Abstract
Breast cancer is one of the most commonly diagnosed malignancies worldwide, while the triple negative breast cancer (TNBC) is the most aggressive and virulent subtype in breast cancers. Compared with luminal type breast cancers, which could be well controlled by endocrine treatment, TNBC is worse in prognosis and lack of effective targeted therapy. Thus, it would be interesting and meaningful to identify novel therapeutic targets for TNBC treatments. Recent genomic data showed the activation of Hippo/YAP signaling in TNBC, indicating its critical roles in TNBC carcinogenesis and cancer progression. Hippo/YAP signaling could subject to several kinds of protein modifications, including ubiquitination and phosphorylation. Quite a few studies have demonstrated these modifications, which controlled YAP protein stability and turnover, played critical role in Hippo signaling activation In our current study, we identified ZNF213 as a negative modifier for Hippo/YAP axis. ZNF213 depletion promoted TNBC cell migration and invasion, which could be rescued by further YAP silencing. ZNF213 knocking down facilitated YAP protein stability and Hippo target gene expression, including CTGF and CYR61. Further mechanism studies demonstrated that ZNF213 associated with YAP and facilitated YAP K48‐linked poly‐ubiquitination at several YAP lysine sites (K252, K254, K321 and K497). Besides, the clinical data showed that ZNF213 negatively correlated with YAP protein level and Hippo target gene expression in TNBC samples. ZNF213 expression correlated with good prognosis in TNBC patients. Our data provided novel insights in YAP proteolytic regulation and TNBC progression.
Keywords: breast cancer, stability, ubiquitin, YAP, ZNF213
The triple negative breast cancer (TNBC) is the most aggressive and virulent subtype in breast cancers.In our current study, we identified ZNF213 as a negative modifier for Hippo/YAP axis in TNBC. Our data provided novel insights in YAP proteolytic regulation and TNBC progression.
Abbreviations
- ER
Estrogen receptor
- HER2
Human growth factor receptor 2
- LATS
large tumor suppressor kinase
- TEAD
TEA domain transcriptional factor
- TNBC
triple negative breast cancer
- YAP
yes‐associated protein
- ZNF213
Zinc finger domain protein 213
1. INTRODUCTION
Breast cancer is the most common cancer type and caused the second leading of malignancy‐related deaths in females. 1 Once the pathological diagnosis is made, the molecular classification will be performed according to several molecular markers, including Estrogen Receptor (ER), Progesterone Receptor (PR) and Human Epidermal Growth Factor Receptor 2 (HER2). 2 If breast cancer lacks all the three receptors, it will be defined as triple negative breast cancer. 3 Compared with Luminal or HER2 positive types, which could be effectively controlled by anti‐estrogen therapy or anti‐HER2 therapy, triple negative type is more aggressive and mostly depends on poly‐chemotherapy. 4 Recent genomics studies revealed that TNBC contains higher genomic mutation, gene amplifications and deletions, compared with other types. For example, several key tumor suppressor genes, such as P53 and Rb, get mutated in most of TNBC samples. 5 Based on the fact that effective “targeted” therapy in TNBC is not available, it is necessary and urgent to explore novel therapeutic targets for cancer biology research and cancer treatments in TNBC.
Recent studies showed that Hippo signaling played critical roles in both normal tissue regeneration and cancer progression. 6 The Hippo signaling, which was firstly identified in Drosophila from genetic screening of tumor suppressors. 7 When the Hippo signaling is activated, the upstream kinases MST1/2 binds to SAV1 and subsequently phosphorylate MOB, which is an adaptor protein. MOB interacts with LATS1/2 and promotes LATS1/2 auto‐phosphorylation. If LATS1/2 get activated, it binds to the WW domains of YAP and promotes YAP poly‐phosphorylation. In the final, the phosphorylated YAP associates with 14‐3‐3 protein and gets degraded by the proteasomes. However, if the Hippo pathway is turned off, which leaves YAP un‐phosphorylated, YAP protein could translocate into the nuclear and bind to several transcriptional factors, such as TEADs and SMADs. 8 , 9 , 10 As a transcriptional factor co‐activator, YAP could facilitate the transcription of several pro‐survival and invasion genes, such as CTGF and CYR61. 11
The hyper‐activation of YAP could due to several mechanisms including silencing of Hippo signaling inhibitors and the copy number amplification of YAP, which has been implicated in several human cancers, such as gastric cancer and TNBC. 12 One population‐based study showed that YAP activation related to breast cancer risk, while YAP copy number amplification is common in TNBC, but not in luminal type breast cancers (https://www.cbioportal.org). Several studies implicated that YAP modulated several aspects of TNBC, such as cancer invasion, migration and “stemness”. For example, YAP depletion prohibited TNBC cell invasion and migration in vitro and in vivo. 13 , 14 Based on this targeting YAP might be an effective strategy for TNBC treatments. However, the direct blocker, which inhibited YAP association with transcriptional factors are still premature in clinics. Our research group focuses on uncovering the YAP modifiers, which controls YAP protein stability and YAP turnover, for potential therapeutic targets in TNBC. Our current study showed that ZNF213 (Zinc Finger Protein 213) as one modifier in Hippo/YAP axis in TNBC. ZNF213 belongs to zinc finger family protein member and is composed of 459 amino acids. 15 Although ZNF proteins were shown to bind DNA or RNA, which modulated gene expression directly, they were also implicated to modulate poly‐ubiquitination process and protein degradation. 16 , 17 In our study, we found ZNF213 is elevated in breast cancer, but favors the prognosis in TNBC patients. The further data show that ZNF213 suppresses the transcriptional function of YAP on Hippo target genes in TNBC cells and inhibits breast cancer cell progression, possibly via promoting YAP protein degradation. Our study provides novel knowledge of ZNF family members in modulating Hippo signaling activity and TNBC progression. ZNF213 could be a potential therapeutic target for TNBC treatments.
2. MATERIALS AND METHODS
2.1. Cell culture
BT549, MDA‐MB‐231, and HEK293 cells were acquired from American Type Culture Collection (ATCC). MDA‐MB‐231 and HEK293 cells grown in Dulbecco's Modified Eagle's Medium that contains 4.5 g/L glucose and 4 mM L‐glutamine (DMEM, 41 965, Life Technologies) supplemented with 10% Fetal Bovine Serum (FBS, 10 270, Life Technologies). BT549 cells were cultured in RPMI‐1640 (42 401, Life Technologies) supplemented with 2 mM L‐glutamine (25030, Life Technologies) and 10% FBS. All cell lines were certified by cell line authentication. The cell line authentication via Short Tandem Repeat (STR) was performed via PowerPlex 21 system. We found that The STR data of BT549, MDA‐MB‐231, and HEK293 cell lines were consistent with STR data in ATCC.
2.2. Plasmids and siRNA
The Myc‐ZNF213 plasmid and the Flag‐ZNF213 plasmid were acquired from Origene Company (https://www.origene.com). The ZNF213 deletion constructs were sub‐cloned from the ZNF213 full‐length plasmid. The HA‐Ub, HA‐K48, and HA‐K63 plasmids were used in previous study. The Lipofectamin 2000 (1662298, Invitrogen) was used for the transfection of plasmids. We used the small interfering RNAs to knockdown the specific gene. The ZNF213 siRNA sequences were: siRNA#1 GGCAUUGGGAGACAUCCCAdTdT;UGGGAUGUCUCCCAAUGCCdTdT and siRNA#2 GCCUUUCAGUGGGCGUGGAdTdT;UCCACGCCCACUGAAAGGCdTdT. The YAP siRNA sequences were GCUCAUUCCUCUCCAGCUU; AAGCUGGAGAGGAAUGAGC. The negative control siRNA sequences were: UUCUCCGAACGUGUCACGUTT; ACGUGACACGUUCGGAGAATT. The RNA iMAX reagent (13778150, invitrogen) was used for the transfection of siRNA. For lenti‐virus based ZNF213 over‐expression, ZNF213 was cloned into the vector pLKO.1, which was co‐transfected with pMD2.G and psPAX2 envelop plasmids into HEK293 cells. The ZNF213 expression lenti‐virus was obtained after 48 hours. We incubated the TNBC cells including MDA‐MB‐231 and BT549 cells with 2 ml antibiotic‐free medium containing 200 μL lentivirus. Lentivirus packaging cells were transfected with Lenti‐X™ shRNA Expression Systems (clontech) containing either the ZNF213 knockdown (shZNF213), YAP knockdown (shYAP) or a negative control sequence (shControl). The shRNA sequences were as follows: ZNF213:5’‐GTCCATGGACCTGTGGCATT‐3’; YAP: 5’‐GCCACCAAGCTAGATAAAGAA‐3’; negative control: 5’‐UUCUCCGAACGUGUCACGU −3’.
2.3. RNA extraction and qPCR analysis
We extracted total RNA by RNeasy plus mini kits according to the protocol (Tiangen). Real‐time PCR was performed as previously described. 18 qPCR was performed in a QuantStudio 5 Fast Real‐Time PCR System (Applied Biosystems) with KOD SYBR® qPCR Mix (TOYOBO) according to conditions specified by the manufacturer.36B4 served as the internal reference, with the 2‐ΔΔCt values normalized to 36B4 levels. The primer sequences were demonstrated here. ZNF213: F: gcg acc ctg gag tac aca tc; R: tca tgc tgg gca gat tcc tg. 36B4: F: ggcgac ctg gaa gtc caa ct; R: cca tca gca cca cag cct tc. CTGF: F: ctc gcg gct tac cga ctg; R: ggc tct gct tct cta gcc tg. CYR61: F: agc agc ctg aaa aag ggc aa; R: agc ctg tag aag gga aac gc. The specificity of all primer pairs was checked by melting curve analysis.
2.4. Quantification of cell viability
BT549 and MDA‐MB‐231 cells were transfected with siZNF213 or siControl in 24‐well plates. About 24h later, the cell number was countered and 4000 cells were seeded into 96‐well plates. The relative cell viability was measured at indicated time points. Cell viability was determined by using the WST‐1 cell proliferation reagent.
2.5. Wound healing assay
Cells were seeded into 12 well plates with 1%FBS. When the cells were 100% fused, we scratched the cells with the tips of yellow pipette. The wound distance was measured at the specified time points and normalized with starting time point. The recovery rate of wound healing was expressed as follows: [1 ‐(Wound width at a given time/wound width at t = 0)] × 100%.
2.6. Trans‐well assay
We used the modified two‐chamber plates to measure cell migration and invasion capacity. For performing the migration assay, cells in serum‐free medium were seeded into the upper chambers. For performing the invasion assay, the upper chambers were coated with matrigel (BD Biocoat, USA) For stimulating invasion, the bottom wells were filled with the medium containing 20% FBS, while the cells in the upper chambers were filled with FBS‐free medium. About 12h later, we removed the cells carefully and fixed the cells that invaded through the membrane and stained them with Crystal Violet Staining solution. Then the cells were photographed with a microscope and counted by ImageJ.
2.7. Western blotting
The cells were harvested and lysed with RIPA extraction reagent (Beyotime). Proteins were separated by electrophoresis on SDS‐polyacrylamide gel electrophoresis (PAGE) and electro‐transferred to PVDF membrane. The following antibodies were used in the study: Anti‐ZNF213 (HPA035000, Sigma); Anti‐YAP (SC‐101199, Santa Cruz); Anti‐β‐Actin (A5441, Sigma); Anti‐HA (MMS‐101R, COVANCE); Anti‐Myc (Ab9106, Abcam); Anti‐Flag (20543‐1‐AP, Proteintech); Anti‐GFP (Ab290, Abcam). Then Membranes were washed with PBS for three times and incubated with secondary antibodies Peroxidase‐Conjugated AffiniPure Goat Anti‐Mouse IgG or Goat Anti‐Rabbit IgG. Protein signals were detected with an ECL kit (Millipore Co, Billerica) Fluorescent signals were visualized with ECL system (Bio‐rad ChemiDoc).
2.8. Luciferase assay
The luciferase activity of TEAD was detected using the Dual‐Luciferase Reporter kit (Promega). The cells were transfected with the TEAD luciferase reporter and the Renilla plasmid. About 24h later, Cells were harvested and Luciferase activity was measured.
2.9. Co‐immunoprecipitation assay
Immunoprecipitation was performed as described in the previous study. 19 The BT549 cells total cell lysis was precleared with rabbit IgG or mouse IgG for 2 h and subsequently immunoprecipitated with ZNF213 antibody (HPA035000, Sigma) or YAP antibody (SC101199, Santa Cruz) over night, and rabbit IgG (Santa Cruz) or mouse IgG (Santa Cruz) was used as the negative control. The isotype control was matched with both host species and isotype of the primary antibody. Anti‐YAP antibody (SC‐101199, Santa Cruz) or Anti‐ZNF213 antibody (HPA035000, Sigma) were used for analysis of the bound protein. For the overexpression experiment, HEK293 cells were transfected with 5 μg GFP‐ZNF213 (full length or deletion domains) and YAP plasmid (full length or deletion domains) in the 10 cm dish. Cell lysates were pre‐cleared with IgG and subsequently incubated with GFP (Ab290, Abcam) antibody or Myc (Ab9106, Abcam) antibody, and rabbit IgG or mouse IgG was used as the negative control. The isotype control was matched with both host species and isotype of the primary antibody. Western blotting was used for analysis of the bound proteins.
2.10. Protein stability assays
About 105 HEK293 cells were cultured in 24 well plates and transfected with 0.5 µg Myc‐ZNF213 or Myc‐tag. About 48h later, cells were treated with 100 µM cycloheximide (C7698, Sigma) for the indicated time points. Western blotting was used for the samples to detect YAP degradation. For BT549 cells, 105 cells were cultured in 24 well plates and transfected with 50 nM siZNF213 or siControl. About 24h later, cells were treated with 100 µM cycloheximide (C7698, Sigma) for the indicated time points. Western blotting was used for the samples to detect YAP degradation.
2.11. Analysis of protein ubiquitination
HEK293 cells were transfected with 2 µg YAP plasmid together with 2 µg Myc‐ZNF213 or Myc‐tag. About 48h later, cells were treated with 10 µM MG132 (474 787, Sigma) for 6h. Then the cells were harvested directly. Western blotting was used for the detection of the polyubiquitination of YAP. 20
2.12. Poly‐ubiquitination detection assay
To detect the enriched K48‐ubiquitinated and K63‐ubiquitinated YAP from the cell extracts directly, HEK293 cells were transfected with 0.5 µg K48 Ubi or 4 µg K63 Ubi plasmids, 2 µg YAP together with 0.5 µg Myc‐ZNF213 or Myc‐tag. About 48h later, the total protein was extracted and precleared with 20 µL protein A (santa cruz, SC‐2001) for 2 h. The supernatant was collected and immunoprecipitated by YAP antibody. Western blotting with HA antibody was used to detect K48 or K63 poly‐ubiquitinated YAP. 21
2.13. Immunofluorescence assay
MDA‐MB‐231 cells and BT549 cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.25% Triton X‐100 for 5 min, and blocked by 3% BSA for 1 h. The rabbit anti‐ZNF213 polyclonal antibody (HPA035000, Sigma) and mouse anti‐YAP monoclonal antibodies (SC‐101199, Santa Cruz) were used, followed by Alexa Flour 647 (Invitrogen) anti‐rabbit antibody and FITC‐conjugated anti‐mouse antibodies (Jackson ImmunoResearch, West Grove, PA). As negative controls, the samples were incubated with the secondary antibodies without primary antibodies. Images were captured by a confocal laser‐scanning microscope (Leica TCS SP8 STED). The acquired pictures were further processed and analyzed by ImageJ.
2.14. Publical available clinical data analysis
The analysis of ZNF213 and YAP correlation with clinical prognosis was carried out through KMPLOT database (https://kmplot.com). Analysis of ZNF213 correlation with YAP target gene (CTGF and CYR61) was carried out by TCGA database with 1080 breast cancer samples. The acquired data were analyzed and calculated by Prism 7.0 (GraphPad).
2.15. Clinical breast tumor samples
Thirty triple negative breast cancer samples were provided by Qilu Hospital of Shandong University. The ER, PR, and HER2 status of all these samples were examined. According to standard method, the immuno‐histochemisty of ZNF213 and YAP were detected. The IHC results of ZNF213 and YAP were examined through pathological specialists. The rabbit anti‐ZNF213 polyclonal antibody (HPA035000, Sigma) and mouse anti‐YAP monoclonal antibodies (SC‐101199, Santa Cruz) were used for IHC analysis. The size of the FFPE section was prepared in 4 μm. The results of YAP and ZNF213 staining were determined by two independent certified pathologists.
2.16. In vivo tumorigenesis essay
For vivo tumorigenic experiment, we used the 5‐week‐old female BALB/c nude mice in each group. 3X106 MDA‐MB‐231 cells were injected subcutaneously into each mouse. Tumor formation in nude mice was monitored about 5 weeks. The tumor volume was calculated by the formula: tumor volume = length × width2/2.
2.17. Statistics
Student's t‐test, Pearson correlation coefficient, and Cox regression analysis were used for comparisons. P < .05 was considered as a significant difference. All statistical tests were performed with Prism 7.0 (GraphPad).
3. RESULTS
3.1. ZNF213 functions to inhibit TNBC cell migration and invasion
In order to uncover the role of ZNF213 in triple negative breast cancer, the BT549 and MDA‐MB‐231 cell lines were used as models for most experiments. In order to avoid off‐target effect, two independent siRNAs were used in the study. The knockdown efficiencies of ZNF213 were confirmed through western blotting and real‐time PCR (Figure 1A and 1B). To evaluate the depletion effect of ZNF213 in TNBC cells, we performed trans‐well assay. In BT549 and MDA‐MB‐231 cells, ZNF213 depletion promoted breast cancer cell invasion and migration (Figure 1E‐H). Besides, the Wound‐healing assay showed that ZNF213 depletion facilitated TNBC cell migration (Figure 1I‐L). However, we did not observe any effect of ZNF213 in TNBC cell proliferation (Figure 1C‐D). We further investigated the effect of ZNF213 in TNBC cells via lentivirus‐based ZNF213 over‐expression. ZNF213 over‐expression inhibited cancer cell invasion and migration by trans‐well assay in BT549 and MDA‐MB‐231 cells (Figure 2A‐B; Figure 2E‐F). Besides, elevated ZNF213 also inhibited cancer cell migration by Wound healing assay in BT549 and MDA‐MB‐231 cells (Figure 2C‐D; Figure 2G‐H). The in vivo tumor growth assay showed that ZNF213 overexpression cells inhibited growth speed in MDA‐MB‐231 cell models (Figure 2I‐K).
FIGURE 1.
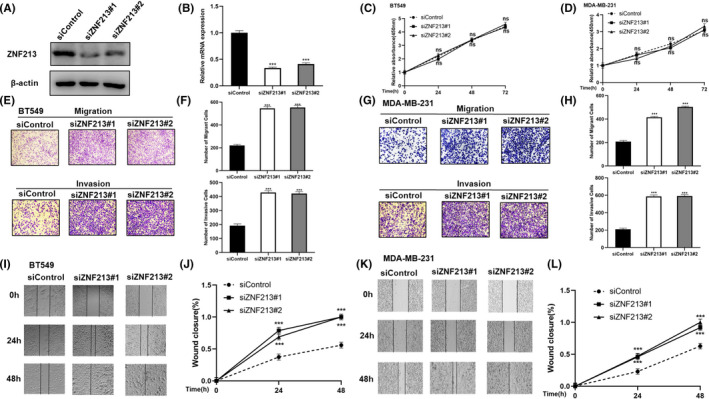
ZNF213 depletion promotes migration and invasion in triple negative breast cancer cells. A‐B, ZNF213 depletion efficiency by two different siRNA oligos in TNBC cell lines. BT549 cells were transfected with ZNF213 siRNA or siControl. About 48h later, ZNF213 protein and mRNA levels were determined by Western blotting and QPCR. C‐D, ZNF213 depletion has no effect on TNBC cancer cell proliferation. BT549 and MDA‐MB‐231 cells were transfected with siControl or siZNF213. About 24h later, the WST assay was used to determine the cellar metabolic activity at indicated time points after infection. Experiments were done in triplicates. The data are showed as ±SD.***P < .001 (student's t‐test). E‐F, ZNF213 depletion promotes TNBC cell migration and invasion in BT549 cells. We used two independent siRNA in the study. What's more, we used the trans‐well assay to check the migration and invasion capacity. The cell number was counted and data are showed as ±SD.***P < .001 (student's t‐test). G‐H, ZNF213 depletion promotes TNBC cell migration and invasion in MDA‐MB‐231 cells. We used two independent siRNA in the study. What's more, we used the trans‐well assay to check the migration and invasion capacity. The cell number was counted and data are showed as ±SD. ***P < .001 (student's t‐test). I‐J Wound healing assay of BT549 cells was transfected with indicated 50 nM ZNF213 siRNA or siControl. Quantification of wound closure at the specified time points. Data are showed as ±SD. ***P < .001 (student's t‐test). K‐L, Wound healing assay of MDA‐MB‐231 cells were transfected with indicated 50 nM ZNF213 siRNA or siControl. Quantification of wound closure at the specified time points. Data are showed as ±SD. ***P < .001 (student's t‐test)
FIGURE 2.
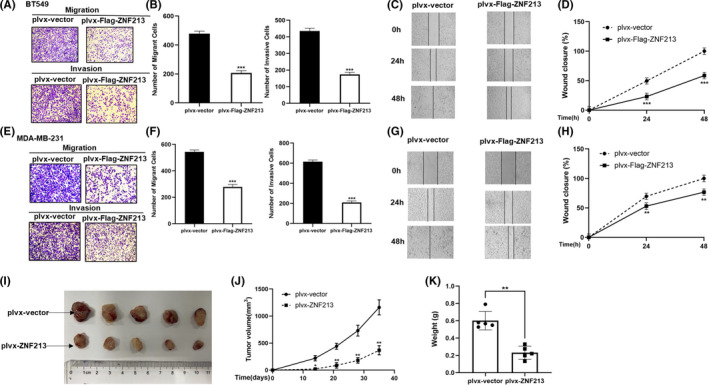
ZNF213 inhibits the migration and invasion of triple negative breast cancer cells. A‐B, ZNF213 inhibits cell migration and invasion in BT549 cells. We used the trans‐well assay to check the migration and invasion capacity. The cell number was counted and data are showed as ±SD. ***P < .001 (student's t‐test). C‐D, Wound healing assay of BT549 cells with stable expression of ZNF213 or empty vector. Quantification of wound closure at the specified time points. Data are showed as ±SD. ***P < .001 (student's t‐test). E‐F, ZNF213 inhibits cell migration and invasion in MDA‐MB‐231 cells. We used the trans‐well assay to check the migration and invasion capacity. The cell number was counted and data are showed as ±SD. ***P < .001 (student's t‐test). G‐H, Wound healing assay of MDA‐MB‐231 cells with stable expression of ZNF213 or empty vector. Quantification of wound closure at the specified time points. Data are showed as ±SD. **P < .01, ***P < .001 (student's t‐test). I‐K, ZNF213 overexpression inhibited the tumor growth of MDA‐MB‐231 cells in a xenograft model. The growth of xenografts was monitored over 5 weeks. Then the size and weight of xenograft tumors were measured. Data are showed as ±SD. *P < .05,**P < .01 (student's t‐test)
3.2. ZNF213 regulates TNBC cell progression via Hippo/YAP axis
We further evaluated the role of ZNF213 in Hippo signaling. The western blotting analysis showed that ZNF213 depletion increased YAP protein level in two independent ZNF213 siRNAs in both BT549 and MDA‐MB‐231 cells (Figure 3A and D). Besides, ZNF213 depletion had little influence on the mRNA expression of YAP. (Figure 3B and E). However, silencing of ZNF213 promoted the YAP target gene expression, including CTGF and CYR61 in BT549 and MDA‐MB‐231 cells (Figure 3C and F). We further evaluated the effect of ZNF213 in YAP‐mediated transcriptional function. The luciferase reporter assay showed that ZNF213 silencing promoted TEAD response element activity (Figure 3G). Consistently, ZNF213 overexpression in BT549 cells showed decreased YAP protein level, YAP target gene expression (CTGF and CYR61) and the inhibited luciferase activity of TEAD response elements (Figure 3H‐J). In order to investigate the logic link between TNBC progression and Hippo/YAP axis in ZNF213 function, a series of rescue experiments were carried out. The western blotting data showed that YAP protein level, which was increased by ZNF213 depletion, could be further inhibited by YAP knocking‐down in BT549 cells (Figure 4A). Consistently, the YAP target genes level, which increased by ZNF213 silencing, could also been rescued by further YAP depletion (Figure 4B). In the trans‐well assay, the increased cancer cell invasion caused by ZNF213 depletion could be further rescued by YAP depletion in BT549 cells (Figure 4C‐D). Besides, the Wound‐healing assay also indicated the migration phenotype could be rescued by YAP interfering (Figure 4E‐F). Consistently, the rescue experiments were also carried out in MDAMB231 cells. The data showed that further depletion of YAP could also rescue the increased invasion and migration caused by ZNF213 silence in MDAMB231 cells (Figure S1).
FIGURE 3.
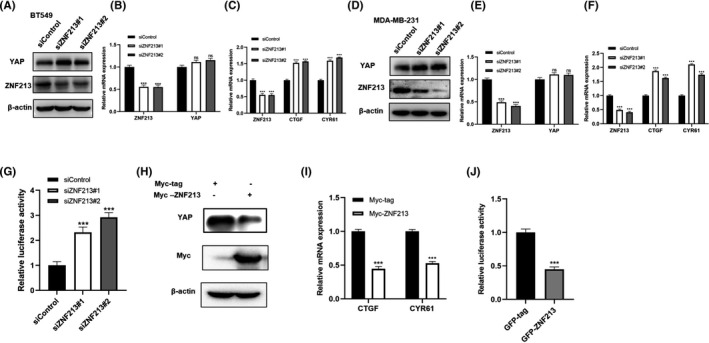
ZNF213 inhibits Hippo signaling in triple negative breast cancer cells. A, ZNF213 depletion increased the levels of YAP protein in BT549 cells. BT549 cells were transfected with siControl or siZNF213. About 48h later, cells were harvested for western blotting analysis. ZNF213 and YAP protein levels were determined by western blotting.β‐Actin was used as internal reference. B‐C, ZNF213 depletion increased the expression of YAP target gene in BT549 cells. But ZNF213 depletion had no influence on the mRNA expression of YAP. BT549 cells were transfected with siControl or siZNF213. About 48h later, total RNA was extracted for the analysis of gene expression. Each group was repeated three times. ***P < .001 for target gene expression comparison. D, ZNF213 depletion increased the levels of YAP protein in MDA‐MB‐231 cells. MDA‐MB‐231 cells were transfected with siControl or siZNF213. About 48h later, cells were harvested for western blotting analysis. ZNF213 and YAP protein levels were determined by western blotting. β‐Actin was used as internal reference. E‐F, ZNF213 depletion increased the expression of YAP target gene in MDA‐MB‐231 cells. But ZNF213 depletion had no influence on the mRNA expression of YAP. MDA‐MB‐231 cells were transfected with siControl or siZNF213. About 48h later, total RNA was extracted for the analysis of gene expression. Each group was repeated three times. ***P < .001 for target gene expression comparison. G, ZNF213 depletion increased TEAD Luciferase activity in BT549 cells. BT549 cells were transfected with siControl or siZNF213. About 24h later, cells were transfected with the YAP luciferase reporter plasmid and Renilla plasmid. Another 24h later, cells were harvested for the detection of luciferase activity. H, ZNF213 over‐expression decreased the levels of YAP protein in BT549 cells. BT549 cells were transfected with Myc‐ZNF213 or Myc‐tag plasmids. About 48h later, cells were harvested for the analysis of western blotting. Myc‐ZNF213 and YAP protein levels were determined by western blotting.β‐Actin was used as internal reference. I, ZNF213 overexpression decreased the expression of YAP target gene in BT549 cells. BT549 cells were transfected with Myc‐ZNF213 or Myc‐tag plasmids. About 48h later, total RNA was extracted for the analysis of gene expression. Each group was repeated three times. ***P < .001 for target gene expression comparison. J, ZNF213 decreased TEAD Luciferase activity in BT549 cells. BT549 cells were transfected with GFP‐ZNF213 or GFP plasmids. About 24h later, cells were transfected with the YAP luciferase reporter plasmid and Renilla plasmid. Another 24h later, cells were harvested for the detection of luciferase activity
FIGURE 4.
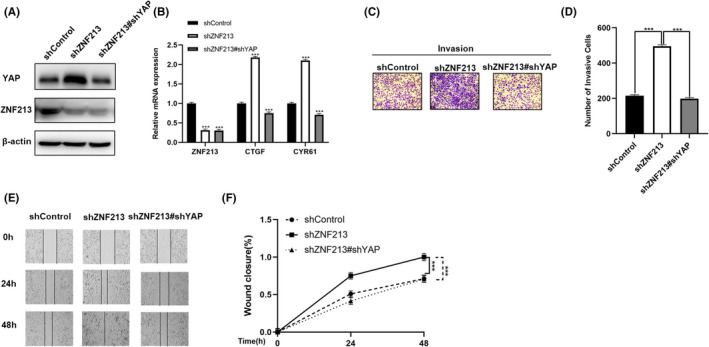
ZNF213 inhibits the migration and invasion of triple negative breast cancer cells through Hippo/YAP signaling. A, ZNF213 depletion increased the level of YAP protein, which can be reversed after YAP knocking‐down. ZNF213 and YAP protein levels were determined by western blotting.β‐Actin was used as internal reference. B, ZNF213 depletion increased the level of Hippo target gene, which can be reversed after YAP knocking‐down. ZNF213 and YAP protein levels were determined by western blotting.β‐Actin was used as internal reference. C‐D, ZNF213 depletion increased the invasion capacity of TNBC cells, which can be reversed after YAP knocking‐down. The cancer cells were seeded into the chamber for trans‐well assay. The cell number was counted and data are showed as ±SD. ***P < .001 (student's t‐test). E‐F, Wound healing assay demonstrated that ZNF213 depletion increased the migration capacity of TNBC cells, which can be reversed after YAP knocking‐down. Quantification of wound closure at the specified time points. Data are showed as ±SD. ***P < .001 (student's t‐test)
3.3. ZNF213 is elevated in breast cancer, but reversely correlates with Hippo/YAP signaling in TNBC samples
We further investigated ZNF213 expression in clinical samples. From the TCGA data, we observed that ZNF213 was elevated in breast cancer compared with normal breast tissues (Figure 5A). When we analyzed the ZNF213 expression in each subtype of breast cancer, we found that ZNF213 was increased in every subtype compared with normal breast tissues (https://tcga‐data.nci.nih.gov/tcga/) (Figure 5B). Interestingly, ZNF213 expression correlated with longer relapse survival in both total breast cancers and TNBC groups (Figure 5C and D). In contrast, YAP expression related to poor survival in TNBC patients (Figure 5E). The further bio‐informatics analysis from TCGA database showed that ZNF213 expression was reversely correlated with YAP target genes, including CTGF and CYR61 in triple negative breast cancers (Figure 5F‐G). In order to validate this relation, we collected thirty TNBC sample for immunohistochemistry analysis. The IHC data showed that ZNF213 located both in the cytosol and nuclear, while YAP mainly located in the nuclear. The statistical analysis showed that ZNF213 expression negatively correlated with YAP protein level (Figure 5H and Table 1).
FIGURE 5.
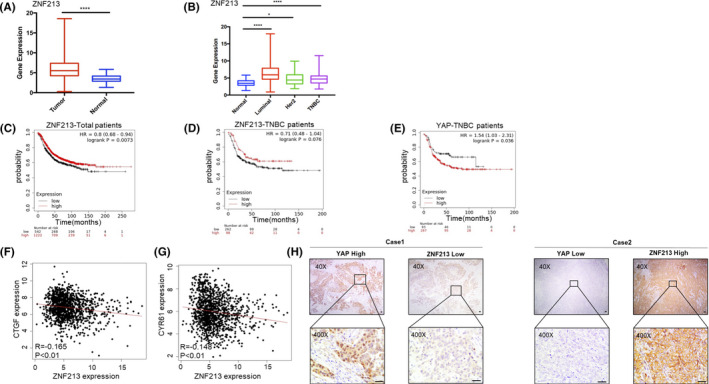
ZNF213 reversely correlates with Hippo/YAP signaling in TNBC tumor samples. A, ZNF213 gene expression was significantly increased in tumor tissues compared with normal tissues. The data were obtained from TCGA database. Data are showed as ±SD. ****P < .001(student's t‐test). B, Comparison of expression levels of ZNF213 in normal tissues and different types of breast cancer. The data were obtained from TCGA database. Data are showed as ±SD. *P < .05, ****P < .001 (student's t‐test). C, Kaplan–Meier graph of progression‐free survival demonstrates that ZNF213 relates to good prognosis in all breast cancer patients. D, Kaplan–Meier graph of progression‐free survival demonstrates that ZNF213 relates to good prognosis in triple negative breast cancer patients. E, Kaplan–Meier graph of progression‐free survival demonstrates that YAP relates to poor prognosis in triple negative breast cancer patients. F‐G, TCGA database shows that the expression of ZNF213 is negatively correlated with Hippo/YAP target genes: CTGF and CYR61. H, Example tumor cases show that ZNF213 protein correlates with YAP protein negatively in IHC. Scale bar, 50 μm
TABLE 1.
Correlation between ZNF213 and YAP in TNBC samples
| ZNF213 IHC staining | P values | |||
|---|---|---|---|---|
| + | − | |||
| YAP IHC staining | + | 8 | 13 | .046 |
| − | 7 | 2 | ||
Bold value is the statistic P value.
3.4. ZNF213 associates with YAP and modulates YAP protein stability
In order to explore the mechanism of ZNF213 in Hippo/YAP signaling, we explored the subcellular localization in TNBC cells. The immuno‐staining showed that YAP was located mainly in the nuclear, while ZNF213 was localized both in cytosol and nucleus (Figure 6A). Besides, we found that ZNF213 knockdown didn't enhance the nuclear accumulation of YAP (Figure S3). ZNF213 over‐expression could suppress YAP protein level, which effect could be rescued via proteasome inhibitor MG132 (Figure 6B). The protein stability assay indicated that ZNF213 depletion could enhance YAP protein stability in TNBC cells (Figure 6C, D and Figure S2), while ZNF213 over‐expression could shorten YAP protein half‐life in HEK293 cells (Figure 6E‐F). The endogenous immuno‐precipitation assay (IP) showed that ZNF213 associated with YAP in BT549 cells (Figure 7A). We further investigated the associated domain of ZNF213 and YAP for such interaction. YAP protein is composed of transcriptional activation domain (TBD), WW domain and TEAD‐binding domain (TA) in the C‐terminal. ZNF213 is composed of LeR/SCAN domain, KRAB‐A domain and ZF domain (Figure 7B). We made these deletion constructs and further investigated the associated domain by IP assay. The data showed that KRAB‐A domain was required for ZNF213 to interact with YAP, while the WW domain of YAP was responsible domain for interaction with ZNF213 (Figure 7C and D).
FIGURE 6.
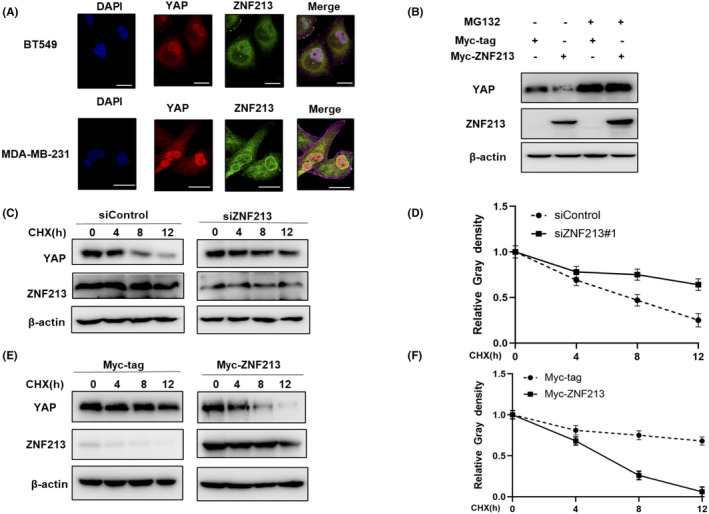
ZNF213 modulates YAP protein stability. A, Intracellular localization analysis of ZNF213 and YAP through the immunofluorescence assay. BT549 cells and MDA‐MB‐231 cells grown in normal medium before fixation. Intracellular localization of YAP (red) and ZNF213 (green) were shown in the pictures. Nuclei (blue) were stained with 40,6‐diamidino‐2‐phenylindole (DAPI). Scale bar, 20 μm. B, If the cells were treated with the proteasome inhibitor MG132, the degradation effect of ZNF213 on YAP did not increase the levels of YAP protein any more. HEK293 cells were transfected with 0.5 mg Myc‐tag or Myc ‐ZNF213 plasmids. About 24h later, cells were treated with 10 mM MG132 or DMSO for 6 h. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. C‐D, ZNF213 depletion increased the half‐life of YAP in BT549 cells. BT549 cells were transfected with 50 nM siControl or siZNF213. About 24h later, cells were treated with 100 mM cycloheximide or vehicle for the indicated times. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. Image J software was used to measure the protein density. E‐F, ZNF213 decreased YAP half‐life in HEK293 cells. HEK293 cells were transfected with 0.5 mg Myc‐tag or Myc‐ZNF213 plasmids. About 24h later, cells were treated with 100 mM cycloheximide or vehicle for the indicated times. The cell lysates were used for the analysis of western blotting. The results represent three independent experiments. Image J software was used to measure the protein density
FIGURE 7.
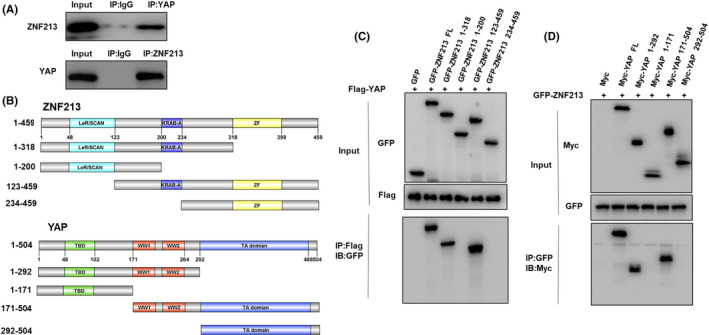
ZNF213 associates with YAP WW domain through its KRAB‐A domain. A, Co‐IP assay revealed the association between endogenous ZNF213 and YAP protein in BT549 cells. BT549 cells were harvested with RIPA lysis buffer. CO‐IP was performed using antibody as indicated. B, ZNF213 domain structure and deletion mutants were used in the study (full length, ΔZF, ΔKRAB‐A+ΔZF, ΔLeR/SCAN, ΔLeR/SCAN+ΔKRAB‐A). YAP full‐length and deletion mutants are used in the study (full length, ΔTA domain, ΔWW+ΔTA domain, ΔTBD, ΔTBD+ΔWW). C, ZNF213 interacts with YAP through its KRAB‐A domain. HEK293 cells were transfected with 2 µg Flag‐YAP together with GFP‐ZNF213 full length or mutants (ΔZF, ΔKRAB‐A+ΔZF, ΔLeR/SCAN, ΔLeR/SCAN+ΔKRAB‐A). After 24 h, cells were harvested with NP‐40 lysis buffer. Co‐IP was performed using Flag antibody. The possible interacted ZNF213 domains were detected by GFP antibody. D, WW domain is required for YAP to interaction with ZNF213. HEK293 cells were transfected with 2 µg GFP‐ZNF213 together with Myc‐YAP full length or mutants (ΔTA domain, ΔWW+ΔTA domain, ΔTBD, ΔTBD+ΔWW). After 24 h, cells were harvested with NP‐40 lysis buffer. Co‐IP was performed using GFP antibody. The possible interacted YAP domains were detected by Myc antibody
3.5. ZNF213 promotes YAP K48‐linked ubiquitination at multiple lysine sites
Further co‐expression with ZNF213 full length or domain variants in HEK293 cells showed that although KRAB‐A domain is responsible for ZNF213 interaction with YAP, the YAP stabilization effect could only be observed in ZNF213 full length (Figure 8A). This might indicate that the necessity of all protein domains of ZNF213 to exert its function on YAP protein stability. We further explored the effect of ZNF213 on YAP ubiquitination, while the data showed that ZNF213 could promote YAP poly‐ubiquitination (Figure 8B). We further explored which ubiquitination manners were dominant. The ubiquitination‐based IP showed that ZNF213 could promote K48‐linked ubiquitination level of YAP, but inhibited K63‐linked ubiquitination of YAP (Figure 8C and D). The further analysis showed that the integrity of ZNF213 was required for promoting K48‐linked YAP ubiquitination and inhibiting K63‐linked YAP ubiquitination (Figure 9A‐B). We further investigated the exact ubiquitin sites of YAP by ZNF213. It is known that there are 13 lysine sites located in YAP protein. The ubiquitination‐based IP showed that ZNF213 could facilitate YAP ubiquitination at multiple sites (K252, K254, K321 and K497) (Figure 9C‐D).
FIGURE 8.
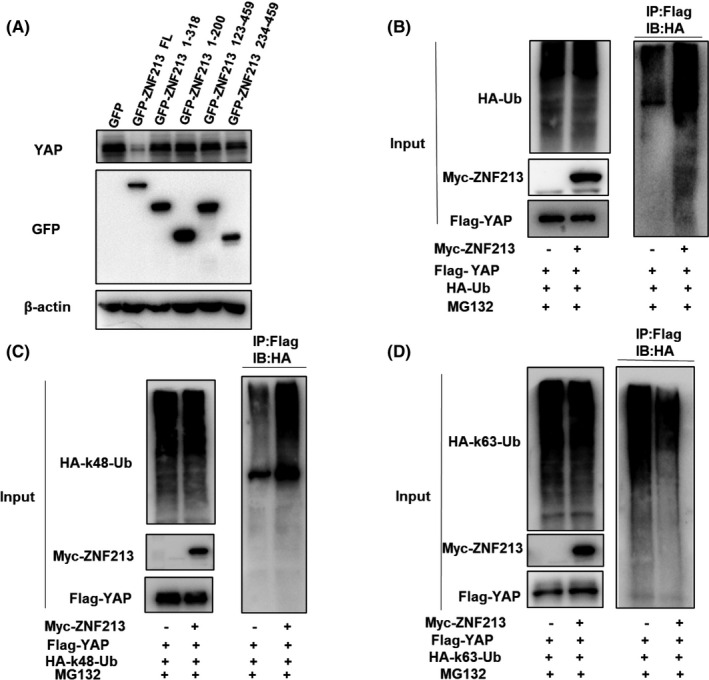
ZNF213 destabilizes YAP possibly through K48‐linked poly‐ubiquitination. A, ZNF213 prohibits YAP degradation through its full length. HEK293 cells were transfected with 2 µg GFP‐ZNF213 full length or mutant plasmids (ΔZF, ΔKRAB‐A+ΔZF, ΔLeR/SCAN, ΔLeR/SCAN+ΔKRAB‐A). About 24h later, cells were treated with 10 µM MG132 for 6 h. Cells were harvested directly and used for the analysis of western blotting. B, ZNF213 increases the overall poly‐ubiquitination of YAP. HEK293 cells were transfected with 1 mg Myc‐ZNF213 or Myc‐tag plasmid. About 24h later, cells were transfected with 1 mg HA‐Ub plasmid. Another 24h later, the cell extracts were immunoprecipitated with HA antibody. The poly‐ubiquitinated YAP was detected through the analysis of western blotting. C, ZNF213 increases K48‐linked poly‐ubiquitination of YAP. HEK293 cells were transfected with 1 mg Myc‐ZNF213 or Myc‐tag plasmid, together with 1 mg HA‐K48 Ubi plasmid. The cell extracts were immunoprecipitated with HA antibody. The K48 specific poly‐ubiquitinated YAP was detected through the analysis of western blotting. D, ZNF213 decreases K63‐linked poly‐ubiquitination of YAP. HEK293 cells were transfected with 1 mg Myc‐ZNF213 or Myc‐tag plasmid, together with 1 mg HA‐K63 Ubi plasmid. The cell extracts were immunoprecipitated with HA antibody. The K63 specific poly‐ubiquitinated YAP was detected through the analysis of western blotting
FIGURE 9.
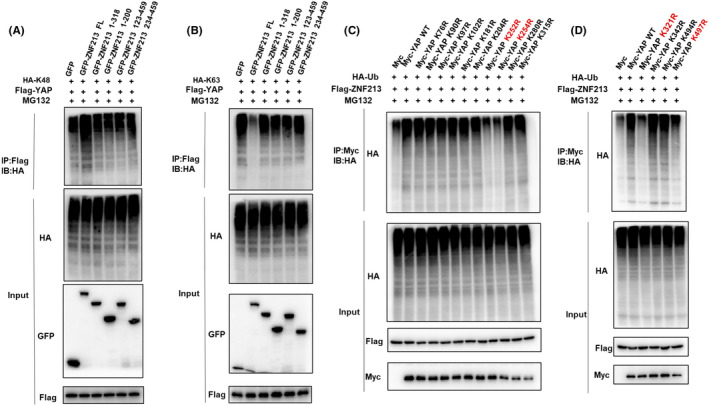
ZNF213 promotes YAP poly‐ubiquitination at K252, K254, K321 and K497 sites. A, The intact ZNF213 protein is necessary for it to facilitate K48‐linked ubiquitination on YAP. HEK293 cells were transfected with 2 µg Flag‐YAP, 1 µg HA‐K48 Ubi plasmid and 1 µg GFP‐ZNF213 full length or mutants (ΔZF, ΔKRAB‐A+ΔZF, ΔLeR/SCAN, ΔLeR/SCAN+ΔKRAB‐A). The K48‐specific polyubiquitinated YAP was detected through the analysis of western blotting. B, The intact ZNF213 protein is necessary for its inhibition effect on K48‐linked ubiquitination on YAP. HEK293 cells were transfected with 2 µg Flag‐YAP, 1 µg HA‐K63 Ubi plasmid and 1 µg GFP‐ZNF213 full length or mutants (ΔZF, ΔKRAB‐A+ΔZF, ΔLeR/SCAN, ΔLeR/SCAN+ΔKRAB‐A). The K63‐specific polyubiquitinated YAP was detected through the analysis of western blotting. C‐D, K252, K254, K321 and K497 mutations (K252R, K254R, K321R and K497R) largely abolished ubiquitination of YAP by ZNF213. HEK293 cells were transfected with indicated vectors for ubiquitination assays. The poly‐ubiquitinated YAP was detected through the analysis of western blotting
4. DISCUSSION
In our study, we identified one zinc finger protein member ZNF213, which was elevated in breast cancer, but related to good prognosis in TNBC patients, which correlated with YAP protein and Hippo target gene negativity in TNBC samples. ZNF213 inhibited YAP signaling and TNBC cell invasion, possibly via regulating YAP protein K48‐linked ubiquitination and protein degradation (Figure 10). Our study explored an interesting regulator for Hippo/YAP signaling in TNBC invasion, which provided novel targets for TNBC therapeutics.
FIGURE 10.
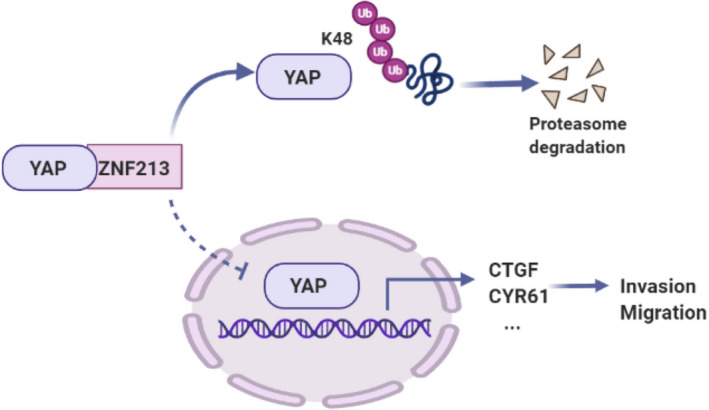
The hypothetical model for ZNF213 regulating Hippo/YAP signaling in triple negative breast carcinoma. ZNF213 protein associated with YAP and promoted YAP degradation via inducing YAP K48‐linked poly‐ubiquitination, which inhibited the activation of Hippo/YAP axis and the invasion and migration of triple negative breast cancer cells
Several factors could contribute to the carcinogenesis of breast cancer, such as loss of function of tumor suppressors or gain of function of oncogenes, which lead to uncontrolled proliferation and cancer metastasis. 22 , 23 , 24 Several studies showed the aberrant Hippo pathway could promote tumor progression since its important roles in cell proliferation and organ size control. 25 In breast cancer, the genomic amplification of YAP was observed in breast cancer, which might indicate enhanced transcription of Hippo signaling target genes, such as CTGF and CYR61. 26 However, YAP was elevated in TNBC, but decreased in ER alpha positive breast cancer, which might indicate different activation status among subtype of breast cancers. In TNBC, YAP acted as a transcriptional cofactor and facilitated signaling transduction of several transcriptional factors, including TEADs and RUNX. 27 Besides, one CHIP‐sequence analysis showed that Hippo/YAP axis could synchronize with AP1 transcription factors in TNBC cells, which subsequently promoted cell proliferation, invasion and anti‐apoptosis. 28 Based on the importance of Hippo signaling in breast cancer, targeting YAP protein could be a promising strategy in treating triple negative breast cancers.
YAP protein played center role in controlling Hippo signaling activity, which was identified to contain three functional domains: TEAD interaction domain, WW domain and transcriptional activation domain. The TEAD domain was the effector, which mediated the associations with several transcriptional factors. 29 , 30 , 31 Thus, the blockage of YAP‐TEAD interaction was a plausible way for inhibition Hippo/YAP axis. However, the structure study showed the extensive interactive interface between YAP and TEAD, which became obstacles in targeting YAP‐TEAD interaction. 32 Thus, our research team proposed to target YAP protein by modulating protein stability. Besides the phosphorylation control by several serine/threonine kinases, such as MST1/2 and LATS1/2, which controlled YAP phosphorylation (S61, S109, S127 and S381) and degradation, quite a few studies have shown the importance of YAP protein ubiquitination and degradation. 33 We, and others identified several ubiquitin ligases or modulators, such as FBW7, SCFb‐TRCP and RNF187, which induced YAP protein K48‐linked poly‐ubiquitination and degradation. 34 , 35 , 36 Here, we showed ZNF213 as a new modulator for Hippo/YAP signaling. ZNF213, which was negatively correlated with YAP protein and Hippo target genes in TNBC samples, could inhibit TNBC cell progression possibly via promoting YAP protein poly‐ubiquitination and degradation at multiple lysine sites. Such finding provided novel knowledge in understanding Hippo signaling in TNBC, but also promising targets to Hippo/YAP axis blockage in TNBC treatments.
ZNF213 belonged to zinc finger domain family member, which contained around fifty amino acids. 15 Zinc finger domains acted to mediate protein‐protein interactions. A few studies showed that ZNF protein could directly bind to nuclear acids and regulate gene expression. 37 However, a few ZNF family members were also shown to modulate protein ubiquitination and degradation. 38 , 39 As one of the ZNF member, ZNF213 was firstly found to associate with DNA and regulate gene expression. However, our study showed that ZNF213 could interact with YAP protein and promote YAP degradation in TNBC cells, but not directly in gene regulation. This provided a novel insight in ZNF213 function in modulating Hippo signaling and breast cancer progression via post‐translational mechanism. ZNF213, as an adaptor protein, could be a novel target for TNBC therapeutics.
DISCLOSURE STATEMENT
Authors declare no conflicts of interest for this article.
ETHICAL APPROVAL
This study was reviewed and approved by the Ethical Board at Xinxiang Medical University. This usage of clinical samples was reviewed and approved by the Ethical Board at the Qilu Hospital of Shandong University with written informed consents from all the patients.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the members of Laboratory of Molecular Oncology in Xinxiang University for sharing valuable material and research support. We thank Qilu Hospital for the valuable cancer sample support.
Liu Y, Su P, Zhao W, et al. ZNF213 negatively controls triple negative breast cancer progression via Hippo/YAP signaling. Cancer Sci. 2021;112:2714–2727. 10.1111/cas.14916
Funding information
The project was supported from the National Science Foundation for Young Scientists of China (No. 81702725, Ting Zhuang); the Joint Fund of the National Natural Science Foundation of China (No.U1604190, Jian Zhu); Shandong Provincial National Natural Science Foundation (ZR2016HQ44, Peng Su); Key R&D programs in Shandong (2019GSF108229, Yinlu Ding); The National Natural Science Foundation of China (Grant No. U1804167, NO.81770721; NO.81570624, Qingsong Huang); Key Scientific and Technological Projects of Henan Province (Grant No. 202102310024, Qingsong Huang); Key Scientific Research Projects of Higher Education Institutions in Henan Province (Grant No.18A320004).
Contributor Information
Yinlu Ding, Email: dingyinlu@126.com.
Jian Zhu, Email: zhujian1204@yahoo.com.
Zhiguo Niu, Email: niuzhiguo@xxmu.edu.cn.
Ting Zhuang, Email: ting.zhuang@xxmu.edu.cn.
DATA AVAILABILITY STATEMENT
The public available data are in the supplementary materials.
REFERENCES
- 1. Vaz‐Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node‐negative breast cancer: a multi‐institutional study. J Clin Oncol. 2014;32(20):2142‐2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747‐752. [DOI] [PubMed] [Google Scholar]
- 4. Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsang JYS, Tse GM. Molecular classification of breast cancer. Adv Anatomic Pathol. 2020;27(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 6. Hao J, Hang Y, Hing D, Li Y, Li J, Zhao Z. The Role of Hippo signaling in cancer stem cells. Cell Physiol. 2014;29:66‐70. [DOI] [PubMed] [Google Scholar]
- 7. Snigdha K, Gangwani KS, Lapalikar GV, Singh A, Kango‐Singh M. Hippo Signaling in Cancer: Lessons From Drosophila Models. Front Cell Dev Biol. 2019;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14(3):377‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sudol M, Shields DC, Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin Cell Dev Biol. 2012;23(7):827‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246‐257. [DOI] [PubMed] [Google Scholar]
- 13. Basu‐Roy U, Bayin NS, Rattanakorn K, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ling HH, Kuo CC, Lin BX, Huang YH, Lin CW. Elevation of YAP promotes the epithelial‐mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp Cell Res. 2017;350(1):218‐225. [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Hamon M, Deng Z, et al. Identification and characterization of a zinc finger gene (ZNF213) from 16p13.3. Biochim Biophys Acta. 1999;1444(2):218‐230. [DOI] [PubMed] [Google Scholar]
- 16. Bomar MG, Pai MT, Tzeng SR, Li SS, Zhou P. Structure of the ubiquitin‐binding zinc finger domain of human DNA Y‐polymerase eta. EMBO Rep. 2007;8(3):247‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 18. Yang H, Yu N, Xu J, et al. SMURF1 facilitates estrogen receptor ɑ signaling in breast cancer cells. J Exp Clin Cancer Res. 2018;37(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang H, Yu S, Wang W, et al. SHARPIN facilitates p53 degradation in breast cancer cells. Neoplasia. 2017;19(2):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhuang T, Yu S, Zhang L, et al. SHARPIN stabilizes estrogen receptor α and promotes breast cancer cell proliferation. Oncotarget. 2017;8(44):77137‐77151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xue M, Zhang K, Mu K, et al. Regulation of estrogen signaling and breast cancer proliferation by an ubiquitin ligase TRIM56. Oncogenesis. 2019;8(5):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen CDK, Yi C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer. 2019;5(5):283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tufail R, Jorda M, Zhao W, Reis I, Nawaz Z. Loss of Yes‐associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131(3):743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X, Abdelrahman A, Vollmar B, Zechner D. The ambivalent function of YAP in apoptosis and cancer. Int J Mol Sci. 2018;19(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li YW, Xu J, Zhu GY, et al. Apigenin suppresses the stem cell‐like properties of triple‐negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018;4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu‐Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD‐YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies CC, Chakraborty A, Diefenbacher ME, Skehel M, Behrens A. Arginine methylation of the c‐Jun coactivator RACO‐1 is required for c‐Jun/AP‐1 activation. Embo j. 2013;32(11):1556‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen YA, Lu CY, Cheng TY, Pan SH, Chen HF, Chang NS. WW domain‐containing proteins YAP and TAZ in the hippo pathway as Key regulators in stemness maintenance, tissue homeostasis, and tumorigenesis. Front Oncol. 2019;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crawford JJ, Bronner SM, Zbieg JR. Hippo pathway inhibition by blocking the YAP/TAZ‐TEAD interface: a patent review. Expert Opin Ther Pat. 2018;28(12):867‐873. [DOI] [PubMed] [Google Scholar]
- 31. Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103(33):12405‐12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian W, Yu J, Tomchick DR, Pan D, Luo X. Structural and functional analysis of the YAP‐binding domain of human TEAD2. Proc Natl Acad Sci U S A. 2010;107(16):7293‐7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sugihara T, Werneburg NW, Hernandez MC, et al. YAP Tyrosine phosphorylation and nuclear localization in cholangiocarcinoma cells are regulated by LCK and independent of LATS activity. Mol Cancer Res. 2018;16(10):1556‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26(4):455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Z, Kong Q, Su P, et al. Regulation of Hippo signaling and triple negative breast cancer progression by an ubiquitin ligase RNF187. Oncogenesis. 2020;9(3):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta‐TRCP). Genes Dev. 2010;24(1):72‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandevenne M, Jacques DA, Artuz C, et al. New insights into DNA recognition by zinc fingers revealed by structural analysis of the oncoprotein ZNF217. J Biol Chem. 2013;288(15):10616‐10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berrocal‐Lobo M, Stone S, Yang X, et al. ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin‐ and NADPH oxidase‐mediated defense responses. PLoS One. 2010;5(12):e14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu SH, Zhu S, Wang Y, et al. ECD promotes gastric cancer metastasis by blocking E3 ligase ZFP91‐mediated hnRNP F ubiquitination and degradation. Cell Death Dis. 2018;9(5):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The public available data are in the supplementary materials.


