Abstract
Mitochondria are key cytoplasmic organelles. Their activation is critical for the generation of T cell proliferation and cytotoxicity. Exhausted tumor‐infiltrating T cells show a decreased mitochondrial function and mass. 5‐Aminolevulinic acid (5‐ALA), a natural amino acid that is only produced in the mitochondria, has been shown to influence metabolic functions. We hypothesized that 5‐ALA with sodium ferrous citrate (SFC) might provide metabolic support for tumor‐infiltrating T cells. In a mouse melanoma model, we found that 5‐ALA/SFC with a programmed cell death‐ligand 1 (PD‐L1) blocking Ab synergized tumor regression. After treatment with 5‐ALA/SFC and anti‐PD‐L1 Ab, tumor infiltrating lymphocytes (TILs) were not only competent for the production of cytolytic particles and cytokines (granzyme B, interleukin‐2, and γ‐interferon) but also showed enhanced Ki‐67 activity (a proliferation marker). The number of activated T cells (PD‐1+Tim‐3−) was also significantly increased. Furthermore, we found that 5‐ALA/SFC activated the mitochondrial functions, including the oxygen consumption rate, ATP level, and complex V expression. The mRNA levels of Nrf‐2, HO‐1, Sirt‐1, and PGC‐1α and the protein levels of Sirt‐1 were upregulated by treatment with 5‐ALA/SFC. Taken together, our findings revealed that 5‐ALA/SFC could be a key metabolic regulator in exhausted T cell metabolism and suggested that 5‐ALA/SFC might synergize with anti‐PD‐1/PD‐L1 therapy to boost the intratumoral efficacy of tumor‐specific T cells. Our study not only revealed a new aspect of immune metabolism, but also paved the way to develop a strategy for combined anti‐PD‐1/PD‐L1 cancer immunotherapy.
Keywords: cancer immunotherapy, exhausted T cell, mitochondrial function, PD‐1/PD‐L1 blockade, tumor infiltrating lymphocyte
Exhausted T cells have decreased effector and proliferation function. We found that the mRNA levels of the Nrf‐2, HO‐1, Sirt‐1, and PGC‐1α and the protein levels of Sirt‐1 were upregulated by treatment with 5‐aminolevulinic acid (5‐ALA)/sodium ferrous ion (SFC). The enforced expression of PGC‐1α in activated T cells rescued their effector function and enhanced the efficacy of the mitochondrial function. Our findings revealed that 5‐ALA/SFC could be a key metabolic regulator in exhausted T cell metabolism and suggested that 5‐ALA/SFC might synergize with anti‐programmed cell death‐1/programmed cell death‐ligand 1 therapy to boost the intratumoral efficacy of tumor‐specific T cells.

Abbreviations
- 5‐ALA
5‐aminolevulinic acid
- APC
antigen‐presenting cell
- FMO
fluorescence minus one
- GrB
Granzyme B
- HO‐1
heme oxygenase‐1
- IFN‐γ
γ‐interferon
- IL
interleukin
- MFI
mean fluorescent intensity
- NCCHD
National Center for Child Health and Development
- Nrf‐2
nuclear factor‐erythroid factor 2‐related factor‐2
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- PD‐1
programmed cell death‐1
- PD‐L1
programmed cell death ligand‐1
- PGC‐1α
peroxisome proliferator‐activated receptor‐gamma coactivator‐1α
- SFC
sodium ferrous citrate
- Sirt‐1
Sirtuin‐1
- TIL
tumor‐infiltrating lymphocyte
- Tim‐3
T‐cell immunoglobulin and mucin domain‐3
- TME
tumor microenvironment
1. INTRODUCTION
Programmed cell death‐1/PD‐L1 checkpoint blockade therapy is a means to reinvigorate the effector function of T cells by inhibition of the PD‐1/PD‐L1 pathway. This treatment increases the proliferation of effector cells in TILs and the continuous release of cytokines, and leads to a more clonal T‐cell receptor repertoire within the T cell population directed against the tumor. These effects ultimately provide cancer patients with significant immune responses against their malignancy. Although PD‐1/PD‐L1 checkpoint blockade can result in a dramatic therapeutic response, this therapy is only effective in a subset of patients, and many patients only show a partial response to the therapy. 1 , 2 Approximately 30‐50% of patients remain unresponsive or show a low response to PD‐1/PD‐L1 blockade therapy. 3 Mitochondria are considered the powerhouse of cells. Mitochondria generate most of the cell’s supply of ATP, which is used as a source of chemical energy, and thus are considered as bioenergetics. The maintenance and activation of T cells are energy‐demanding activities that require the use of bioenergy in the form of ATP; thus, mitochondria are considered to be bioenergetic. 4 T cells rely on the function of mitochondria during their migration, activation, proliferation, differentiation, memory phase, and exhaustion. 5 In the TME, intratumoral T cells show a repressed mitochondrial metabolism and decreased mitochondrial mass, due to the low nutrient availability. While restoring the T cell mitochondrial function can improve the function of TILs, in the TME, TILs can also become exhausted due to the low nutrient availability. 6 Restoring the T cell glucose uptake and glycolytic intermediates or mitochondrial mass can improve the function of TILs. Furthermore, enhancing the mitochondrial function might represent a therapeutic strategy for reviving defective CD8+ T cells in diverse settings. 7
5‐Aminolevulinic acid, an endogenous nonprotein amino acid, is the first compound in the porphyrin synthesis pathway leading to heme synthesis. 5‐Aminolevulinic acid is present in virtually all cells and forms a vital part of mitochondrial activity, ATP production, and energy metabolism. 8 , 9 , 10 5‐Aminolevulinic acid supplementation in mice enhances Complex IV (cytochrome c oxidase, COX) activity and ATP production levels, and combined treatment with 5‐ALA and SFC was reported to upregulate the expression of electron transfer chain complexes III, IV, and V in the white adipose tissue of obese mice. 10 These study results suggest the possibility of a definitive therapy against mitochondrial disease by an “enzyme reinforcing treatment” that reinforces the activity of remaining mitochondrial respiratory chain enzymes to improve the mitochondrial function. 11 , 12
In the present study, we used 5‐ALA/SFC combined with anti‐PD‐L1 mAb to increase the mitochondrial function of TILs, and thereby enhance the anti‐PD‐L1 mAb effect, and reverse T cell exhaustion. These findings will pave the way for the development of combination therapies for cancer patients who are less responsive to PD‐1/PD‐L1 blockade treatment. Furthermore, mitochondrial activation could be considered as a biomarker of the effectiveness of PD‐1/PD‐L1 blockade therapy.
2. MATERIALS AND METHODS
2.1. Mice, cell lines, Abs, and tumor studies
Eight‐week‐old female C57BL/6NCrSlc (B6) mice and BALB/c mice were purchased from Japan SLC. All cultures were mycoplasma‐free and were maintained in the recommended culture media. Mice were housed with ad libitum access to filtered water and food. Light and temperature were automatically controlled. Mice were cared for in accordance with the NCCHD guidelines on laboratory animal welfare.
The murine B16F10 melanoma cell line (RCB2630, RRID: CVCL 0159; Cell Bank, RIKEN), B16BL6 melanoma cell line (RCB2638, RRID: CVCL 0157; Cell Bank, RIKEN) and the murine colon carcinoma cell line C26, gifted from Dr Jun Fang, Faculty of Pharmaceutical Sciences, Sojo University, were routinely cultured in RPMI‐1640 media (Wako) supplemented with 10% FBS (Thermo Fisher Scientific) in a 10‐cm2 dish at 37°C under 5% CO2. B16F10/B16BL6 cells were resuspended at 3 × 106 cells/mL in PBS and injected (3 × 105/100 μL s.c.) into the shaved right flank of B6 mice. C26 cells were resuspended at 5 × 106 cells/mL in PBS and injected (5 × 105/100 μL s.c.) into the shaved right flank of BALB/c mice. Tumors were measured every 2 days (length and width) with a caliper from day 8 after transplantation. Tumor volume growth was monitored by measurement of the perpendicular tumor diameter and calculated using the formula (mm3) = 1/2 (length × width2). Mice with tumors for which 1/2 (length + width) exceeded 12 mm were killed in accordance with NCCHD guidelines (permission number: A2017‐005‐C03).
Tumor‐bearing (tumor size 10 mm3 or larger) mice were intraperitoneally treated on days 8 and 12 with anti‐PD‐L1 mAb (200 μg/head; Clone: MIH5; gift from Dr Azuma, Tokyo Medical and Dental University), 5‐ALA (100 mg/kg; neo ALA Co. Ltd), and SFC (157 mg/kg; Komatsuya) for 8 days from day 8, or anti‐PD‐L1 mAb with 5‐ALA/SFC.
2.2. Preparation of TILs and flow cytometry
Excised tumors were washed with PBS, dissected into smaller fragments using a scalpel, and further dissociated into single cell suspension using a Miltenyi Tumor Dissociation Kit (130‐096‐730; Miltenyi) and a GentleMACS Octo dissociator (130‐093‐235; Miltenyi). The digested tumors were filtered through 70‐μmol/L preseparation filters (Thermo Fisher Scientific) and washed with PBS. Mononuclear cells were isolated by centrifugation (600 g, 20°C, 20 minutes) through a Percoll gradient (55% and 70%; GE Healthcare).
2.3. Intracellular cytokine staining
After isolation from the tumor, TILs and spleen cells were stimulated with 50 ng/mL PMA (Sigma‐Aldrich) and 500 ng/mL ionomycin (Sigma‐Aldrich) in the presence of Brefeldin A Solution (Thermo Fisher Scientific) for 4 hours at 37°C, and then stained and analyzed by flow cytometry, as described in the following section. For each sample, 1 × 106 cells were treated with anti‐mouse CD16/32 (BioLegend) and then stained with a defined panel containing Live/Dead stain (L34957; Thermo Fisher Scientific) and eight different labeling Abs (BioLegend): PerCp‐Cy5.5‐conjugated anti‐CD3 (145‐2C11), APC‐conjugated anti‐CD4 (RM4‐5), AF700‐conjugated anti‐CD8 (53‐6.7), APC‐Cy7‐conjugated CD45 (30‐F11), PE‐Cy7‐conjugated Ki67 (16A8), APC‐conjugated Tim‐3 (RMT3‐23), PE‐Cy7‐conjugated CD8 (53‐6.7), AF700‐conjugated CD4 (RM4‐5), PE‐Cy7‐conjugated IFN‐γ (XMG1.2), PE‐conjugated IL‐2 (JES6‐5H4), PE‐conjugated GrB (QA16A02), FITC‐conjugated anti‐Ly‐6G (1A8), PE‐conjugated anti‐Ly‐6C (HK1.4), APC‐conjugated anti‐NKp46 (29A1.4), PE‐Cy7‐conjugated anti‐B220 (RA3‐6B2), APC‐Cy7‐conjugated anti‐CD11b (M1/70), AF700‐conjugated anti‐CD11c (N418), and BV570‐conjugated anti‐CD45 (30‐F11) from BioLegend, and FITC‐conjugated PD‐1 (MIH‐4) from Thermo Fisher Scientific. Intracellular Fixation & Permeabilization Buffer (00‐5523‐00; Thermo Fisher Scientific) was used to permeabilize and facilitate intracellular staining. All samples were acquired on BD FACS LSR Fortessa (Becton Dickinson), and analyzed by the FlowJo software (version 10.5.0; BD Biosciences).
2.4. Western blot analysis
Spleen cells were isolated from the spleens of B6 mice and suspended in RPMI‐1640 (Wako) supplemented with 10% FBS and 50 μmol/L 2‐ME (Sigma‐Aldrich). They were plated at 1 × 107 cells per well in 2‐mL 6‐well flat‐bottomed tissue culture plates coated with anti‐CD3 mAb (Clone 17A2, 1 μg/mL; Becton Dickinson). Soluble anti‐CD28mAb (Clone 37.51, 1 μg/mL; BioXCell) was also added. The spleen cells were cultured in an incubator at 37°C under 5% CO2 and 95% air for 2 days, and then subjected to western blotting.
The cells were treated with RIPA lysis buffer (Wako) containing phosphatase inhibitor cocktail and protease inhibitor cocktail from different groups at the indicated time points. Proteins were subjected to electrophoresis and transferred onto PVDF membranes (Bio‐Rad). The PVDF membranes were blocked with 3% skin milk for 1 hour at room temperature, and then incubated with the primary Abs overnight at 4°C. Appropriate HRP‐conjugated secondary Abs were applied for 1 hour at room temperature. Proteins were extracted and blotted with anti‐β‐actin Ab (1:4000; Abcam), anti‐Sirt1 Ab (1:1000; Abcam), and total OXPHOS Rodent Ab (Abcam). The proteins were detected with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The relative optical density of each specific band obtained was measured using the ImageJ software program (NIH).
2.5. Measurement of Complex IV activity
After 48 hours, stimulated spleen cells (with or without 5‐ALA/SFC) were stored frozen at −80°C until use in a Complex IV activity assay. The Complex IV activity was measured by a Complex IV Rodent Enzyme Activity Micro assay kit (ab83355; Abcam), according to the manufacturer’s instructions. The measurement conditions were as follows: absorbance 550 nm, 30°C, 120 minutes. Three independent experiments were carried out. The sample volume used for testing in the first experiment in each group was 10 μg. The sample volume used in the second and third experiments was 8 μg.
2.6. Extracellular OCR
Spleen cells were isolated from the spleens of B6 mice and suspended in RPMI‐1640 medium supplemented with 10% FBS and 50 μmol/L 2‐ME. They were plated at 1 × 107 cells per well in 2‐mL 6‐well flat‐bottomed tissue culture plates coated with anti‐CD3 mAb. Soluble anti‐CD28 mAb was also added. The spleen cells were cultured in an incubator with at 37°C under 5% CO2 and 95% air for 2 days. They were then applied subjected to an extracellular OCR assay. In another experiment, spleen cells were isolated from the treated or untreated tumor‐bearing mice, and then subjected to an extracellular OCR assay without CD3/CD28 stimulation.
The extracellular OCR was assayed using an O2‐sensitive phosphorescent probe MitoXpress Xtra (Agilent). Cells were seeded in a 96‐well plate coated with anti‐CD3 mAb at a density of 1 × 106 cells/well in 90 μL fresh media supplemented with anti‐CD28 mAb. For stimulation with 5‐ALA and SFC, 1000 μmol/L 5‐ALA and 500 μmol/L SFC was added to the cell suspension. Ten microliters of MitoXpress Xtra reagent was added to each well. The wells were immediately sealed with two drops of mineral oil prewarmed to 37°C. The plate was placed in an ARVO X5 microplate reader (Perkin Elmer) at 37°C. The samples were monitored at every 3 minutes for 180 minutes, by measuring the fluorescence intensity at 340/642 nm excitation/emission at delay times of 30 and 70 μs. The intensity signals of time‐resolved fluorescence measurement for each sample were converted into fluorescence lifetime (τ) values. From the resulting τ profiles, the initial slopes were calculated (μs/min), which reflected the OCR of the samples.
2.7. Quantitative RT‐PCR
RNA samples were reverse transcribed into cDNA using oligo (dT) primers and Super Script reverse transcriptase (Invitrogen). The primers and specific probes were synthesized by Biosearch Technologies (Novato). Fifty quantitative PCR cycles (95°C for 30 s and 60°C for 1 minute) were carried out after initial denaturation (95°C for 15 minutes) on an Applied Biosystem PRISM 7700 instrument (Thermo Fisher Scientific). 18S mRNA was used as the housekeeping gene. The final result was analyzed by the −△△Ct method. The target‐specific primers and probes sequences are shown in Table 1.
TABLE 1.
Primer sequences and probes used in the present study
| Gene | Forward (5′–3′) primer | Reverse (5′–3′) primer | Probe |
|---|---|---|---|
| 18S | ATGAGTCCACTTTAAATCCTTTAACGA | CTTTAATATACGCTATTGGAGCTGGAA | ATCCATTGGAGGGCAAGTCTGGTGC |
| HO‐1 | CAGGGTGACAGAAGAGGCTAAGAC | TCTTTGTGTTCCTCTGTCAGCAGT | TCCTGCTCAACATTGAGCTGTTTGAGGA |
| Nrf‐2 | GCCCTCAGCATGATGGACTTG | TGCCTCCAAAGGATGTCAATCAA | AGTTGCCACCGCCAGGACTACAGTCC |
| PGC‐1α | CATTTGATGCACTGACAGATGGA | CCGTCAGGCATGGAGGAA | CCGTGACCACTGACAACGAGGCC |
| Sirt1 | CAGCATCTTGCCTGATTTGTAAATAC | CACCGAGGAACTACCTGATTAAAAA | TCTCCACGAACAGCTTCACAATCAACT |
2.8. Measurement of ATP level
After 48 hours, the stimulated spleen cells (with/without 1000 μmol/L 5‐ALA/500 μmol/L SFC) were stored frozen at −80°C until used in the biochemical analysis. The ATP levels were measured by an ATP assay kit (ab83355; Abcam) according to the manufacturer’s instructions and expressed as micromoles per gram of tissue.
2.9. Statistical analysis
The results are expressed as the mean ± SD. All data were analyzed using the GraphPad Prism software program (version 7.0; GraphPad Software). One‐tailed unpaired (in one independent sample) or paired (in three independent experiments) Student’s t test were used to compare two groups (normal distribution). One‐tailed Wilcoxon matched‐pairs signed rank test were used to compare two groups (abnormal distribution). One‐way ANOVA analysis with Tukey’s multiple comparisons test were used to compare multiple groups. P values of <.05 were considered to indicate statistical significance.
3. RESULTS
3.1. Combination of 5‐ALA/SFC and anti‐PD‐L1 mAb treatment synergistically enhanced antimelanoma response
In comparison to 5‐ALA/SFC only, anti‐PD‐L1 mAb only, and no‐treatment control groups, the treatment 5‐ALA/SFC and anti‐PD‐L1mAb resulted in the potent inhibition of tumor growth in B6 mice bearing an established subcutaneous B16F10 tumor (Figure 1A). Combination treatment with 5‐ALA/SFC and anti‐PD‐L1 mAb increased the antitumor response in comparison to anti‐PD‐L1 mAb therapy alone. We observed the differences in tumor growth curves of each mouse in each group (Figure 1B) and the changes in tumor size on day 16 in each group (Figure 1C). To examine whether this tumor growth inhibition was also shown in other murine syngeneic tumor models, B16BL6 melanoma and C26 colon tumor models were examined in C57BL/6NCrSlc and BALB/c mice, respectively. We have also confirmed similar results in the B16BL6 and C26 models, as the data show in Figure S1.
FIGURE 1.
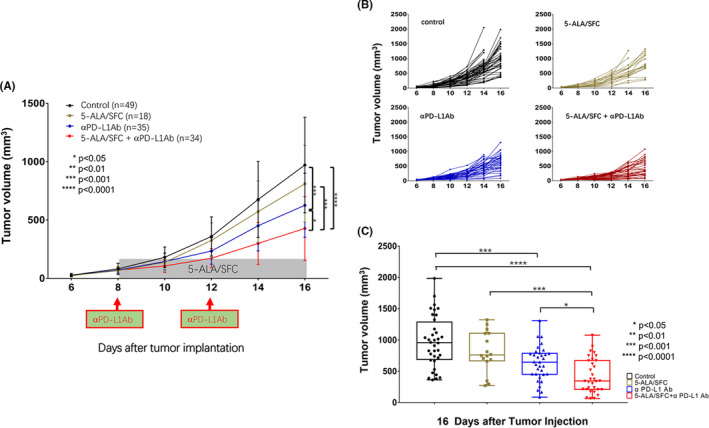
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) and anti‐ programmed cell death‐ligand 1 (PD‐L1) mAb treatment synergistically enhanced the antimelanoma response. A, Effect of combination treatment with 5‐ALA/SFC and anti‐PD‐L1 mAb on B16F10 tumor growth curves in mice. B, Individual tumor growth curves from control (untreated), anti‐PD‐L1 mAb‐treated, 5‐ALA/SFC‐treated, and 5‐ALA/SFC + anti‐PD‐L1 mAb‐treated mice. Each line represents one mouse. C, Effect of combination treatment with 5‐ALA/SFC and anti‐PD‐L1 mAb on day 16 (end‐point) after tumor implantation. Data represent the mean ± SD. *P < .05; ***P < .001; ****P < .0001 by one‐way ANOVA with Tukey’s multiple comparisons test
3.2. 5‐Aminolevulinic acid/SFC increased tumor‐infiltrating activated PD‐1+Tim‐3− T lymphocyte population
Until recently, PD‐1 has been the main marker for T cell exhaustion. However, other studies have shown that PD‐1 single‐positive tumor‐infiltrating T lymphocytes likely include bona fide effector T cells that produce IFN‐γ, IL‐2, and other cytokines. 13 We analyzed tumors and spleens on day 16. The 5‐ALA/SFC and anti‐PD‐L1 mAb treatment strongly induced the expression of PD‐1+Tim‐3− T cells in TILs, whereas the PD‐1+Tim‐3− T cell population in TILs from tumor bearing mice after anti‐PD‐L1 mAb treatment was nearly the same as that in control (Figure 2). These data clearly showed that TILs were activated after treatment with 5‐ALA/SFC and anti‐PD‐L1mAb. During treatment, the numbers of PD‐1+Tim‐3− T cells significantly increased in TILs (Figure 2A) but not in spleen cells (Figure 2B). Thus, our data suggested that 5‐ALA/SCF and anti‐PD‐L1mAb enhanced the effector T cell population.
FIGURE 2.
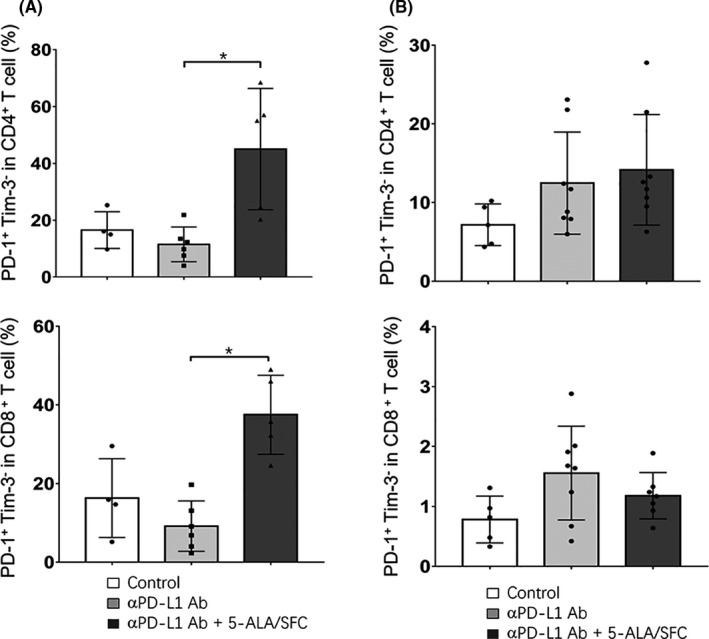
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) increased the tumor‐infiltrating activated programmed cell death‐1 (PD‐1)+Tim‐3− T lymphocyte population. A, Mean percentages of CD4+PD‐1+Tim‐3− and CD8+PD‐1+Tim‐3− cells among CD3+ tumor‐infiltrating lymphocyte T cells. B, Mean percentages of CD4+PD‐1+Tim‐3− and CD8+PD‐1+Tim‐3− cells among CD3+ spleen cells. Data represent the mean ± SD. *P < .05, by a one‐way ANOVA with Tukey’s multiple comparisons test
3.3. 5‐Aminolevulinic acid/SFC increased TIL proliferation and cytokine and cytolytic enzyme expression
The functionality of the T cells in TILs was evaluated based on proliferation ability, cytokine production, and the ability to degranulate. To assess T cell activation, we examined the intracellular expression of Ki‐67, which is a cell cycle marker expressed by circulating or recently dividing cells. Among the TILs in B16F10 tumor‐bearing mice, the CD4+ and CD8+ T cells expressed higher levels of Ki‐67 relative to control (no treatment) and tumor‐bearing mice that received anti‐PD‐L1 mAb treatment alone. The freshly isolated lymphocytes were stimulated with PMA and ionomycin for 4 hours, and intracellular cytokines were examined in gated CD3+CD4+/CD8+ T cells. The production of multiple effector cytokines, such as IFN‐γ and IL‐2, is a well‐established indicator of the activation of the T cell function, and it has been suggested that failure to produce multiple cytokines could be related to the stage of exhaustion. The amounts (as reflected by the mean fluorescent intensity) of IFN‐γ and IL‐2 produced by CD4 and CD8 TILs treated with 5‐ALA/SFC and anti‐PD‐L1 mAb were significantly higher in comparison to those treated with anti‐PD‐L1 mAb alone. We also investigated the degranulation of CD8+ TILs using GrB as a marker of the cytotoxic activity of CD8+ T cells. The high expression of GrB was observed in CD8+ TILs in tumors of the 5‐ALA/SFC and anti‐PD‐L1 mAb treatment group (Figure 3). These data indicate that treatment with 5‐ALA/SFC combined with anti‐PD‐L1 mAb led to the recovery of part of the lost effector function of TILs in the TME and markedly enhanced the antitumor efficacy in vivo. Representative contour plots showing the general gating strategy was used to differentiate purified T cells (CD3+CD4+/CD8+) from mouse TILs (Figure S2). Furthermore, as data shows in Figure S3, splenocyte proliferation and cytokine and cytolytic enzyme expression showed no difference among the groups, meaning that anti‐PD‐L1 mAb and 5‐ALA/SFC have no effect on the immune status of the whole body except TILs.
FIGURE 3.
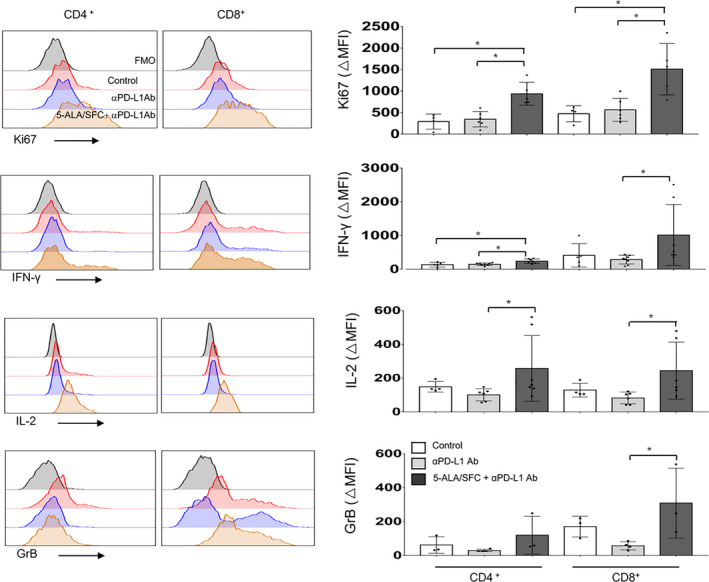
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) increased tumor‐infiltrating lymphocyte (TIL) proliferation, and the expression levels of cytokines and cytolytic enzymes. Representative FACS data of the gated population. Intracellular staining of Ki‐67, interleukin‐2 (IL‐2), γ‐interferon (IFN‐γ), and Granzyme B (GrB) (left) and the quantified data (right) of TILs from B16F10‐bearing mice. Data represent the mean ± SD. *P < .05 by one‐way ANOVA with Tukey’s multiple comparisons test. Δmean fluorescent intensity (MFI) = MFI− fluorescence minus one (FMO) MFI
3.4. 5‐Aminolevulinic acid/SFC enhanced mitochondrial function by OXPHOS
The OXPHOS system consists of five mitochondrial complexes and provides cellular energy by generating ATP from ADP. First, we found that the linked expression of Complex V (ATP synthesis) was significantly increased in CD3/CD28 stimulated spleen cells treated with 5‐ALA/SFC and that the complexes I‐IV were also increased, but not to a statistically significant extent (Figure 4A). We also found that the enzymatic activity of complex IV was consistently increased in three independent experiments (Figure 4B). Complexes I‐IV are electron acceptors in the respiratory chain that are involved in the reduction of O2 to H2O. Complex V is the last of five mitochondrial complexes that carries out a multistep process called OXPHOS, from which much of the cellular energy is derived. Furthermore, we next sought to determine the bioenergetic effects of 5‐ALA/SFC on this respiratory capacity. The ATP levels in cells are relatively stable in nonstress situations, and we found that anti‐CD3/CD28 mAb‐activated cells treated with 5‐ALA/SFC had much higher intracellular levels of ATP in comparison to nontreated cells, suggesting that these cells contained mitochondria that were energetically primed to produce ATP swiftly upon demand (Figure 4C). Taken together, these results indicated that 5‐ALA/SFC could increase the mitochondrial functions in CD3/CD28 stimulated spleen cells.
FIGURE 4.
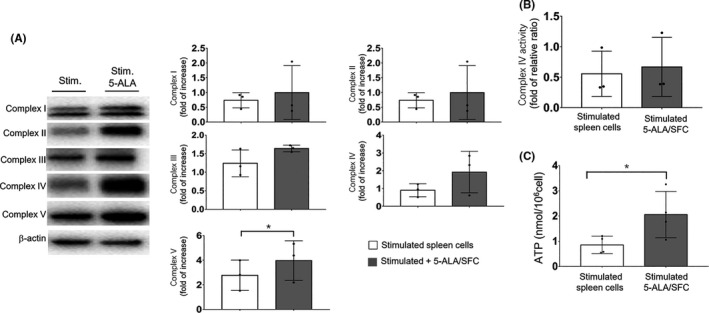
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) enhanced the mitochondrial function by oxidative phosphorylation. A, Stimulated spleen cells were treated with or without 5‐ALA/SFC (1 mmol/L/0.5 mmol/L) for 72 h, and then the expression levels of complex I‐V proteins were detected by immunoblotting and analyzed by a one‐tailed unpaired Student’s t test. B, Enzymatic activity of Complex IV in stimulated spleen cells with or without 5‐ALA/SFC was detected by microplate assay kits was analyzed by a one‐tailed Wilcoxon matched‐pairs signed rank test. C, ATP levels in stimulated spleen cells with or without 5‐ALA/SFC measured by an ATP assay kit were analyzed by a one‐tailed unpaired Student’s t test. Each dot represents one independent experiment. Data represent the mean ± SD. *P < .05
3.5. 5‐Aminolevulinic acid/SFC enhanced the OCR
As 5‐ALA/SFC enhanced the expression of mitochondrial complex proteins in immune cells, we assessed the effect of 5‐ALA/SFC on their metabolic features. The OCR is recognized as a fundamental measure of the mitochondrial metabolic function. Thus, we used the extracellular OCR as an index of the cellular metabolism. First, we evaluated the effect of 5‐ALA/SFC on the OCR of mouse spleen cells in three independent experiments. The spleen cells isolated from B6 mice were activated with anti‐CD3/CD28mAbs, and then subjected to an OCR assay. The oxygen consumption was significantly enhanced by the addition of 5‐ALA/SFC (Figure 5A), suggesting that 5‐ALA/SFC energized the spleen cells through the activation of mitochondrial function. Next, we assessed the mitochondrial features of immune cells in the context of combination tumor therapy with 5‐ALA/SFC and anti‐PD‐L1 mAb in vivo. We isolated spleen cells from untreated, anti‐PD‐L1‐treated, and 5‐ALA/SFC + anti‐PD‐L1 mAb‐treated tumor‐bearing mice, and compared the OCRs of spleen cells among these treatment groups. As shown in Figure 5B, anti‐PD‐L1 mAb treatment alone had no effect on the oxygen consumption of the immune cells. The addition of 5‐ALA/SFC to the Ab treatment upregulated the oxygen consumption of the immune cells, although the upregulation was not statistically significant. These results suggested that the activation of the mitochondrial function by 5‐ALA/SFC enhanced the rejection of the tumor burden induced by PD‐L1 Ab therapy.
FIGURE 5.
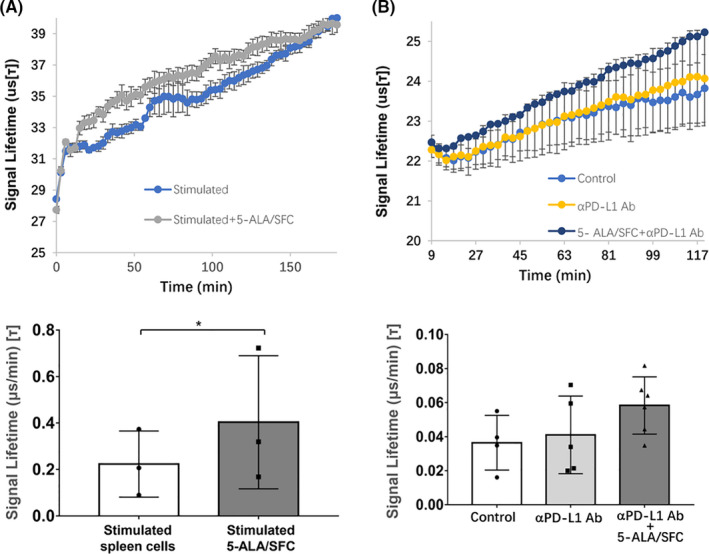
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) enhanced the oxygen consumption rate (OCR). A, A representative OCR trace and quantification of the spare respiratory capacity of T cells activated by anti‐CD3/CD28mAbs in the presence of 5‐ALA/SFC for 48 h in vitro. Data represent the mean ± SD, *P < .05 by a one‐tailed paired Student’s t test. Each dot represents one independent experiment. B, A representative OCR trace and quantification of the spare respiratory capacity of spleen cells of mice treated with anti‐programmed cell death‐ligand 1 (PD‐L1) mAb with or without 5‐ALA/SFC or untreated mice. Data represent the mean ± SD. Data were analyzed by one‐way ANOVA with Tukey’s multiple comparisons test. Each dot represents one mouse
3.6. 5‐Aminolevulinic acid/SFC enhanced mitochondrial function through the Nrf‐2, HO‐1, Sirt‐1, and PGC‐1α pathways
Next, we wanted to determine the mechanism by which 5‐ALA/SFC promotes the increase of the mitochondrial function. To do this, we used spleen cells stimulated for 48 hours with anti‐CD3/CD28 mAbs in vitro with or without 5‐ALA/SFC. We sought to determine how 5‐ALA/SFC signaling might promote increased biogenesis. We found that 5‐ALA/SFC significantly enhanced the mRNA expression of HO‐1 and Nrf‐2 (Figure 6A). HO‐1 and Nrf‐2 could activate the transcription factor Sirt‐1 (Figure 6B) to promote mitochondrial fusion and biogenesis, in part, through an increase in the mRNA expression of PGC‐1α (Figure 6A), a regulator of mitochondrial biogenesis, which is important for the development of T cell‐mediated antitumor immunity. Thus, these data indicated that the activation of PGC‐1α by 5‐ALA/SFC through the enhanced mitochondrial function resulted in the improvement of the efficacy of PD‐1/PD‐L1 blockade.
FIGURE 6.
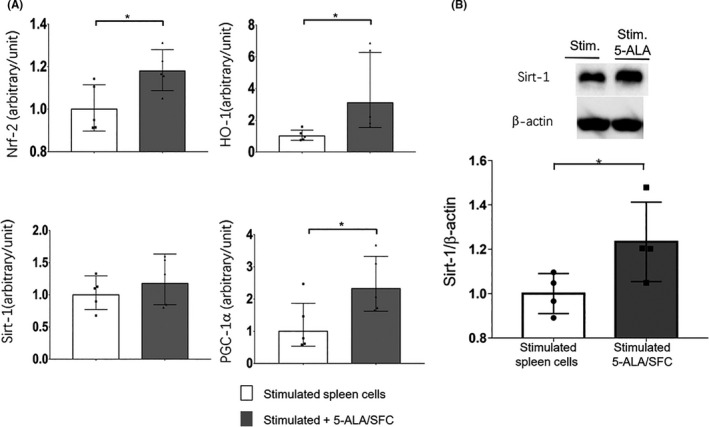
5‐Aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) enhanced the mitochondrial function through the nuclear factor‐erythroid factor 2‐related factor‐2 (Nrf‐2)/heme oxygenase‐ 1 (HO‐1)/Sirtuin 1 (Sirt‐1)/peroxisome proliferator‐activated receptor‐gamma coactivator‐1α (PGC‐1α) pathway. A, Stimulated spleen cells were treated with or without 5‐ALA/SFC (1 mmol/L/0.5 mmol/L) for 72 h, and then a quantitative RT‐PCR assay was used to analyze the HO‐1, Nrf‐2, Sirt‐1, and PGC‐1α mRNA content. *P < .05 by a one‐tailed unpaired Student’s t test. B, Stimulated spleen cells were treated with or without 5‐ALA/SFC for 72 h, and then immunoblotting was used to analyze the Sirt‐1 protein content. *P < .05 by a one‐tailed unpaired Student’s t test. Each dot represents one independent experiment. Data represent the mean ± SD
4. DISCUSSION
Programmed cell death‐1/PD‐L1 pathway blockade‐based cancer immunotherapy has significantly changed cancer treatment because it targets more cancers and is associated with longer responses with fewer side effects in comparison to other cancer therapies. 1 , 2 However, many patients show no response or a low response to this therapy. 3 , 14 One of the main causes of a poor response is the absence (or insufficiency) of functional effector T cells at tumor sites, due to T cell exhaustion. 2 , 15 , 16 After PD‐1/PD‐L1 checkpoint blockade, initially there is a robust beneficial response for a growing subset of patients. However, after a while, the patients to experience T cell exhaustion and a decreased effector function and proliferative capacity. 17 In exploring the causes of T cell exhaustion, we have gradually discovered that activated CD8+ T cells were exhausted by persistent chronic stimulation with cancer antigens. Reinvigorated T cells became re‐exhausted with the persistent chronic stimulation with cancer antigens. Exhausted CD8+ T cells in cancer frequently express high levels of markers of exhaustion, including PD‐1, Tim‐3, and Lag‐3. 18 , 19 , 20
The state of exhaustion is mainly characterized by the sequential loss of the T cell effector functions, and IL‐2, TNF‐α, IFN‐γ, and other cytokines are dramatically reduced, resulting in the inactivation of cytotoxic activity. 2 , 17 , 21 Severely exhausted T cells appear to undergo apoptosis and are eliminated, leading to a marked decline in tumor‐specific T cells. In a cancer model of the TME, exhausted T lymphocytes showed a reduced mitochondrial mass and function and reduced oxidative OXPHOS. Mitochondria are key cytoplasmic organelles that efficiently supply the ATP necessary for the rapid proliferation and differentiation of T cells. Mitochondria must provide proliferative cells with a metabolic advantage. 22 The maintenance and activation of T cells are energy‐demanding activities that require the use of bioenergy in the form of ATP. In addition to ATP production, mitochondria are involved in calcium homeostasis, lipid synthesis, apoptosis, signaling, and cell cycle progression. Mitochondria have recently been reported to be the biosynthetic and bioenergetic centers of organisms. 3 , 23 , 24 Mitochondrial diseases are clinically and genetically heterogeneous disorders caused by defects of energy production through OXPHOS. 11 , 25 Mitochondria could be considered as the master metabolic regulators of T cells, wherein they control their own metabolism. T cell exhaustion could also be considered a mitochondrial disease. In the present study, we attempted to control the lymphocyte fate and function to reverse T cell exhaustion. In recent years, studies have shown that 5‐ALA/SFC can be used to treat various mitochondrial diseases. 9 , 10 , 11 , 12 , 26 As a mitochondria‐activating substance, 5‐ALA is synthesized from glycine and succinyl‐CoA by the action of ALA synthase in mitochondria. It serves as the first compound in the porphyrin synthesis pathway, which subsequently consists of two elements of mitochondrial electron carriers, heme and cytochrome C (Complex IV). Complex IV is the key electron carrier in the mitochondrial electron transport system, and 5‐ALA supplementation with SFC has been shown to promote mitochondrial electron transport and increase ATP production. 5‐Aminolevulinic acid was shown to increase the expression of PGC‐1α. 27 , 28 It is the master regulator of mitochondrial biogenesis and could enhance the mitochondrial function; 3 , 16 , 29 however, its effects on mitochondrial electron transport vary from one organ to another.
Here, we showed for the first time that the combination of 5‐ALA/SFC with anti‐PD‐L1 mAb synergistically inhibited the growth of a poorly immunogenic tumor model (B16F10) in syngeneic B6 mice, 30 in which 5‐ALA/SFC treatment alone did not show any therapeutic effect (Figure 1). We found that 5‐ALA/SFC with anti‐PD‐L1 mAb therapy activated the upregulation of the PD‐1+Tim‐3− T cell population in TILs (Figure 2). This cell population was considered an activated T cell population. 13 However, there was no difference between the anti‐PD‐L1 mAb group and the control group. We hypothesized that this was related to the poor immunogenicity of the tumor model and T cell exhaustion. 31
We then evaluated the effects of 5‐ALA/SFC with anti‐PD‐L1 mAb on T cell proliferation, cytokines, and cytolytic enzymes in TILs (Figure 3). In the present study, we showed for the first time the synergistic effect of 5‐ALA/SFC with anti‐PD‐L1 mAb to increase the T cell expression of Ki‐67, which is a proliferation marker and a commonly measured indicator of T cell function, in TILs. 32 We then found that the IFN‐γ+ expression on CD4+ and CD8+ T cells was significantly increased in TILs, which had direct effects on tumor cells that could restrain tumorigenesis (eg, enhanced susceptibility to killing or apoptosis and slowed proliferation). 33 , 34 , 35 For immune cells, IFN‐γ signaling activates APCs to upregulate the expression of cytokines (IL‐12 and IL‐18) and costimulatory molecule CD86, which enhance T helper type 1 cell differentiation and CTL function. However, IFN‐γ signaling also promotes tumor elimination by inhibiting the functions of some suppressive immune cells in tumors, such as regulatory T cells, myeloid‐derived suppressor cells, and tumor‐associated macrophages. 36 , 37 Furthermore, we found that the expression of IL‐2 by the T cells was also significantly increased in TILs. Interleukin‐2 is a T‐cell growth factor. Interleukin‐2 not only regulates T helper cell differentiation but also promotes the development of naïve CD8+ T cells into effector CTLs, depending on the IL‐2/IL‐2R signal strength, and it is crucial for the secondary expansion of memory CD8+ T cells. 38 Finally, the expression of GrB increased in TILs. Granzyme B is a serine protease that is most commonly found in the granules of CTLs, and is secreted by these cells along with the pore forming protein to mediate apoptosis in target cells. 39 , 40 Thus, the combination of 5‐ALA/SFC with anti‐PD‐L1 mAb represented a mechanistically rational combination to maximize the antitumor potential.
We thus focused our research on the effects of 5‐ALA/SFC on T cell mitochondria. We stimulated T cells in spleen cells and detected its function and determined the mechanism. As shown as in Figure 6, 5‐ALA/SFC resulted in the upregulation of PGC‐1α through the Nrf‐2, HO‐1, Sirt‐1, and PGC‐1α pathways, which enhanced the mitochondrial function in vitro. Consistent with this finding, we found that 5‐ALA/SFC plus anti‐PD‐L1mAb‐treatment enhanced the CTL function in vivo. The proliferation of primed CTLs generated more effector T cells and secreted a large number of cytokines and toxic granules (Figure 3). This indicated that 5‐ALA/SFC could reverse the exhaustion of effector T cells via the HO‐1, Sirt‐1, and PGC‐1α pathways. We found that HO‐1, Sirt‐1, and PGC‐1α activation by 5‐ALA/SFC enhanced the OCR (Figure 5), explaining the upregulation of Complex V and ATP production, which are necessary for the induction of effector CTLs. Although the increases in complexes I, II, III, and IV were not significant, these showed a tendency to increase (Figure 4). Following activation, the exhausted T cells proliferate and acquire their effector function, a process that is called OXPHOS. 7 , 41 This requires energy generation (ATP) by the mitochondrial respiratory chain complex. Our data from cancer immunotherapy models support the notion that 5‐ALA/SFC can provide metabolic support to T cells and reverse the exhausted T cell functions. This metabolic support, characterized most prominently by elevated mitochondrial sufficiency, enables immunotherapeutic responses. We compared the composition of TILs and splenocytes, apart from T cells, in the B16F10 tumor model. Treatment with 5‐ALA/SFC did not alter the immune signature of TILs, except neutrophil cells. Treatment with 5‐ALA/SFC did not alter the immune signature of splenocytes in tumor‐bearing mice (Figure S4).
In conclusion, the new discovery that 5‐ALA/SFC could help build a stronger immune system is exciting. In the untreated TME, the T cell functions and their mitochondrial functions are very weak, and it is difficult for T cells to exert antitumor effects. After applying PD‐1/PD‐L1 blockade therapy to tumor‐bearing mice, the T cell functions were enhanced but the mitochondrial functions were not fully restored. T cells were prone to exhaustion and the loss of their functions. 5‐Aminolevulinic acid/SFC combined with PD‐1/PD‐L1 blockade therapy can enhance the T cell functions and increase the mitochondrial functions to reverse T cell exhaustion (Figure 7). 5‐Aminolevulinic acid/SFC combined with PD‐1/PD‐L1 blockade therapy could improve the T‐cell effector function and prevent cancer from evading the immune system. Taken together, these data revealed a mechanism by which 5‐ALA/SFC works on the mitochondria of T cells and promotes the production of ATP by mitochondria, and restores the function of exhausted T cells, and then supports T cell antitumor activities in PD‐1/PD‐L1 checkpoint blockade cancer immunotherapy. This synergistic effect is energy efficient and enables tumor‐targeting CD8+ T cells to make maximal use of their available bioenergy supply and carry out antitumor actions under metabolically stressful conditions. Notably, the oral administration of 5‐ALA/SFC has already been tested in a phase III clinical trial for mitochondrial disease therapy 42 after completion of the preclinical and phase I and II studies. It will be applicable to clinical studies by convenient oral administration in the combination with PD‐1/PD‐L1 blockade therapy. Our study not only opens a new aspect of immune metabolism but also paves the way for the development of a treatment strategy using the combination of PD‐1/PD‐L1 cancer immunotherapy and 5‐ALA/SFC.
FIGURE 7.
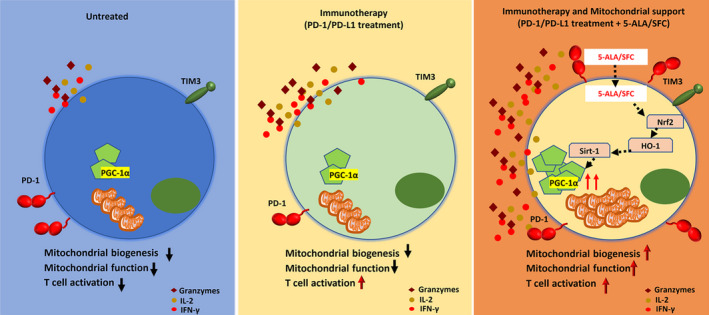
Proposed mechanisms as to how 5‐aminolevulinic acid (5‐ALA)/sodium ferrous citrate (SFC) could synergize with anti‐programmed cell death‐1 (PD‐1)/PD‐ligand 1 (PD‐L1) therapy to boost intratumoral efficacy of tumor‐specific T cells. Left panel, In the untreated tumor microenvironment, the T cell function and mitochondrial function are very weak, and it is difficult to exert antitumor effects. Middle panel, After applying PD‐1/PD‐L1 blockade therapy to tumor‐bearing mice, the T cell functions were enhanced but the mitochondrial function was not fully restored. T cells were prone to exhaustion and lost their function. Right panel, 5‐ALA/SFC combined with PD‐1/PD‐L1 blockade therapy could enhance the T cell function and increase their mitochondrial function to reverse T cell exhaustion. HO‐1, heme oxygenase‐1; IFN‐γ, γ‐interferon; IL‐2, interleukin‐2; Nrf‐2, nuclear factor‐erythroid factor 2‐related factor‐2; PGC‐1α, peroxisome proliferator‐activated receptor‐gamma coactivator‐1α; Sirt‐1, Sirtuin 1; Tim‐3, T‐cell immunoglobulin and mucin domain‐3
CONFLICT OF INTEREST
NN, TI, MI, HI, KT, and MN are employees of SBI Pharmaceuticals Co., Ltd. TT is employee Neopharma Japan Co., Ltd.
ETHICAL APPROVAL
Mice were cared for in accordance with the NCCHD guidelines on laboratory animal welfare.
Supporting information
Fig S1‐S4
Supplementary Material
Hu X, Que W, Hirano H, et al. 5‐Aminolevulinic acid/sodium ferrous citrate enhanced the antitumor effects of programmed cell death‐ligand 1 blockade by regulation of exhausted T cell metabolism in a melanoma model. Cancer Sci. 2021;112:2652–2663. 10.1111/cas.14930
Hu, Que, and Hirano contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Chen G, Huang AC, Zhang W, et al. Exosomal PD‐L1 contributes to immunosuppression and is associated with anti‐PD‐1 response. Nature. 2018;560(7718):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD‐1/PD‐L1 blockade. Pharmacol Res. 2019;145:104258. [DOI] [PubMed] [Google Scholar]
- 3. Chamoto K, Chowdhury PS, Kumar A, et al. Mitochondrial activation chemicals synergize with surface receptor PD‐1 blockade for T cell‐dependent antitumor activity. Proc Natl Acad Sci U S A. 2017;114(5):E761‐E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desdin‐Mico G, Soto‐Heredero G, Mittelbrunn M. Mitochondrial activity in T cells. Mitochondrion. 2018;41:51‐57. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Lee LF, Fisher TS, et al. Combination of 4–1BB agonist and PD‐1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3(2):149‐160. [DOI] [PubMed] [Google Scholar]
- 6. Molon B, Cali B, Viola A. T Cells and cancer: How metabolism shapes immunity. Front Immunol. 2016;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scharping NE, Menk AV, Moreci RS, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Omori C, Motodate R, Shiraki Y, et al. Facilitation of brain mitochondrial activity by 5‐aminolevulinic acid in a mouse model of Alzheimer's disease. Nutr Neurosci. 2017;20(9):538‐546. [DOI] [PubMed] [Google Scholar]
- 9. Fujii C, Miyashita K, Mitsuishi M, et al. Treatment of sarcopenia and glucose intolerance through mitochondrial activation by 5‐aminolevulinic acid. Sci Rep. 2017;7(1):4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hara T, Koda A, Nozawa N, et al. Combination of 5‐aminolevulinic acid and ferrous ion reduces plasma glucose and hemoglobin A1c levels in Zucker diabetic fatty rats. FEBS Open Bio. 2016;6(6):515‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ota U, Hara T, Nakagawa H, et al. 5‐aminolevulinic acid combined with ferrous ion reduces adiposity and improves glucose tolerance in diet‐induced obese mice via enhancing mitochondrial function. BMC Pharmacol Toxicol. 2017;18(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki H, Masuki S, Morikawa A, et al. Effects of 5‐aminolevulinic acid supplementation on home‐based walking training achievement in middle‐aged depressive women: randomized, double‐blind, crossover pilot study. Sci Rep. 2018;8(1):7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim‐3 and PD‐1 pathways to reverse T cell exhaustion and restore anti‐tumor immunity. J Exp Med. 2010;207(10):2187‐2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boussiotis VA. Molecular and Biochemical Aspects of the PD‐1 Checkpoint Pathway. N Engl J Med. 2016;375(18):1767‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canale FP, Ramello MC, Nunez N, et al. CD39 expression defines cell exhaustion in tumor‐infiltrating CD8(+) T Cells. Cancer Res. 2018;78(1):115‐128. [DOI] [PubMed] [Google Scholar]
- 16. Bengsch B, Johnson AL, Kurachi M, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD‐1 are an early driver of CD8(+) T Cell exhaustion. Immunity. 2016;45(2):358‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saeidi A, Zandi K, Cheok YY, et al. T‐Cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. 2018;9:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He QF, Xu Y, Li J, Huang ZM, Li XH, Wang X. CD8+ T‐cell exhaustion in cancer: mechanisms and new area for cancer immunotherapy. Brief Funct Genomics. 2019;18(2):99‐106. [DOI] [PubMed] [Google Scholar]
- 19. Jing W, Gershan JA, Weber J, et al. Combined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myeloma. J Immunother Cancer. 2015;3(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD‐1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juneja VR, McGuire KA, Manguso RT, et al. PD‐L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Menk AV, Scharping NE, Rivadeneira DB, et al. 4–1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215(4):1091‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8(+) T Cells. Cancer Immunol Res. 2019;7(3):476‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siska PJ, Beckermann KE, Mason FM, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI. Insight. 2017;2(12):e93411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mansilla N, Racca S, Gras DE, Gonzalez DH, Welchen E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int J Mol Sci. 2018;19(3):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li S, Takahara T, Li XK, et al. 5‐Aminolevulinic acid combined with ferrous iron ameliorate ischemia‐reperfusion injury in the mouse fatty liver model. Biochem Biophys Res Commun. 2016;470(4):900‐906. [DOI] [PubMed] [Google Scholar]
- 27. Yamashita K, Hagiya Y, Nakajima M, Ishizuka M, Tanaka T, Ogura S. The effects of the heme precursor 5‐aminolevulinic acid (ALA) on REV‐ERBalpha activation. FEBS Open Bio. 2014;4:347‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu H, Zhang M, Ma Y, et al. 5‐ALA ameliorates hepatic steatosis through AMPK signaling pathway. J Mol Endocrinol. 2017;59(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 29. Hou X, Xu S, Maitland‐Toolan KA, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP‐activated protein kinase. J Biol Chem. 2008;283(29):20015‐20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baird JR, Byrne KT, Lizotte PH, et al. Immune‐mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190(1):469‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mansour M, Pohajdak B, Kast WM, et al. Therapy of established B16–F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax. J Transl Med. 2007;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gettinger SN, Choi J, Mani N, et al. A dormant TIL phenotype defines non‐small cell lung carcinomas sensitive to immune checkpoint blockers. Nat Commun. 2018;9(1):3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotredes KP, Gamero AM. Interferons as inducers of apoptosis in malignant cells. J Interferon Cytokine Res. 2013;33(4):162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsushita H, Hosoi A, Ueha S, et al. Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNgamma‐dependent cell‐cycle arrest. Cancer Immunol Res. 2015;3(1):26‐36. [DOI] [PubMed] [Google Scholar]
- 35. Yin H, Jiang Z, Wang S, Zhang P. IFN‐gamma restores the impaired function of RNase L and induces mitochondria‐mediated apoptosis in lung cancer. Cell Death Dis. 2019;10(9):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feld M, Shpacovitch VM, Ehrhardt C, et al. Agonists of proteinase‐activated receptor‐2 enhance IFN‐gamma‐inducible effects on human monocytes: role in influenza A infection. J Immunol. 2008;180(10):6903‐6910. [DOI] [PubMed] [Google Scholar]
- 37. Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7(9):4509‐4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liao W, Lin JX, Leonard WJ. IL‐2 family cytokines: new insights into the complex roles of IL‐2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23(5):598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mouchacca P, Schmitt‐Verhulst AM, Boyer C. Visualization of cytolytic T cell differentiation and granule exocytosis with T cells from mice expressing active fluorescent granzyme B. PLoS One. 2013;8(6):e67239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tian H, Shi G, Wang Q, et al. A novel cancer vaccine with the ability to simultaneously produce anti‐PD‐1 antibody and GM‐CSF in cancer cells and enhance Th1‐biased antitumor immunity. Signal Transduct Target Ther. 2016;1:16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR‐Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor‐Reactive CD8(+) T Cells and Facilitates Anti‐PD‐1 Therapy. Cancer Immunol Res. 2018;6(11):1375‐1387. [DOI] [PubMed] [Google Scholar]
- 42. Shimura M, Nozawa N, Ogawa‐Tominaga M, et al. Effects of 5‐aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci Rep. 2019;9(1):10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Supplementary Material
Data Availability Statement
Data are available upon reasonable request.


