Abstract
Adjuvant chemotherapy has reduced the risk of tumor recurrence and improved survival in patients with resected colorectal cancer. Potential utility of circulating tumor DNA (ctDNA) prior to and post surgery has been reported across various solid tumors. We initiated a new type of adaptive platform trials to evaluate the clinical benefits of ctDNA analysis and refine precision adjuvant therapy for resectable colorectal cancer, named CIRCULATE‐Japan including three clinical trials. The GALAXY study is a prospectively conducted large‐scale registry designed to monitor ctDNA for patients with clinical stage II to IV or recurrent colorectal cancer who can undergo complete surgical resection. The VEGA trial is a randomized phase III study designed to test whether postoperative surgery alone is noninferior to the standard therapy with capecitabine plus oxaliplatin for 3 months in patients with high‐risk stage II or low‐risk stage III colon cancer if ctDNA status is negative at week 4 after curative surgery in the GALAXY study. The ALTAIR trial is a double‐blind, phase III study designed to establish the superiority of trifluridine/tipiracil as compared with placebo in patients with resected colorectal cancer who show circulating tumor–positive status in the GALAXY study. Therefore, CIRCULATE‐Japan encompasses both “de‐escalation” and “escalation” trials for ctDNA‐negative and ‐positive patients, respectively, and helps to answer whether measuring ctDNA postoperatively has prognostic and/or predictive value. Our ctDNA‐guided adaptive platform trials will accelerate clinical development toward further precision oncology in the field of adjuvant therapy. Analysis of ctDNA status could be utilized as a predictor of risk stratification for recurrence and to monitor the effectiveness of adjuvant chemotherapy. ctDNA is a promising, noninvasive tumor biomarker that can aid in tumor monitoring throughout disease management.
Keywords: adaptive clinical trial design, adjuvant chemotherapy, circulating tumor DNA, colorectal cancer, trifluridine
CIRCULATE‐Japan encompasses both “de‐escalation” and “escalation” trials for circulating tumor DNA–negative and –positive patients, respectively, and helps to answer whether measuring circulating tumor DNA postoperatively has prognostic and/or predictive value. Our circulating tumor DNA–guided adaptive platform trials will accelerate clinical development toward further precision oncology in the field of adjuvant therapy.
![]()
1. INTRODUCTION
Circulating tumor DNA (ctDNA) is a promising, noninvasive tumor biomarker that can aid in tumor monitoring throughout disease management. Results from GOZILA, a large‐scale nationwide registry for comprehensive ctDNA sequencing of metastatic colorectal cancer (mCRC), reinforced the relevance of matching targetable oncogenic drivers to the appropriate targeted therapy for individual patients. Thus, ctDNA sequencing can potentially accelerate precision treatment of cancer. 1
The monitoring of ctDNA levels in the blood has shown to accurately detect molecular residual disease (MRD) and aid in measuring therapeutic effects after curative treatment. Signatera™ (Natera, Inc) is a novel, patient‐specific, custom‐built ctDNA monitoring assay for MRD detection (bespoke, mPCR‐NGS) that tracks 16 patient‐specific somatic single‐nucleotide variants in the patient's plasma, according to the variants identified via whole‐exome sequencing of the tumor tissue. This assay has shown >95% sensitivity at 0.01% variant allele frequency with high specificity. 2 Among 122 patients with stages I to III CRC, ctDNA was preoperatively detectable in 108 (88.5%). After definitive treatment, longitudinal ctDNA analysis identified 14 (87.5%) of 16 relapses. Furthermore, at postoperative day 30, ctDNA‐positive patients are significantly more likely to relapse than ctDNA‐negative patients (hazard ratio, 7.2; 95% confidence interval, 2.7‐19.0; P < .001), regardless of stage. In addition, serial ctDNA analyses revealed disease recurrence up to 16.5 months ahead of standard‐of‐care radiologic imaging (mean, 8.7 months). 3
We launched a large platform, enrolling patients with resectable CRC to evaluate the clinical utility of ctDNA analysis, named the CIRCULATE‐Japan project (Figure 1). Here, we provide an overview of CIRCULATE‐Japan, composed of one observational study and two randomized phase III trials. This project aims to detect MRD and measure treatment responsiveness in resectable CRC using ctDNA testing. Ultimately, CIRCULATE‐Japan aims to use ctDNA to guide the administration of more precise adjuvant therapy treatment regimens in patients.
FIGURE 1.
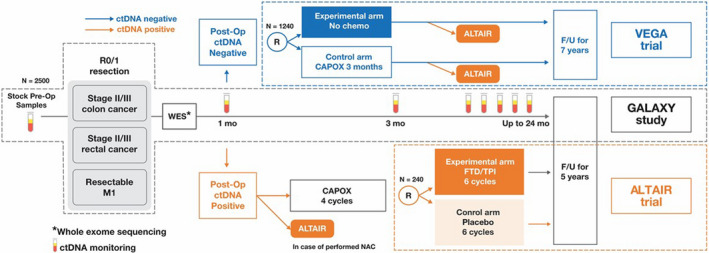
CIRCULATE‐Japan overview. ctDNA, circulating tumor DNA; F/U, follow up; FTD/TPI, trifluridine/tipiracil; mo, month; NAC, neoadjuvant chemotherapy; Op, operative; WES, whole‐exome sequencing
2. GALAXY STUDY
The GALAXY study is a prospectively conducted large‐scale nationwide registry designed to monitor ctDNA status for patients with clinical stage II to IV CRC who can undergo complete surgical resection. Key eligibility criteria are shown in Table 1. A personalized, tumor‐informed ctDNA assay from Natera, Inc, (bespoke, mPCR‐NGS), is used in this study. The blood samples will be collected before surgery and 4, 12, 24, 36, 48, 72, and 96 weeks after surgery. Computed tomography (CT) will be performed every 6 months after surgery for 7 years. Investigators will receive the results of ctDNA assay in a timely manner and, based on ctDNA status, can consider patients for enrollment into the VEGA or ALTAIR trials. Residual blood and frozen and formalin‐fixed tissue samples will be collected for further analyses, including RNA sequencing. A total of 2500 patients will be enrolled.
TABLE 1.
Eligibility criteria of the GALAXY trial
| Inclusion criteria |
|
| Exclusion criteria |
*Patients with clinical stage Tis or T1a colorectal cancer judged to be cured by local treatment may be included in this study
*However, patients with a relapse‐free survival period of 5 y or longer or patients with skin basal cell or spinocellular cell carcinoma which has been considered cured by local treatment, superficial bladder cancer, cervical cancer, carcinoma in situ (intraepithelial cancer) that can be treated endoscopically, lesions equivalent to intramucosal cancer, or nonmetastatic prostate cancer that does not require systemic treatment may be enrolled
*Patients with positive SARS‐CoV‐2 PCR or suspected COVID‐19 based on clinical symptoms; patients with confirmed negative SARS‐CoV‐2 PCR or other tests and no symptoms of COVID‐19 may be included in this study. However, if the physician deems that the patients will affect the evaluation of this study, the patients are ineligible (COVID‐19 testing is not required)
|
3. VEGA TRIAL
The VEGA trial is a randomized phase III study designed to test whether postoperative surgery alone is noninferior to the standard CAPOX therapy. Key eligibility criteria are (a) primary tumor location in the colon; (b) R0 resection performed with colectomy with D2 or D3 lymph node dissection, (c) high‐risk stage II or low‐risk stage III (T1‐3 and N1) colon cancer, and (d) ctDNA‐negative status at week 4 after surgery in the GALAXY study (Table 2). Patients are randomly assigned in a 1:1 ratio to either undergo surgery alone (observational group) or receive CAPOX therapy for 3 months (control group: 1‐14 days of capecitabine, 2000 mg/m2/d and oxaliplatin, 130 mg/m2/d once every 3 weeks). Randomization is stratified by age (<70 vs ≥70 years), stage (high‐risk stage II vs low‐risk stage III), primary tumor location (right‐sided vs left‐sided vs rectosigmoid colon), and RAS status (mutant vs wild type). The primary endpoint is disease free survival (DFS). Key secondary endpoints include time to treatment failure, overall survival, adverse events, relative dose intensity, and ctDNA status at each timepoint. Contrast medium–enhanced CT is performed once every 6 months for up to 7 years after enrollment.
TABLE 2.
Eligibility criteria of the VEGA trial
| Inclusion criteria |
*Includes the rectosigmoid part defined in the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, Ninth Edition
*N1c (UICC TNM Classification, 8th Edition) is also considered to be eligible (tumor deposits, or satellite nodules, are seen in the adjacent soft tissues of the colon or rectum without subserosal layer or peritoneal coat, but no regional lymph node metastasis). (a) T4 (SE/SI/AI), (b) Intestinal tract obstruction (clinical), (c) Intestinal tract perforation/penetration (clinical), (d) <12 dissected lymph nodes, (e) Poorly differentiated adenocarcinoma, signet‐ring cell carcinoma, or mucinous carcinoma, (f) Positive for lymphatic invasion, venous invasion, or neuroinvasion
*The results of ctDNA testing are based on the test results in the GALAXY study (UMIN000039205)
|
| Exclusion criteria |
*Men and women who agreed to use contraception during and up to 30 d after the treatment with CAPOX and understand the risks with pregnancy may be enrolled
|
Abbreviations: ctDNA, circulating tumor DNA; DPD, dihydoropyrimidine dehydrogenase.
The 3‐year DFS rate among standard CAPOX patients is assumed to be 85%. However, the reported DFS hazard ratio for postoperative ctDNA‐positive vs ctDNA‐negative patients is 7.2 4 , 5 , and the ctDNA‐positive rate is assumed to be 10%. Based on these previous data, we assume that the 3‐year DFS rate among ctDNA‐negative patients will be 91%. With an acceptable 3% decrease in 3‐year DFS (ie, from 91% to 88%) associated with switching to no chemotherapy, which corresponds to a noninferiority margin of 1.355, a total of 1240 (620 per arm) will provide a statistical power of 70% to test the noninferiority hypothesis at a one‐sided significance level of 10%, with enrollment and follow‐up periods of 2 and 3 years, respectively. This trial has been registered in the Japan Registry of Clinical Trials (jRCT1031200006).
4. ALTAIR TRIAL
The ALTAIR trial is a randomized, double‐blind, phase III study designed to establish the superiority of trifluridine/tipiracil (FTD/TPI) as compared with placebo in patients with CRC who show ctDNA‐positive status with the Signatera® assay at any time after curative resection for up to 2 years after surgery. Key eligibility criteria are (a) having undergone radical resection of primary and/or metastatic tumors, (b) a history of standard adjuvant chemotherapy, (c) positive ctDNA status within the previous 3 months at any time postoperatively, and (d) no obvious relapse confirmed by chest, abdominal, and pelvic CT scans (Table 3). Patients will be randomly assigned in a 1:1 ratio to receive either 6 months of oral FTD/TPI (35 mg/m2 twice daily on days 1‐5 and days 8‐12 in a 28‐day cycle) or a matching course of placebo. Randomization is stratified by age (<70 vs ≥70 years), stage (stage II or lower vs stage III vs stage IV or M1), primary tumor location (right‐sided vs left‐sided colon vs rectum), ctDNA status at 1 month (positive vs negative or unmeasurable), and institution. The primary endpoint is DFS. Key secondary endpoints include rate of conversion from positive to negative ctDNA status, overall survival, adverse events, and quality of life.
TABLE 3.
Eligibility criteria of the ALTAIR trial
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviations: CT, computed tomography; ctDNA, circulating tumor DNA; FTD/TPI, trifluridine/tipiracil.
The mean time from ctDNA‐positive status to detectable recurrence on CT has been reported to be 8.7 months. 3 Based on the data, we assume that the median DFS in the placebo group will be approximately 8 months. A total of 240 patients (120 per arm) will provide 80% power to detect an expected DFS hazard ratio of 0.667 at two‐sided significance level of .05, with an enrollment period of 2 years and a follow‐up period of 1 year. This trial has been registered in the Japan Registry of Clinical Trials (JapicCTI‐205363) and at Clinicaltrials.gov (NCT04457297).
5. DISCUSSION
The CIRCULATE‐Japan study provides multilayer testing platforms, comprising a large‐scale patient‐screening registry (GALAXY) followed by two ctDNA‐guided phase III trials (VEGA and ALTAIR), which aim to refine adjuvant therapy in patients with resectable CRC. In the GALAXY study, ctDNA testing is performed for MRD detection. Patients with stage II or III colon cancer and negative MRD status 4 weeks after resection are enrolled in the VEGA study, whereas patients with positive MRD status at any time after standard adjuvant therapy are enrolled in the ALTAIR study. CIRCULATE‐Japan will thus encompass both “de‐escalation” and “escalation” trials for ctDNA‐negative and ‐positive patients, respectively, and help to answer whether measuring ctDNA postoperatively has prognostic and/or predictive value in patients with resectable CRC.
The GALAXY trial also aims to generate a large dataset with high‐quality clinical data and comprehensive genomic profiling (whole‐exome sequencing) of resected tumor tissue. Real‐world evidence based on high‐quality registries and longitudinal health care databases outside of randomized control trials (RCTs) has recently been used as an alternative to RCTs for regulatory decision making, especially for a biomarker‐guided therapy, in a small population. 6 The data collected in the GALAXY study could be used as a reference, especially in new molecularly stratified treatments and/or to further investigate the MRD‐positive space.
The clinical question of the VEGA trial is whether or not to eliminate an immediate adjuvant chemotherapy for patients who are less likely to benefit from it. If the noninferiority of surgery alone against chemotherapy is proven, it will become the new standard of care in patients with MRD‐negative status 4 weeks after surgery for high‐risk stage II or low‐risk stage III colon cancer. This would represent a substantial treatment paradigm shift. The key objective of the VEGA trial is to provide individual patient data into a multinational collaborative project, called “Circulate IDEA,” which we will plan to launch to compare surgery alone vs adjuvant CAPOX in this population. We will design the Circulate IDEA to prospectively combine and analyze data from several trials as in the original IDEA Collaboration and to provide more than 80% of statistical power to test noninferiority of surgery alone against adjuvant CAPOX at a one‐sided significance level of 2.5%.
The aim of the ALTAIR trial is to establish the clinical significance of early intervention in patients with MRD at an early stage by monitoring ctDNA status during the surveillance period. FTD/TPI exhibits antitumor effects against 5‐fluorouracil–resistant tumors that are similar to those exerted in 5‐fluorouracil–sensitive tumors and has demonstrated a survival benefit in chemotherapy‐refractory mCRC even when disease has been refractory to 5‐fluorouracil–containing regimens. 7 Thus, we expect FTD/TPI to have antitumor effect on any existing MRD, even on tumors refractory to standard adjuvant therapy, including 5‐fluorouracil. This trial will be of great value because there is no confirmative prospective trial developing therapy for resected CRC patients with ctDNA‐positive status.
In summary, using a ctDNA assay that has high sensitivity and specificity for detecting MRD is most likely to enable suitable patients to receive appropriate adjuvant therapy. Our ctDNA‐guided adaptive platform trials will accelerate clinical development toward further precision oncology in the field of adjuvant therapy. In addition, the resulting CIRCULATE‐Japan database, consisting of multiomics data, ctDNA results, and clinical outcomes, will serve as a reference in further development of adjuvant therapy but also contribute to the understanding of the nature of cancer itself or through international harmonization with other data platforms.
DISCLOSURE
Alexey Aleshin, Paul R Billings, Matthew Rabinowitz are from Natera Inc, San Carlos, CA, USA. Hiroya Taniguchi received lecture fees from Taiho and Chugai. Yoshiaki Nakamura received research funds from Taiho and Chugai. Eiji Oki received lecture fees from Taiho and Chugai. Daisuke Kotani received lecture fees from Taiho and Chugai. Takeshi Kato received lecture fees from Taiho and Chugai. Masaki Mori received research funds from Taiho and Chugai, and received lecture fee from Taiho and Chugai. Takayuki Yoshino received lecture fees from Taiho and Chugai, and research funds from Taiho and Chugai.
ACKNOWLEDGMENTS
CIRCULATE‐Japan receives financial supports from the Japan Agency for Medical Research and Development (AMED; Grant Number JP19ck0106447) and from Taiho Pharmaceutical Co., Ltd., through Alpha‐A Inc. Investigational drugs, FTD/TPI, and placebo were provided by Taiho Pharmaceutical Co., Ltd. The funders had no role in the study design; in data collection, analysis, or interpretation; or in the writing of the report.
Taniguchi H, Nakamura Y, Kotani D, et al. CIRCULATE‐Japan: Circulating tumor DNA–guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112:2915–2920. 10.1111/cas.14926
[Correction added on 2 July 2021, after first online publication: the surname of the eleventh author has been corrected from ‘Bllings’ to ‘Billings’.]
REFERENCES
- 1. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM‐Japan GI‐SCREEN and GOZILA studies. Nat Med. 2020;26:1859‐1864. [DOI] [PubMed] [Google Scholar]
- 2. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25:4255‐4263. [DOI] [PubMed] [Google Scholar]
- 3. Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell‐free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5:1124‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshino T, Yamanaka T, Oki E, et al. Efficacy and long‐term peripheral sensory neuropathy of 3 vs 6 months of oxaliplatin‐based adjuvant chemotherapy for colon cancer: the ACHIEVE phase 3 randomized clinical trial. JAMA Oncol. 2019;5:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khozin S, Blumenthal GM, Pazdur R. Real‐world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109:djx187. [DOI] [PubMed] [Google Scholar]
- 7. Mayer RJ, Van Cutsem E, Falcone A, et al. RECOURSE Study Group: randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909‐1919. [DOI] [PubMed] [Google Scholar]


