Abstract
Several therapeutic regimens, including neoadjuvant chemoradiation therapy (NACRT), have been reported to serve as anticancer immune effectors. However, there remain insufficient data regarding the immune response after NACRT in pancreatic ductal adenocarcinoma (PDAC) patients. Data from 40 PDAC patients that underwent surgical resection after NACRT (NACRT group) and 30 PDAC patients that underwent upfront surgery (US group) were analyzed to examine alterations in immune cell counts/distribution using a multiplexed fluorescent immunohistochemistry system. All immune cells were more abundant in the cancer stroma than in the cancer cell nest regardless of preoperative therapy. Although the stromal counts of CD4+ T cells, CD20+ B cells, and Foxp3+ T cells in the NACRT group were drastically decreased in comparison with those of the US group, counts of these cell types in the cancer cell nest were not significantly different between the two groups. In contrast, CD204+ macrophage counts in the cancer stroma were similar between the NACRT and US groups, while those in the cancer cell nests were significantly reduced in the NACRT group. Following multivariate analysis, only a high CD204+ macrophage count in the cancer cell nest remained an independent predictor of shorter relapse‐free survival (odds ratio = 2.37; P = .033). NACRT for PDAC decreased overall immune cell counts, but these changes were heterogeneous within the cancer cell nests and cancer stroma. The CD204+ macrophage count in the cancer cell nest is an independent predictor of early disease recurrence in PDAC patients after NACRT.
Keywords: fluorescent immunohistochemistry, immune response, macrophage, pancreatic ductal adenocarcinoma, preoperative chemoradiation
This study sought to investigate any potential alterations in the distribution and clinical impact of immune cells in patients with pancreatic ductal adenocarcinoma (PDAC) treated with neoadjuvant chemoradiation therapy (NACRT). The present analysis revealed that NACRT for PDAC decreased overall immune cell counts, but these changes were heterogeneous within the cancer cell nests and cancer stroma. The CD204+ macrophage count in the cancer cell nest is an independent predictor of early disease recurrence in PDAC patients after NACRT.
Abbreviations
- BR
borderline resectable
- LA
locally advanced
- NACRT
neoadjuvant chemoradiation therapy
- NCCHE
National Cancer Center Hospital East
- PDAC
pancreatic ductal adenocarcinoma
- R
resectable
- RFS
relapse‐free survival
- RT
radiation therapy
- S‐1
tegafur, gimeracil, and oteracil potassium
- TAM
tumor‐associated macrophage
- TIICs
tumor‐infiltrating immune cells
- US
upfront surgery
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal disease, highlighted by the close parallel between disease incidence and mortality rates. 1 Surgical resection is the only potentially curative therapy, but only 15% to 20% of PDAC patients are considered to be candidates for this treatment. 2 Furthermore, several studies have reported that the rate of microscopic or macroscopic margin involvement (R1 or R2) was approximately 19% to 50%, and the resection margin status was an essential prognostic factor of patients who underwent curative surgery for PDAC. 3 , 4 , 5 , 6 Given the significance of the resection margin status, PDAC is classified as resectable (R), borderline resectable (BR), locally advanced (LA), and metastatic (M). BR‐PDAC was described as PDAC involving the mesentericoportal or arterial axis, 7 indicating that it was too close to achieve a constant negative margin with immediate surgery but had the potential to improve to margin‐negative (R0) after neoadjuvant therapy. 8 Theoretically, neoadjuvant therapy has potential advantages in BR‐ or LA‐PDAC: (a) it can result in tumor regression that maximizes the potential of R0 resection, (b) it can provide early treatment for micrometastatic disease, (c) it can offer an interval of time to estimate the aggressiveness of the tumor, and (d) it can be better tolerated by patients than adjuvant therapy because poor recovery from surgical stress often disrupts adjuvant therapy. 9 Although a standardized therapy for patients with BR‐ or LA‐PDAC has not yet been established, National Comprehensive Cancer Network (NCCN) guidelines also recommend neoadjuvant therapy for patients with BR‐PDAC. 10 Therefore, our study group (Japan Adjuvant Study Group of Pancreatic Cancer [JASPAC]) also conducted a phase II trial to evaluate the efficacy of neoadjuvant chemoradiation therapy (NACRT) for BR‐PDAC patients (JASPAC05 study). 11 Additionally, accompanying research using the samples in this study was planned.
Recently, it has been reported that several therapeutic regimens have anticancer immune effects in addition to their cytotoxic effects. These can be achieved directly or indirectly by targeting immunosuppressive mechanisms. 12 For example, cyclophosphamide selectively depletes and/or inhibits regulatory T cells, which have immunosuppressive functions. 13 Taxane can stimulate T cell blastogenesis and natural killer cell cytotoxicity in breast cancer patients. 14 Similarly, a 5‐fluorouracil and cisplatin regimen is useful to induce CD4+ and CD8+ T cells in esophageal squamous cell carcinoma. 15 Radiation was also shown to be an immune adjuvant therapy through the enhancement of both innate and adaptive immunity in a melanoma cell line. 16 Immune cells infiltrating into cancer cell nests are referred to as tumor‐infiltrating immune cells (TIICs) and act as major determinants of the host immune response to tumor cells. Accordingly, TIICs have previously been found to influence patient prognosis in a number of solid cancers. 17 A meta‐analysis in gastric cancer suggested CD8+ TIICs as a potential prognostic biomarker. 18 In ovarian cancer, CD8+ TIICs can also be used as a prognostic factor for increased survival, whereas regulatory T cells of TIICs are associated with poor outcomes. 19 However, there are insufficient data regarding the immune response following NACRT in PDAC patients.
The objectives of this study were to investigate any potential alterations in the counts and distribution of immune cells following NACRT and clarify the clinical impact of TIICs in PDAC patients treated with NACRT. This work may provide clues to better comprehend biological alterations in the tumor immune microenvironment and help identify robust prognostic factors in PDAC patients treated with NACRT.
2. MATERIALS AND METHODS
2.1. Patients
We originally included 20 PDAC patients who underwent surgical resection after NACRT from 2006 to 2016 at National Cancer Center Hospital East (NCCHE). These cases consisted of two LA‐, three R‐, and 15 BR‐PDAC patients. Next, 23 BR‐PDAC patients who had taken part in the JASPAC05 trial (curative resection after CRT) at six institutions were also included. After excluding three patients for whom clinical data were not available, we analyzed the clinicopathological and immunohistochemical data in the remaining 40 patients (NACRT group). To estimate the therapeutic effect on immune cell distribution, we retrospectively reviewed medical records and included 16 BR‐PDAC and 14 randomly selected R‐PDAC patients who underwent surgical resection without preoperative therapy as the upfront surgery (US) group. The treatment effect was assessed according to the Evans grading system. 20 Tumor‐node‐metastasis staging classification was assessed according to the criteria outlined in the 8th edition of the Union for International Cancer Control. 21
2.2. Definition of BR‐PDAC and the NACRT regimen
The definitions of BR‐PDAC and the NACRT regimen were the same as in the JASPAC 05 trial, as described previously. 11 According to the 2009 NCCN guidelines, BR‐PDAC was defined with some modifications 22 as follows: (a) reconstructible bilateral impingement of the superior mesenteric vein or portal vein, except occlusion by tumor thrombus; (b) tumor contact with the superior mesenteric artery of ≤180°; (c) tumor contact with the common hepatic artery of ≤180° without extension to the coeliac axis or bifurcation of the hepatic artery; or (d) tumor contact with the coeliac axis of ≤180°. The NACRT regimen was S‐1 (tegafur, gimeracil, and oteracil potassium) plus radiation. S‐1 was administered orally at 40 mg, twice daily on the day of irradiation during radiation. For RT, 50.4 Gy was delivered in 28 fractions over 5.5 weeks. Some patients in the NCCHE cohort underwent additional treatment, such as the administration of gemcitabine, before or after CRT based on the discretion of the attending doctor.
2.3. Multiplexed fluorescent immunohistochemistry
Multiplexed fluorescent immunohistochemistry (IHC) was performed manually using the tyramide signal amplification (TSA) method with an Opal IHC kit (Perkin Elmer) in accordance with the manufacturer's instructions. This analysis uses microwave treatment to remove primary and secondary antibodies while retaining the fluorescent signal. This process is repeated until all antigens have been stained with their respective fluorophores. Formalin‐fixed, paraffin‐embedded (FFPE) tissue blocks were generated from the most representative tumor areas for all samples in the NACRT and US groups. Tissue sections were cut at 4 µm thickness from each FFPE tumor specimen and then baked at 60°C onto adhesive glass slides for 30 minutes before deparaffinization using xylene. All information regarding the primary antibodies, activation conditions, and dilutions are listed in Table S1. We performed conventional IHC with single antibodies to confirm that the multiplexed fluorescent IHC procedure was adequately performed (Figure S2). For staining nuclei, spectra DAPI (Perkin Elmer) was used. The antibodies were grouped into two sets for staining: set 1 (cytokeratin, CD3, CD4, CD8, Foxp3, and CD20) and set 2 (cytokeratin, CD3, CD204, and PD‐L1) (Figure 1). Fluorophore‐conjugated tyramide (Opal fluorophores, Perkin Elmer) was used for the detection of each primary antibody. A horseradish peroxidase–labeled secondary detection system (EnVision Plus, DAKO) was used as a catalyst for Opal fluorophores. Microwave heating (95°C for 15 minutes) was performed to unmask the primary antigens and remove antibodies after each fluorescent labeling step.
FIGURE 1.
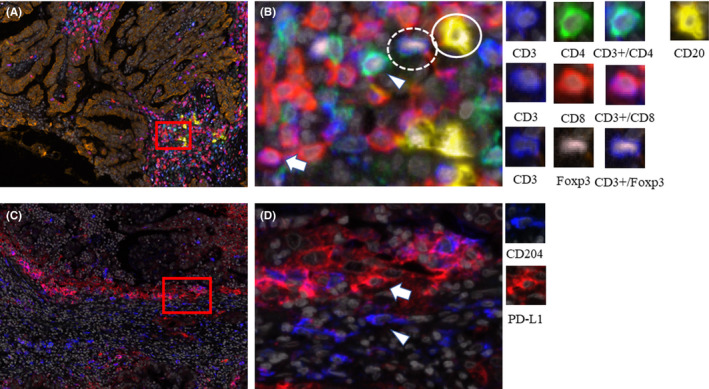
Representative images of multiplexed fluorescently labeled pancreatic ductal adenocarcinoma (PDAC) sections. The same tumor specimen was stained for cytokeratin, CD3, CD4, CD8, Foxp3, and CD20 (A, low magnification, B, high magnification), and CD204 and PD‐L1 staining were also performed using the same specimen (C, low magnification, D, high magnification). CD3+/CD4+ lymphocytes were stained cyan (arrowhead), CD3+/CD8+ lymphocytes were purple (arrow), CD20+ B cells were yellow (lined circle), Foxp3+ cells were peach (dashed circle), cytokeratin (CK) was brown, and DAPI (nuclei) was gray (A and B). CD204+ cells were blue (arrowhead), and PD‐L1+ cells were red (arrow) (C and D)
2.4. Multispectral imaging analysis and quantification
Multiplexed fluorescently labeled images (669 × 500 µm each) of the tumor margin (20 fields) and center (20 fields) were captured with an automated imaging system (Vectra ver. 3.0, Perkin Elmer). Image analysis software (InForm, Perkin Elmer) was used to automatically segment each image into the cancer cell nest (intraepithelial region) and cancer stroma (stromal region) and to detect immune cells with specific phenotypes. Tissue segmentation and phenotype recognition were repeated until the algorithm reached at least 90% accuracy of confidence before assessment, as recommended by the program supplier. The number and density (cells/mm2) of infiltrating immune cells were quantified automatically using an analytic software program (Spotfire, TIBCO). Using Spotfire software, the CD3 positive population in CD4, CD8, and Foxp3 T cell subsets was divided according to the fluorescence signal intensity. Immune cells in cancer cell nests were defined as TIICs in this study (including macrophages). Clinicopathological associations of TIICs, as well as any observed alterations to these following NACRT, were estimated.
2.5. Statistical analysis
Statistical analysis was performed using the IBM SPSS software (ver. 23.0 SPSS Inc) for Windows. The medians and ranges of continuous data were compared using the Mann‐Whitney U test. Categorical data were compared using the Pearson chi‐squared test or the Fisher exact test, as appropriate. Relapse‐free survival (RFS) was defined as the period from the date of surgery to that of tumor recurrence or death of any cause, whichever came first. The date of tumor recurrence was determined as the day when the diagnostic examination or procedure for recurrence was performed. Survival curves were generated using the Kaplan‐Meier method and compared using a log‐rank test. To identify risk factors for early recurrence, recurrence‐related factors from univariate analyses (P ≤ .1) were entered in the multivariate Cox proportional hazards model. The level of significance was set at P < .05.
3. RESULTS
3.1. Patient demographics
Table 1 summarizes the basic characteristics of the 70 patients (NACRT and US groups). In the NACRT group, the median age of the patients was 66 years, and 24 of them (60%) were male. NACRT was performed in three patients with R‐PDAC, 35 patients with BR‐PDAC, and two patients with LA‐PDAC. A pancreatoduodenectomy was performed in 31 patients (79%). The NACRT group had a higher proportion of pretreatment diagnosis for BR‐ or LA‐PDAC (93% vs 53%, P < .01) and a lower percentage of lymph node metastasis (40% vs 80%, P < .01) compared with the US group. However, the differentiation status of tumors, tumor size, and proportion of resection margin–negative were similar between the two groups.
TABLE 1.
Basic characteristics of analyzed patients with pancreatic cancer
| Characteristics | NACRT | US | NACRT vs US |
|---|---|---|---|
| N = 40 | N = 30 | P value | |
| Age (y) | 66 (51‐78) | 68 (52‐84) | .44 |
| Male gender | 24 (65%) | 16 (53%) | .28 |
| Pretreatment diagnosis: R/BR/LA | 3 (8%)/35 (88%)/2 (5%) | 14 (47%)/16 (53%)/0 | <.01 |
| Procedure: SSPPD(TP)/DP | 31 (79%)/9 (22%) | 19 (63%)/11 (37%) | .15 |
| Poor differentiation | 4 (10%) | 0 | .07 |
| ypTS | 2.7 (0.9‐5.5) | 2.9 (1.0‐4.3) | .14 |
| Nodal metastasis | 12 (40%) | 24 (80%) | <.01 |
| Stage IA/IB/IIA/IIB/III | 10 (25%)/17 (43%)/1 (3%)/9 (23%)/3 (8%) | 3 (10%)/5 (17%)/0/16 (53%)/6 (20%) | .01 |
| Treatment effect, Evans grade I/IIA/IIB/III | 9 (19%)/20 (50%)/8 (20%)/3 (8%) | ‐ | ‐ |
| Resection margin–negative | 34 (85%) | 23 (77%) | .28 |
| Recurrence | 28 (70%) | 20 (67%) | .77 |
Abbreviations: BR, borderline resectable; DP, distal pancreatectomy; LA, locally advanced; NACRT, neoadjuvant chemoradiotherapy; R, resectable; SSPPD, subtotal stomach‐preserving pancreatoduodenectomy; TP, total pancreatectomy; US, upfront surgery.
3.2. Immune cell distribution according to preoperative treatment
As shown in Figure 2, all immune cells were present in both the cancer stroma and the cancer cell nests of PDAC samples. These cells were found to be more abundant in the cancer stroma than in the cancer cell nest regardless of preoperative therapy. Figure 2 shows a comparison of immune cell distributions between the NACRT and US groups. Although the cancer stromal counts of CD4+ T cells, CD20+ B cells, and Foxp3+ T cells in the NACRT group were drastically decreased compared with those in the US group, these counts in the cancer cell nests were not different between the two groups. In contrast, CD204+ macrophage counts in the cancer stroma were similar between the NACRT and US groups, whereas those in the cancer cell nests were significantly reduced in the NACRT group. PD‐L1+ carcinoma cell counts in the NACRT group were substantially lower in comparison with the US group (Table 2). These results suggest that alterations in TIICs following NACRT appear to be very different from those in cancer stromal immune cells.
FIGURE 2.
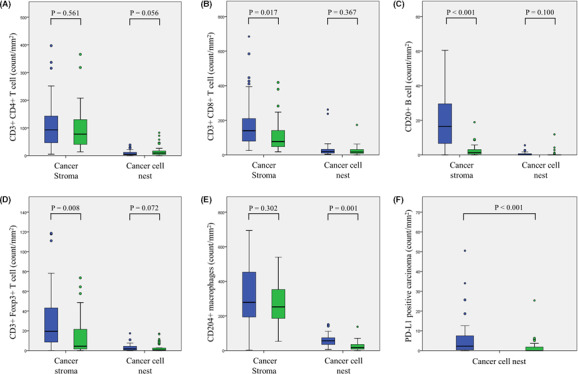
Therapeutic changes in each immune cell count (blue color, control group; green color, chemoradiation group). A, CD3+/CD4+ T cells; B, CD3+/CD8+ T cells; C, CD20+ B cells; D, CD3+/Foxp3+ T cells; E, CD204+ cells; and F, PD‐L1+ carcinoma cells
TABLE 2.
Pathological characteristics of patients with pancreatic cancer classified by preoperative treatment
| Characteristics (count/mm2) | NACRT | US | NACRT vs US |
|---|---|---|---|
| N = 40 | N = 30 | P value | |
| CD3+ CD4+ T cell (stroma) | 77.5 (13.5‐365.7) | 92.9 (4.9‐397.0) | .561 |
| CD3+ CD4+ T cell (cancer cell nest) | 9.0 (0.0‐82.6) | 4.4 (0.0‐38.2) | .056 |
| CD3+ CD8+ T cell (stroma) | 77.3 (17.9‐418.8) | 139.6 (25.2‐684.3) | .017 |
| CD3+ CD8+ T cell (cancer cell nest) | 16.4 (0.0‐173.3) | 19.7 (3.5‐262.1) | .367 |
| CD20+ B cell (stroma) | 1.3 (0.0‐18.9) | 16.5 (0.0‐253.4) | < .001 |
| CD20+ B cell (cancer cell nest) | 0.0 (0.0‐11.9) | 0.0 (0.0‐5.6) | .100 |
| CD3+ Foxp3+ T cell (stroma) | 4.4 (0.0‐73.5) | 19.4 (0.1‐118.6) | .005 |
| CD3+ Foxp3 + T cell (cancer cell nest) | 0.5 (0.0‐17.0) | 1.8 (0.0‐17.4) | .072 |
| CD204+ cell (stroma) | 252.4 (53.0‐959.6) | 278.6 (2.7‐693.8) | .302 |
| CD204+ cell (cancer cell nest) | 16.7 (0.0‐137.6) | 56.3 (6.1‐150.0) | .001 |
| PD‐L1 high carcinoma | 0.0 (0.0‐25.4) | 2.2 (0.0‐521.3) | < .001 |
Abbreviations: NACRT, neoadjuvant chemoradiation therapy; US, upfront surgery.
3.3. Association between TIICs and early recurrence of disease in the NACRT group
The count of each immune cell found in carcinomas was divided into low and high groups according to the cutoff value (set as the median amount). Kaplan‐Meier curve analysis demonstrated that only patients with high CD204+ macrophage counts in the cancer cell nest (>16.7 counts/mm2) had significantly shorter RFS times compared with patients with low CD204+ macrophage counts in the cancer cell nest (Figure 3). Univariate and forest plot analyses suggested that high PD‐L1 expression and the presence of CD204+ macrophages in the cancer cell nest were associated with shorter RFS (Table 3 and Figure S2). Following multivariate analysis, only high CD204+ macrophage counts in the cancer cell nest remained an independent predictor of shorter RFS (Table 3). There were no significant differences in the basic characteristics between the groups with high and low CD204+ macrophage counts in the cancer cell nest (Table 4).
FIGURE 3.
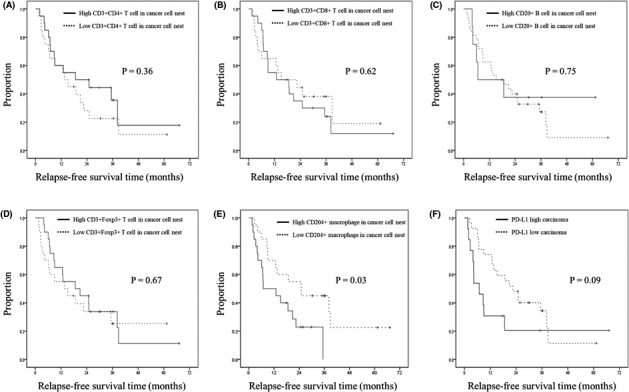
Kaplan‐Meier curve of post–neoadjuvant chemoradiation resections for pancreatic ductal adenocarcinoma (PDAC) patients. Relapse‐free survival time classified by the counts of CD3+/CD4+ T cells (A), CD3+/CD8+ T cells (B), CD20+ B cells (C), CD3+/Foxp3+ T cells (D), CD204+ macrophages (E), and PD‐L1+ carcinoma cells (F)
TABLE 3.
Risk factors for early relapse in pancreatic ductal adenocarcinoma (PDAC) patients who underwent neoadjuvant chemoradiotherapy
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| MRFS (months) | P value | OR (95% CI) | P‐value | ||
| CD3+ CD4+ T cell (cancer cell nest) | <9.0 | 13.5 | .360 | ||
| >9.0 | 18.7 | ||||
| CD3+ CD8+ T cell (cancer cell nest) | <16.4 | 15.2 | .622 | ||
| >16.4 | 12.9 | ||||
| CD20+ B cell (cancer cell nest) | <0.0 | 15.2 | .747 | ||
| >0.0 | 6.6 | ||||
| CD3+ Foxp3+ T cell (cancer cell nest) | <0.5 | 13.5 | .667 | ||
| >0.5 | 18.7 | ||||
| CD204+ cell (cancer cell nest) | <16.7 | 25.0 | .032 | 2.366 (1.074‐5.215) | .033 |
| >16.7 | 6.9 | ||||
| PD‐L1 high carcinoma (cancer cell nest) | <0.0 | 22.5 | .091 | 2.001 (0.912‐4.390) | .084 |
| >0.0 | 6.9 | ||||
Abbreviations: CI, confidence interval; MRFS, median relapse free survival time; OR, Odds ratio.
TABLE 4.
Comparison of basic characteristics between pancreatic ductal adenocarcinoma (PDAC) patients with high or low macrophage infiltration in cancer cell nests
| Characteristics | High CD204+ (cancer cell nest) | Low CD204+(cancer cell nest) | High vs low |
|---|---|---|---|
| N = 20 | N = 20 | P‐value | |
| Age (y) | 66 (51‐78) | 66 (54‐78) | .34 |
| Male gender | 14 (70%) | 10 (50%) | .17 |
| Pretreatment diagnosis: R/BR/LA | 2 (10%)/16 (80%)/2 (10%) | 1 (5%)/19 (95%)/0 | .27 |
| Procedure: SSPPD(TP)/DP | 31 (79%)/9 (22%) | 19 (63%)/11 (37%) | .15 |
| Poor differentiation | 2 (10%) | 2 (10%) | 1.00 |
| ypTS | 2.5 (0.9‐5.5) | 3.0 (1.0‐4.0) | .51 |
| Nodal metastasis | 4 (20%) | 8 (40%) | .17 |
| Resection margin–negative | 3 (15%) | 3 (15%) | 1.00 |
| Recurrence | 16 (80%) | 12 (60%) | .15 |
Abbreviations: BR, borderline resectable; DP, distal pancreatectomy; LA, locally advanced; R, resectable; SSPPD, subtotal stomach‐preserving pancreatoduodenectomy; TP, total pancreatectomy; ypTS, pathological tumor size.
4. DISCUSSION
In this study, we investigated potential alterations in the counts and distribution of immune cells in PDAC cases following NACRT and any clinical impacts of these alterations. Together, our results show an immunosuppressive effect in the cancer stroma after NACRT. However, our results revealed heterogeneous alterations in immune cell distributions whereby NACRT reduced immune cell counts in the cancer stroma but not in the cancer cell nests, with the exception of CD204+ macrophages. In addition, although CD204+ macrophage counts in the cancer cell nests decreased following NACRT, they were an independent predictive factor for early disease recurrence (Figure 2 and Table 3).
Several studies have evaluated the impact of immune cell numbers in PDAC tumors after NACRT. Tsuchikawa et al reported no significant differences in the overall CD4+ and CD8+ T cell counts, but the number of Foxp3+ T cells was lower in the NACRT group compared with that in the US group. 23 However, Murakami et al and Homma et al reported that the counts of CD4+ and CD8+ TIICs in the NACRT group were significantly higher than those in the non‐NACRT group. 24 , 25 Based on our intensive measurements from a total of 40 fields from tumor margins and centers, as well as detailed counts of immune cells from the cancer cell nests and stroma, we first showed that immune cell alterations following NACRT were strongly dependent on their position in the tumor microenvironment. The discrepancies between previously published results may be attributed to this variability in immune cell alterations after NACRT. The different assessment methods may have also contributed to the variability between these studies. We represented the average number of immune cells in the whole tumor as a variable, whereas others measured some areas with the most abundant distribution of immune cells in tumors, known as hot spots. Radiation can also reportedly attenuate tumor blood vessel function, which decreases the infiltration of CD8+ T cells into tumors. 26 Our results were compatible with this previous report. Functional differences in immune cells in the cancer stroma and cancer cell nests pre‐ and post‐NACRT should be biologically investigated and compared in the future.
Tumor‐associated macrophages (TAMs) play an important role in tumor‐related inflammation. TAMs are classified into two major phenotypes: M1 (antitumor immunity) and M2 (protumoral). 27 CD204+ macrophages are known as M2‐type TAMs. A previous report suggested that CD204 expressed on TAMs played an important role in suppressing the production of nitric oxide (NO) and interferon (IFN)‐γ. 28 NO induces apoptosis, and IFN‐γ has antitumor effects via the inhibition of cancer cell progression and metastasis. Furthermore, M2‐type TAMs preferentially produce immune inhibitory cytokines, such as IL‐10. Thus, M2‐type TAMs are associated with enhanced tumorigenesis and metastasis. 28 , 29 The predominant distribution of M2‐type TAMs in cancer patients with a poor prognosis has been previously reported in breast cancer. 30 , 31 M2‐type TAMs were also reportedly associated with overall survival in untreated PDAC patients. 32 However, the distribution and clinical impact of M2‐type TAMs during NACRT have not been well established in solid tumors.
In this study, similar to previous reports, M2‐type TAMs were distributed more predominantly in the stroma compared with the cancer cell nest with or without NACRT. 30 , 31 Nonetheless, we found that NACRT leads to a significant decrease only in the density of M2‐type TAMs in cancer cell nests, resulting in a favorable prognosis. We classified patients in the NACRT group by the median value of immune cell counts, and the results revealed that patients with a low density of M2‐type TAMs showed significantly longer RFS. In the US group, almost all patients (28 of 30 patients) were classified as M2‐type TAM high and showed rapid recurrence after resection; however, we could not perform the statistical analysis because only two patients remained as M2‐type TAM low. These were important observations in PDAC and suggested a strong association of M2‐type TAMs located in cancer cell nests with tumor progression followed by a poor prognosis. Our findings also demonstrated the effect of NACRT on tumor microenvironments. Unlike the density of other immune cells and M2‐type TAMs in cancer cell nests, there was no decrease in M2‐type TAMs in the stroma after NACRT. This finding may be associated with the resistance of M2‐type TAMs to radiation therapy. A previous study reported that M2‐type TAMs were more radioresistant than other immune cells, B cells, T cells, regulatory T cells, and M1‐type TAMs. 33 , 34 In a previous report, irradiation reprogrammed TAMs towards an M1 (proinflammatory) phenotype. 35 In contrast, other reports showed that radiation therapy induced M2 polarization (suppressed immune responses) in mice with pancreatic cancer. 36 Wu et al and others described that during the early phases of irradiation, macrophages differentiate into the M1 type. However, during later phases following vascular disruption and tumor hypoxia, macrophages from the bone marrow accumulate in the irradiated tumor and differentiate into M2‐type TAMs, resulting in tumor recurrence. 37 , 38 During later phases after NACRT, stromal M2‐type TAMs may rapidly recover due to de novo differentiation of M2‐type TAMs followed by their accumulation via blood vessels, whereas irradiation depletes M2‐type TAMs during the early phases. In cancer cell nests, the de novo accumulation may be disturbed because of the vascular disruption induced by NACRT, resulting in a decrease in M2‐type TAMs. However, as described above, the effect of radiation on TAMs remains controversial. Therefore, further study is needed to investigate the effect of radiation on TAMs.
Our results and previous reports suggest that M2‐type TAMs can be a novel therapeutic target in the future. 39 In pancreatic cancer, several antitumor strategies target M2‐type TAMs by blocking monocyte recruitment into tumor tissues, 40 decreasing the population of M2‐type TAMs, 41 and inducing the transformation of M2‐type TAMs into M1‐type cells. 42 These reports suggested that normalization of the tumor microenvironment enhanced proinflammatory tumor immunity, which suppressed tumor growth and metastasis. Our results demonstrate that NACRT reduces immunosuppressive cells in cancer cell nests, including regulatory T cells, PD‐L1+ carcinoma cells, and M2‐type TAMs. Moreover, in addition to M2‐type TAM depletion in cancer cell nests, the combination of approaches to preferentially deplete or prevent stromal M2‐type TAM recovery may improve the tumor microenvironments, resulting in a good prognosis after NACRT.
The limitations of the present study include its retrospective nature and selected patient population. As NCCN guidelines recommend preoperative treatment for BR‐PDAC cases, few participants underwent US for BR‐PDAC. Therefore, it was quite difficult to adjust the basic characteristics, including pretreatment PDAC classification, between the NACRT and control groups. However, local tumor characteristics, including tumor size and tumor differentiation, showed no remarkable changes between the NACRT and control groups. Therefore, the results of this study were considered meaningful and significant.
In conclusion, we first revealed that immune cell alterations following NACRT were heterogeneous and strongly dependent on their position in the PDAC tumor microenvironment. In addition, the CD204+ macrophage count in the cancer cell nest was identified as an independent predictor of early disease recurrence in PDAC patients after NACRT. Our results may warrant future studies investigating the efficacy of NACRT combined with anti–M2‐type TAM therapies for PDAC. An alternative therapeutic strategy that enhances tumor immunity may provide a more effective neoadjuvant therapy in PDAC patients.
5. ETHICS APPROVAL
This study was conducted with the approval of the National Cancer Ethical Review Board (No. 2017‐358), and the analyses and data management were performed in accordance with the ethical guidelines for clinical studies in Japan.
DISCLOSURE
All authors declare that they have no conflict of interest.
Supporting information
Figure S1
Figure S2
Table S1
ACKNOWLEDGMENTS
This work is supported by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) and the National Cancer Center Research and Development Fund (25‐B‐8). We thank the timely help given by Kazumasa Takenouchi for performing fluorescent immunohistochemistry. We also thank J. Iacona, PhD, and Melissa Crawford, PhD, from Edanz Group (https://en‐author‐services.edanz.com/ac) for editing a draft of this manuscript.
Okubo S, Suzuki T, Hioki M, et al. The immunological impact of preoperative chemoradiotherapy on the tumor microenvironment of pancreatic cancer. Cancer Sci. 2021;112:2895–2904. 10.1111/cas.14914
REFERENCES28
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA A Cancer J Clin. 2014;64(1):9‐29. [DOI] [PubMed] [Google Scholar]
- 2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039‐1049. [DOI] [PubMed] [Google Scholar]
- 3. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas‐616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567‐579. [DOI] [PubMed] [Google Scholar]
- 4. Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC‐1 randomized controlled trial. Ann Surg. 2001;234(6):758‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long‐term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25‐year experience. World J Surg. 2003;27(3):324‐329. [DOI] [PubMed] [Google Scholar]
- 6. Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40(4):549‐558. [DOI] [PubMed] [Google Scholar]
- 7. Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977‐988. [DOI] [PubMed] [Google Scholar]
- 8. Gilbert JW, Wolpin B, Clancy T, et al. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image‐based classification. Ann Oncol. 2017;28(9):2067‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035‐1046. [DOI] [PubMed] [Google Scholar]
- 10. Tempero MA, Malafa MP, Al‐Hawary M, et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(8):1028‐1061. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi S, Ohno I, Ikeda M, et al. Neoadjuvant S‐1 with concurrent radiotherapy followed by surgery for borderline resectable pancreatic cancer: study protocol for an open‐label, multicentre, prospective phase II trial (JASPAC05). BMJ Open. 2017;7(10):e018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151‐160. [DOI] [PubMed] [Google Scholar]
- 13. Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carson WE 3rd, Shapiro CL, Crespin TR, Thornton LM, Andersen BL. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res. 2004;10(10):3401‐3409. [DOI] [PubMed] [Google Scholar]
- 15. Tsuchikawa T, Miyamoto M, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19(5):1713‐1719. [DOI] [PubMed] [Google Scholar]
- 16. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome. 2018;29(11–12):843‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor‐infiltrating lymphocytes in gastric cancer: a systematic review and meta‐analysis. Medicine. 2018;97(32):e11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanton SE, Disis ML. Clinical significance of tumor‐infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santoiemma PP, Powell DJ Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335‐1339. [DOI] [PubMed] [Google Scholar]
- 21. Brierley JD, Gospodarowicz MK, Wittekind C. UICC TNM Classification of Malignant Tumours, 8th edn. Hoboken, NJ: Wiley‐Blackwell; 2016. [Google Scholar]
- 22. Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8(9):972‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuchikawa T, Hirano S, Tanaka E, et al. Novel aspects of preoperative chemoradiation therapy improving anti‐tumor immunity in pancreatic cancer. Cancer Sci. 2013;14(5):531‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murakami T, Hommma Y, Matsuyama R, et al. Neoadjuvant chemoradiotherapy of pancreatic cancer induces a favorable immunogenic tumor microenvironment associated with increased major histocompatibility complex class I‐related chain A/B expression. J Surg Oncol. 2017;116:416‐426. [DOI] [PubMed] [Google Scholar]
- 25. Homma Y, Taniguchi K, Murakami T. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21(2):670–676. [DOI] [PubMed] [Google Scholar]
- 26. Jarosz‐Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci. 2019;20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komohara Y, Takemura K, Lei XF, et al. Delayed growth of EL4 lymphoma in SR‐A‐deficient mice is due to upregulation of nitric oxide and interferon‐ɤ production by tumor‐associated macrophages. Cancer Sci. 2009;100(11):2160‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petty AJ, Yang Y. Tumor‐associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9(3):289‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang M, Li Z, Ren M, et al. Stromal infiltration of tumor‐associated macrophages conferring poor prognosis of patients with basal‐like breast carcinoma. J Cancer. 2018;9(13):2308‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan Y, Lu F, Fei Q, et al. Single‐cell RNA sequencing reveals compartmental remodeling of tumor‐infiltrating immune cells induced by anti‐CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jarosz‐Biej M, Smolarczyk R, Cichon T, Kulach N. Tumor microenvironment as a ‘Game Changer’ in cancer radiotherapy. Int J Mol Sci. 2019;20(13):3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leblond MM, Peres EA, Helaine C, et al. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget. 2017;8(42):72597‐72612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klug F, Prakash H, Huber PE, et al. Low‐dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589‐602. [DOI] [PubMed] [Google Scholar]
- 36. Seifert L, Werba G, Tiwari S, et al. Radiation therapy induces macrophages to suppress T‐Cell responses against pancreatic tumors in mice. Gastroenterology. 2016;150(7):1659‐1672.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini JL. Macrophage biology plays a central role during ionizing radiation‐elicited tumor response. Biomed J. 2017;40(4):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown JM, Thomas R, Nagpal S, Recht L. Macrophage exclusion after radiation therapy (MERT): A new and effective way to increase the therapeutic ratio of radiotherapy. Radiother Oncol. 2020;144:159‐164. [DOI] [PubMed] [Google Scholar]
- 39. Sawa‐Wejksza K, Kandefer‐Szerszeń M. Tumor‐associated macrophages as target for antitumor therapy. Arch Immunol Ther Exp (Warsz). 2018;66(2):97‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor‐infiltrating macrophages decreases tumor‐initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neyen C, Pluddemann A, Mukhopadhyay S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190(7):37898‐43805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng M, Huang B, Qi Z, et al. Embelin inhibits pancreatic cancer progression by directly inducing cancer cell apoptosis and indirectly restricting IL‐6 associated inflammatory and immune suppressive cells. Cancer Lett. 2014;354(2):407‐416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1


