCGβV102G;R104K/α was markedly lower in the culture supernatant. This indicated that the amino acid residue 102 of the eCG β-subunit markedly influence the secretion of eCG. The levels of rec-eCGβV102G;F103P;R104 K/α (398 ± 41 mIU/mL) in the culture supernatant were slightly higher than those of rec-eCGβV102G;R104 K/α. Although the levels of rec-eCG mutants in the culture supernatant were not analyzed over time post-transfection, the residue 102 of the eCG β-subunit appears to be more important for eCG secretion into the culture medium than the residues 103 and 104.
Figure 2.
Quantification of rec-eCGβ/α mutants. The culture media were collected at 72 h post-transfection and centrifuged. The levels of rec-eCGβ/α proteins were analyzed using enzyme-linked immunosorbent assay as described in the Methods section. The data are represented as mean ± standard error of mean from at least three independent experiments. Values with different superscripts are significantly different (P < 0.05).
Western blot analysis of rec-eCGβ/α proteins
Next, the molecular weight of rec-eCGβ/α proteins was determined using western blotting. The molecular weight of rec-eCG was approximately 40–46 kDa (Figure 3). Additionally, the molecular weight of the mutants was similar. The appearance of the broad protein band is attributed to the oligosaccharide modifications in rec-eCG. The band density of the mutant proteins was slightly lower than that of the eCG-wt. However, we suggest that this is due to the differential binding of the antibody to the rec-eCG mutants as the structure of rec-eCG mutants is modified with the amino acid substitution.
Figure 3.
Western blot analysis of rec-eCGβ/α mutants. The proteins in the conditioned media were concentrated 5–10 times. The rec-eCGβ/α proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a blotting membrane. The proteins were detected using anti-myc-tag primary antibodies and horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibodies.
In vitro LH-like activity of rec-eCG mutants in cells expressing rLH/CGR
The in vitro LH-like activity of the rec-eCG mutants was analyzed using CHO-K1 cells expressing rLH/CGR. The ability of the rec-eCG mutants to elicit a cAMP response is shown in Figure 4. The EC50 and Rmax values of rec-eCGβ/α-wt were 0.020 nM/mL and 43.9 ± 0.9 nM/104 cells, respectively (Table 1). This indicated that rec-eCGβ/α-wt exhibited potent LH-like activities. The dose-response curves of the seven mutants for eliciting cAMP response through LH-like activity shifted slightly to the right (Figure 4). The EC50 values of rec-eCGβV102G/α, rec-eCGβF103P/α, and rec-eCGβR104 K/α were approximately 0.015, 0.018, and 0.033 ng/mL, respectively. The LH-like activity of rec-eCGβR104 K/α was 61% of that of eCGβ/α-wt. This indicated that the amino acid residue 104 of the eCG β-subunit was critical for the LH-like activity. The EC50 values of rec-eCGβV102G;F103P/α, rec-eCGβV102G;R104 K/α, and rec-eCGβF103P;R104 K/α were 0.016, 0.038, and 0.037 ng/mL, respectively (Figure 4A–C). The LH-like activity of rec-eCGβV102G;F103P/α was 125% of that of eCGβ/α-wt (Figure 4D). However, the LH-like activities of rec-eCGβV102G;R104 K/α and rec-eCGβF103P;R104 K/α were 52% and 54% of that of eCGβ/α-wt, respectively (Figure 4E and F). The LH-like activity of rec-eCGβV102G;F103P;R104 K/α was 153% of that of eCGβ/α-wt. The Rmax values of all mutants were 61.9%–78.8% of those of eCGβ/α-wt (Table 1). eCGβR104 K/α exhibited the lowest Rmax value (58.9% of that of eCGβ/α-wt) (Figure 5). Thus, the amino acid residue 104 of the eCG β-subunit plays a pivotal role in signal transduction for eliciting cAMP response through rLHR.
Table 1.
LH-like bioactivity of rec-eCG mutants in cells expressing rat LH/CG receptor.
| rec-eCG mutants | cAMP responses | ||
|---|---|---|---|
| Basala (nM/104 cells) | EC50b (ng/mL) | Rmaxc (nM/104 cells) | |
| rec-eCGβ/α-wt | 2.7 ± 1.2 | 0.020 (100%) | 43.9 ± 0.9 (100%) |
| rec-eCGβV102G/α | 2.1 ± 1.0 | 0.015 (133%) | 30.1 ± 0.7 (68.6%) |
| rec-eCGβF103P/α | 2.6 ± 0.9 | 0.018 (111%) | 27.2 ± 0.7 (61.9%) |
| rec-eCGβR104K/α | 1.8 ± 0.8 | 0.033 (61%) | 25.9 ± 0.6 (58.9%) |
| rec-eCGβV102G;F103P/α | 2.1 ± 0.9 | 0.016 (125%) | 33.2 ± 0.6 (75.6%) |
| rec-eCGβV102G;R104K/α | 3.1 ± 0.9 | 0.038 (52%) | 33.8 ± 0.7 (76.9%) |
| rec-eCGβF103P;R104K/α | 2.0 ± 0.9 | 0.037 (54%) | 34.6 ± 0.7 (78.8%) |
| rec-eCGβV102G;F103P;R104K/α | 2.3 ± 0.9 | 0.013 (153%) | 28.2 ± 0.6 (64.2%) |
Values are the means ± SEM of triplicate experiments. The half maximal effective concentration (EC50) values were determined from the concentration-response curves from in vitro bioassays. The cAMP responses of the basal and Rmax in rec-eCG β/α-wild type were shown as 100%.
Basal cAMP level average without agonist treatment.
Half maximal effective concentration.
Rmax average cAMP level/104 cells.
In vitro FSH-like activity of rec-eCG mutant in cells expressing rFSHR
The in vitro FSH-like activity of rec-eCG mutants was assessed using CHO-K1 cells expressing rFSHR. Rec-eCGβ/α-wt dose-dependently increased cAMP level. However, the dose-response curves of all mutants markedly shifted to the right (Figure 6). The EC50 and Rmax values of rec-eCGβ/α-wt were 0.16 ng/mL and 55.5 ± 1.3 nM/104 cells, respectively, which indicated potent activity (Table 2). The FSH-like activity of all mutants was significantly lower than that of eCGβ/α-wt. The EC50 values of rec-eCGβV102G/α, rec-eCGβF103P/α, and rec-eCGβR104K/α were approximately 0.79, 0.99, and 0.98 ng/mL, respectively (Figure 6A–C). The FSH-like activities of single mutants were approximately 16%–20% of those of eCGβ/α-wt. Meanwhile, the FSH-like activities of rec-eCGβV102G;F103P/α, rec-eCGβV102G;R104K/α, and rec-eCGβF103P;R104K/α were 9.1%, 2.9%, and 2.5% of those of rec-eCGβ/α-wt (Figure 6D–F). Similarly, the EC50 value of rec-eCGβV102G;F103P;R104K/α was 5.6% of that of eCGβ/α-wt (Figure 6G). The activities of the double mutant and triple mutant were less than 10% of those of eCGβ/α-wt. This suggested that the amino acid residues 102–104 of the eCG β-subunit co-operatively mediate signal transduction in cells expressing rFSHR. The Rmax values of all mutants were not affected even though they exhibited markedly decreased biological activity (Table 2). Thus, we suggest that the role of the amino residues 102–104 of the eCG β-subunit in FSH-like activity is more important than that in LH-like activity.
Figure 6.
Effect of recombinant equine chorionic gonadotropin (rec-eCG) β-subunit mutants on cyclic adenine monophosphate (cAMP) production in Chinese hamster ovary (CHO)-K1 cells expressing rat follicle-stimulating hormone receptor (rFSHR). Rec-eCGβ/α proteins dose-dependently increased cAMP production in CHO-K1 cells transiently expressing rFSHR. The cAMP levels in cells were measured at 48–72 h post-transfection (see Methods for details). The cAMP values were calculated using GraphPad Prism. The open circle was shown the eCG β/α-wt and black circles were indicated each eCG mutants in the 102–104 amino residue of eCG β-subunit. (A) eCGβV102G/α; (B) eCGβF103P/α; (C) eCGβR104K/α; (D) eCGβV102G;F103P/α; (E) eCGβV102G;R104K/α; (F) eCGβF103P;R104K/α; (G) eCGβV102G;F103P;R104K/α.
Table 2.
FSH-like bioactivity of rec-eCG mutants in cells expressing rat FSH receptor.
| rec-eCG mutants | cAMP responses | ||
|---|---|---|---|
| Basala (nM/104 cells) | EC50b (ng/mL) | Rmaxc (nM/104 cells) | |
| rec-eCGβ/α-wt | 2.3 ± 1.9 | 0.16 (100%) | 55.5 ± 1.3 (100%) |
| rec-eCGβV102G/α | 1.9 ± 1.6 | 0.79 (20%) | 58.6 ± 1.5 (105.6%) |
| rec-eCGβF103P/α | 1.2 ± 1.1 | 0.99 (16%) | 48.1 ± 1.1 (86.7%) |
| rec-eCGβR104K/α | 1.9 ± 1.2 | 0.98 (16%) | 54.7 ± 1.2 (98.5%) |
| rec-eCGβV102G;F103P/α | 2.9 ± 1.2 | 1.75 (9.1%) | 60.4 ± 1.3 (108.8%) |
| rec-eCGβV102G;R104K/α | 1.4 ± 1.2 | 5.45 (2.9%) | 51.2 ± 1.5 (92.3%) |
| rec-eCGF103P;R104K/α | 1.2 ± 0.7 | 6.40 (2.5%) | 47.9 ± 0.9 (86.3%) |
| rec-eCGV102G;F103P;R104K/α | 2.4 ± 1.5 | 2.86 (5.6%) | 48.3 ± 1.7 (87.0%) |
Values are the means ± SEM of triplicate experiments. The half maximal effective concentration (EC50) values were determined from the concentration-response curves from in vitro bioassays. The cAMP responses of the basal and Rmax in rec-eCG β/α-wild type were shown as 1-fold.
Basal cAMP level average without agonist treatment.
Half maximal effective concentration.
Rmax average cAMP level/104 cells.
Discussion
This study examined both LH-like and FSH-like activities of single-chain rec-eCG proteins with mutated amino acid residues 102–104 of the eCG β-subunit. The findings of this study indicated that the amino acid residues 102–104 of the eCG β-subunit are critical for the secretion of eCG into the culture medium of CHO-S cells. Additionally, the amino acid residue 104 of the eCG β-subunit played a pivotal role in signal transduction to elicit cAMP response through rLHR. Furthermore, we suggest that the role of the amino acid residues 102–104 of the eCG β-subunit in FSH-like activity is more important than that in LH-like activity.
Previously, we had reported that the glycosylation site mutations in dimeric and tethered rec-eCGs affected the secretion of eCG into the culture medium. In particular, the mutations at the glycosylation sites Asn 82 of the α-subunit and Asn 13 of the β-subunit resulted in impaired secretion of eCG into the culture supernatant of the transfected cells (Min et al. 2019). Additionally, CTP region of the eCG β-subunit, which contains O-linked glycosylation sites, was not involved in the secretion of eCG. The deletion of the amino acid residue 87 at the COOH-terminal region of the eCG α-subunit almost completely inhibited the secretion of the protein. Interestingly, the mRNA levels of wild-type and mutant eCG were similar. However, the deletion of the amino acid residue 87 completely inhibited the secretion of the mutant protein into the culture medium or the intracellular fraction of cell lysates (Jeoung et al. 2010). The expression levels of five other mutants (β43–45, β54–56, β94–96, α33–35, and α42–44) of tethered-eCGβ/α were determined using the alanine scanning method. The β94–96 mutant exhibited the same expression pattern as wild-type eCG (Park et al. 2010). Consistent with these observations, the levels of rec-eCGβV102G/α, rec-eCGβR104K/α, and rec-eCGβV102G;R104K/α mutants in the culture supernatant were lower than those of other mutants in this study. This indicated that the amino acid residues 102 and 104 of the eCG β-subunit play an important role in the efficient secretion of eCG. The molecular weight of wild-type eCG was approximately 40–46 kDa (Park et al. 2010). However, the digestion of N-linked oligosaccharides markedly decreased the molecular weight of wild-type eCG to 30 kDa (Park et al. 2009; Lee et al. 2017). This indicated that glycosylation increased the molecular weight of single-chain rec-eCG protein by approximately 10–15 kDa in CHO-K1 cells. However, post-translational modifications do not involve O-linked glycosylation in mammalian cells, such as CHO-K1. Recently, we reported that the molecular weight of rec-eCGβ/α was approximately 40–46 kDa and that the molecular weight of rec-eCGβ/αΔ56 mutant, which lacks one N-linked glycosylation site (Asn56) at the α-subunit, decreased by approximately 3–4 kDa (Min et al. 2019). Thus, these results suggest that the glycosylation patterns were markedly modified in the rec-eCG proteins produced in CHO-K1 or S cells.
Several studies have reported that the in vitro LH-like activity of CG mutants lacking glycosylation sites is 5–10-fold lower than that of CG with glycosylation sites (Sairam and Manjunath 1983; Thoakura et al. 1990; Thoakura and Blithe 1995). In contrast, the LH-like activity of Val102-Phe103→Gly102-Pro103 eCG β-subunit mutant was reported to be approximately two times higher than that of wild-type eCG in the COS-7 and rat Leydig cells (Chopineau et al. 2001). The study also reported that the LH-like activity of G102-P103-K104→V102-R103-R104 hCG β-subunit mutant was 75% of that of wild-type hCG. However, the activity of the hCG β-subunit mutant (V102-R103-R104) with the equine α-subunit was 1.48 times higher than that of wild-type equine α-subunit/wild-type hCG β-subunit. These results are consistent with those of this study, which demonstrated that the LH-like activities of mutants were similar. However, the EC50 value of the rec-eCGβR104K/α mutant for the LH-like activity was approximately 0.033 ng/mL. Thus, the LH-like activity of rec-eCGβR104K/α was 61% of that of eCGβ/α-wt. This indicated that the R104 residue (but not V102 and F103 residues) of the eCG β-subunit mediates the LH-like activity in cells expressing rLHR. Previously, we had reported that the LH-like activity of the Gln94-Ile95-Lys96→Ala94-Ala95-Ala96 eCG β-subunit mutant was similar to that of single-chain wild-type rec-eCGβ/α (Park et al. 2010). The LH-like activity of eCGβ-CTP/α (with mutant O-linked glycosylation sites) mutant was slightly lower (77.2%) than of wild-type eCG. However, the dose-response curve of eCGβ-CTP/α was similar to that of wild-type eCG (Min et al. 1996, 2004). The glycosylation site at Asn56 of the eCG α-subunit is critical for the LH-like activity of dimeric rec-eCGα/β and single-chain rec-eCGβ/α. Thus, specific glycosylation sites of the eCG α-subunit mediate the LH-like activity of eCG in cells expressing rat LHR and rat Leydig cells.
The activity of single chain eCG protein produced in the milk of transgenic rabbits was 2.4-fold higher than that of standard eCG (FL652, 5000 IU/mg) in rat Leydig cells. The administration of standard eCG in vivo dose-dependently increased ovarian weight. In contrast, the administration of rec-eCGβ/α protein produced from transgenic rabbit milk did not affect ovarian weight (Galet et al. 2000). Thus, we suggest that the differential activity of eCG in vivo and in vitro is due to the modified oligosaccharide chains. The amino acid residues 104–109 of β-subunit are critical for the secretion of correctly folded tethered eCGβ/α (Galet et al. 2009). The rec-eCG mutants and wild-type eCG exhibit similar activities in COS-7, αT3, and MLTC-1 cells. Thus, these results are consistent with those of this study, which suggested that the amino acid residues 102–104 are not essential for LH-like activity. The single-chain rec-eCG protein produced from the milk of transgenic rabbits exhibited potent FSH-like activity in Y1 cells expressing hFSHR (Galet et al. 2000). However, this single-chain rec-eCG protein did not significantly affect ovarian weight in vivo.
We suggest the deglycosylated glycoproteins were rapidly cleared from the blood. Proteins treated with small molecular weight glycosylation inhibitor exhibited lower FSH-like activity than native or wild-type glycoproteins. The FSH-like activity of Val102-Phe103→Gly102-Pro103 eCG β-subunit mutant was approximately 15% of that of wild-type eCG in the Y1 cells (Chopineau et al. 2001). Meanwhile, the FSH-like activity of the Gly102-Pro103→Val102-Phe103 donkey CG β-subunit mutant was slightly higher than that of wild-type donkey CG. The FSH-like activity of the triple mutant (Val102-Phe103-Gly104) of the donkey CG β-subunit was 5-fold to 8-fold lower than that of the wild-type CG in Y1 cells expressing human FSHR (Chopineau et al. 2001). These results are consistent with those of this study, which demonstrated that the FSH-like activity of the triple mutant was 90% of that of wild-type eCG in cells expressing rFSHR. The results of alanine scanning revealed that the secretion of correctly folded rec-eCG with mutated β104–109 sequence was 90% lower than that of correctly folded rec-eCGβ/α (Galet et al. 2009). The FSH-like activity of this mutant was 70% lower than that of wild-type eCG. Thus, the authors suggested that the amino acid residues 104–109 of the eCG β-subunit are essential for secretion and FSH-like activity and that the FSH-like activity of the mutant was 25% of that of eCG wild-type. These results are consistent with those of this study, which demonstrated that the EC50 values of the single, double, and triple mutants were only 2.5–20% of that of wild-type eCG.
In conclusion, the LH-like activity of rec-eCG mutants was slightly lower than that of wild-type eCG in cells expressing rLHR. However, the FSH-like activity for eliciting cAMP response of eCG mutants was markedly lower than that of wild-type eCG. The EC50 values of the double and triple mutants of rec-eCGβ/α to elicit the cAMP response through FSH-like activity was approximately 2.5% to 9.1% of that of rec-eCGβ/α-wt. We suggest that the amino acid residues 102–104 of the eCG β-subunit are critical for the structural and functional studies of the FSHR. Thus, these mutants may be useful for further analysis of eCG interactions with mammalian FSH receptors in vitro and in vivo. The findings of this study enable the development of novel rec-eCGβ/α mutants with potent biological activity to induce oocytes in vivo.
Funding Statement
This work was financially supported by the Korean Research Foundation Program [grant number 2021R1A2B01001602], Republic of Korea. The funder’s role was providing funding for the study, while all other aspects of the project (design, collection, analysis, and interpretation of data and writing of the manuscript) were executed by the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aggarwal BB, Papkoff H.. 1981. Relationship of sialic acid residues to in vitro biological and immunological activities of equine gonadotropins. Biol Reprod. 24(5):1082–1087. [PubMed] [Google Scholar]
- Apparailly F, Combarnous Y.. 1994. Role of sialic acid residues in the in vitro superactivity of human choriogonadotropin (hCG) in rat Leydig cells. Biochim Biophys Acta. 1224(3):559–565. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Matzuk MM, Boime I.. 1989. Site-specific processing of the N-linked oligosaccharides of the human chorionic gonadotropin α subunit. J Biol Chem. 264(29):17113–17118. [PubMed] [Google Scholar]
- Bishop LA, Robertson DM, Cahir N, Schofield PR.. 1994. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 8(6):722–731. [DOI] [PubMed] [Google Scholar]
- Bousfield GR, Sugino H, Ward DN.. 1985. Demonstration of a COOH-terminal extension on equine lutropin by means of a common acid-labile bond in equine lutropin and equine chorionic gonadotropin. J Biol Chem. 260(17):9531–9533. [PubMed] [Google Scholar]
- Byambaragchaa M, Kim JS, Park HK, Kim DJ, Hong SM, Kang MH, Min KS.. 2020. Constitutive activation and inactivation of mutations inducing cell surface loss of receptor and impairing of signal transduction of agonist-stimulated eel follicle-stimulating hormone receptor. Int J Mol Sci. 21(19):7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopineau M, Martint N, Galet C, Guillou F, Combarnous Y.. 2001. β-subunit 102-104 residues are crucial to confer FSH activity to equine LH/CG but are not sufficient to confer FSH activity to human CG. J Endocrinol. 169(1):55–63. [DOI] [PubMed] [Google Scholar]
- Crispo M, Meikle MN, Schlapp G, Menchaca A.. 2021. Ovarian superstimulatory response and embryo development using a new recombinant glycoprotein with eCG-like activity in mice. Theriogenology. 164:31–35. [DOI] [PubMed] [Google Scholar]
- Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC.. 1994. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 269(19):14015–14020. [PubMed] [Google Scholar]
- Galet C, Bourhis CML, Chopineau M, Griec GL, Perrin A, Magallon T, Attal J, Viglietta C, Houdebine LM, Guillou F.. 2000. Expression of a single β/α chain protein of equine LH/CG in milk of transgenic rabbits and its biological activity. Mol Cell Endocrinol. 174:31–40. [DOI] [PubMed] [Google Scholar]
- Galet C, Guillou F, Foulon-Gauze F, Combarnous Y, Chopineau M.. 2009. The β104-109 sequence is essential for the secretion of correctly folded single-chain βα horse LH/CG and for its activity. J Endocrinol. 203(1):167–174. [DOI] [PubMed] [Google Scholar]
- Garcia-Ispierto I, Lopez-Helguera I, Martino A, Lopez-Gatius F.. 2012. Reproductive performance of anoestrous high-producing dairy cows improved by adding equine chorionic gonadotrophin to a progesterone-based oestrous synchronizing protocol. Reprod Domest Anim. 47(5):752–758. [DOI] [PubMed] [Google Scholar]
- Grasso P, Rozhavskaya M, Reichert LE Jr.. 1997. A synthetic peptide corresponding to amino acid residues 34 to 37 of human follicle-stimulating hormone β-subunit accelerates the onset of puberty in male and female mice. Endocrinology. 138(10):4215–4219. [DOI] [PubMed] [Google Scholar]
- Huth JR, Mountjoy K, Perini F, Ruddon RW.. 1992. Intracellular folding pathway of human chorionic gonadotropin beta subunit. J Biol Chem. 267(13):8870–8879. [PubMed] [Google Scholar]
- Jameson JL, Hollenberg AN.. 1993. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 14(2):203–221. [DOI] [PubMed] [Google Scholar]
- Jeoung YH, Yoon JT, Min KS.. 2010. Biological functions of the COOH-terminal amino acids of the (-subunit of tethered equine chorionic gonadotropin. Reprod Dev Biol. 34:47–53. [Google Scholar]
- Keutmann HT, Mason KA, Kitzmann K, Ryan RJ.. 1989. Role of the beta 93-100 determinant loop sequence in receptor binding and biological activity of human luteinizing hormone and chorionic gonadotropin. Mol Endocrinol. 3(3):526–531. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim CH, An MJ, Lee JH, Shin GS, Song MS, Kim JW.. 2020. Ethylparaben induces apoptotic cell death in human placenta BeWo cells via the caspase-3 pathway. Animal Cells Syst. 24(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(5259):680–685. [DOI] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW.. 1994. Crystal structure of human chorionic gonadotropin. Nature. 369(6480):455–461. [DOI] [PubMed] [Google Scholar]
- Lundberg PS, Moskowitz GJ, Bellacose C, Demirel E, Trau HA, Duffy DM.. 2020. Granulosa cell proliferation is inhibited by PGE2 in the primate ovulatory follicle. Animal Cells Syst. 24(3):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Mizuochi T, Titani K, Okinaga T, Hoshi M, Bousfield GR, Sugino H, Ward DN.. 1994. Structural analysis of N-linked oligosaccharides of equine chorionic gonadotropin and lutropin β-subunits. Biochemistry. 33(47):14039–14048. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Keene JL, Boime I.. 1989. Site specificity of the chorionic gonadotropin N-linked oligosaccharides in signal transduction. J Biol Chem. 264(5):2409–2414. [PubMed] [Google Scholar]
- Min KS, Hattori N, Aikawa JI, Shiota K, Ogawa T.. 1996. Site-directed mutagenesis of recombinant equine chorionic gonadotropin/luteinizing hormone: differential role of oligosaccharides in luteinizing hormone- and follicle-stimulating hormone-like activities. Endocrine J. 43(5):585–593. [DOI] [PubMed] [Google Scholar]
- Min KS, Hiyama T, Seong HH, Hattori N, Tanaka S, Shiota K.. 2004. Biological activities of tethered equine chorionic gonadotropin (eCG) and its deglycosylated mutants. J Reprod Dev. 50(3):297–304. [DOI] [PubMed] [Google Scholar]
- Min KS, Park JJ, Byambaragchaa M, Kang MH.. 2019. Characterization of tethered equine chorionic gonadotropin and its deglycosylated mutants by ovulation stimulation in mice. BMC Biotechnol. 19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KS, Park JJ, Lee YS, Byambaragchaa M, Kang MH.. 2020. Comparative gene expressing profiling of mouse ovaries upon stimulation with natural equine chorionic gonadotropin (N-eCG) and tethered recombinant-eCG (R-eCG). BMC Biotechnol. 20(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BD, Martinuk SD.. 1991. Equine chorionic gonadotropin. Endocr Rev. 12(1):27–44. [DOI] [PubMed] [Google Scholar]
- Pacala N, Corin N, Bencsik I, Dronca D, Cean A, Boleman A, Caraba V, Papp S.. 2010. Stimulation of the reproductive function at cyclic cows by ovsynch and PRID/eCG. Anim Sci Biotech. 43:317–320. [Google Scholar]
- Park JJ, JarGal N, Yoon JT, Min KS.. 2009. Function of the tethered rec-eCG in rat and equine receptors. Reprod Dev Biol. 33:229–236. [Google Scholar]
- Park JJ, JarGal N, Yoon JT, Min KS.. 2010. β-subunit 94-96 residues of tethered recombinant equine chorionic gonadogropin are important sites luteinizing hormone and follicle stimulating hormone like activities. Reprod Dev Biol. 34:33–40. [Google Scholar]
- Park JJ, Seong HK, Kim JS, Byambaragchaa M, Kang MH, Min KS.. 2017. Internalization of rat FSH and LH/CG receptors by rec-eCG in CHO-K1 cells. Dev Reprod. 21(2):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF.. 1981. Glycoprotein hormones: structure and function. Ann Rev Biochem. 50:465–495. [DOI] [PubMed] [Google Scholar]
- Roth KE, Dias JA.. 1996. Follitropin conformational stability mediated by loop 2β effects follitropin-receptor interaction. Biochemistry. 35(24):7928–7935. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Manjunath P.. 1983. Hormonal antagonistic properties of chemically deglycosylated human choriogonadotropin. J Biol Chem. 258(1):445–449. [PubMed] [Google Scholar]
- Saneyoshi T, Min KS, Ma JX, Nambo Y, Hiyama T, Tanaka S, Shiota K.. 2001. Equine follicle-stimulating hormone: molecular cloning of beta subunit and biological role of the asparagine-linked oligosaccharide at asparagine56 of alpha subunit. Biol Reprod. 65(6):1686–1690. [DOI] [PubMed] [Google Scholar]
- Sherman GB, Wolfe NW, Farmerie TA, Clay CM, Threadgill DS, Sharp DC, Nison JH.. 1992. A single gene encodes the β-subunit of equine luteinizing hormone and chorionic gonadotropin. Mol Endocrinol. 6(6):951–959. [DOI] [PubMed] [Google Scholar]
- Sim BW, Min KS.. 2014. Production of cloned mice by aggregation of tetraploid embryo. Animal Cells Syst. 18:324–332. [Google Scholar]
- Sim BW, Park CW, Kang MH, Min KS.. 2017. Abnormal gene expression in regular and aggregated somatic cell nuclear transfer placentas. BMC Biotechnol. 17(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Bousfield GR, Kumar S, Fiete D, Baenziger JU.. 1993. Equine lutropin and chorionic gonadotropin bear oligosaccharides terminating with SO4-4-GalNAc and Sia(2,3Gal, respectively. J Biol Chem. 268(2):795–802. [PubMed] [Google Scholar]
- Stewart F, Allen WR.. 1981. Biological functions and receptor binding activities of equine chorionic gonadotropins. J Reprod Ferti. 62(2):527–536. [DOI] [PubMed] [Google Scholar]
- Sugino H, Bousfiedl GR, Moore WT Jr, Ward DN.. 1987. Structural studies on equine glycoprotein hormones: amino acid sequence of equine chorionic gonadotropin β-subunit. J Biol Chem. 262(18):8603–8609. [PubMed] [Google Scholar]
- Thoakura NR, Blithe DL.. 1995. Glycoprotein hormones: glycobiology of gonadotropins, thyrotrophin and free α subunit. Glycobiology. 5(1):3–10. [DOI] [PubMed] [Google Scholar]
- Thoakura NR, Weintraub BD, Bahl OP.. 1990. The role of carbohydrate in human choriogonadotropin (hCG) action. Effects of N-linked carbohydrate chains from hCG and other glycoproteins on hormonal activity. Mol Cell Endocrinol. 70(3):263–272. [DOI] [PubMed] [Google Scholar]
- Valove FM, Finch C, Anasti JN, Froehlich J, Flack MR.. 1994. Receptor binding and signal transduction are dissociable functions requires different sites on follicle-stimulating hormone. Endocrinology. 135(6):2657–2661. [DOI] [PubMed] [Google Scholar]